Abstract
BACKGROUND AND PURPOSE:
Percutaneous vertebral body perforation is a new technique for treating painful VCFs. Herein, we compare the therapeutic effect of vertebral perforation and conventional vertebroplasty for treating VCFs.
MATERIALS AND METHODS:
One hundred eight patients with single painful VCFs were assigned to undergo vertebral perforation (perforation group) or vertebroplasty (PVP group). Clinical outcomes were assessed by using the VAS. The associations of analgesic effect and clinical factors were also analyzed by multivariate regression. Plain radiographs were used to quantify the progression of vertebral body compression after surgery and to evaluate cement leakage and new vertebral fractures. The median follow-up time was 10 months.
RESULTS:
Baseline characteristics were similar in the 2 groups. No factors correlated with analgesic effects in the PVP group. The analgesic effect of vertebral perforation was, however, related to the preoperative severity of vertebral compression and was low in patients with severe deformity (P < .05). Among patients with preoperative vertebral percentage of compression below 30%, there were no significant differences between the 2 groups in analgesic effect at any postoperative intervals. Progression of vertebral compression after surgery occurred in 22.2% and 16.0% of treated vertebrae in the perforation and PVP groups, respectively (P = .38). Respectively, 3.7% and 20.0% of the perforation and PVP groups had new postoperative fractures during follow-up (P < .05). There were no other complications.
CONCLUSIONS:
Vertebral perforation was safe and effective for painful VCFs with slight compression. However, vertebroplasty should be considered for patients with marked vertebral body compression.
Vertebroplasty using bone cement has been performed worldwide to control pain due to osteoporotic VCFs resistant to conservative treatment.1–8 While marked analgesic effect and improvement in ADL are apparent immediately after vertebroplasty, problems including postoperative fractures of adjacent vertebral bodies, pulmonary embolism due to leakage of bone cement out of the vertebral body, and symptoms of spinal cord compression have been reported.9–11 In particular, postoperative fractures have been reported to occur in 41%–67% of patients, suggesting that the treatment itself may induce new fractures.12–14 Because no effective method of avoiding such fractures has been established, to our knowledge, this complication is currently a major limitation of vertebroplasty. Thus, breakthroughs to overcome this problem are awaited.
Fracture pain and increased intraosseous pressure have long been regarded as being closely related. In fact, there have been a number of reports evaluating increased intraosseous vertebral pressure in VCFs.15–23 Intraosseous decompression for the treatment of fracture pain is thought to have therapeutic benefits and is covered by medical insurance in Japan. It is well-known that there is no dose-escalation effect of bone cement used for the treatment of VCFs.24,25 Furthermore, we have actually experienced some patients who obtained a remarkable analgesic effect despite injection of a small amount of bone cement into painful fractured vertebrae. We aimed at reducing increased intraosseous vertebral pressure, which was a cause of pain in this study. We prospectively performed percutaneous vertebral body perforations without bone cement in patients with VCFs resistant to conservative treatment. We evaluated the therapeutic effects, complications, and factors affecting its analgesic effect and compared them with those of conventional vertebroplasty.
Materials and Methods
The study protocol was approved by our institutional review board. Before surgery, informed consent was obtained from all patients after a full explanation of the therapeutic procedure, including the lack of bone cement infusion in the perforation group, had been provided.
Subjects
We assessed 108 patients with single painful VCFs who had not responded to conservative treatment in a pain clinic or at an orthopedic clinic. These patients were treated with either vertebroplasty or vertebral perforation at our institution from 2007 through 2010. The first 50 patients (50 vertebrae) were treated with vertebroplasty (PVP group); the last 58 (58 vertebrae), with vertebral perforation (perforation group). We retrospectively reviewed clinical and imaging data from these patients. To avoid a learning-curve effect, we excluded 232 vertebrae treated with vertebroplasty before 2007 at our institution.
The perforation group included 48 women and 10 men, ranging from 61 to 100 years of age (mean age, 76.7 years). The PVP group included 42 women and 8 men, ranging from 59 to 90 years of age (mean age, 77.5 years). Patients were evaluated before surgery on the basis of a complete history, physical examination, and neuroimaging evaluations (x-ray, CT, and MR imaging). Radiographs (anteroposterior and lateral views) of the thoracic and lumbar spine were obtained, preferably in a standing position, if the patient was able, or in a sitting position if not.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1) VCF with 0%–90% loss of VBH on x-ray of the spine; 2) severe back pain related to a single VCF refractory to analgesic medication for at least 2 weeks; 3) pain with a VAS score of 5 or higher interfering with ADL, tapping pain at the spinal process of the fractured vertebral body; and 4) on MR imaging, the affected vertebral body showed a high signal intensity on short TI inversion recovery imaging and low signal intensity on T1WI.
