Abstract
BACKGROUND AND PURPOSE:
Children with new-onset seizures may have antecedent neurobiologic alterations that predispose them to developing seizures. Our aim was to evaluate hippocampal and thalamic volumes and lobar cortical thickness of children with new-onset seizures.
MATERIALS AND METHODS:
Twenty-nine children with new-onset seizures and normal MR imaging findings were recruited. Ten patients had generalized seizures, 19 had partial seizures, and 15 were on antiepileptic medications. Twenty-three age-matched healthy controls were also recruited. Hippocampal and thalamic volumes and lobar cortical thickness, including frontal, medial temporal, lateral temporal, parietal, cingulate, and occipital cortical thickness, were assessed by using volumetric T1-weighted imaging and were compared between patients and controls.
RESULTS:
There were no significant differences in hippocampal and thalamic volumes of patients with new-onset seizures, including the subgroups with generalized and partial seizures and those on and off antiepileptic medications, compared with controls (P > .01). There was significant reduction in cortical thickness in right cingulate (P = .004), right medial temporal (P = .006), and left frontal (P = .007) cortices in patients with new-onset seizures. Patients with generalized seizures did not demonstrate a significant reduction in cortical thickness (P > .01). Patients with partial seizures demonstrated a significant reduction in cortical thickness in the right frontal (P = .008), right parietal (P = .003), and left frontal (P = .007) cortices. There were no significant differences in cortical thickness among patients on or off antiepileptic medications (P > .01).
CONCLUSIONS:
We found reduced cortical thickness in children with new-onset seizures. Further studies are necessary to elucidate the neurobiologic relevance of these structural changes.
Patients with chronic localization-related epilepsy, including adults and children, have demonstrated structural abnormalities, including cortical thickness and volume reduction and reduced hippocampal volume.1–7 Volumetric abnormalities have been reported in neuronal regions involved in the generation and propagation of seizures, including the hippocampus,8–11 amygdala,12 entorhinal cortex,13 thalamus,14 and also extratemporal regions15,16 in patients with chronic temporal lobe epilepsy. Children with idiopathic generalized epilepsies have also shown distributed patterns of abnormality affecting the thalamus and frontal lobe.17–21 It is not known whether the structural alterations developed during a vulnerable phase of cerebral development or are the cumulative effects of recurrent seizures22 or are a combination of these factors acting synergistically.11 To determine whether antecedent neurobiologic alterations are present, assessment could be performed in a population of patients with new-onset seizures, which would exclude the confounding effects of recurrent seizures and long-term antiepileptic medications on the brain.
Children with new-onset seizures or new-onset epilepsy are known to have neuropsychological deficits and academic underachievement,23–26 even when they are intellectually healthy. It is possible that there is an antecedent neurobiologic alteration that predisposes to both seizures and neuropsychological deficits. Volumetric imaging of the brain has been used to quantify total and lobar gray and white matter volumes in children with new-onset epilepsy but did not identify overall differences in total cerebral or lobar gray and white matter volumes.24 It is possible that voxel-based morphometry of total and lobar volumes is not sufficiently sensitive to assess subtle changes in brain structures. Alternatively, other structures such as the hippocampi or thalami could be more susceptible to the neurobiologic alterations and are, therefore, more likely to manifest structural changes. In this study, we evaluated the hippocampal and thalamic volumes and lobar cortical thickness of the cerebral hemispheres of children with new-onset seizures. Our hypothesis was that antecedent structural alterations, including changes in cortical thickness and other gray matter structures, were present in children with new-onset seizures, which predisposed the patients to seizures.
Materials and Methods
This study had the approval of the research ethics board. We retrospectively recruited children between 5 and 18 years of age with new-onset seizures who had undergone an MR imaging from 2008 to 2010. The exclusion criteria were children with febrile seizures and those with a mass or focal or diffuse abnormality on MR imaging as assessed by the pediatric neuroradiologist. Twenty-three consecutive children with unprovoked seizures and normal MR imaging findings were recruited. The type of seizures, either focal or generalized seizures, was obtained from the MR imaging requisitions and patient charts and was available in the EEG records. We also noted whether the patients were on antiepileptic medications. Twenty-three healthy age-matched controls with no neurologic or psychiatric disorders who participated in another epilepsy study were also included in this study. All 23 controls had normal MR imaging findings.
