Abstract
Purpose of Review
Forest managers have long suggested that forests can be made more resilient to insect pests by reducing the abundance of hosts, yet this has rarely been done. The goal of our paper is to review whether recent scientific evidence supports forest manipulation to decrease vulnerability. To achieve this goal, we first ask if outbreaks of forest insect pests have been more severe in recent decades. Next, we assess the relative importance of climate change and forest management–induced changes in forest composition/structure in driving these changes in severity.
Recent Findings
Forest structure and composition continue to be implicated in pest outbreak severity. Mechanisms, however, remain elusive. Recent research elucidates how forest compositional and structural diversity at neighbourhood, stand, and landscape scales can increase forest resistance to outbreaks. Many recent outbreaks of herbivorous forest insects have been unprecedented in terms of duration and spatial extent. Climate change may be a contributing factor, but forest structure and composition have been clearly identified as contributing to these unprecedented outbreaks.
Summary
Current research supports using silviculture to create pest-resistant forest landscapes. However, the precise mechanisms by which silviculture can increase resistance remains uncertain. Further, humans tend to more often create pest-prone forests due to political, economic, and human resistance to change and a short-sighted risk management perspective that focuses on reactive rather than proactive responses to insect outbreak threats. Future research efforts need to focus on social, political, cultural, and educational mechanisms to motivate implementation of proven ecological solutions if pest-resistant forests are to be favoured by management.
Keywords: Unprecedented outbreaks, Forest management, Structural and compositional diversity, Forest resistance to insect pests, Climate change, Social barriers to management
Introduction
Outbreaks of forest insect pests have been identified in all major forested ecosystems [1, 2]. Economic damage due to the widespread extent and massive mortality of trees is most important in high-latitude forests [3–5]. Over a 10-year period ending in 2012, more than 70 million ha of boreal and temperate forests around the world were attacked by forest insect pests [6]. North America may have been the hardest hit, but large areas of the forest were affected in jurisdictions from across the world: in 2005, forest insects affected 17.3 million hectares (Mha) of forests in Canada and 5.6 Mha in the USA, whereas elsewhere in the world insects affected 3.2 Mha in China, 1.7 Mha in Russia, and 1.3 Mha in Romania [7]. The cost of such outbreaks in terms of wood loss is staggering. Liu et al. (2019) showed that wood volume losses due to a forest insect in a 2.4-Mha area in eastern Canada could amount to $25 to $35 billion CAD [8]. For forest managers, there is thus great incentive to limit the damage caused by these infestations.
It is not surprising that forest pest managers have long proposed ways of mitigating the risk of loss to outbreaks (i.e. enhancing resistance, sensu [9]). Peirson in 1925 remarked “that it seems wholly unnecessary to point out the obvious place that protection from insects must take in forest policy” (p. 372 ) [10]. Since this early assertion, many authors have proposed that increasing forest resistance should be the central goal of forest pest management [11–13]. Here, resistance refers to the capacity of a forest ecosystem to withstand disturbance (i.e. insect outbreaks) without changing state [14]. As most major forest pests have species-specific host requirements, often restricted to a single tree genus or family, recommendations for improving forest resistance to outbreaks have focused on reducing the quantity and quality of host trees within and among stands and in general, avoiding monocultures. Almost 50 years ago, Baskerville proposed favouring mixed stands of host and non-host species, mixing vulnerable and less vulnerable age classes, and breaking up (at the landscape scale) large contiguous forest blocks of host species to increase forest resistance [15]. In part motivated by these early ideas, there has been a strong development, in the intervening decades, of decision support tools to identify at-risk stands that need to be targeted for interventions against defoliators [16] and bark beetles [17, 18]. Successful application of these decision support tools has demonstrated the value of investment in research dedicated to modelling and predicting forest susceptibility and vulnerability.
Despite this knowledge and these tools, recent decades have borne witness to some of the most extreme outbreaks since forest management has been practiced at large scales [19••]. This increase can be observed in many forest types around the Northern Hemisphere and for both defoliators and bark beetles that generally cause the most extensive damage worldwide [2]. Outbreaks of multiple species of bark beetles (mostly Dendroctonus spp., Ips spp., and Scolytus spp.) have affected historically unprecedented spatial extents of forest throughout western North America and Europe [20–22]. In terms of defoliators, outbreaks of conifer-feeding budworms (Choristoneura spp.) as well as forest tent caterpillar (Malacosoma disstria Hbn. (Lepidoptera: Lasiocampidae)) [23, 24], balsam fir sawfly (Neodiprion abietis (Harris); Hymenoptera: Diprionidae) [25], and oak processionary moth (Thaumetopoea processionea L. (Lepidoptera: Thaumetopoeidae) [26] have increased in extent, duration, and intensity during the second part of the twentieth century [27–30] or have increased their range [31] (Table 1). A paradox thus emerges from these observations: despite a general consensus in forest pest management regarding what needs to be done to decrease forest vulnerability and increase resistance, empirical evidence suggests that outbreaks are getting worse.
Table 1.
Major insect pests, their most vulnerable hosts, silvicultural knowledge on controlling outbreak severity, severity of most recent outbreaks, and causal factors identified in explaining recent outbreaks
| Insect pest | Primary susceptible host | Proposed silvicultural management | Recent history | Major causal factors |
|---|---|---|---|---|
| Spruce budworm (Choristoneura fumiferana) | Mature balsam fir (Abies balsamea) (Johns et al., 2019) [43] |
Reduce contiguous blocks of fir Increase hardwood content Increase secondary host (black spruce (Picea mariana)) (Bognounou et al., 2017) [61••] |
1980s outbreak most severe in known history 2010 outbreak less severe but suggests range expansion (Bognounou et al., 2017; Johns et al., 2019) [43] |
1980s outbreak more fir (primary host) than previously due to forest management and reduced fires Currently less fir except in North (expanded range) and East |
| Mountain pine beetle (Dendroctonus ponderosae) | >25cm DBH lodgepole pine (Pinus contorta) (Safranyik & Carroll, 2006) |
Reduce contiguous blocks of large mature lodgepole pine Mix age and size classes (Björklund, N., & Lindgren, 2009) |
2000s outbreak worst in recorded history (Taylor et al 2006) |
Primary factor—large expanses of large mature pine Contributing factor—lower mortality due to warmer winters; drought facilitates increase of beetle population (Taylor et al, 2006; Creeden, et al, 2014) |
| European spruce bark beetle (Ips typographus) | Mature spruce, wounded or damaged or windthrown trees (Wermelinger, 2004; Raffa et al., 2008) [35, 20] |
Reduce large expanses of spruce plantations Salvage log windthrown sites Heterogeneous landscape (Wermelinger, 2004; Fettig et al., 2007) [35, 45] |
Current outbreak unprecedented in terms of damage (Jenkins et al., 2008) |
Large area of spruce monocultures due to plantations (Fettig et al., 2007; Mezei et al., 2017) Contributing factor—warmer temperatures and long-term drought (Raffa et al., 2008) [20] |
| Forest tent caterpillar (Malacosoma disstria) | Trembling aspen, (Populus tremuloides) paper birch, (Betula papyrifera) sugar maple, (Acer saccharum) etc. (Cooke and Lorenzetti, 2006; Uelmen et al., 2016) | Thinning of vulnerable lower canopy trees, clone selection, reduce large expanses of old forest (Man et al., 2008) | 1992–1999 outbreak longer and more severe (Cooke, et al, 2012) [24] |
Outbreak rebounded stands hit multiple times, abundant old aspen trees Duration of 1990s outbreak rebounded increased by large-scale forest fragmentation (Roland, 1993) [106] |
| Gypsy moth (Lymantria dispar) | Mostly oak (Quercus spp) species. Apple (Malus), basswood (Tilia), hawthorn (Crataegus), hazelnut (Corylus), hornbeam (Carpinus), larch (Larix), mountain-ash (Sorbus), river birch (Betula nigra), serviceberry (Amelanchier), willow (Salix), etc. (Gottschalk, 1993; Gottschalk, et al, 1998) |
Thinning (1–3 years before defoliation): removing most vulnerable trees Sanitation thinning: preventing spread by eliminating infested trees (Gottschalk, 1993) |
Higher rate of spread observed from 1960 to 1990 (Sharov and Liebhold, 1998) | Contributing factor—abundance of food species, structural features, or refuges for larvae. Stress conditions (drought, fire, cutting, etc.) (Gottschalk, 1993) |
| Oak processionary moth (Thaumetopoea processionea) | Oak trees (Quercus sp) (Godefroid et al. 2012) | Reduce oak monocultures (Castagneyrol et al. 2012) |
Range expansion throughout much of Europe Increase in frequency and severity in Central Europe (Groenen & Meurisse 2012) |
Climatic suitability, plus abundant oak in new ranges (Groenen & Meurisse 2012, Godefroid et al. 2020) |
| Larch casebearer (Coleophora laricella) | Larches from all age classes in pure and mixed stands (Tabakovic-Tosic et al., 2011) | Cutting branches above 1.3m may help (Ward & Aukema, 2019) | Quebec, late 1970s population infestation caused severe defoliation (Langor et al., 2014) | Climatic suitability (dry warm) (Tabakovic-Tosic et al., 2011) (Ward & Aukema, 2019) |
| Balsam woolly adelgid (Adelges piceae) | Fir (Abies) species (Hrinkevich, et al 2017) | Regulations restricting wood movement from infested areas (Hrinkevich, et al 2017) | Recent outbreak in 2014 (Hrinkevich,et al, 2017) | Varies by site quality and species of fir (Hrinkevich et al 2016) |
References: Björklund and Lindgren [178], Bognounou [61••], Cooke et al. [24], Creeden et al. [179], Fettig et al. [45], Gottschalk [180], Gottschalk et al. [181], Hrinkevich et al. [182], Hrinkevich et al. [183], Jenkins et al. [184], Johns et al.[43], Langor et al. [185], Man et al. [186], Mezei et al. [22], Raffa et al. [20], Roland [106], Safranyik and Carroll [192], Sharov and Liebhold [187], Tabakovic-Tosic et al. [188], Taylor et al. [189], Uelmen et al. [190], Ward and Aukema [191], Wermelinger [35]
In the 1990s, researchers questioned why, given the relative consistency of stand-scale hazard analyses, there were still no large-scale experiments to clearly evaluate the efficacy of silvicultural treatments [32]—a fact that remains essentially true today. There has also been increasing recognition that insect outbreaks are subject to nonlinear threshold effects—now more widely recognized as cross-scale interactions [33]—where the constraints on system behaviour at one scale can be overwhelmed by the relaxation of constraints at another. For example, the combination of warming temperatures and drought in the North American West has been implicated in the population release of multiple bark beetle species that either abated once the drought receded or became amplified by the sheer numbers of beetles overpowering the defences of healthy trees [20]. Such nonlinear dynamics complicate the effective scaling of stand-scale forest resistance to landscapes and regions. Likewise, such dynamics can contribute to the “ecological surprise” that plagues forest land managers when past experience in insect dynamics no longer applies [34]. Yet there is a common theme to virtually all such surprises with respect to insect outbreaks: namely, that there must be sufficient host available to sustain outbreaks, regardless of climatic constraints.
