Because of the potential benefits of flat panel CT in assessing perfusion and the brain parenchyma, these investigators correlated studies with multisection and perfusion CT. All 4 techniques were used to study 16 patients with MCA occlusions posttreatment. There was a high correlation for CBV obtained with both flat panel and standard techniques. The authors concluded that this new flat panel CT application allows assessment of CBV in acute stroke patients. Their initial results indicated that these measurements may predict final infarct volume. The ability to assess this key parameter of cerebral perfusion within the angiographic suite may improve the management of these patients.
Abstract
BACKGROUND AND PURPOSE:
A new FPCT application offers the possibility of perfusion (FPCT CBV) and parenchymal (FPCT) imaging within the angiography suite. We tested the hypothesis that findings in FPCT CBV and FPCT would correlate with those obtained using MSCT and PCT.
MATERIALS AND METHODS:
In 16 patients with acute MCA occlusion, FPCT CBV was performed immediately posttreatment. The volume of tissue having abnormal CBV values was determined by FPCT CBV and PCT images. Stroke volume on follow-up MSCT was determined, CBV values in the effected parenchyma were measured, and FPCT images were reviewed.
RESULTS:
In 6 cases, we found a FPCT CBV value identical or higher (hyperemia) in comparison with the contralateral side. In 10 cases, we found CBV lesions with values lower (oligemia) than the contralateral brain tissue. We found a high correlation of CBV lesion volume on FPCT CBV images to stroke volume on follow-up MSCT (r = 0.9, P < .05) in the oligemia group. Absolute FPCT CBV and PCT CBV values were comparable and showed good correlation (r = 0.9, P < .05). In 8 patients, contrast medium extravasation was visible.
CONCLUSIONS:
The new FPCT application allows assessment of CBV in acute stroke patients. Our initial results indicate that these measurements may predict final infarct volume. The ability to assess this key parameter of cerebral perfusion within the angiographic suite may improve the management of these patients.
Flat panel detector–equipped angiography systems that allow acquisition of CT-like images (FPCT) are now widely available and are used in many institutions for different purposes.1–10 Although the temporal resolution of FPCT is inadequate for dynamic perfusion imaging equivalent to that provided by PCT, it has been shown that it is possible to measure the CBV and then display these values as a CBV map.11,12 Recent publications describe the first results in humans.13,14
This FPCT application is now commercially available (syngo Neuro PBV IR; Siemens, Erlangen, Germany). Here we report our initial results in a series of patients with acute ischemic stroke secondary to MCA occlusion. The hypothesis for this study was that, by using FPCT, it would be possible to measure CBV values, detect parenchymal hemorrhage, and/or or contrast medium extravasation after endovascular stroke treatment, and that these findings would correlate those using MSCT and PCT.
Materials and Methods
Under an institutionally approved protocol (ethics committee approval was obtained), 18 patients admitted for acute MCA occlusion between January and December 2010 were identified. Because of technically inadequate studies (motion artifact), 2 patients were excluded. In the other 16 patients, 10 women and 6 men, all imaging studies were suitable for evaluation. Mean age was 69.6 ± 12.4 years (On-line Table).
According to institutional guidelines, patients seen within 3 hours of symptom onset are routinely investigated by MSCT, including CT, CTA, and PCT. Patients seen beyond 3 hours after symptom onset are examined primarily by a multimodal stroke MR imaging protocol. If large vessel occlusion is identified, intravenous thrombolytic therapy is initiated and the patients are then transferred to the angiography suite for subsequent endovascular treatment.9,15 In our practice, FPCT is routinely used after all interventional procedures. In these 16 patients, the FPCT application syngo Neuro PBV IR was used, allowing the creation of both a FPCT CBV map and a FPCT. Within 24 hours of treatment follow-up, imaging including MSCT, CTA, and PCT was routinely performed.
Imaging and Treatment Protocol
Initial MSCT Imaging.
In 14 of the 16 patients, initial imaging was performed using either a 64-section CT (Somatom 64; Siemens [patients 4, 9, 11, 12]) or a 128-section CT (Somatom AS+ [patients 2, 3, 6–8, 10, 13–16]). First, we obtained a MSCT with sections of 4.8-mm thickness. Using the Somatom 64, PCT imaging was performed using a standard protocol, with section positioning at the level of the basal ganglia. Acquisition of 1 image per second over a period of 40 seconds was initiated, resulting in 3 sections with a thickness of 9.6 mm each. The Somatom AS+ provides the possibility of obtaining a perfusion dataset (section thickness 5 mm) in the z-axis of 96 mm. We included the entire hemispheres and accepted if parts of the cerebellum were not included. The perfusion imaging was started 10 seconds after injection of 40 mL contrast medium (Imeron 350; Bracco Imaging, Konstanz, Germany) at 6 mL/s, followed by 60 mL saline flush at 6 mL/s. Acquisition time was 46 seconds (80 kV, 200 mAs, time resolution 1.5 seconds). Semiautomated analysis of the PCT data was performed on a dedicated workstation (syngo MMWP, Siemens, software version VE36A) using standard perfusion CT software and algorithm (Ostergaard).