The exclusion criteria were as follows: 1) uncorrected coagulopathy, 2) local or systemic infection, 3) secondary osteoporosis, 4) inability to give informed consent, 5) impaired cardiopulmonary function, 6) dementia, 7) painless VCF, 8) spinal metastatic cancer, and 9) neurologic symptoms.
Surgical Procedures
Percutaneous Vertebral Body Perforation (Perforation Group).
All patients were operated on by 1 of the authors (M.K.) who had performed 282 vertebroplasties. Surgery was performed with the patient in the prone position under local anesthesia. Under C-arm guidance, a 13-ga biopsy needle (K-project, 13 ga × 150 mm; TSK Laboratory, Tochigi-Shi Tochigi-Ken, Japan) was inserted via the bilateral transpedicular routes into the anterior third of the vertebral body. The presence or absence of a communication between the bilateral needle holes was checked, and blood or effusion in the vertebral body was aspirated. Next, a contrast medium (iodixanol, Visipaque 270; Daiichi-Sankyo, Tokyo, Japan) was injected through the bilateral needles; the position of the tip of each needle, its communication with the vertebral vein, and the efflux pattern of the contrast medium were then checked. Finally, irrigation with 50 mL of saline was performed via each needle. Surgery was completed by withdrawing both needles. The patients were discharged on the same day after approximately 1 hour of bed rest.
PVP Group.
All patients were operated on by 1 of the authors (M.K.). Surgery was performed with the patient in the prone position under local anesthesia. Under C-arm guidance, a 13-ga biopsy needle (K-project, 13 ga × 150 mm, TSK Laboratory) was inserted via a unilateral transpedicular route into the anterior third of the vertebral body. A contrast medium (Visipaque 270, Daiichi-Sankyo) was injected through the needle; the position of the tip of the needle and the efflux pattern of the contrast medium were checked. A polymethylmethacrylate mixture was injected into the vertebral body. Surgery was completed by withdrawing the needle. The patients were discharged on the same day after approximately 2 hours of bed rest. During cement injection, fluoroscopic monitoring with a C-arm unit was used in both planes.
Outcome Evaluation and Comparison of Parameters.
Pain was evaluated before and 2 days (next day), 1 week, 1 month, and 3 months after surgery. Pain was evaluated by using a VAS from 10 for maximum pain to 0 for no pain. We compared postoperative VAS score changes between the perforation and PVP groups.
For each patient, the recovery rate with treatment on day 2 was evaluated. The recovery rate (percentage) was expressed as the VAS score improvement rate [(preoperative VAS–postoperative VAS on day 2)/preoperative VAS × 100].
For multivariate analysis of pain reduction, the items evaluated included age, sex, symptom duration, preoperative vertebral body collapse (%), presence of an intravertebral cleft on preoperative MR imaging, pre-existing vertebral fractures, preoperative kyphotic change on radiography (lateral view), preoperative posterior wall destruction on radiography (lateral view), and communication between the bilateral needle holes (only in the perforation group).
In each group, we analyzed the association between the recovery rate (day 2) and the aforementioned factors. In this analysis, we adopted the day 2 recovery rate because the analgesic effect after 7 days might be affected by the natural course of VCF.
The degree of vertebral body collapse (percentage) was calculated as the loss of vertebral body height in comparison with the vertebra above it (referent vertebra) in all patients. Intravertebral clefts were judged by examining sagittal T2WI MR images of the spine.
Statistical Analysis
Statistical analyses were performed by using StatView 5.0 software (SAS Institute, Cary, North Carolina). For comparison of demographic characteristics, VAS scores on follow-up, and complications between the 2 groups, we applied the Mann-Whitney U test or the Fisher exact test. Relationships between parameters and pain outcomes were assessed by multivariate analysis. All data are presented as mean ± SD, and differences were considered statistically significant at a P value <.05.