The major insect pests affecting large areas of North American and European forests have been studied intensively, with many reviews available [2, 34, 35]. Many of these reviews recommended that landscape diversification in some form (e.g. species composition, age class diversity, thinning regimes) should be part of the overall mitigation strategy, but references to the primary literature are either superficial or indirect. Yet the number of empirical studies investigating the landscape ecology of forest insect disturbance across a variety of systems has greatly expanded over the last decade (e.g. [36, 37•]), and their implications for outbreak mitigation are only beginning to be explored. Likewise, many reviews address the growing concern of climate change effects on outbreak dynamics, but without teasing apart the effects of forest structure from the effects of climate change [31, 39–42].
In this paper, we briefly review the processes that affect the relative effectiveness of silvicultural mitigation strategies across different insect/forest systems and work to clarify ambiguities in how methods such as species diversification and thinning affect forest resistance to influence stand-scale insect damage. Next, we review how multiple ecological processes scale up and interact to influence insect outbreak dynamics and consequent large-scale forest damage. We further explore how human land management legacies influence resistance to pest damage. We then examine the role of climate vs. forest management in recent unprecedented outbreaks. Finally, we explore the barriers that have constrained implementation of proactive management for resistance.
Stand-Scale Mitigation Strategies
Forest entomologists generally classify pest control strategies as either direct or indirect [19••]. Direct methods focus on population control of the outbreaking insects through a wide variety of techniques (such as spraying of chemical or biological insecticides and use of pheromone interruption) that target hotspots with high population densities [19••]. Indirect methods focus on manipulations of forest habitat and food resources for both the insect pest species and their natural enemies to dampen the amplitude of pest populations and limit their relative impacts on the forest resource. Direct methods are typically applied tactically on relatively fast time scales with a focus on the target organism [8, 43]. Indirect methods rely on longer-term strategic manipulations of forest composition, age structure, stem densities, and spatial configurations. Such indirect interventions and proactive planning must also balance the sustainability of other ecosystem services such as timber and pulp supplies, water quality, wildlife habitat, and biodiversity. Indirect pest control strategies using silvicultural interventions are the focus of this review.
Thinning is a common practice applied to enhance stand resistance to insect outbreaks in many conifer systems of North America and Europe [35, 44–46]. Thinning reduces competition and promotes the growth, vigour, and defensive capacity of the residual trees. Thinning has been recommended as a tool against European spruce beetles (Ips typographus) [47]. Well-timed thinning implemented prior to a mountain pine beetle (Dendroctonus ponderosae) outbreak in the western USA resulted in notable reductions in beetle-induced mortality of ponderosa pine (Pinus ponderosa): 34% in unthinned vs. 4% in thinned stands [19••]. Likewise, increased spacing and consistent thinning have drastically reduced the incidence of southern pine beetle (Dendroctonus frontalis) outbreaks in recent decades in the south-eastern USA [48]. Greater spacing as a result of thinning has also been shown to modify within-stand microclimatic conditions that can affect tree defences and accelerate insect development such that life stages susceptible to freezing occur during overwinter periods. Increased spacing between trees can also influence tree phenology (see below), increase airflow, and interrupt the effectiveness of mass attack aggregation pheromones, and increased inter-tree distances may decrease insect dispersal success [19••, 45].
By contrast, the effects of thinning on defoliator impacts are more equivocal. For example, thinning many years before a spruce budworm outbreak may increase tree vigour and forest resistance but when conducted during an outbreak, may increase vulnerability as the insect population is concentrated on a smaller number of trees [49–51]. Thinning may reduce overall foliage biomass, leading to greater damage to residual trees if defoliating insect populations do not decrease and are thus concentrated on fewer stems.
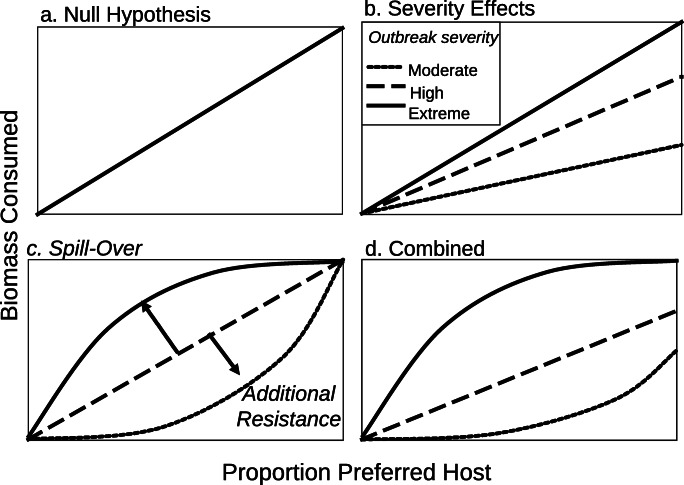
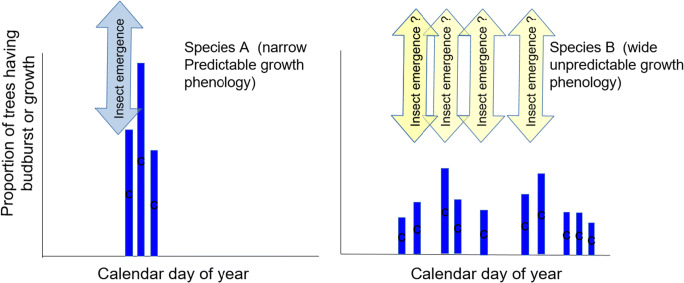
Many studies suggest that homogeneous stands are more susceptible to insect outbreaks than heterogeneous stands [52]. If forest susceptibility were a simple function of the availability of host to a given insect pest, then susceptibility would scale as a simple linear function of the proportion of host in a stand (Fig. 1). Yet most ecological situations deviate from this simple null model through association with the other tree species within stand mixtures. “Associational resistance” occurs when species mixtures offer additional protection for host species, “bending the curve” downwards in the relationship between host abundance and herbivore impacts (Fig. 1). Two general processes underlie associational resistance within forested stands: (1) resource dilution and (2) enhancement of natural enemy communities [53]. Insects may have difficulty locating rare hosts (resource dilution) because insects relying on chemical cues may be confused by non-host volatiles or because rare hosts are hard to detect visually. For example, overstory aspen have been observed to visually hide subdominant white spruce and thus protect them from white pine weevil (Pissodes strobi) damage to their leaders [54]. Many prominent defoliator species are early-season feeders, are dependent on the poorly defended and nutrient-rich emergent foliage, and therefore must match their phenology with that of their primary hosts [55]. In a stand containing several host species or genotypes with different bud-burst phenologies, there is no single optimal time for larvae to emerge (Fig. 2). Varying microclimates within a forest (e.g. southern vs. northern exposure, thinned vs. unthinned) can also affect the synchronization of tree and insect phenology.
Fig. 1.
Combined effects of tree species diversity expressed as proportion of preferred host and outbreak severity. Panel (a) shows that there is an allometric linear decrease in biomass consumed as the proportion of hosts decreases. Panel (b) shows that this decrease in the severity of an outbreak also influences biomass consumed. Panel (c) illustrates that biomass consumed is either greater or less than expected if associational effects are present (see text). Panel (d) integrates severity and associational effects
Fig. 2.
Synchronism of insect emergence facilitated when tree phenology occurs predictably over a short time window in panel 1 rather than for a tree species with a less predictable or more variable growth phenology (panel 2)
Associational resistance can also occur when generalist natural enemies more effectively control outbreaks in complex environments. For example, fir and spruce in mixtures with hardwoods have been found to be less damaged by spruce budworm than in pure conifer stands [56, 57] due, at least in part, to increased rates of parasitism. Similarly, populations of European sawfly (Neodiprion sertifer) experience greater rates of parasitism in mixed forests [58]. Finally, increased forest heterogeneity and structural diversity increase parasitoid diversity and abundance in the boreal forest [59]. Diverse stands can thus increase forest resistance by hiding host trees and supporting natural enemies [52]. Indeed, meta-analyses of experimental and observational studies from diverse forest-insect systems from around the world have consistently shown that mixtures of phylogenetically distant trees increase associational resistance [52, 60].