In 2 patients (patients 1 and 5), initial imaging was performed using MR imaging (Sonata; Siemens) and follow-up was done with CT (Somatom AS+). Briefly, the initial MR imaging was performed using standard MR imaging applications, including FLAIR, GRE, DWI, and PWI.
We used sequences proposed by the manufacturer with the following parameters:
1) FLAIR: 25 sections, section thickness 5 mm, distance factor 20%, TR 8430 ms, TE 109 ms, TI 2500 ms, scan time 2 minutes 33 seconds, flip angle 150 degrees.
2) DWI: 25 sections, section thickness 5 mm, distance factor 20%, TR 3800 ms, TE 84 ms, scan time 1 minute 25 seconds, 3 b-values (0, 500, 1000), EPI factor 128.
3) GRE: 25 sections, section thickness 5 mm, distance factor 20%, TR 858 ms, TE 26 ms, scan time 2 minutes 26 seconds, flip angle 20 degrees.
4) PWI: 25 sections, section thickness 5 mm, distance factor 20%, TR 2610 ms, TE 40 ms, scan time 2 minutes 17 seconds, flip angle 90 degrees, 50 measurements, EPI factor 128.
PWI was performed by scanning the entire brain after paramagnetic contrast material injection (Gadovist; Bayer Schering Pharma, Leverkusen, Germany). Postprocessing of the PWI data was performed using a dedicated workstation (syngo MMWP) using standard perfusion software to calculate the CBV maps.
Endovascular Treatment.
All patients were transferred to the angiography suite within 1 hour after initial imaging evaluation. Following recent publications, we attempted to avoid general anesthesia and investigate the patients under sedation.16,17 Revascularization was attempted via an intra-arterial approach using a reperfusion catheter (Penumbra Stroke System PSS; Penumbra, Alameda, California).9 Procedures were terminated if satisfactory recanalization was achieved (TIMI 2 or 3) or if the target vessel was still occluded after 60 minutes of intra-arterial intervention. Each series was performed using 5–6 mL of contrast material, meaning that with an average of 12 series (range 7 to 20), a mean volume of 60–72 mL was used.
FPCT.
Both the revascularization procedures and FPCT imaging were performed on a biplane or a monoplane flat panel detector angiographic system (Axiom Artis dBA/Axiom Artis ZeeGo; Siemens). All FPCT acquisitions were performed immediately after termination of endovascular treatment. As previously described, the FPCT acquisition consists of 2 rotations—an initial mask run followed by a second rotation after contrast medium injection (fill run).13,14 The following parameters were used: rotation time per run of 8 seconds, 70 kV, 616 × 480 matrix, projection on 30 × 40 cm flat panel size, 200° total angle, 0.5°/frame, 400 frames total, dose 0.36 μGy/frame. The effective patient dose of this program is 2.3 mSv (information from the manufacturer). Determination of CBV values and creation of a FPCT CBV map is only possible in a steady state of contrast medium in the brain parenchyma during the acquisition of the fill run.12 To recognize the steady state, we used the “bolus watching” method.13,14 We used 60 mL of contrast material (Imeron 350; Bracco Imaging), which was injected at a rate of 5 mL/s followed by 60 mL saline flush.
Postprocessing of FPCT CBV Imaging.
Postprocessing of the FPCT CBV acquisitions was performed using a dedicated workstation (syngo XWP, Siemens). The software is an improved version of the prototype software described previously.11–14,18 For review of the FPCT CBV maps, 20 sections with 6-mm thickness were reconstructed using MPR. To visualize the brain parenchyma, we used DynaCT software (Siemens) to reconstruct the mask run from the FPCT CBV acquisition.13 Reconstructions were performed using kernel type “HU,” image impression “smooth,” field of view 18 cm, and reconstruction mode “native mask.” Postprocessing resulted in a volume dataset with a batch of approximately 400 sections, 0.36-mm thickness, in a 512 × 512 matrix format. The dataset was then further processed as axial MPR reconstructions with 6-mm section thickness.
Follow-Up MSCT Imaging.
Follow-up MSCT examinations were performed in all patients 24 hours after treatment. The Somatom 64 was used in 4 (patients 4, 9, 11, 12), and the Somatom AS+ was used in 12 (patients 1–3, 5–8, 10, 13–16). PCT was performed in 11 patients (1–3, 5, 6, 9–11, 13, 14, 16).
Data Analysis
All imaging data were stored anonymously on a workstation and were reviewed by 3 experienced neuroradiologists. The reviewers were blinded to the clinical presentation of the patient and were not informed if they were evaluating initial or follow-up imaging. Reviewer 2 was not involved in any treatment procedure.