MR Imaging and Image Processing
MR imaging was performed on 3T scanner (Achieva, Philips Medical Systems, Best, the Netherlands) by using an 8-channel phased-array head coil in patients and controls. The imaging in patients consisted of axial and coronal FLAIR (TR/TE = 10,000/140 ms, section thickness = 3 mm, FOV = 22 cm, matrix = 316 × 290), T2 and PD (TR/TE = 4200/80/40 ms, section thickness = 3 mm, FOV = 22 cm, matrix = 400 × 272), and volumetric 3D T1 (TR/TE = 4.9/2.3 ms, section thickness = 1 mm, FOV = 22 cm, matrix = 220 × 220). The imaging in controls included axial FLAIR (TR/TE = 10,000/140 ms, section thickness = 3 mm, FOV = 22 cm, matrix = 316 × 290), T2 and PD (TR/TE = 4200/80/40 ms, section thickness = 3 mm, FOV = 22 cm, matrix = 400 × 272), and volumetric 3D T1 (TR/TE = 4.9/2.3 ms, section thickness = 1 mm, FOV = 22 cm, matrix = 220 × 220).
FreeSurfer (version 3.05, https://surfer.nmr.mgh.harvard.edu) was used for hippocampal and thalamic volume assessment, as well as cortical surface reconstruction and cortical thickness estimation of the patients and controls.27–34 Nonuniform intensity-correction was performed. Nonuniform nonparametric intensity normalization was applied. The data were skull-stripped and linear and nonlinear normalized to the Montreal Neurological Institute 305 atlas within FreeSurfer. Segmentation of the white matter was obtained by using a connected-components algorithm. Subcortical structures, including the basal ganglia, thalamus, amygdala, hippocampus, and the ventricles were labeled by using a probabilistic atlas and Bayesian classification rule for label assignment. The hippocampal and thalamic volumes were obtained.
To assess the cortical thickness, we divided the brain into 2 hemispheres and filled the white matter region and ventricles to obtain a single white matter volume for each hemisphere, which was then covered with a polygonal tessellation and smoothed to reduce metric distortions. The obtained surface was inflated and topologic defects were automatically corrected. Subsequently, the gray-white matter boundary was reconstructed by segmenting all white matter voxels in the MR imaging, and the resulting white matter surface was refined to obtain submillimeter accuracy in delineating the gray-white matter surface. This surface was then outward deformed to identify the gray-CSF boundary. The cortical thickness at each vertex across the cortical mantle was defined by calculating the average of the following: 1) the shortest distance between the gray-white boundary and the gray-CSF boundary, and 2) the shortest distance between the gray-CSF boundary and the gray-white boundary at each vertex on the tessellated surface. Thickness measures were then mapped to the inflated surface of each brain reconstruction, allowing optimal visualization in both sulcal and gyral regions across the entire neocortex without being obscured by cortical folding. Sulcal and gyral features across individual subjects were aligned by morphing each subject's brain to an average spheric representation by using a nonrigid high-resolution surface based an averaging method that allowed accurate matching of cortical locations among subjects while minimizing metric distortion.
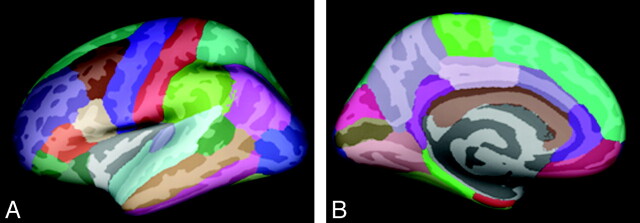
The data were then smoothed on the tessellated surface by using a 10-mm full width half maximum Gaussian kernel to improve the signal intensity–to-noise ratio. An automated parcellation technique was used to subdivide each hemisphere into 34 gyral labels (11 frontal, 4 medial temporal, 5 lateral temporal, 5 parietal, 4 occipital, and 5 cingulate) (Fig 1).35 Six lobar regions of interest were subsequently defined in each hemisphere: frontal, medial temporal, lateral temporal, parietal, cingulate, and occipital.36 The mean thickness of each lobe was obtained by using the weighted average of the thickness within each gyral-based region of interest—that is, mean regional thickness multiplied by the number of vertices for that region and divided by the total number of vertices.
Fig 1.