On the other hand, it appears that in some cases, stand diversity can increase the impact of forest insects. The phenomenon is referred to as “associational susceptibility”: when an insect population exploits the main part of its principal resource, it can then “spill over” to an associated, but normally less desirable host [53, 61••, 62], “bending the curve” upwards in the relationship between host abundance and herbivore impacts (Fig. 1). Some experimental studies have even suggested that some mixed forests may be equally or even more susceptible to insect herbivores than pure stands [63–67••]. These varying results regarding associational effects have resulted in much debate and active research in both agricultural and forest ecosystems [68]. Different findings of associational resistance and susceptibility may be related to weaknesses related to observational vs. experimental studies [63–67, 69••] that merit further careful examination. Meta-analyses of experimental and observational studies from diverse forest-insect systems from around the world have consistently shown that mixtures of phylogenetically distant trees increase associational resistance [52, 60]. Associational effects are, however, sensitive to insect population density, which can span several orders of magnitude in outbreaking systems. For example, spill-over typically occurs following depletion of the primary host resource, which means that non-preferred tree species may be attacked when outbreaks are severe, but not under less severe conditions [61••, 62]. Such spill-over effects were especially evident during the recent outbreak of mountain pine beetle in British Columbia (Canada), where beetles attacked small pine trees and even the occasional spruce tree—hosts that do not normally support successful brood production [70]. During severe defoliation events, spruce budworm larvae also switch from their primary host, balsam fir (Abies balsamea), when it becomes severely defoliated, to a secondary host, black spruce (Picea mariana), where food resources are not yet depleted [61••, 71]. This context dependence related to outbreak stage can make it difficult to clearly observe the mechanisms underlying observed associational relationships. While both susceptibility and resistance are possible, their relative importance may vary with outbreak context.
Nonetheless, important generalities emerge from this current body of work with respect to the role of tree species diversification and resistance to insect outbreaks. First, the species identity within species mixtures matters, as some species invest more resources in defence than others [72], and specific insect pests rely on a restricted set of hosts. Second, the likelihood of associational resistance versus susceptibility is contingent in part on the diet breadth of the insect [52, 60]. Third, spill-over (i.e. associational susceptibility) most commonly occurs on phylogenetically proximal trees and under peak outbreak levels [60–62, 73••]. Fourth, forest age also matters such that associational resistance is more common in mature stands [74]. Collectively, these findings demonstrate that it is not the number of tree species per se that enhances forest resistance to insect damage. Tree species identity (phylogenetically proximal vs. distant) within the mixture and age of the forest matters a great deal, as does the identity of the insect species capable of reaching outbreak levels within a given region. Further, any stand-level mitigation strategy, be it thinning or diversification, will both contribute to and be influenced by broader-scaled population dynamics, as addressed in the next section.
Scaling-up Resistance to Landscapes and Regions
Knowledge of how stand-scale compositional and structural diversity affects resistance to insect outbreaks has permitted the successful development of stand-level insect hazard ratings across the globe [16, 17, 75]. For example, hazard ratings (i.e. stand risk) to the spruce budworm incorporated into the SBW-Decision Support System are based on knowledge that the insect causes greater damage to mature balsam fir forests than to either immature fir stands or spruce stands of any age. Thus, managers can use this information to target interventions based on the risk of different stand types (composition and age) to spruce budworm–caused defoliation. Because insect outbreaks often affect large areas of forest beyond single stands, these stand-scale hazard ratings have frequently been scaled up to inform pest mitigation strategies at landscape and regional scales [19••, 35, 36]. Although hazard rating systems perform well at the stand scale, there remains significant scepticism regarding our ability to use these tools to inform landscape-scale mitigation strategies [32, 76, 77]. In this section, we review the insect-forest interactions that affect landscape-scale insect outbreak dynamics. We distinguish between bark beetle and defoliator systems due to the fundamentally different drivers underlying their dynamics.
Unlike defoliators, intergenerational success of bark beetles depends on connectivity between the source trees killed by one generation and the vulnerable host trees receiving the next generation of insects. Likewise, the relative vulnerability of a tree to beetle attack is dynamically linked to the numbers of beetles available to aggregate and overcome tree defences [78]. Healthy host trees are generally able to defend against beetle attacks and therefore act as population sinks, unless attack density is sufficiently high to overwhelm those defences, at which point healthy trees transition to powerful source resources [19••]. The concept of “beetle pressure” (i.e. the number or area of infestations in the neighbourhood of any tree or stand) is therefore necessary to gauge the relative risk of a new infestation [36, 79–81]. These landscape-scale features of bark beetle ecology make their populations highly sensitive to spatial, population, and environmental contexts constraining their irruptive dynamics.
Landscape-scale abundance and connectivity of host trees can also shape bark beetle outbreak impacts. Specific examples demonstrating such effects include the European spruce beetle across Europe [80, 82–85], spruce bark beetle (Dendroctonus rufipennis) in AK (USA) [86], and mountain pine beetle in BC [77]. In the mountain pine beetle system, fragmentation of pine host stands enhanced the likelihood of infestation early in an outbreak, but in later years as the outbreak gained momentum, more connected host forests experienced the greatest impacts [77]. Similarly, a study in Bavaria National Park (Germany) found that spruce concentration was either uncorrelated or negatively correlated with infestation in the first few years of the outbreak, but positively correlated with damage in all subsequent years as the insect population increased and affected all available host trees [87].
Transitions between endemic and epidemic populations are often triggered by other landscape-level events that weaken or kill trees [36]. Outbreaks of European spruce beetle have been triggered by large-scale wind events, [22, 81, 87] that may also synchronize small infestations across broader areas [84, 85]. Drought triggered extensive outbreaks across multiple bark beetle species in the American West; in some cases, the outbreaks receded as the drought receded, and in others, beetle pressure was sufficiently high to transition to successful attack against healthy trees even though the trees were no longer weakened by water stress [20]. Once beetle populations exceed local thresholds due to these larger-scale stress events (i.e. other disturbances and climate forcing), outbreaks can be amplified by large well-connected tracts of mature host [20].
The insect-forest interactions of the southern pine beetle in the south-eastern USA provides an interesting contrast to the previous examples. The region had initiated pine plantation forestry starting in the 1930s that rapidly expanded following World War II, reaching 5 Mha by the end of the twentieth century [48]. Starting in the mid-1950s, southern pine beetle epidemics switched from rare and isolated events to widespread, irrupting outbreaks over large areas every 6–10 years through that same period. Yet damage in the twenty-first century has been limited to largely unmanaged pine forest, with barely detectable damage in the last decade [48]. The recent decline in southern pine beetle outbreaks have been primarily attributed to high-intensity forestry practices initiated in the 1980s that included greater spacing and thinning activities within plantations and reintroduction of fire through controlled burn programs to reduce competition [48, 88]. Methods were also developed for efficient and rapid control of emerging infestation spots [89]. This southern pine beetle/pine plantation forestry case is analogous to the intensive spruce forestry practiced through much of Europe, including institutionalized control of beetle outbreaks primarily through direct methods. It remains to be seen whether such practices can be sustained in this region or if (as in the European spruce beetle example) environmental change such as drought frequency and severity might reduce the efficacy of these approaches [84, 85]. Indeed, southern pine beetle remains a serious problem in Central America where such intensive forestry is not yet possible [90].
Unlike bark beetles, defoliators feed on a renewable resource (foliage), such that their population outbreaks are less dependent on landscape-level host connectivity [91]. Although insect population growth is not as strongly linked to landscape-scale forest connectivity, patterns of defoliation, including spatial extent and severity, are expected to be strongly associated with host availability and concentration [92]. Several recent landscape studies are consistent with these predictions [93–95].
Since tree impacts including growth reduction and mortality are related to cumulative annual defoliation across years, outbreak dynamics in terms of frequency, duration, and intensity ultimately determine the relative resistance of forests to defoliator outbreaks [96]. Spatial synchrony, the degree to which outbreaks are correlated in space [97] , also has implications for forest managers seeking to mitigate or salvage impacted forests. There is evidence that defoliators require some minimum amount of host for outbreaks to emerge at all (e.g. [98]). Naturalized gypsy moth (Lymantria dispar dispar) outbreaks in eastern North America cycle more frequently with increasing abundance of the primary host (i.e. oak, Quercus spp.; [99]). Spatial synchrony in outbreak dynamics poses significant challenges to forest managers as the underlying mechanisms that give rise to synchrony can vary [100]. Greater understanding of the processes that determine synchrony is essential to developing effective proactive forest management strategies, such as the “Early Intervention” program being implemented in Canada’s boreal and Acadian forests [43]. A recent landscape study examined outbreak histories in an area with divergent land use histories in central North America. Research determined that spruce budworm outbreaks were more synchronous in unmanaged regions with higher host concentration, while outbreaks were less synchronous with higher frequency in managed regions with greater hardwood content [37•]. Other studies have documented that spread from outbreak epicentres is contingent on host concentration and thus connectivity of adjacent forest [93, 101]. Dispersal is also implicated in landscape-scale spatial synchrony and has been recently confirmed using modern weather radar- [102] and genetic-based approaches [103]. Challenging questions regarding how host availability and connectivity interact with dispersal and outbreak context are an area of ongoing investigation and are unlikely to be resolved using traditional, field-based approaches; spatially explicit simulation modelling is required to address these complex landscape-scale questions (e.g. [104, 105]).