Results
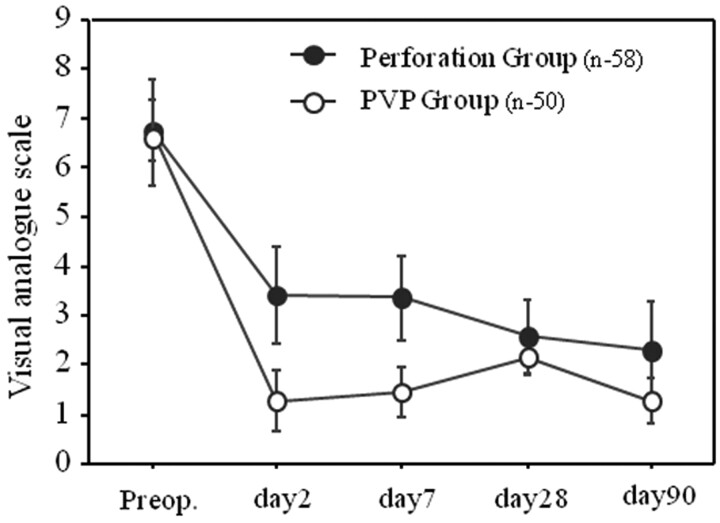
Table 1 shows demographic data from the perforation and PVP groups. Baseline characteristics were similar in the 2 groups. Preoperative VAS scores did not differ between the perforation and PVP groups (7.0 ± 2.1 versus 7.0 ± 1.4). Figure 1 shows VAS score reductions during follow-up in both groups. The VAS score decrease after vertebroplasty was significantly higher than that after vertebral perforation at all time points (∼day 7, P < .05; day 7 ∼ day 90, P < .3). The improved pain relief in the 2 groups was apparent 1 day after the procedure and on day 2, with the VAS score decreasing from 7.0 ± 2.1 to 3.0 ± 1.0 in the perforation group and from 7.0 ± 1.4 to 1.5 ± 0.7 in the PVP group. The day 2 recovery rate was 53.9 ± 62.2% in the perforation group and 78.6 ± 8.5% in the PVP group. Pain relief was sustained in both groups for 90 days. The multivariate analysis resulted in day 2 recovery rates of the 2 groups. In the perforation group, the vertebral percentage of compression before surgery was statistically significant (P = .03). The therapeutic effect was lower in patients with a more severe vertebral percentage of compression. The symptom duration (days) was shorter in patients showing a high therapeutic effect (P = .09). On the other hand, in the PVP group, no constitutional factors correlated with the therapeutic effect.
Table 1:
Baseline characteristics of 108 patients treated for VCFsa
| Characteristic | Perforation Group | PVP Group | P Value |
|---|---|---|---|
| No. of patients | 58 | 50 | |
| Mean age (yr) (range) | 76.7 ± 4.2 | 77.5 ± 8.4 | .35 |
| No. of women (%) | 48 | 42 | |
| Preop. VAS score | 7.0 ± 2.1 | 7.0 ± 1.4 | .82 |
| Mean duration of symptoms (day) | 127.8 ± 105 | 145.3 ± 112 | .68 |
| Posterior destruction of VB | (+) 19 | (+) 10 | .09 |
| (–) 39 | (–) 40 | ||
| No. of patients with preexisting VCFs | (+) 34 | (+) 31 | .42 |
| (–) 24 | (–) 19 | ||
| Distribution of treated VCFs | T7-L5 | T6-L4 | |
| Presence of kyphotic change | (+) 15 | (+) 18 | .15 |
| (–) 43 | (–) 32 | ||
| Preop. vertebral percentage of compression (%) | 45.9 ± 29.7 | 42.5 ± 14.2 | .64 |
| Preop. intravertebral cleft on MRI | (+) 30 | (+) 15 | .02 |
| (–) 28 | (–) 35 | ||
| Communication of puncture holes | (+) 15 | ||
| (–) 43 |
Note:—preop indicates preoperative; VB, vertebral body.
Statistical analyses were performed with the Mann-Whitney U test or the Fisher exact test.
Fig 1.
Comparison of reductions in postoperative VAS scores during follow-up between the perforation and PVP groups. The VAS score decrease after vertebroplasty was significantly greater than with vertebral perforation at all time points.