Regional labeling of neocortical structures as provided by FreeSurfer35 on lateral (A) and medial (B) views of the inflated brain.
Statistical Analysis
Statistical analysis was performed by using the Statistical Package for the Social Sciences, Version 15 (SPSS, Chicago, Illinois). The hippocampal volume, thalamic volume, and lobar cortical thickness of patients with new-onset seizures and controls were compared by using the Mann-Whitney U test. The hippocampal volume, thalamic volume, and lobar cortical thickness of subgroups of patients with generalized and partial seizures and those who were on or off antiepileptic medications were compared with those in controls by using analysis of variance and subsequently the Mann-Whitney U test. To minimize the likelihood of type I error, a P value of <.01 was considered statistically significant.
Results
Subjects
Twenty-nine patients with new-onset seizures and normal MR imaging findings (17 males and 12 females; mean age, 9.2 ± 2.2 years) were included. Ten patients had generalized seizures, including tonic clonic, absence, and myoclonic seizures, and 19 had partial seizures. Fifteen patients were on antiepileptic medications at the time of the MR imaging, 8 patients were on carbamazepine, 6 patients were on valproic acid, and 1 was on oxcarbazepine. Thirteen patients were not on antiepileptic medications at the time of MR imaging; in 1 patient, the medication status could not be obtained. The time interval between seizure onset and MR imaging varied from 9 days to 1.1 years, with a mean of 5.8 months. Twenty-three healthy controls with normal MR imaging findings (14 males and 9 females; mean age, 10.0 ± 2.7 years) were included. There was no significant difference in the age of patients and controls.
Hippocampal and Thalamic Volumes
There were no significant differences in hippocampal and thalamic volumes of patients with new-onset seizures and controls (P > .01 for all) (On-line Table).
Subgroup analysis of patients with generalized or partial seizures also did not show any significant differences in hippocampal and thalamic volumes of both patient groups compared with controls (P > .01 for all) or between patients with generalized and those with partial seizures (P > .01 for all).
Subgroup analysis among patients on or off antiepileptic medications did not show any significant differences in hippocampal and thalamic volumes of both patient groups compared with controls (P > .01 for all) or between the 2 groups of patients on and off antiepileptic medications (P > .01 for all).
Lobar Cortical Thickness
Patients with new-onset seizures demonstrated a significant reduction in cortical thickness in the right cingulate (P = .004), medial temporal (P = .006), and left frontal (P = .007) cortices and a tendency to reduced cortical thickness in the right frontal (P = .015), parietal (P = .018), and left cingulate (P = .054) cortices (On-line Table).
Subgroup analysis of patients with generalized seizures demonstrated a tendency toward reduced cortical thickness in the right cingulate (P = .028) and medial temporal (P = .042) cortices compared with controls. Subgroup analysis of patients with partial seizures demonstrated significant reduction in cortical thickness in the right frontal (P = .008), right parietal (P = .003), and left frontal (P = .007) cortices, and a tendency toward reduced cortical thickness in the right cingulate (P = .010) and medial temporal (P = .013) cortices. There were no significant differences in lobar cortical thicknesses of patients with generalized seizures and those with partial seizures (P > .01 for all).
Subgroup analysis of patient who were on antiepileptic medications showed a tendency toward reduced cortical thickness in the right cingulate (P = .035), right medial temporal (P = .041), right parietal (P = .044), and left frontal (P = .030) cortices compared with controls. Patients who were not on antiepileptic medications showed a tendency toward reduced cortical thickness in the right frontal (P = .031), right cingulate (P = .012), right medial temporal (P = .020), and left frontal (P = .034) cortices. There were no significant differences in the cortical thickness of patients on antiepileptic medications and those not on antiepileptic medications (P > .01 for all).
Discussion
We found that children with new-onset seizures and normal routine MR imaging findings demonstrated significant reduction in cortical thickness in the right cingulate, medial temporal, and left frontal cortices. However, we did not detect a significant alteration in thalamic and hippocampal volumes. The significance of the reduction in cortical thickness in the cingulate, medial temporal, and frontal cortices remains to be elucidated. A previous study in children with new-onset epilepsy found impairment in several cognitive domains, including intelligence, executive function, psychomotor speed, and language.24 The reduction in cortical thickness in the frontal lobe could be related to impairment in executive function and psychomotor speed, and the reduced cortical thickness in the cingulate may reflect impairment in executive function.