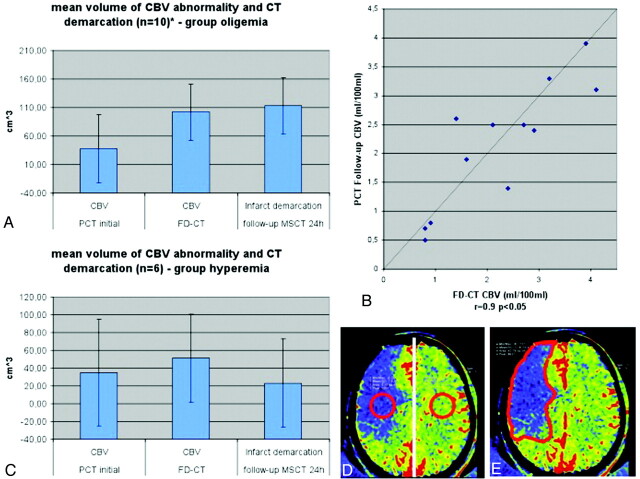
The first reviewer evaluated the initial imaging. The MSCT/MR images were assessed for hemorrhage or ischemic stroke. The CBV maps were assessed for the presence of qualitative abnormalities. Regarding the CBV maps, the threshold for manually outlining the abnormalities was set according to the color code purple to blue, corresponding to decreased (oligemia), and yellow to red, corresponding to increased (hyperemia) CBV. This method has been described before and has proved to be practicable under clinical conditions.19 Volume of CBV abnormalities was calculated using a previously described method.19,20 Briefly, the area of the CBV abnormality in each section was measured and this value was multiplied by the thickness of the section. The sum volume of the values for each section was then taken as the volume of the lesion (Fig 1E).
Fig 1.
In the oligemia group (A), CBV abnormality volume increased from the initial measurement to the FPCT CBV measurement performed immediately at the end of treatment. The FPCT CBV abnormality volume was nearly identical to the infarct volume, as determined on the 24-hour follow-up CT. Comparison of CBV values (B) of the oligemia group revealed a high correlation on FPCT and follow-up PCT. In the hyperemia group (C), the abnormality volume increased only slightly, but not significantly, from initial to posttreatment FPCT CBV measurement. Infarct volume was lower than FPCT CBV abnormality volume. Only 2 patients in this group of 6 patients presented with stroke demarcation. (D) The region of interest for measurement of the absolute CBV values is displayed. (E) The region of interest for measurement of the volume is displayed.
The second reviewer evaluated the imaging during the angiographic procedure (FPCT CBV map, FPCT, angiographic images). If a CBV abnormality was detected, measurement of CBV values within the abnormality and in the contralateral hemisphere was performed (Fig 1D). We used a standard tool of the workstation, applying a region of interest in the CBV abnormality and in a symmetrical area in the contralateral hemisphere. To compare the values, we calculated the difference in percent. The volumes of the abnormalities were also determined.19,20 The FPCT images were reviewed for hemorrhage or early signs of ischemic stroke.21 Angiographic results pre- and postendovascular treatment were recorded according to the TIMI classification.9,22
The third reviewer assessed follow-up images. The MSCT scans were reviewed for signs of hemorrhage, contrast extravasation, or ischemic stroke.21 If ischemic stroke was detected, the volume was calculated.19,20 Stroke areas were defined as hypoattenuated asymmetric regions.19,23 If PCT was available, measurement of absolute CBV values within the area of abnormality and in the contralateral hemisphere were performed.
Correlation coefficients of initial PCT CBV, and FPCT CBV abnormality volume after treatment, were calculated. Correlation coefficients of FPCT CBV abnormality volume and stroke volume on follow-up MSCT were also calculated. Correlation of the absolute CBV values measured on FPCT CBV and on control PCT was also completed.
Results
Initial Imaging
There were no hemorrhages observed in any of the patients. In 6 patients, there was no evidence of a CBV abnormality (patients 4, 7–9, 14, 16). In 10 patients, CBV areas with decreased CBV values were obvious. In 8 of these patients, the volumes of the abnormalities were calculated (patients 1–3, 5, 6, 10, 13, 15); in the other 2 (patients 11, 12), volumes could not be determined, as the abnormalities extended outside of the coverage provided by the scan.
FPCT and Angiographic Imaging
Review of the angiographic images revealed a pretreatment TIMI score of 0 in 15 patients, and of 1 in one patient. In 12 patients, treatment was successful (TIMI 2/3), and in 4 patients, recanalization was not achieved (TIMI 0/1). The FPCT images revealed hyperattenuated lesions in 8 patients (patients 1, 5, 10–12, 14–16). None of these showed mass effect and all were in treatment-related regions; therefore, they were considered to be secondary to contrast medium extravasation.9 Signs of ischemic stroke were not visible in any case.