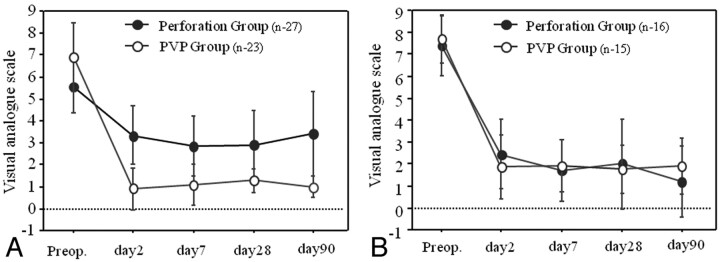
Next, we investigated VAS scores during follow-up in patients with marked vertebral compression (>70%) and in those with slight vertebral compression (<30%) (Figure 2). In patients with marked vertebral compression (>70%), the VAS score decrease after vertebroplasty was markedly higher than that with vertebral perforation at all time points. On the other hand, in patients with slight vertebral compression (<30%), there were no significant differences between the 2 groups in analgesic effect at any postoperative intervals examined in this study.
Fig 2.
Correlation between pain relief for 3 months and vertebral percentage of compression. We compared pain relief between the perforation and PVP groups. A, Patients with marked vertebral percentage of compression (>70%). B, Patients with slight vertebral percentage of compression (<30%).
Complications of vertebral perforation and vertebroplasty are shown in Table 2.
Table 2:
Comparison of complications between the perforation and PVP groupsa
| Complication | Perforation Group | PVP Group | P Value |
|---|---|---|---|
| New fracture after surgery (%) | 1/27 (3.7) | 10/42 (23.8) | .042 |
| (median, 10.1 mo) | (follow-up, 10.0 mo) | ||
| 16/42 (38.1) | |||
| (median, 15.0 mo) | |||
| Progression of vertebral collapse after surgery (%) | 6/27 (22.2) | 8/50 (16.0) | .380 |
| Pulmonary embolism | 0 | 0 | |
| Infection | 0 | 0 | |
| Pneumothorax | 0 | 0 |
Statistical analysis was performed by the Fisher exact test.
After a mean follow-up of 10 months, only 1 new fracture was reported in 27 patients (3.7%) treated with vertebral perforation, while there were 10 new fractures in 10 of 42 patients (23.8%) who underwent vertebroplasty. This difference was statistically significant (P = .04). However, 39 patients refused or could not undergo follow-up radiographs. Analysis of the progression of vertebral body collapse during follow-up in the perforation and PVP groups revealed no statistically significant differences (perforation group, 6 cases [22.2%] versus PVP group, 8 cases [19.0%, P = .38]). Perioperative cement leakage outside the vertebral body was observed in 6 patients (12.0%) in the PVP group. No patients in either group had other complications such as pulmonary embolism, infection, and pneumothorax.
Discussion
Fracture pain and increased intraosseous pressure have long been regarded as being closely related, and numerous reports have been published on this topic.15–23 Arnoldi15,16 measured intraosseous pressure of vertebral bodies in patients with new VCFs and demonstrated that pressure is significantly higher in fractured than in normal vertebral bodies. They also revealed that pressures vary within different areas of the same fractured vertebral body. Esses and Moro23 measured intraosseous pressure of the lumbar vertebral bodies in healthy subjects and found variations depending on posture and body position. They suggested that pain during body movement due to acute VCFs may be associated with variations among the increased intraosseous pressures. In addition, Ogihara,26 a group of anesthesiologists, reported that according to data collected in Japan, performing the vertebral perforation procedure on patients with painful lumbar compression fractures achieved marked analgesic effects. They described the analgesic mechanism of the procedure as possibly being attributable to improved intraosseous blood flow, equalization of intraosseous pressure, decreased stimulation of pain nerve fibers, elimination of pain substances, relief of periosteal pain, and so forth. If these reports are accurate, the vertebral perforation procedure performed in our study is not simply a sham procedure simulating vertebroplasty without using bone cement. This procedure may, in fact, have effects on the intraosseous environment completely different from those of vertebroplasty.
In this study, we compared analgesic effects between the vertebral perforation procedure and vertebroplasty in patients with painful compression fractures. The effect was generally superior in the PVP group compared with the perforation group. However, as shown in Fig 2B, the analgesic effect of the vertebral perforation procedure could be considered similar to that of vertebroplasty in patients with the vertebral percentage of compression 30% or less of the original height, and the effect was maintained for 3 months. The fractured vertebral bodies without progression of collapse were often in a relatively early stage and less affected by instability due to pseudoarthrosis. In such cases, pain may be caused by increased intraosseous pressure as previously described. Thus, we believe that the vertebral perforation procedure either suppresses the increased intraosseous pressure or changes the intraosseous blood flow in the early stage of fracture, thereby achieving the analgesic effect. Moreover, given that the half-life of local anesthetics is approximately 3.5 hours, an analgesic effect manifesting immediately after the procedure and then lasting 3 months cannot simply be explained by the action of anesthetics.