In contrast to spruce budworm outbreaks, forest tent caterpillar (Malacosoma disstria) outbreaks have been supposed to increase in duration with an increase in host fragmentation [106, 107]. The hypothesis that fragmentation limits the capacity of natural enemies to respond to and dampen outbreaks spurred some of the most detailed landscape-scale investigations of natural enemy communities in the world [108]. Ongoing investigation in this area has produced mixed results that vary among observational and experimental studies, scales of observation, and different natural enemy species in different regions [94, 106, 107, 109–111]. The diversity of findings in this regard demonstrates the complexities of disentangling the spatial dynamics of trophic interactions. Recent analysis of a long outbreak time series within a single heterogeneous landscape suggested that the results of shorter-term correlational studies may be misleading [38••]. This work found that outbreak dynamics are spatially and temporally heterogeneous and characterized by travelling waves. Portions of these waves may correlate well with some landscape attributes within a single outbreak, but the relationships are not consistent among outbreaks [38••]. These spatiotemporal outbreak patterns are influenced by host spatial structure. In the case of the above study, forest tent caterpillar outbreaks were found to be more synchronous within hardwood-dominated forests than in conifer-dominated forests. Although this result is in direct contrast with what was observed for spruce budworm, it is consistent with the hypothesis that host abundance is an important driver of outbreak dynamics.
Although it remains challenging to clearly identify and quantify the landscape-scale processes that influence tree mortality during insect outbreaks, some generalities emerge. Both bark beetle and defoliator systems exhibit synchronous, nonlinear outbreak dynamics that are influenced by the availability of large, well-connected areas of susceptible host trees. The roles of host abundance, spatial extent, and connectivity have been consistently demonstrated in many different systems in both North America and Europe [20, 37•, 84, 85, 98]. This matters because the availability of such contiguous tracts of forest often falls under the purview of human forest management agencies. Although the cross-scale interactions that are required to allow insect populations to grow from endemic to epidemic levels are based on a myriad of factors, we know that if host stands are small and isolated this build-up and transition to an outbreak is generally not possible (e.g. [98]). Recent modelling work has confirmed the importance of concentration and connectivity of large blocks of host forest in outbreak build-up [84, 85, 112, 113].
Global Change as a Driver of Increased Insect Outbreaks
Climate change is expected to influence outbreak dynamics through direct changes in insect population dynamics and indirectly through changes in the range of host species [19••]. Warmer climate may drive future changes in host species distributions, such as illustrated by the northern migration of lodgepole pine (Pinus contorta var. latifolia) [114] or of balsam fir [31]. Warmer temperatures are also expected to increase the reproductive potential and winter survival of insects at the northern and high-elevation parts of their range [19••, 41••, 42, 115] which could lead to considerable changes in the spatial distributions of outbreaks. However, Jactel et al. [116] and Pureswaran et al. [41••] caution that responses will be variable as extreme heat or late or early season frosts and other events can also have negative impacts on outbreaks. Indeed, climate warming that shifted the thermal optimum of the larch budmoth (Zeiraphera diniana) beyond where the host tree species currently occurs is in part responsible for the lack of budmoth cycling in recent decades [117]. Nonetheless, the mountain pine beetle has recently spread eastwards across the Rocky Mountains into northern Alberta (Canada), in part because mild winter temperatures have increased winter survival [118–120]. It has been suggested that with warmer winters, the beetle may continue spreading throughout the range of available host (i.e. all pine species), which potentially could include much of Canada’s boreal forest [118–120].
There is also a danger of complacency in assuming that forests of potential host species that historically have not been defoliated will not be defoliated in the future either. As climate change modifies insect pest ranges, foresters should be aware that large extents of previously unaffected host forest will be increasingly vulnerable to severe outbreaks [31, 115]. This range expansion of indigenous insect pests also raises the question of the response of invasive non-indigenous insect pests. Invasive pests usually have been introduced from forests in one continent to those in another, either intentionally or more often unintentionally. The hosts that are affected are often “naive”—i.e. with no defences against the previously unknown pest—and are thus more vulnerable [121]. However, climate may also play a role in limiting or allowing invasive non-indigenous pests to outbreak. As with indigenous pests, climate change may increase the resource niche available for non-indigenous invasive pests [122].
Increased warming and drying associated with climate change also increase tree stress and have been linked to a worldwide increase in background tree mortality rates [123–125]. Such stress may also render surviving trees more vulnerable to future outbreaks [126–128]. For example, because forest tent caterpillar defoliation combined with drought causes aspen decline [23], high temperatures and dry conditions aggravate the consequences of defoliation for this tree species [129]. Throughout the North American West, drought stress has drastically reduced the capacity of conifers to defend against bark beetle infestations [20]. However, as compelling as the evidence is to support drought effects on bark beetle–caused mortality, empirical studies have not been able to consistently identify a causal link between drought stress and defoliators [130]. A recent study by Itter et al. [131] of two outbreaking defoliators found that both drought and defoliators have immediate and lagged effects on tree growth but that there was no interaction between drought and defoliation. To illustrate the complexity of the relationships, one study on tree mortality suggested that climate stress occurring before an outbreak may have been a contributing factor to insect-caused mortality of trees [132], whereas another study showed that insect defoliation, by reducing photosynthetic and transpiring surfaces, may protect trees from drought [133]. The temporal sequence of events and the measurement of effect (growth loss and/or mortality) are thus factors that need to be considered.
Given that both climate and forest management are changing concurrently through many high-latitude forests, the relative influence of each driver of change on insect outbreaks is difficult to determine. While weather stations have documented climatic changes for over a century, remote sensing methods necessary to comprehensively monitor broad-scale forest changes are limited to a few decades. In addition, the taxonomic and ecological diversity of outbreak-prone insect species contributes to the inherent complexity of outbreak systems, even in apparently simple forest ecosystems, consisting of interactions between climate, forest conditions, predators, and parasites (i.e. in complex food webs [134, 135]) over a range of spatial and temporal scales [117].
In summary, climate change contributes to increasing outbreak severity when suitable conditions for insect population growth increase, while the opposite is equally true (i.e. decreased outbreak severity where climate conditions have become suboptimal) [41••]. Climate change may be an important trigger in increasing the ranges of outbreaking species or in allowing insect pests to move into forested areas previously unaffected [136], although the number of documented range expansions remains limited [137]. When the range of a pest species expands, this can lead to severe outbreaks as the pest moves into naive hosts [138] or escapes (at least temporarily) predators [139] and moves into forested areas with abundant hosts [31]. More favourable climate may also allow some insects to escape controls and expand to outbreak conditions.
Climate thus is a contributing factor as it may allow range expansion or greater survival and growth of some insect populations. Yet without vast expanses of primary or secondary host forests, large outbreaks would be limited [98]. Although there is strong agreement on decreasing the proportion of vulnerable stands to reduce risk of timber loss to insect pests, these recommendations have rarely been heeded (Fig. 3), especially in the context of a climate potentially more favourable for many insect pests. In the next sections, we explore some of the potential barriers to change and speculate on whether it will ever be possible to achieve what many have advocated for decades.
Fig. 3.
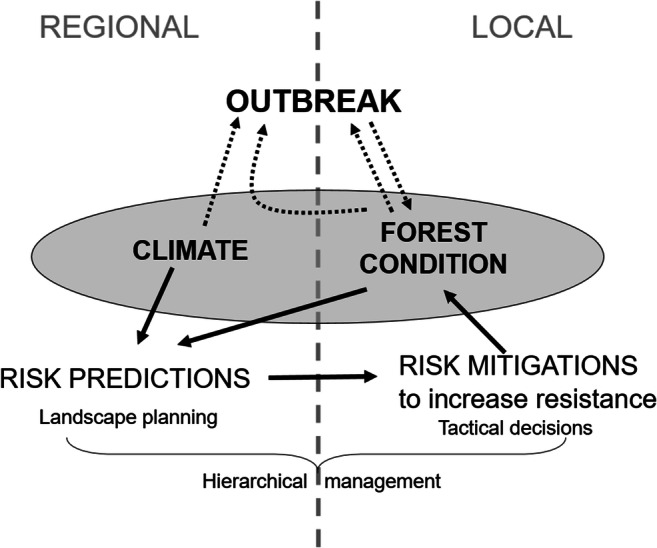
Local and regional factors affecting outbreak severity
Barriers to Implementing Host Reduction
So far, we have validated the ecological foundation for applying preventive or indirect treatments to increase forest resistance based on silvicultural treatments that reduce host abundance and host concentration in stands and landscapes. Nonetheless, larger and more severe outbreaks have been occurring in multiple insect pests over recent decades [19••]. Climate change may be a contributing factor to some of this increased severity, but large forest extents of concentrated hosts are necessary for outbreaks to expand and grow to unprecedented levels. As some insect pest ranges expand, complacency to the effect of outbreaks in historically unaffected areas undoubtedly contributes to the lack of management in these hitherto unaffected forests. However, in the central parts of an outbreaking pest’s range, relevant knowledge is arguably not a limiting factor, and we thus evaluate other potential barriers to creating more resistant and resilient forests to insect pests. The barriers are economic, political, and linked to interactions between natural disturbances (Table 2).
Table 2.
Barriers to implementing successful silvicultural management
| Barriers | Examples |
|---|---|
| Economic | Costly to manage mixed species stands |
| Multiple pests | Shifting from one tree species to another also shifts vulnerability to a different insect |
| Natural history | Reduced fires increase proportion of fir (spruce budwom host), increase lodgepole pine size (mountain pine beetle host) |
| Misguided management |
Overplanting spruce plantations in Europe Overharvesting large blocks of black spruce and not controlling advance fir regeneration |
| Political will | Difficult to convince populations to spend money on something that may not happen |
In some systems, favouring landscapes of non-host trees to increase resistance to one insect may render the forest vulnerable to a different outbreaking insect [135]. For example, increasing hardwood content to reduce stand vulnerability to the spruce budworm will ironically increase stand vulnerability to the forest tent caterpillar [136]. Still, concurrent and sequential outbreaks of these two species are rare, and severe outbreaks of alternative insect pests also have limited overlap.