Review of the FPCT CBV maps revealed abnormalities in all patients except patient 15. According to the findings of the FPCT CBV map, the patients could be divided into 2 groups. Group 1 consisted of 10 patients (patients 2–5, 7–9, 11–13) in whom the CBV abnormalities showed decreased CBV values (measured by the regions of interest) compared with the contralateral side (oligemia). In 8 of these patients, the CBV values were less than 50%, and in 2 patients (patients 8, 13), the CBV value was slightly higher than 50% of the contralateral side. Group 2 consisted of 6 patients (patients 1, 6, 10, 14–16) in whom the CBV abnormalities showed identical (patient 15) or increased (patients 1, 6, 10, 14, 16) CBV values compared with the contralateral side (hyperemia). In this group, all of the patients had been treated successfully (TIMI 3 after treatment).
Follow-Up MSCT Imaging
All patients in Group 1 had evidence of stroke demarcation on the MSCT correlating in size to FPCT CBV abnormalities. Three patterns were found. The first was where the initial CBV map was normal, with a new CBV abnormality observed on the FPCT CBV map, which then continued to be present on the follow-up PCT CBV map (Fig 2). The second was where the posttreatment FPCT CBV showed enlargement of an abnormality observed on the initial pretreatment PCT CBV map, which then remained stable on the follow-up PCT CBV map (Fig 3). The third was where the initial pretreatment PCT CBV study findings matched those on both the immediate posttreatment FPCT CBV study as well as the follow-up PCT CBV study (Fig 4).
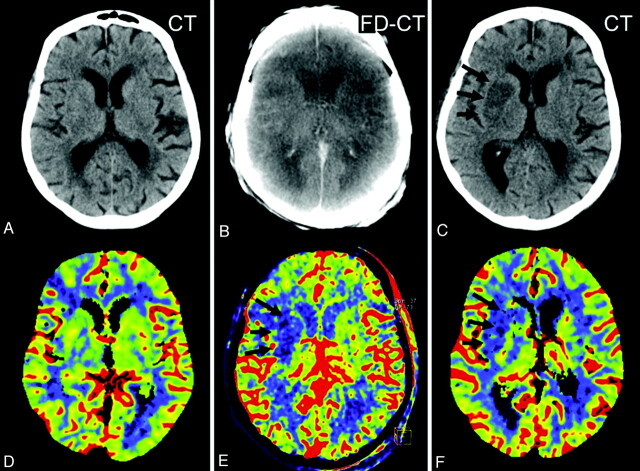
Fig 2.
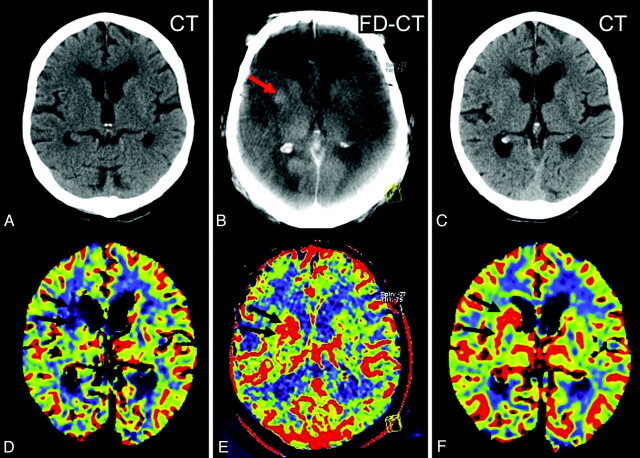
Patient 9. The initial CT (A) and PCT CBV map (D) showed no abnormality. After successful revascularization, there was a small lesion (oligemia) identified on the FPCT CBV map (E, black arrows). This corresponded well with the lesion identified on the follow-up PCT CBV map (F, black arrows). The CBV abnormality observed on the FPCT CBV map (E) matches the sizes of the infarct observed on the follow-up MSCT (C, black arrows). Brain parenchyma reconstruction of the FPCT (B) was without findings.
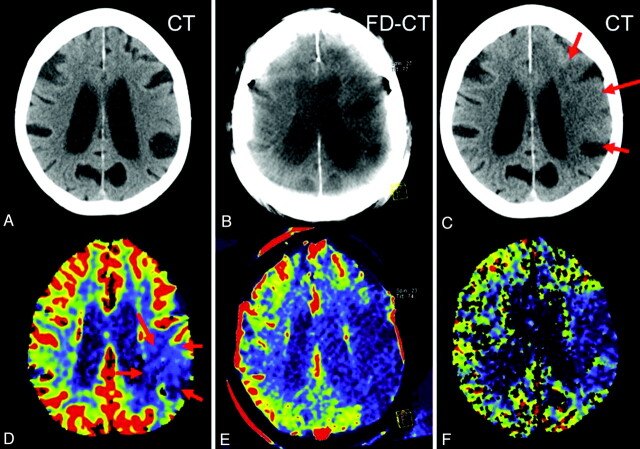
Fig 3.
Patient 2. A CBV lesion (oligemia) is observed on the initial PCT CBV map (D, red arrows); the CT scan (A) was without findings. Revascularization was not successful, and the abnormality had increased in size on the FPCT CBV map perform at the end of treatment (E). No evidence of hemorrhage or contrast extravasation was seen on the corresponding FPCT (B). On the 24-hour follow-up, the infarct observed on the MSCT (C, red arrows) corresponds to the CBV abnormality identified on both the FPCT CBV map (E) and the follow-up PCT CBV map (F).