Few studies have examined the structural consequences of predominantly adults with new-onset seizures.37,38 Liu et al37 evaluated the hippocampal, cerebellar, total brain, gray and white matter, and intracranial volumes and hippocampal T2 relaxation times in patients with new-onset seizures, and they found no significant change in brain volumes and hippocampal T2 relaxation times in patients compared with controls at baseline. The observed lack of alteration in total gray and white matter volumes and cerebral volume may be due to a variety of reasons. Volume loss may occur within specific brain regions rather than diffusely in the whole brain. Measurements using total brain and cerebral gray and white matter volumes may not be sufficiently sensitive to identify volumetric changes within specific regions of the brain. In a subsequent study by the same group, the authors found a reduction in hippocampal volume in a subgroup of patients with new-onset temporal lobe epilepsy.38 We also found no significant differences in hippocampal volume in children with new-onset seizures compared with controls, similar to findings in the earlier study by Liu et al.37 Although subgroup analysis was performed in patients with generalized and partial seizures and did not demonstrate a difference in hippocampal or thalamic volume, we did not distinguish the different subtypes of partial seizures due to the relatively small sample size. Liu et al included patients with normal structural imaging findings as well as those with lesions such as focal cortical dysplasia, cavernoma, and dysembryoplastic neuroepithelial tumor. We excluded patients with lesions due to potential for these lesions to affect cortical thickness measurements.
Hermann et al24 evaluated children with new-onset epilepsy and found no significant change in total cerebral gray and white matter volumes and also no significant change in frontal, parietal, temporal, and occipital volumes within 1 year of the diagnosis of epilepsy. The authors also found no significant differences in total gray and white matter and lobar volumes in patients with partial and generalized epilepsy. However, the subgroup with academic underachievement revealed significantly lower gray matter volumes in the parietal and occipital regions compared with controls and patients without academic problems. We have not distinguished patients with or without academic problems because such data were not available in this retrospective study. The reduction in cortical thickness in our patients may be related to differences in population characteristics or methodologic assessment of the cortex, in that thickness measures by using surface-based morphometry are more sensitive than volume measures by using voxel-based morphometry. Pulsipher et al39 also assessed pediatric patients with new-onset idiopathic generalized epilepsy and found a smaller right thalamic volume but no change in the left thalamic volume within 1 year of the diagnosis of epilepsy. They also found that the frontal gray matter volume was decreased in patients with new-onset idiopathic generalized epilepsy compared with controls. We have not found a reduction in frontal cortical thickness in patients with generalized seizures. Failure to identify such a reduction in may be related to the sample size.
One of the potential confounding factors of our study was that approximately half of the patients were on antiepileptic medications at the time of MR imaging. However, subgroup analysis results of those who were on antiepileptic medication were not significantly different compared with those who were not on antiepileptic medications. Another potential confounding factor was the time interval between seizure onset and MR imaging. This is partly related to wait times for a pediatric neurologist consult and also for MR imaging, in particular if the MR imaging had to be performed with the patient under general anesthesia. In the prospective studies by Hermann et al24 and Pulsipher et al,39 the time interval between the diagnosis of epilepsy and imaging was also within 1 year of diagnosis, similar to that in our study. Further study that evaluates structural changes in the brain within a shorter time interval between the diagnosis of new seizures or epilepsy and neuroimaging is needed to exclude any potential effects of these ongoing changes from as-yet-unknown predisposing factors.
We excluded children with a mass or focal or diffuse abnormalities on MR imaging as assessed by the pediatric neuroradiologist. However, it is possible that some patients with subtle focal cortical dysplasia were not identified and that some cases with focal cortical dysplasia may have had decreased cortical thickness, which confounded the results. We have minimized the likelihood of missing a lesion by performing the MR imaging on a 3T system with a high-resolution epilepsy protocol in all patients. We have analyzed children with generalized and partial seizures as a group due to the size of the study cohort, similar to the analysis in the study by Hermann et al24 and Tosun et al.40 Subsequently, subgroup analysis was performed in patients with generalized and partial seizures. Details of the specific seizure localization were not available in all patients with partial seizures because short EEG recordings were obtained in patients with new-onset seizures and none had prolonged video-EEG recordings. Also the EEG recordings were not available for review in some patients who were referred by external neurologists.