DeRose and Long further caution that some stand-level treatments offering short-term advantages may be disadvantageous in the long term [139]. Pre-emptive management strategies in which trees are removed before attaining an age or size threshold (e.g. mountain pine beetle primarily attacks trees 20 cm DBH and greater) limit trees from attaining greater size and reproductive potential but also reduce a forest’s economic and ecological value. Pre-emptive harvesting is also common for invasive pests, where the goal of harvesting trees before they are infested may also lead to the removal of genetically resistant individuals that could be used to develop future resistant forests [140]. Another example of treatments that could have both positive and negative impacts is thinning treatments that are used to increase tree vigour in order to resist European spruce beetle or mountain pine beetle attack which will also shorten the time before trees become big enough to become susceptible to infestation [139]. Plot-level resistance will also be overcome if landscape- and regional-scale outbreaks vastly exceed incremental levels of increased resistance. It is also worth noting that changing forest composition, as well as age and size class structure is a long-term process, whereas insect response to climate change can occur on much shorter time scales. Initiatives implemented to control insect populations or reduce damage may only be as good as the weakest link that allows an outbreak to overcome control measures.
Although guidelines for forest pest management seem unequivocal, they do not occur in a vacuum; rather, forest structure and composition are a legacy of past land use, forest management practices, and natural disturbances [141]. Forest management policies and practices have a lasting effect on the composition, age structure, and landscape patterns of forests [142, 143]. For example, fire suppression, where temporarily successful, has led to large blocks of mature timber that may be more susceptible to many insect pests. Fire suppression in interior forests of BC has resulted, on average, in forests older than under historical conditions and more vulnerable to mountain pine beetle [144]. An increasing use of harvesting that protects advance regeneration in eastern Canada also led to an increase in balsam fir, the primary host of the spruce budworm [145]. The widespread establishment of Norway spruce (Picea abies) plantations outside its native range in Europe since the 1850s has been a precipitating factor in recent large beetle infestations [146].
Forest management in many temperate and boreal regions, instead of increasing forest resistance to pests, has tended to replace diverse and uneven-aged stands with uniform, monospecific plantations [144]. In Europe, large-scale forestry has increased homogenization of forest composition and structure over broad regions [147, 148]. In North America, many commercially managed northern forested landscapes are more homogeneous in terms of patch size, stand age, and plant species composition than they were prior to European settlement [149–151]. The relative composition of tree species has also changed: those that tend to prevail after logging, such as trembling aspen or balsam fir, have increased in abundance in eastern boreal forests of North America [29, 152, 153] (see [154] for contrasting results). In western Canada, the area covered by mature lodgepole pine tripled over the last century [144]. All these forest changes have been driven or amplified by land use changes and forest management practices [147, 154–156].
In multi-owner landscapes of small forest blocks, stakeholder platforms are necessary to structure practice through the inclusive and sustainable governance of landscapes [157]. For example, European landscapes are highly fragmented belonging to a variety of landowners [158]. Over recent decades, 15 million small-scale forestry holdings in Europe covered more than 37 Mha of the area [159]. These many private forest owners often have multiple and different goals in forest management, hampering the implementation of European Union (EU) policies. In fact, sustainable land management practices were recommended under the EU Community strategic guidelines for rural development from 2007 to 2013 [160] and the Common Agricultural Policy that prioritized the preservation and development of valuable farming and forestry landscapes [161]. Many jurisdictions in the USA are also characterized by forest management on small land holdings and are subject to the same decision pressures. It could be argued that few individual landowners would be willing to reduce high-profit monocultures for a common good. Thus, up until now, the promise of coordinated landscape management has rarely been put into practice (although see the above examples of southern pine beetle in the USA and spruce beetle in Europe) due to the complex decision-making processes between landowners, industry, and the government.
As has been previously noted, the forest products industry in many parts of the world, especially where harvesting still occurs in natural forests, has been, and continues to be, characterized by a lack of significant expenditure on silviculture and forest management [32, 162, 163]. The industrial forest structure in the boreal zone of North America was built on the premise that the resource was unlimited. Once it became clear that the forest resource was limited, suppression or control of disturbances was attempted to minimize timber losses [164, 165]. This resulted in significant investments in direct interventions against pests and fires rather than preventive strategies. It was expected that interest and investments in silviculture would reverse this trend as natural forests disappeared. However, in regions of Canada and Russia, where forest expanses are large, productivity is low, road networks are expensive to maintain, and distance to mills is large, the incentive for major investments is still low [166, 167]. Insecure forest tenure arrangements also act as an impediment to silvicultural investments [151, 152]. In other regions without these constraints (e.g. New Brunswick in eastern Canada and much of central Europe and Fennoscandia), some transformation has occurred, sometimes to less susceptible hosts, but usually as even-aged monocultures rather than mixed stands. Spiecker [151, 168] notes that after 150 years of forest management in Europe, the shift to coniferous monocultures has increased forest productivity but has also increased landscape-scale vulnerability to climate change and insect outbreaks as many of the cultivated trees species are being planted outside their natural range. The current problems with spruce mortality due to European spruce bark beetle in Europe is at least partially a legacy of many single-species spruce plantations established decades ago [166]. Despite demonstrations of overyielding in mixedwood stands and greater resistance to insects compared to single-species stands [169], there is still a widespread perception that monocultures are more profitable [170].
Increasing stand composition diversity may remain economically unattractive despite increasing forest resistance to insect pests. For a company whose mills run on softwoods, shifting forest composition to hardwoods would immediately affect timber supply. A study in eastern Canada showed that replacing balsam fir stems (the primary host of the spruce budworm) with hardwoods would reduce total conifer yield while increasing resistance to the spruce budworm [171]. In this work, the protective effect of hardwoods on residual fir was less than the softwood timber supply lost to hardwoods. In other words, managers may opt to try to protect timber during an outbreak rather than undertaking the opportunity cost of planting or recruiting non-desired tree species. This same sentiment was voiced decades earlier in Europe when it was noted that changes to current silvicultural practices will be limited unless it could be demonstrated that promoting diversity outweighs costs [172]. More recently, Armstrong [173] showed that policy vision within the forest industry (i.e. silvicultural expenditures are viewed as a cost of current harvesting rather than as an investment in the future forest) also acts as a brake on silvicultural investment in mixedwoods [173]. In human systems, the same phenomena is observed, as the burden of outbreaking diseases may be as high as 490 billion dollars per year [174] (and even higher during the COVID-19 pandemic) [175, 176], despite the knowledge to prepare for and limit outbreaks being well known [175]. Yet until an outbreak occurs and people (or trees) start to die, there is limited political incentive to spend money on prevention and preparation for something that may not happen [177].
Conclusions
Despite repeated suggestions over at least the last 100 years to use silviculture to manipulate forest composition and structure to increase forest resistance to insect pest outbreaks, this has not or rarely been done. We questioned whether this was due to equivocal scientific support but found, on the contrary, that recent literature provides more and more support, as well as explanatory mechanisms, for decreasing large expanses of hosts and increasing tree structural and compositional diversity (especially of phylogenetically distant species) as effective approaches to increasing forest resistance to pests. In both bark beetle and defoliator systems, pest management solutions to increase forest resistance all point to increasing stand and landscape diversity and reducing large connected blocks of host species. Despite this knowledge, recent decades have witnessed unprecedented outbreaks in many forest systems in terms of area affected and severity. Although climate change plays some role in unprecedented outbreaks (especially in cases where insect ranges are expanding), these extreme outbreaks could not occur without large tracts of continuous forests dominated by susceptible host species. The barriers to creating resistant forests are not knowledge based but rather, we argue, due to too much inertia in using traditional forest management systems, potentially short-sighted views of economics and the forest, and reliance on reactive measures due to a recurring inability to generate the political will to spend money on preparation and prevention for something that is perceived as unlikely to occur. Advancement and innovation in the forestry sector will require new longer-term perspectives that embrace our current understanding of the role of forest diversity and view insect outbreaks as an integral part of forest systems.
Acknowledgments
The authors would like to extend special thanks to Dr. Barry Cooke, Dr. Dave MacLean, Dr. Michael Papaik, Dr. Marie-Josée Fortin, Dr. Josie Hughes and Patti Sonntag for stimulating early discussions on themes addressed in this paper as well as to César Gabillot for his support. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official Canadian Forest Service, USDA, or US or Canadian Government determination or policy.
Funding
Dr. Kneeshaw and co-authors received funding from the Sustainable Forest Management Network to begin this review. Subsequent funding to Dr. Kneeshaw was provided by SERG International and by the Natural Sciences and Engineering Research Council of Canada.
Compliance with Ethical Standards
Conflict of Interest
Dr. Kneeshaw, Dominique Tardif, Dr. Enrique Doblas-Miranda, Dr. Patrick James, and Dr. Phil Burton declare that they have no conflict of interest. Dr. Sturtevant works for the USDA Forest Service but has no conflict of interest. Dr. DeGrandpré works for the Canadian Forest Service but has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Forest Entomology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel D. Kneeshaw, Email: Kneeshaw.daniel@uqam.ca
Brian R. Sturtevant, Email: brian.r.sturtevant@usda.gov
Louis DeGrandpé, Email: louis.degrandpre@canada.ca.
Enrique Doblas-Miranda, Email: e.doblas@creaf.uab.cat.
Patrick M. A. James, Email: patrick.james@utoronto.ca
Dominique Tardif, Email: dominique.trdf@gmail.com.
Philip J. Burton, Email: phil.burton@unbc.ca
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Dyer LA, Carson WP, Leigh EG. Insect outbreaks in tropical forests: patterns, mechanisms, and consequences. Insect Outbreaks Revisit. 2012. 10.1002/9781118295205.ch11.
- 2.Kneeshaw D, Sturtevant BR, Cooke B, Work T, Pureswaran D, De Grandpre L, et al. Insect disturbances in forest ecosystems. In: Routledge Handb. For. Ecol; 2015. p. 93–113.