Fig 4.
Patient 11. The initial PCT CBV map (D) shows a large area of CBV abnormality (oligemia). No clearly defined infarct was identified on the initial CT (A). Revascularization was not successful. On the FPCT CBV map generated immediately after treatment, the CBV abnormality (E) was unchanged from that observed on the inital PCT study. A small area of hyperattenuation was seen on the FPCT performed immediately after treatment (B, black arrow). This was felt to be due to contrast medium extravasation. The follow-up PCT CBV map showed an abnormality corresponding to those observed the initial study and in the study performed immediately after treatment (F). The follow-up CT showed no evidence of the previously observed area of hyperattenuation. The area of infarction seen on the follow-up MSCT (C) matches that seen on the 3 CBV studies.
Of the 6 patients in group 2, 4 had normal (patients 6, 10, 14, 16) follow-up MSCT. Three of these (patients 10, 14, 16) had an area of elevated CBV values on follow-up PCT, corresponding in size and value to that shown on the FPCT CBV map (Fig 5). In only 2 patients, stroke demarcation was visible on follow-up MSCT. One (patient 1) had an area of infarction matching that observed on both the pretreatment MR imaging CBV map and the FPCT CBV map. In the other (patient 15), there was no FPCT CBV abnormality visible, but there was stroke demarcation corresponding to the initial pretreatment PCT abnormality. Hemorrhage was not observed in any of the patients.
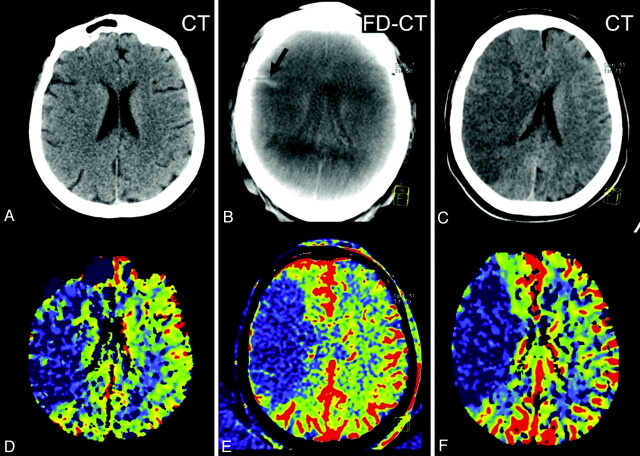
Fig 5.
Patient 10. On the initial PCT CBV map, a small area of decreased CBV (oligemia) is present, adjacent to the lateral aspect of the right frontal horn (D, black arrows). After successful treatment, a hyperattenuated lesion (B, red arrow) within the basal ganglia, without space occupying effect, is obvious on the FPCT. The FPCT CBV map generated immediately after treatment shows an area of increased CBV (hyperemia) in this same area (E, black arrows). The follow-up PCT CBV map (F, black arrows) shows persistence of the area of elevated CBV. No clear area of infarct is seen on the corresponding noncontrast CT (C). In addition, the hyperattenuated abnormality seen on the FPCT (B, red arrow) is no longer visible. This represented contrast extravasation.
Analysis of CBV Abnormality Volume
Calculation of abnormality volumes of Group 1 revealed that the CBV volumes increased from the pretreatment CBV studies to the immediate posttreatment FPCT CBV and the follow-up MSCT stroke lesion volume (37.5 cm3, SD 49.1 cm3 versus 101.8 cm3, SD 84.6 cm3 versus 112.9 cm3, SD 90.8 cm3). Correlation between the volumes observed on the pretreatment PCT CBV studies and those on the immediate posttreatment FPCT CBV studies was poor (r = 0.6, P > .05). Comparison of the volumes observed on the immediate posttreatment FPCT CBV studies and the infarct volume observed on the follow-up MSCT, however, showed a significant correlation (r = 0.9, P < .05) (Fig 1A). There was a strong correlation between of the absolute CBV values (patients 2, 3, 5, 9, 11, 13) measured on immediate posttreatment FPCT CBV and on follow-up PCT (r = 0.9, P < .05) in Group 1 (Fig 1B).
Mean abnormality volumes of Group 2 revealed that the volumes observed on the pretreatment CBV studies were lower than those on the posttreatment FPCT CBV maps (35.3 cm3, SD 60.0 cm3 versus 51.1 cm3, SD 49.4 cm3). Mean infarct volume, as measured on the follow-up MSCT scans, was slightly lower than was the volume of the CBV abnormality observed on the immediate posttreatment FPCT CBV maps (35.3 cm3, SD 60.0 cm3 versus 23.2 cm3, SD 49.8 cm3). There was no correlation between the volume observed on the pretreatment CBV studies and that on the posttreatment FPCT CBV studies (r = 0.7, P > .05). Volumes of the FPCT CBV studies and the infarct volume on follow-up MSCT also showed no correlation (r = 0.7, P > .05) (Fig 1C).