However, the placebo effect can also never be denied because we explained the analgesic effect without the cement to a patient beforehand. On the other hand, the analgesic effect of the vertebral perforation procedure was clearly inferior to that of vertebroplasty in patients with advanced vertebral collapse. In many of these cases, a long time had passed since the onset of fracture. The origin of VCF pain may be strongly associated with vertebral instability due to pseudoarthrosis rather than increased intraosseous pressure. Thus, vertebroplasty that restabilizes a vertebral body with bone cement may be necessary for patients with advanced vertebral compression.
As for vertebroplasty with bone cement, new postoperative adjacent vertebral fracture, pulmonary embolism due to extravertebral leakage of bone cement used during surgery, onset of spinal cord compression symptoms, and so forth are regarded as important complications.9–11 Most notably, the frequency of new postoperative adjacent vertebral fractures reportedly ranges from 41% to 67%; thus, the nature of the procedure itself is considered to possibly induce new fractures.12–14 In this study, intraoperative extravertebral leakage of bone cement was observed in 12% of patients in the PVP group, and new postoperative fractures occurred in 20% of these subjects during the mean follow-up period of 10 months. The frequency of both events was as high as that described in past reports. On the other hand, the frequency of new postoperative fractures was only 3.7% for the vertebral perforation procedure, markedly lower than that with vertebroplasty. In addition, no intraoperative complications occurred in the perforation group.
Moreover, exacerbation of kyphotic deformity due to progression of postoperative vertebral collapse has been a concern with the vertebral perforation procedure. Although the frequency of exacerbation was 22%, slightly higher than the 19% for vertebroplasty, the difference did not reach statistical significance. Thus, the risk of this deformity does not seem to increase substantially. Although it was seen in a few cases, the risk does not appear be much different from that in the natural course of VCF. Although we performed bilateral puncture in every case for the vertebral perforation procedure, there have been no reports evaluating the difference in analgesic effect between 1-sided puncture and bilateral puncture. We consider bilateral puncture to be necessary for the vertebral perforation procedure because greater therapeutic effects can be achieved. However, with sufficient experience using the C-arm or performing conventional vertebroplasty, the frequency of complications is not anticipated to increase with puncture alone.
As to the limitations of this study, although we compared vertebroplasty with the vertebral perforation procedure in patients with VCFs resistant to conservative treatment, the subjects were not randomly assigned to these 2 treatment groups. However, because patients were consecutively assigned to undergo 1 of these procedures, the treatment assignments were not biased.
Although the comparison of patients with vertebral compression of 30% or less of the original height revealed a significant difference in treatment outcomes, the number of such patients was small in both groups (ie, 16 in the perforation group and 15 in the PVP group). In general, vertebroplasty is often indicated for patients in the chronic stage, and the proportion of those with mild vertebral compression is relatively small. Thus, we consider the number of patients who are good candidates for the vertebral perforation procedure to be limited.
In this study, we followed changes in VAS for 3 months. This may be regarded as a short follow-up period. However, because VAS scores would be affected by other factors including new fractures at 3 months postoperatively or later, especially in the PVP group, the follow-up period was set at 3 months.
Conclusions
VCFs are minimally associated with instability in the early stage, and pain may be caused by increased intraosseous pressure. Thus, early-stage VCFs are different in pathology and pain mechanism from those with severe vertebral collapse in the chronic stage, which is often associated with instability. The vertebral perforation procedure can prevent complications associated with bone cement and is as effective as vertebroplasty for treating the pain of early-stage VCFs with mild vertebral compression. If pain due to instability develops after the procedure, bone cement can also be injected later. However, vertebroplasty with bone cement should be considered in patients with severe vertebral compression associated with instability in the chronic stage.