- 3.Berner LT, Law BE, Meddens AJH, Hicke JA. Tree mortality from fires, bark beetles, and timber harvest during a hot and dry decade in the western United States (2003-2012). Environ Res Lett. 2017;12. 10.1088/1748-9326/aa6f94.
- 4.Liebhold AM, Brockerhoff EG, Kalisz S, Nuñez MA, Wardle DA, Wingfield MJ. Biological invasions in forest ecosystems. Biol Invasions. 2017;19:3437–3458. doi: 10.1007/s10530-017-1458-5. [DOI] [Google Scholar]
- 5.Mezei P, Jakuš R, Pennerstorfer J, Havašová M, Škvarenina J, Ferenčík J, Slivinský J, Bičárová S, Bilčík D, Blaženec M, Netherer S. Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—an infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agric For Meteorol. 2017;242:85–95. doi: 10.1016/j.agrformet.2017.04.004. [DOI] [Google Scholar]
- 6.van Lierop P, Lindquist E, Sathyapala S, Franceschini G. Global forest area disturbance from fire, insect pests, diseases and severe weather events. For Ecol Manag. 2015;352:78–88. doi: 10.1016/j.foreco.2015.06.010. [DOI] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations (FAO) State of the World’s Forests. 2014;2014:119. [Google Scholar]
- 8.Liu EY, Lantz VA, MacLean DA, Hennigar C. Economics of early intervention to suppress a potential spruce budworm outbreak on crown land in New Brunswick, Canada. Forests. 2019;10:481. doi: 10.3390/f10060481. [DOI] [Google Scholar]
- 9.Westman WE. Measuring the Inertia and Resilience of Ecosystems. Bioscience. 1978;28:705–710. doi: 10.2307/1307321. [DOI] [Google Scholar]
- 10.Peirson H. The place of entomology in silviculture. J For. 1925;23:372–375. [Google Scholar]
- 11.Salman K, Bongberg J. Logging high-risk trees to control insects in the pine stands of northeastern California. J For. 1942;40:533–539. [Google Scholar]
- 12.Mott D. Future pest management systems. In Pest Manag. 21st century~ Idaho Found Nat. Res Ser. 1973;2:73–92. [Google Scholar]
- 13.Dahlsten DL, Dreistadt SH. Forest pest management sociopolitics. For Ecol Manag. 1991;39:289–297. doi: 10.1016/0378-1127(91)90184-W. [DOI] [Google Scholar]
- 14.Grimm V, Wissel C. Babel, or the ecological stability discussions: an inventory and analysis of terminology and a guide for avoiding confusion. Oecologia. 1997;109(3):323–334. doi: 10.1007/s004420050090. [DOI] [PubMed] [Google Scholar]
- 15.Baskerville GL. Spruce budworm: the answer is forest management: or is it? For Chron. 1975;51:157–160. doi: 10.5558/tfc51157-4. [DOI] [Google Scholar]
- 16.MacLean DA, Erdle TA, MacKinnon WE, Porter KB, Beaton KP, Cormier G, Morehouse S, Budd M. The spruce budworm decision support system: forest protection planning to sustain long-term wood supply. Can J For Res. 2001;31:1742–1757. doi: 10.1139/x01-102. [DOI] [Google Scholar]
- 17.Shore TL, Riel WG, Safranyik L. A decision support system for the mountain pine beetle in lodgepole pine stands. Work Jt Meet Entomol Soc Canada Br Columbia. 1996;260:25–30. [Google Scholar]
- 18.Dymond CC, Wulder MA, Shore TL, Nelson T, Boots B, Riel BG. Evaluation of risk assessment of mountain pine beetle infestations. West J Appl For. 2006;21:5–13. doi: 10.1093/wjaf/21.1.5. [DOI] [Google Scholar]
- 19.•• Bentz B, Bonello P, Delb H, Fettig C, Poland T, Pureswaran D, et al. Advances in understanding and managing insect pests of forest trees. In: Stanturf J, editor. Achieving sustainable management of boreal and temperate forests. Cambridge: Burleigh Dodds Science Publishing Ltd; 2019. p. 515–85. This paper is a strong review of many insect pests from around the world and how their behaviour and the damage they have caused has changed with human-caused drivers.
- 20.Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. BioScience. 2008;58:501–517. doi: 10.1641/B580607. [DOI] [Google Scholar]
- 21.Bentz BJ, Régnière J, Fettig CJ, Hansen EM, Hayes JL, Hicke JA, Kelsey RG, Negrón JF, Seybold SJ. Climate change and bark beetles of the western United States and Canada: direct and indirect effects. BioScience. 2010;60(8):602–613. doi: 10.1525/bio.2010.60.8.6. [DOI] [Google Scholar]
- 22.Mezei P, Blaženec M, Grodzki W, Škvarenina J, Jakuš R. Influence of different forest protection strategies on spruce tree mortality during a bark beetle outbreak. Ann For Sci. 2017;74(4):65. doi: 10.1007/s13595-017-0663-9. [DOI] [Google Scholar]
- 23.Candau J-N, Abt V, Keatley L. Bioclimatic analysis of declining aspen stands in northeastern Ontario. In: Northeastern Ontario. Forestry Research Report No. 154., OMNR Sault-Ste-Marie, Ontario; 2002.
- 24.Cooke BJ, Macquarrie CJK, Lorenzetti F. The dynamics of forest tent caterpillar outbreaks across east-central Canada. Ecography. 2012;35:422–435. doi: 10.1111/j.1600-0587.2011.07083.x. [DOI] [Google Scholar]
- 25.Moreau G. Past and present outbreaks of the balsam fir sawfly in western Newfoundland: an analytical review. For Ecol Manag. 2006;221:215–219. doi: 10.1016/j.foreco.2005.09.020. [DOI] [Google Scholar]
- 26.Groenen F, Meurisse N. Historical distribution of the oak processionary moth Thaumetopoea processionea in Europe suggests recolonization instead of expansion. Agric For Entomol. 2012;14:147–155. doi: 10.1111/j.1461-9563.2011.00552.x. [DOI] [Google Scholar]
- 27.Blais JR. Trends in the frequency, extent, and severity of spruce budworm outbreaks in eastern Canada. Can J For Res. 1983;13:539–547. doi: 10.1139/x83-079. [DOI] [Google Scholar]
- 28.Swetnam TW, Lynch AM. Multicentury, regional-scale patterns of western spruce budworm outbreaks. Ecol Monogr. 1993;63:399–424. doi: 10.2307/2937153. [DOI] [Google Scholar]
- 29.Morin H, Jardon Y, Gagnon RA. Relationship between spruce budworm outbreaks and forest dynamics in eastern North America. In: Plant Disturb. Ecol: Academic Press; 2007. p. 555–77.
- 30.Nealis VG, Noseworthy MK, Turnquist R, Waring VR. Balancing risks of disturbance from mountain pine beetle and western spruce budworm. Can J For Res. 2009;39:839–848. doi: 10.1139/X09-014. [DOI] [Google Scholar]
- 31.Pureswaran DS, De Grandpré LD, Paré D, Taylor A, Barrette M, Morin H, Régnière J, Kneeshaw DD. Climate-induced changes in host tree-insect phenology may drive ecological state-shift in boreal forests. Ecology. 2015;96:1480–1491. doi: 10.1890/13-2366.1. [DOI] [Google Scholar]
- 32.Miller A, Rusnock P. The rise and fall of the silvicultural hypothesis in spruce budworm (Choristoneura fumiferana) management in eastern Canada. For Ecol Manag. 1993;61:171–189. doi: 10.1016/0378-1127(93)90197-U. [DOI] [Google Scholar]
- 33.Peters DPC, Pielke RA, Bestelmeyer BT, Allen CD, Munson-McGee S, Havstad KM. Cross-scale interactions, nonlinearities, and forecasting catastrophic events. Proc Natl Acad Sci U S A. 2004;101:15130–15135. doi: 10.1073/pnas.0403822101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holling CS, Meffe GK. Command and control and the pathology of natural resource management. Conserv Biol. 1996;10:328–337. doi: 10.1046/j.1523-1739.1996.10020328.x. [DOI] [Google Scholar]
- 35.Wermelinger B. Ecology and management of the spruce bark beetle Ips typographus - a review of recent research. For Ecol Manag. 2004;202:67–82. doi: 10.1016/j.foreco.2004.07.018. [DOI] [Google Scholar]
- 36.Fettig CJ, Hilszczański J. Management strategies for bark beetles in conifer forests. Bark Beetles Biol Ecol Nativ Invasive Species. In Bark Beetles Academic Press, London 555-584. 2015. 10.1016/B978-0-12-417156-5.00014-9.