Discussion
Our study demonstrates the feasibility and potential value of this new FPCT application to obtain both FPCT CBV maps and FPCT images in patients with acute ischemic stroke during endovascular treatment in the angiography suite.
Previous reports document the ability of FPCT to recognize both areas of parenchymal hemorrhage and contrast medium extravasation.8,9 The FPCT program used in our study was designed and optimized to perform perfusion imaging and is therefore not directly comparable to the DynaCT program. Nonetheless, it was possible to recognize contrast medium extravasation. The sensitivity of this new FPCT application for detection of hemorrhages remains to be determined.
In humans, there is evidence that the perfusion parameter that best describes the infarct is the CBV.24–29 In acutely ischemic, but not yet infarcted, cerebrums, CBV values are usually normal or elevated, whereas infarcted tissue is usually associated with decreased CBV values.30 Recognition of an area of brain parenchyma in which CBV is reduced to a level where infarction has occurred seems best achieved by a comparison with a symmetrical area in the opposite hemisphere (50% difference threshold) rather than by measurement of absolute values.31,32 Although highly variable, depending upon collateral blood flow, among other factors, the size of a CBV abnormality usually increases during the evolution of an acute infarct.30 It is thus not unexpected that, in our series, the correlation of volume between the pretreatment PCT CBV maps and those obtained using FPCT CBV immediately after treatment was poor in Group 1. But, we found a strong correlation between the volume of the CBV abnormalities observed on the immediate posttreatment FPCT CBV map and the volume of the infarct observed on the follow-up MSCT studies. In addition, in 8 of the 10 patients, the CBV values were less than 50% of the opposite site. Only in 2 patients were the CBV values slightly higher than 50% (patients 8, 13). All of these abnormalities were recognized as areas of infarction on follow-up studies. This finding indicates that a FPCT CBV map acquired immediately following revascularization may allow some prediction of the final infarct size if the CBV values are decreased.
The findings in Group 2 are different. In 4 patients (patients 1, 6, 10, 15), the initial CBV study revealed reduced CBV levels, and, in 2 patients (patients 14, 16), an initial perfusion study did not reveal any CBV abnormality. But all of these patients had areas of elevated CBV in the FPCT CBV studies (in patient 15, symmetrical perfusion).33,34 In patients 10, 14, and 16, even the follow-up PCT studies showed persistent hyperemia in the tissue that had been revascularized (Fig 4). The volumes in this group did not show significant correlations. Hyperemia was only visible in successfully treated patients. Only 2 of these 6 patients showed a stroke demarcation. This reflects the well-known fact that reperfusion is the precondition, but not a guarantee, of a good clinical outcome.35
In summary, our study demonstrates the ability, using FPCT, to perform CBV mapping in patients with an acute ischemic stroke. In patients with decreased CBV, the immediate posttreatment FPCT CBV abnormalities matched the volume of infarction observed on follow-up MSCT. We believe that this shows the potential of this technique to predict the size of an infarct and offers the possibility of “monitoring” brain viability during attempts at revascularization. For example, when attempts have gone on for some time without success, then a FPCT CBV study may reveal 1) no change, or 2) no evidence of a new CBV abnormality. Then further attempts at revascularization would seem to be warranted. If, however, as a third possibility, there is either a significant enlargement of a pretreatment or development of a new large CBV abnormality in patients whose initial CBV study was normal, then the treatment might be terminated because brain viability is no longer present and further attempts might harm the patient. Another use of the technique seems to be in patients where there has been a significant delay between initial imaging and arrival at the angiography suite. An update FPCT CBV study may provide information for selecting appropriate patients. This approach offers the possibility of using individual patient-specific physiologic data, rather than artificial time criteria, for decision making.36,37
There are shortcomings in our study. The number of patients was small, but sufficient to demonstrate that CBV abnormalities can be recognized. A larger study is required to confirm our observations, especially concerning the implications of increases and decreases in CBV. Due to subjective definition of the margins of abnormalities, the volume measurements must be considered only as good estimates. We recognize the inherent limitations of only measuring CBV without concomitant measurement of CBF and MTT in determination of brain tissue viability.26 Still, the combination of our findings that measurement of CBV with FPCT provided results that correlated well with ones using standard techniques, and the fact that CBV is perhaps the best perfusion parameter for predicting viability of brain tissue, make us believe that this possibility will add value to the management of acute stroke patients. In 2 patients, an evaluation was not possible due to obvious motion artifacts. Motion artifacts may blur CBV abnormalities, especially in smaller stroke volumes, and this may influence the analysis. Further experience in this field is necessary. Additional to the angiographic procedure, a total dose of 60 mL of contrast material was used. This increase of total contrast material dose did not lead to any renal dysfunction in our series. If renal dysfunction should be obvious, the decision to obtain this dataset should be made carefully. The effective patient dose of this program is 2.3 mSv (according to the manufacturer). The effective patient dose of this dedicated FPCT program is acceptable in comparison to CT. It is an advantage of this FPCT program that additional to the perfusion information, a reconstruction of the brain parenchyma to rule out hemorrhage is possible. 38,39
Finally, a correlation of our imaging findings with the clinical status is warranted.