A longitudinal study of children with new-onset epilepsy has found differences in baseline gray and white matter volumes of patients compared with controls, suggesting that antecedent anomalies in brain development were present.40 In addition to changes in baseline brain volumes, the authors also found slowed white matter expansion and changes in gray matter volume in patients at 2-year follow-up. The image processing was done by using deformation-based morphometry, which is a more sensitive tool for detecting changes in brain volumes because it avoids the need for image segmentation. The authors identified changes in brain volumes in children with new-onset epilepsy40 that were not revealed by using voxel-based morphometry in an earlier study.24
We have assessed cortical thickness rather than cortical volume and have also identified changes in the cortex in children with new-onset seizures, similar to the assessment in the study by Tosun et al,40 again providing support that antecedent anomalies were present in these patients. Janssen et al41 evaluated cortical thickness, surface area, and cortical volume in adolescents with first-episode early-onset psychosis. They found more widespread areas of cortical thinning relative to volume reduction, attributed to changes in surface area, which counteracted volume changes. We have used surface-based morphometry to evaluate cortical thickness because this methodology is less influenced by individual gyral variations than traditional voxel-based morphometry. The FreeSurfer software allows assessment of not only the cortical thickness but also volumetric measurement of other gray matter structures, including the hippocampus and thalamus. However, one of the disadvantages of our method of analysis is that it does not include white matter measurements.
Conclusions
We found reduced cortical thickness in the right medial temporal, cingulate, and left frontal cortices but no significant differences in hippocampal and thalamic volumes in children with new-onset seizures. Structural changes in the gray matter suggest that antecedent developmental anomalies are present and may predispose the patients to seizures. Our findings indicate that cortical thickness is a sensitive measure of gray matter integrity and is compromised in children with new-onset seizures. Further studies are needed to verify the relation between gray matter integrity and neurocognitive function as well as to assess these changes longitudinally.
ABBREVIATIONS:
- EEG
electroencephalography
- PD
proton density
References
- 1. Lawson JA, Cook MJ, Bleasel AF, et al. Quantitative MRI in outpatient childhood epilepsy. Epilepsia 1997;38: 1289–93 [DOI] [PubMed] [Google Scholar]
- 2. Lawson JA, Nguyen W, Bleasel AF, et al. ILAE-defined epilepsy syndromes in children: correlation with quantitative MRI. Epilepsia 1998;39: 1345–49 [DOI] [PubMed] [Google Scholar]
- 3. Lawson JA, Vogrin S, Bleasel AF, et al. Predictors of hippocampal, cerebral, and cerebellar volume reduction in childhood epilepsy. Epilepsia 2000;41: 1540–45 [DOI] [PubMed] [Google Scholar]
- 4. Lawson JA, Vogrin S, Bleasel AF, et al. Cerebral and cerebellar volume reduction in children with intractable epilepsy. Epilepsia 2000;41: 1456–62 [DOI] [PubMed] [Google Scholar]
- 5. Lawson JA, Cook MJ, Vogrin S, et al. Clinical, EEG, and quantitative MRI differences in pediatric frontal and temporal lobe epilepsy. Neurology 2002;58: 723–29 [DOI] [PubMed] [Google Scholar]
- 6. Mueller SG, Laxer KD, Barakos J, et al. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage 2009;46: 353–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDonald CR, Ahmadi ME, Hagler DJ, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 2008;71: 1869–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jack CR, Jr, Sharbrough FW, Cascino GD, et al. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol 1992;31: 138–46 [DOI] [PubMed] [Google Scholar]
- 9. Quigg M, Bertram EH, Jackson T, et al. Volumetric magnetic resonance imaging evidence of bilateral hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsia 1997;38: 588–94 [DOI] [PubMed] [Google Scholar]
- 10. Woermann FG, Barker GJ, Birnie KD, et al. Regional changes in hippocampal T2 relaxation and volume: a quantitative magnetic resonance imaging study of hippocampal sclerosis. J Neurol Neurosurg Psychiatry 1998;65: 656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tasch E, Cendes F, Li LM, et al. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol 1999;45: 568–76 [DOI] [PubMed] [Google Scholar]