- 37.Robert LE, Sturtevant BR, Cooke BJ, James PMA, Fortin MJ, Townsend PA, Wolter PT, Kneeshaw D. Landscape host abundance and configuration regulate periodic outbreak behavior in spruce budworm Choristoneura fumiferana. Ecography. 2018;41:1556–1571. doi: 10.1111/ecog.03553. [DOI] [Google Scholar]
- 38.Robert LE, Cooke B, Surtevant BS, James PMA, Fortin MJ, Kneeshaw DD. Forest landscape structure influences the cyclic-eruptive spatial dynamics of forest tent caterpillar outbreaks. Ecosphere. 2020;11(8):e03096. doi: 10.1002/ecs2.3096. [DOI] [Google Scholar]
- 39.Logan JA, Régnière J, Powell JA. Assessing the impacts of global warming on forest pest dynamics. Front Ecol Environ. 2003;1(3):130–137. doi: 10.1890/1540-9295(2003)001[0130:ATIOGW]2.0.CO;2. [DOI] [Google Scholar]
- 40.Candau JN, Fleming RA. Forecasting the response of spruce budworm defoliation to climate change in Ontario. Can J For Res. 2011;41:1948–1960. doi: 10.1139/x11-134. [DOI] [Google Scholar]
- 41.Pureswaran DS, Roques A, Battisti A. Forest insects and climate change. Curr For Reports. 2018;4:35–50. [Google Scholar]
- 42.Godefroid M, Meurisse N, Groenen F, Kerdelhué C, Rossi JP. Current and future distribution of the invasive oak processionary moth. Biol Invasions. 2020;22:523–534. doi: 10.1007/s10530-019-02108-4. [DOI] [Google Scholar]
- 43.Johns RC, Bowden JJ, Carleton DR, Cooke BJ, Edwards S, Emilson EJS, James PMA, Kneeshaw D, MacLean DA, Martel V, Moise ERD, Mott GD, Norfolk CJ, Owens E, Pureswaran DS, Quiring DT, Régnière J, Richard B, Stastny M. A conceptual framework for the spruce budworm early intervention strategy: can outbreaks be stopped? Forests. 2019;10(10):910. doi: 10.3390/f10100910. [DOI] [Google Scholar]
- 44.Whitehead RJ, Russo GL, Hawkes BC, Armitage OB. A silvicultural assessment of 10 lodgepole pine stands after partial cutting to reduce susceptibility to mountain pine beetle. Victoria: Canadian Forest Service; 2007. [Google Scholar]
- 45.Fettig CJ, Klepzig KD, Billings RF, Munson AS, Nebeker TE, Negrón JF, Nowak JT. The effectiveness of vegetation management practices for prevention and control of bark beetle infestations in coniferous forests of the western and southern United States. For Ecol Manag. 2007;238:24–53. doi: 10.1016/j.foreco.2006.10.011. [DOI] [Google Scholar]
- 46.Six DL, Biber E, Long E. Management for mountain pine beetle outbreak suppression: does relevant science support current policy? Forests. 2014;5:103–133. doi: 10.3390/f5010103. [DOI] [Google Scholar]
- 47.Heidger CM, Lieutier F. Possibilities to utilize tree resistance to insects in forest pest management in central and western Europe. In: Wagner MR, Clancy KM, Lieutier F, Paine TD, editors. Mechanisms and Deployment of Resistance in Trees to Insects. Dordrecht: Springer; 2005. p. 239–63. 10.1007/0-306-47596-0_11.
- 48.Clarke SR, Riggins JJ, Stephen FM. Forest management and southern pine beetle outbreaks: a historical perspective. For Sci. 2016;62(2):166–180. doi: 10.5849/forsci.15-071. [DOI] [Google Scholar]
- 49.Bauce É. One and two years impact of commercial thinning on spruce budworm feeding ecology and host tree foliage production and chemistry. For Chron. 1996;72:393–398. doi: 10.5558/tfc72393-4. [DOI] [Google Scholar]
- 50.MacLean DA, Piene H. Spatial and temporal patterns of balsam fir mortality in spaced and unspaced stands caused by spruce budworm defoliation. Can J For Res. 1995;25:902–911. doi: 10.1139/x95-099. [DOI] [Google Scholar]
- 51.D’Amato AW, Troumbly SJ, Saunders MR, Puettmann KJ, Albers MA. Growth and survival of Picea glauca following thinning of plantations affected by eastern spruce budworm. North J Appl For. 2011;28:72–78. doi: 10.1093/njaf/28.2.72. [DOI] [Google Scholar]
- 52.Jactel H, Brockerhoff EG. Tree diversity reduces herbivory by forest insects. Ecol Lett. 2007;10:835–848. doi: 10.1111/j.1461-0248.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- 53.Jactel H, Brockerhoff E, Duelli P. A test of the biodiversity-stability theory: meta-analysis of tree species diversity effects on insect pest infestations, and re-examination of responsible factors. In: Scherer-Lorenzen M, Körner C, Schulze ED, editors. Forest Diversity and Function. Ecological Studies (Analysis and Synthesis), vol. 176. Berlin, Heidelberg: Springer; 2005. p. 235–62. 10.1007/3-540-26599-6_12.
- 54.Taylor SP, Alfaro RI, DeLong C, Rankin L. The effects of overstory shading on white pine weevil damage to white spruce and its effects on spruce growth rates. Can J For Res. 1996;26(2):306–312. doi: 10.1139/x26-034. [DOI] [Google Scholar]
- 55.van Asch M, Visser ME. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu Rev Entomol. 2007;52:37–55. doi: 10.1146/annurev.ento.52.110405.091418. [DOI] [PubMed] [Google Scholar]
- 56.Su Q, MacLean DA, Needham TD. The influence of hardwood content on balsam fir defoliation by spruce budworm. Can J For Res. 1996;26:1620–1628. doi: 10.1139/x26-182. [DOI] [Google Scholar]
- 57.Campbell EM, MacLean DA, Bergeron Y. The severity of budworm-caused growth reductions in balsam fir/spruce stands varies with the hardwood content of surrounding forest landscapes. For Sci. 2008;54:195–205. [Google Scholar]
- 58.Klapwijk MJ, Björkman C. Mixed forests to mitigate risk of insect outbreaks. Scand J For Res. 2018;33:772–780. doi: 10.1080/02827581.2018.1502805. [DOI] [Google Scholar]
- 59.Rodríguez A, Pohjoismäki JLO, Kouki J. Diversity of forest management promotes parasitoid functional diversity in boreal forests. Biol Conserv. 2019;208:108205. doi: 10.1016/j.biocon.2019.108205. [DOI] [Google Scholar]
- 60.Castagneyrol B, Jactel H, Vacher C, Brockerhoff EG, Koricheva J. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J Appl Ecol. 2014;51(1):134–141. doi: 10.1111/1365-2664.12175. [DOI] [Google Scholar]
- 61.Bognounou F, De Grandprè L, Pureswaran DS, Kneeshaw D. Temporal variation in plant neighborhood effects on the defoliation of primary and secondary hosts by an insect pest. Ecosphere. 2017;8(3):301769. doi: 10.1002/ecs2.1759. [DOI] [Google Scholar]
- 62.White JA, Whitham TG. Associational susceptibility of cottonwood to a box elder herbivore. Ecology. 2000;81:1795–1803. doi: 10.1890/0012-9658(2000)081[1795:ASOCTA]2.0.CO;2. [DOI] [Google Scholar]
- 63.Schuldt A, Baruffol M, Böhnke M, Bruelheide H, Härdtle W, Lang AC, Nadrowski K, von Oheimb G, Voigt W, Zhou H, Assmann T, Fridley J. Tree diversity promotes insect herbivory in subtropical forests of south-east China. J Ecol. 2010;98:917–926. doi: 10.1111/j.1365-2745.2010.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuldt A, Bruelheide H, Härdtle W, Assmann T, Li Y, Ma K, von Oheimb G, Zhang J. Early positive effects of tree species richness on herbivory in a large-scale forest biodiversity experiment influence tree growth. J Ecol. 2015;103:563–571. doi: 10.1111/1365-2745.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haase J, Castagneyrol B, Cornelissen JHC, Ghazoul J, Kattge J, Koricheva J, Scherer-Lorenzen M, Morath S, Jactel H. Contrasting effects of tree diversity on young tree growth and resistance to insect herbivores across three biodiversity experiments. Oikos. 2015;124:1674–1685. doi: 10.1111/oik.02090. [DOI] [Google Scholar]
- 66.Wein A, Bauhus J, Bilodeau-Gauthier S, Scherer-Lorenzen M, Nock C, Staab M. Tree species richness promotes invertebrate herbivory on congeneric native and exotic tree saplings in a young diversity experiment. PLoS One. 2016;11(12):e0168751. doi: 10.1371/journal.pone.0168751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castagneyrol B, Moreira X, Jactel H. Drought and plant neighbourhood interactively determine herbivore consumption and performance. Sci Rep. 2018;8:5930. doi: 10.1038/s41598-018-24299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z. Associational resistance and associational susceptibility: having right or wrong neighbors. Annu Rev Ecol Evol Syst. 2009;40:1–20. doi: 10.1146/annurev.ecolsys.110308.120242. [DOI] [Google Scholar]
- 69.Vehviläinen H, Koricheva J, Ruohomäki K. Tree species diversity influences herbivore abundance and damage: meta-analysis of long-term forest experiments. Oecologia. 2007;152:287–298. doi: 10.1007/s00442-007-0673-7. [DOI] [PubMed] [Google Scholar]
- 70.Huber DPW, Aukema BH, Hodgkinson RS, Lindgren BS. Successful colonization, reproduction, and new generation emergence in live interior hybrid spruce Picea engelmannii × glauca by mountain pine beetle Dendroctonus ponderosae. Agric For Entomol. 2009;11:83–89. doi: 10.1111/j.1461-9563.2008.00411.x. [DOI] [Google Scholar]
- 71.Nealis VG, Régnière J. Insect-host relationships influencing disturbance by the spruce budworm in a boreal mixedwood forest. Can J For Res. 2004;34:1870–1882. doi: 10.1139/x04-061. [DOI] [Google Scholar]
- 72.Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Q Rev Biol. 1992;67(3):283–335. doi: 10.1086/417659. [DOI] [Google Scholar]