Conclusions
CBV mapping by FPCT is feasible during endovascular stroke treatment. FPCT CBV abnormalities with decreased CBV showed a high correlation with infarct volume, as determined on follow-up MSCT scans. Absolute CBV values of FPCT maps performed immediately following treatment compared well with values from standard PCT maps. Image quality of FPCT was limited but was sufficient to visualize contrast medium extravasation. The ability to measure CBV within the angiography suite has the potential to significantly improve the management of patients with acute ischemic strokes.
ABBREVIATIONS:
- FPCT
flat panel detector CT
- GRE
gradient-recalled echo
- MPR
multiplanar reformation
- MSCT
multisection CT
- PBV
parenchymal blood volume
- PCT
perfusion CT
- TIMI
Thrombolysis in Myocardial Infarction
Footnotes
Disclosures: Yu Deuerling-Zheng—Research Support (including provision of equipment or materials): Siemens AG. Martin Köhrmann—Speaker Bureau: Boehringer Ingelheim, Details: Speaker honorarium on 1 occasion; honoraria for contribution to company publication (BI Stroke Newsletter). Charles Strother—Research Support (including provision of equipment or materials): Siemens HealthCare AX, Details: Research support based on a Master Research Agreement with UW Madison; Consultant: Siemens HealthCare AX, Details: Unpaid.
References
- 1. Benndorf G, Strother CM, Claus B, et al. Angiographic CT in cerebrovascular stenting. AJNR Am J Neuroradiol 2005;26:1813–18 [PMC free article] [PubMed] [Google Scholar]
- 2. Benndorf G, Claus B, Strother CM, et al. Increased cell opening and prolapse of struts of a Neuroform stent in curved vasculature: value of angiographic computed tomography: technical case report. Neurosurgery 2006;58:ONS-E380 [DOI] [PubMed] [Google Scholar]
- 3. Benndorf G, Klucznik RP, Strother CM. Images in cardiovascular medicine: angiographic computed tomography for imaging of underdeployed intracranial stent. Circulation 2006;114:e499–500 [DOI] [PubMed] [Google Scholar]
- 4. Struffert T, Richter G, Engelhorn T, et al. Visualisation of intracerebral haemorrhage with flat-detector CT compared to multisection CT: results in 44 cases. Eur Radiol 2009;19:619–25 [DOI] [PubMed] [Google Scholar]
- 5. Struffert T, Eyupoglu IY, Huttner HB, et al. Clinical evaluation of flat-panel detector compared with multisection computed tomography in 65 patients with acute intracranial hemorrhage: initial results. J Neurosurg 2010;113:901–07 [DOI] [PubMed] [Google Scholar]
- 6. Doelken M, Struffert T, Richter G, et al. Flat-panel detector volumetric CT for visualisation of subarachnoid hemorrhage and ventricles: preliminary results compared to conventional CT. Neuroradiology 2008;50:517–23 [DOI] [PubMed] [Google Scholar]
- 7. Kalender W, Kyriakou Y. Flat-detector CT (FPCT). Eur Radiol 2007;17:2767–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engelhorn T, Struffert T, Richter G, et al. Flat-panel detector angiographic CT in the management of aneurysmal rupture during coil embolization. AJNR Am J Neuroradiol 2008;29:1581–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Struffert T, Köhrmann M, Engelhorn T, et al. Penumbra stroke system as an “add-on” for the treatment of large vessel occlusive disease following thrombolysis: first results. Eur Radiol 2009;19:2286–93 [DOI] [PubMed] [Google Scholar]
- 10. Psychogios MN, Buhk JH, Schramm P, et al. Feasibility of angiographic CT in peri-interventional diagnostic imaging: a comparative study with multidetector CT. AJNR Am J Neuroradiol 2010;31:1226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed AS, Zellerhoff M, Strother CM, et al. C-arm CT measurement of cerebral blood volume: an experimental study in canines. AJNR Am J Neuroradiol 2009;30:917–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bley T, Strother CM, Pulfer K, et al. C-arm CT measurement of cerebral blood volume in ischemic stroke: an experimental study in canines. AJNR Am J Neuroradiol 2010;31:536–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat panel detector CT in the evaluation of brain parenchyma, intracranial vasculature and cerebral blood volume: a pilot study in patients with acute symptoms of cerebral ischemia. AJNR Am J Neuroradiol 2010;31:1462–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Struffert T, Deuerling-Zheng Y, Kloska S, et al. Cerebral blood volume imaging by flat detector computed tomography in comparison to conventional multisection perfusion CT. Eur Radiol 2011;21:882–89 [DOI] [PubMed] [Google Scholar]