- 12. Kalviainen R, Salmenpera T, Partanen K, et al. MRI volumetry and T2 relaxometry of the amygdala in newly diagnosed and chronic temporal lobe epilepsy. Epilepsy Res 1997;28: 39–50 [DOI] [PubMed] [Google Scholar]
- 13. Bernasconi N, Bernasconi A, Andermann F, et al. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study. Neurology 1999;52: 1870–76 [DOI] [PubMed] [Google Scholar]
- 14. DeCarli C, Hatta J, Fazilat S, et al. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol 1998;43: 41–45 [DOI] [PubMed] [Google Scholar]
- 15. Marsh L, Morrell MJ, Shear PK, et al. Cortical and hippocampal volume deficits in temporal lobe epilepsy. Epilepsia 1997;38: 576–87 [DOI] [PubMed] [Google Scholar]
- 16. Sandok EK, O'Brien TJ, Jack CR, et al. Significance of cerebellar atrophy in intractable temporal lobe epilepsy: a quantitative MRI study. Epilepsia 2000;41: 1315–20 [DOI] [PubMed] [Google Scholar]
- 17. Betting LE, Mory SB, Li LM, et al. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage 2006;32: 498–502 [DOI] [PubMed] [Google Scholar]
- 18. Betting LE, Mory SB, Lopes-Cendes I, et al. MRI volumetry shows increased anterior thalamic volumes in patients with absence seizures. Epilepsy Behav 2006;8: 575–80 [DOI] [PubMed] [Google Scholar]
- 19. Betting LE, Mory SB, Lopes-Cendes I, et al. MRI reveals structural abnormalities in patients with idiopathic generalized epilepsy. Neurology 2006;67: 848–52 [DOI] [PubMed] [Google Scholar]
- 20. Pardoe H, Pell GS, Abbott DF, et al. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. Neuroimage 2008;42: 611–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pulsipher DT, Seidenberg M, Guidotti L, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia 2009;50: 1210–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pringle CE, Blume WT, Munoz DG, et al. Pathogenesis of mesial temporal sclerosis. Can J Neurol Sci 1993;20: 184–93 [DOI] [PubMed] [Google Scholar]
- 23. Fastenau PS, Johnson CS, Perkins SM, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology 2009;73: 526–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermann B, Jones J, Sheth R, et al. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain 2006;129: 2609–19 [DOI] [PubMed] [Google Scholar]
- 25. Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, et al. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”—a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics 2003;112: 1338–44 [DOI] [PubMed] [Google Scholar]
- 26. Berg AT, Smith SN, Frobish D, et al. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol 2005;47: 749–53 [DOI] [PubMed] [Google Scholar]
- 27. Fischl B, Sereno MI, Tootell RB, et al. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 1999;8: 272–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9: 195–207 [DOI] [PubMed] [Google Scholar]
- 29. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 2000;97: 11050–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 2001;20: 70–80 [DOI] [PubMed] [Google Scholar]
- 31. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33: 341–55 [DOI] [PubMed] [Google Scholar]
- 32. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14: 11–22 [DOI] [PubMed] [Google Scholar]
- 33. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9: 179–94 [DOI] [PubMed] [Google Scholar]
- 34. Segonne F, Grimson E, Fischl B. A genetic algorithm for the topology correction of cortical surfaces. Inf Process Med Imaging 2005;19: 393–405 [DOI] [PubMed] [Google Scholar]
- 35. Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31: 968–80 [DOI] [PubMed] [Google Scholar]
- 36. McDonald CR, Hagler DJ, Jr, Ahmadi ME, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia 2008;49: 794–803 [DOI] [PubMed] [Google Scholar]
- 37. Liu RS, Lemieux L, Sander JW, et al. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol 2002;52: 861, author reply 862 [DOI] [PubMed] [Google Scholar]
- 38. Liu RS, Lemieux L, Bell GS, et al. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia 2005;46: 1482–94 [DOI] [PubMed] [Google Scholar]
- 39. Pulsipher DT, Dabbs K, Tuchsherer V, et al. Thalamofrontal neurodevelopment in new-onset pediatric idiopathic generalized epilepsy. Neurology 2011;76: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tosun D, Dabbs K, Caplan R, et al. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain 2011;134 (Pt 4): 1003–14. Epub 2011 Mar 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Janssen J, Reig S, Aleman Y, et al. Gyral and sulcal cortical thinning in adolescents with first episode early-onset psychosis. Biol Psychiatry 2009;66: 1047–54 [DOI] [PubMed] [Google Scholar]