ABBREVIATIONS:
- ADL
activities of daily living
- PVP
percutaneous vertebroplasty
- VAS
visual analogue scale
- VBH
vertebral body height
- VCF
vertebral body compression fracture
References
- 1. Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (VERTOS II): an open-label randomised trial. Lancet 2010;376: 1085–92. Epub 2010 Aug 9 [DOI] [PubMed] [Google Scholar]
- 2. Rousing R, Hansen KL, Andersen MO, et al. Twelve-months' follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: a clinical randomized study. Spine 2010;35: 478–82 [DOI] [PubMed] [Google Scholar]
- 3. Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009;361: 569–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009;361: 557–68 [DOI] [PubMed] [Google Scholar]
- 5. Rousing R, Andersen MO, Jespersen SM, et al. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months' follow-up in a clinical randomized study. Spine 2009;34: 1349–54 [DOI] [PubMed] [Google Scholar]
- 6. Kallmes DF, Comstock BA, Gray LA, et al. Baseline pain and disability in the Investigational Vertebroplasty Efficacy and Safety Trial. AJNR Am J Neuroradiol 2009;30: 1203–05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures—the VERTOS study. AJNR Am J Neuroradiol 2007;28: 555–60 [PMC free article] [PubMed] [Google Scholar]
- 8. Kawanishi M. Vertebroplasty for vertebral compression fracture: indications, technique, clinical application. Brain Nerve 2009;61: 663–68 [PubMed] [Google Scholar]
- 9. Trout AT, Kallmes DF. Does vertebroplasty cause incident vertebral fractures? A review of available data. AJNR Am J Neuroradiol 2006;27: 1397–403 [PMC free article] [PubMed] [Google Scholar]
- 10. Chen YJ, Tan TS, Chen WH, et al. Intradural cement leakage: a devastatingly rare complication of vertebroplasty. Spine 2006;31: 379–82 [DOI] [PubMed] [Google Scholar]
- 11. Cosar M, Sasani M, Oktenoglu T, et al. The major complications of transpedicular vertebroplasty. J Neurosurg Spine 2009;11: 607–13 [DOI] [PubMed] [Google Scholar]
- 12. Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine 2004;29: 2270–77 [DOI] [PubMed] [Google Scholar]
- 13. Harrop JS, Prpa B, Reinhardt MK, et al. Primary and secondary osteoporosis' incidence of subsequent vertebral compression fractures after kyphoplasty. Spine (Phila Pa 1976) 2004;29: 2120–25 [DOI] [PubMed] [Google Scholar]
- 14. Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol 2006;27: 217–23 [PMC free article] [PubMed] [Google Scholar]
- 15. Arnoldi CC. Intravertebral pressures in patients with lumbar pain. Acta Orthop Scand 1972;43: 109–17 [DOI] [PubMed] [Google Scholar]
- 16. Arnoldi CC. Intraosseous hypertension a possible cause of low back pain. Clin Orthop Relat Res 1976: 30–34 [PubMed] [Google Scholar]
- 17. Spencer DL, Ray RD, Spigos DG, et al. Intraosseous pressure in the lumbar spine. Spine 1981;6: 159–61 [DOI] [PubMed] [Google Scholar]
- 18. Ochia RS, Ching RP. Internal pressure measurements during burst fracture formation in human lumbar vertebrae. Spine (Phila Pa 1976) 2002;27: 1160–67 [DOI] [PubMed] [Google Scholar]
- 19. Arnoldi CC. Venous engorgement and intraosseous hypertension in osteoarthritis of the hip. J Bone Joint Surg 1972;54: 409–21 [PubMed] [Google Scholar]
- 20. Arnoldi CC. Intraosseous hypertension and pain in the knee. J Bone Joint Surg 1975;57: 360–63 [PubMed] [Google Scholar]
- 21. Lemperg RK. The significance of intraosseous pressure in normal and disease states with special reference to the intraosseous pain engorgement syndrome. Clin Orthop Relat Res 1978: 143–56 [PubMed] [Google Scholar]
- 22. Azuma H. Intraosseous pressure as a measure of hemodynamic changes in bone marrow. Angiology 1964;15: 396–406 [DOI] [PubMed] [Google Scholar]
- 23. Esses SI, Moro JK. Intraosseous vertebral pressures. Spine 1992;17: S155–59 [DOI] [PubMed] [Google Scholar]
- 24. Kaufmann TJ, Trout AT, Kallmes DF. The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 2006;27: 1933–37 [PMC free article] [PubMed] [Google Scholar]
- 25. Luo J, Daines L, Charalambous A., et al. Vertebroplasty: only small cement volumes are required to normalize stress distributions on the vertebral bodies. Spine 2009;34: 2865–73 [DOI] [PubMed] [Google Scholar]
- 26. Ogihara M. Core decompression of vertebral body for osteoporotic vertebral compression fracture. Pain Clinic 2006;27: 898–903 [Google Scholar]