- 15. Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary working groups. Stroke 2007;38:1655–711 [DOI] [PubMed] [Google Scholar]
- 16. Abou-Chebl A, Lin R, Hussain MS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke 2010;41:1175–79 [DOI] [PubMed] [Google Scholar]
- 17. Abou-Chebl. An endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke 2010;41:1996–2000 [DOI] [PubMed] [Google Scholar]
- 18. Zellerhoff M, Deuerling-Zheng Y, Strother CM, et al. Measurement of cerebral blood volume using angiographic C-arm systems. In Zellerhoff M, Deuerling-Zheng Y, Strother CM, et al., eds. Medical Imaging 2009: Biomedical Applications in Molecular, Structural, and Functional Imaging. Proceedings of the SPIE, Vol 7262;2009;72620H–72620H-8 [Google Scholar]
- 19. Schramm P, Schellinger PD, Klotz E, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke 2004;35:1652–58 [DOI] [PubMed] [Google Scholar]
- 20. Kwak R, Kadoya S, Suzuke T. Factors affecting the prognosis of thalamic hemorrhage. Stroke 1983;14:493–500 [DOI] [PubMed] [Google Scholar]
- 21. von Kummer R, Bourquain H, Bastianello S, et al. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology 2001;219:95–100 [DOI] [PubMed] [Google Scholar]
- 22. Thrombolysis in Myocardial Infarction (TIMI) Trial: phase 1 findings – TIMI study group. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 23. Silvennoinen HM, Hamberg LM, Lindsberg PJ, et al. CT perfusion identifies increased salvage of tissue in patients receiving intravenous recombinant tissue plasminogen activator within 3 hours of stroke onset. AJNR Am J Neuroradiol 2008;29:1118–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. König M, Banach-Planchamp R, Kraus M, et al. CT perfusion imaging in acute ischemic cerebral infarct: comparison of cerebral perfusion maps and conventional CT findings. Rofo 2000;172:219–26 [DOI] [PubMed] [Google Scholar]
- 25. Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology 1999;210:519–27 [DOI] [PubMed] [Google Scholar]
- 26. Koenig M, Kraus M, Theek C, et al. Quantitative assessment of the ischemic brain by means of perfusion-related parameters derived from perfusion CT. Stroke 2001;32:431–37 [DOI] [PubMed] [Google Scholar]
- 27. König M. Brain perfusion CT in acute stroke: current status. Eur J Radiol 2003;45:S11–22 [DOI] [PubMed] [Google Scholar]
- 28. Parsons MW, Pepper EM, Bateman GA, et al. Identification of the penumbra and infarct core on hyperacute noncontrast and perfusion CT. Neurology 2007;68:730–36 [DOI] [PubMed] [Google Scholar]
- 29. Parsons MW, Pepper EM, Chan V, et al. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol 2005;58:672–79 [DOI] [PubMed] [Google Scholar]
- 30. Nagar VA, McKinney AM, Karagulle AT, et al. Reperfusion phenomenon masking acute and subacute infarcts at dynamic perfusion CT: confirmation by fusion of CT and diffusion-weighted MR images. AJR Am J Roentgenol 2009;193:1629–38 [DOI] [PubMed] [Google Scholar]
- 31. Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol 2009;61:321–30 [DOI] [PubMed] [Google Scholar]
- 32. Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 1999;53:1528–37 [DOI] [PubMed] [Google Scholar]
- 33. Nguyen TB, Lum C, Eastwood JD, et al. Hyperperfusion on perfusion computed tomography following revascularisation for acute stroke. Acta Radiol 2005;46:610–15 [DOI] [PubMed] [Google Scholar]
- 34. Kloska SP, Dittrich R, Fischer T, et al. Perfusion CT in acute stroke: prediction of vessel recanalization and clinical outcome in intravenous thrombolytic therapy. Eur Radiol 2007;17:2491–98 [DOI] [PubMed] [Google Scholar]
- 35. Jansen O, Schellinger P, Fiebach J, et al. Early revascularisation in acute ischemic stroke saves tissue at risk defined by MRI. Lancet 1999;353:2036–37 [DOI] [PubMed] [Google Scholar]
- 36. Mishra NK, Albers GW, Davis SM, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke 2010;41:e25–33 [DOI] [PubMed] [Google Scholar]
- 37. Maulaz A, Piechowski-Józwiak B, Michel P, et al. Selecting patients for early stroke treatment with penumbra images. Cerebrovasc Dis 2005;20:19–24 [DOI] [PubMed] [Google Scholar]
- 38. Cohnen M, Wittsack HJ, Assadi S, et al. Radiation exposure of patients in comprehensive computed tomography of the head in acute stroke. AJNR Am J Neuroradiol 2006;27:1741–45 [PMC free article] [PubMed] [Google Scholar]
- 39. Diekmann S, Siebert E, Juran R, et al. Dose exposure of patients undergoing comprehensive stroke imaging by multidetector-row CT: comparison of 320-detector row and 64-detector row CT scanners. AJNR Am J Neuroradiol 2010;31:1003–09 [DOI] [PMC free article] [PubMed] [Google Scholar]