- 73.Jactel H, Bauhus J, Boberg J, Bonal D, Castagneyrol B, Gardiner B, Gonzalez-Olabarria JR, Koricheva J, Meurisse N, Brockerhoff EG. Tree diversity drives forest stand resistance to natural disturbances. Curr For Reports. 2017;3:223–243. [Google Scholar]
- 74.Guyot V, Castagneyrol B, Vialatte A, Deconchat M, Jactel H. Tree diversity reduces pest damage in mature forests across Europe. Biol Lett. 2016;12(4):20151037. doi: 10.1098/rsbl.2015.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hennigar CR, MacLean DA. Spruce budworm and management effects on forest and wood product carbon for an intensively managed forest. Can J For Res. 2010;40:1736–1750. doi: 10.1139/X10-104. [DOI] [Google Scholar]
- 76.Muzika RM, Liebhold AM, Twery MJ. Dynamics of twolined chestnut borer Agrilus bilineatus as influenced by defoliation and selection thinning. Agric For Entomol. 2000;2:283–289. doi: 10.1046/j.1461-9563.2000.00077.x. [DOI] [Google Scholar]
- 77.Bone C, Wulder MA, White JC, Robertson C, Nelson TA. A GIS-based risk rating of forest insect outbreaks using aerial overview surveys and the local Moran’s I statistic. Appl Geogr. 2013;40:161–170. doi: 10.1016/j.apgeog.2013.02.011. [DOI] [Google Scholar]
- 78.Bentz BJ, Amman GD, Logan JA. A critical assessment of risk classification systems for the mountain pine beetle. For Ecol Manag. 1993;61(3-4):349–366. doi: 10.1016/0378-1127(93)90211-5. [DOI] [Google Scholar]
- 79.Simard M, Powell EN, Raffa KF, Turner MG. What explains landscape patterns of tree mortality caused by bark beetle outbreaks in Greater Yellowstone? Glob Ecol Biogeogr. 2012;21(5):556–567. doi: 10.1111/j.1466-8238.2011.00710.x. [DOI] [Google Scholar]
- 80.Stadelmann G, Bugmann H, Wermelinger B, Meier F, Bigler C. A predictive framework to assess spatio-temporal variability of infestations by the European spruce bark beetle. Ecography. 2013;36(11):1208–1217. doi: 10.1111/j.1600-0587.2013.00177.x. [DOI] [Google Scholar]
- 81.Mezei P, Potterf M, Škvarenina J, Rasmussen JG, Jakuš R. Potential solar radiation as a driver for bark beetle infestation on a landscape scale. Forests. 2019;10(4):604. doi: 10.3390/f10070604. [DOI] [Google Scholar]
- 82.Kärvemo S, Van Boeckel TP, Gilbert M, Grégoire JC, Schroeder M. Large-scale risk mapping of an eruptive bark beetle - importance of forest susceptibility and beetle pressure. For Ecol Manag. 2014;318:158–166. doi: 10.1016/j.foreco.2014.01.025. [DOI] [Google Scholar]
- 83.Kärvemo S, Rogell B, Schroeder M. Dynamics of spruce bark beetle infestation spots: importance of local population size and landscape characteristics after a storm disturbance. For Ecol Manag. 2014;334:232–340. doi: 10.1016/j.foreco.2014.09.011. [DOI] [Google Scholar]
- 84.Seidl R, Donato DC, Raffa KF, Turner MG. Spatial variability in tree regeneration after wildfire delays and dampens future bark beetle outbreaks. Proc Natl Acad Sci U S A. 2016;113(46):13075–13080. doi: 10.1073/pnas.1615263113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seidl R, Müller J, Hothorn T, Bässler C, Heurich M, Kautz M. Small beetle, large-scale drivers: how regional and landscape factors affect outbreaks of the European spruce bark beetle. J Appl Ecol. 2016;53(2):530–540. doi: 10.1111/1365-2664.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hansen WD, Chapin FS, Naughton HT, Rupp TS, Verbyla D. Forest-landscape structure mediates effects of a spruce bark beetle (Dendroctonus rufipennis) outbreak on subsequent likelihood of burning in Alaskan boreal forest. For Ecol Manag. 2016;369:38–46. doi: 10.1016/j.foreco.2016.03.036. [DOI] [Google Scholar]
- 87.Lausch A, Fahse L, Heurich M. Factors affecting the spatio-temporal dispersion of Ips typographus (L.) in Bavarian Forest National Park: a long-term quantitative landscape-level analysis. For Ecol Manag. 2011;262(2):233–245. doi: 10.1016/j.foreco.2010.10.012. [DOI] [Google Scholar]
- 88.Nowak JT, Meeker JR, Coyle DR, Steiner CA, Brownie C. Southern pine beetle infestations in relation to forest stand conditions, previous thinning, and prescribed burning: evaluation of the Southern Pine Beetle Prevention Program. J For. 2015;113(5):454–462. doi: 10.5849/jof.15-002. [DOI] [Google Scholar]
- 89.Asaro C, Nowak JT, Elledge A. Why have southern pine beetle outbreaks declined in the southeastern U.S. with the expansion of intensive pine silviculture? A brief review of hypotheses. For Ecol Manag. 2017;391:338–348. doi: 10.1016/j.foreco.2017.01.035. [DOI] [Google Scholar]
- 90.Gomez DF, Sathyapala S, Hulcr J. Towards sustainable forest management in Central America: review of southern pine beetle (Dendroctonus frontalis Zimmermann) outbreaks, their causes, and solutions. Forests. 2020;11(2):173. doi: 10.3390/f11020173. [DOI] [Google Scholar]
- 91.Cooke BJ, Roland J. Trembling aspen responses to drought and defoliation by forest tent caterpillar and reconstruction of recent outbreaks in Ontario. Can J For Res. 2007;37(9):1586–1598. doi: 10.1139/X07-015. [DOI] [Google Scholar]
- 92.Carson WP, Cronin JP, Long ZT. A general rule for predicting when insects will have strong top-down effects on plant communities: on the relationship between insect outbreaks and host concentration. In: Insects and Ecosystem Function. Berlin: Springer; 2008. p. 193–211. 10.1007/978-3-540-74004-9_10.
- 93.Lindenmayer DB, Wood JT, Cunningham RB, Crane M, Macgregor C, Michael D, Montague-Drake R. Experimental evidence of the effects of a changed matrix on conserving biodiversity within patches of native forest in an industrial plantation landscape. Landsc Ecol. 2009;24(8):1091–1103. doi: 10.1007/s10980-008-9244-5. [DOI] [Google Scholar]
- 94.Charbonneau D, Lorenzetti F, Doyon F, Mauffette Y. The influence of stand and landscape characteristics on forest tent caterpillar (Malacosoma disstria) defoliation dynamics: the case of the 1999-2002 outbreak in northwestern Quebec. Can J For Res. 2012;42(10):1827–1836. doi: 10.1139/x2012-126. [DOI] [Google Scholar]
- 95.Bouchard M, Auger I. Influence of environmental factors and spatio-temporal covariates during the initial development of a spruce budworm outbreak. Landsc Ecol. 2014;29(1):111–126. doi: 10.1007/s10980-013-9966-x. [DOI] [Google Scholar]
- 96.Foster JR. Xylem traits, leaf longevity and growth phenology predict growth and mortality response to defoliation in northern temperate forests. Tree Physiol. 2017;37(9):1151–1165. doi: 10.1093/treephys/tpx043. [DOI] [PubMed] [Google Scholar]
- 97.Liebhold A, Koenig WD, Bjørnstad ON. Spatial synchrony in population dynamics. Annu Rev Ecol Evol Syst. 2004;35:467–490. doi: 10.1146/annurev.ecolsys.34.011802.132516. [DOI] [Google Scholar]
- 98.Hartl-Meier C, Esper J, Liebhold A, Konter O, Rothe A, Büntgen U. Effects of host abundance on larch budmoth outbreaks in the European Alps. Agric For Entomol. 2017;19(4):376–387. doi: 10.1111/afe.12216. [DOI] [Google Scholar]
- 99.Haynes KJ, Liebhold AM, Johnson DM. Spatial analysis of harmonic oscillation of gypsy moth outbreak intensity. Oecologia. 2009;159(2):249–256. doi: 10.1007/s00442-008-1207-7. [DOI] [PubMed] [Google Scholar]
- 100.Peltonen M, Liebhold AM, Bjørnstad ON, Williams DW. Spatial synchrony in forest insect outbreaks: roles of regional stochasticity and dispersal. Ecology. 2002;83(11):3120–3129. doi: 10.1890/0012-9658(2002)083[3120:SSIFIO]2.0.CO;2. [DOI] [Google Scholar]
- 101.Johnson DM, Bjørnstad ON, Liebhold AM. Landscape geometry and travelling waves in the larch budmoth. Ecol Lett. 2004;7(10):967–974. doi: 10.1111/j.1461-0248.2004.00659.x. [DOI] [Google Scholar]
- 102.Boulanger Y, Fabry F, Kilambi A, Pureswaran DS, Sturtevant BR, Saint-Amant R. The use of weather surveillance radar and high-resolution three dimensional weather data to monitor a spruce budworm mass exodus flight. Agric For Meteorol. 2017;234:127–135. doi: 10.1016/j.agrformet.2016.12.018. [DOI] [Google Scholar]
- 103.Larroque J, Legault S, Johns R, Lumley L, Cusson M, Renaut S, Levesque RC, James PMA. Temporal variation in spatial genetic structure during population outbreaks: distinguishing among different potential drivers of spatial synchrony. Evol Appl. 2019;12(10):1931–1945. doi: 10.1111/eva.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.James PMA, Fortin MJ, Sturtevant BR, Fall A, Kneeshaw D. Modelling spatial interactions among fire, spruce budworm, and logging in the boreal forest. Ecosystems. 2011;14:60–75. doi: 10.1007/s10021-010-9395-5. [DOI] [Google Scholar]
- 105.Bouchard M, Aquilué N, Périé C, Lambert MC. Tree species persistence under warming conditions: a key driver of forest response to climate change. For Ecol Manag. 2019;442:96–104. doi: 10.1016/j.foreco.2019.03.040. [DOI] [Google Scholar]
- 106.Roland J. Large-scale forest fragmentation increases the duration of tent caterpillar outbreak. Oecologia. 1993;93(1):25–30. doi: 10.1007/BF00321186. [DOI] [PubMed] [Google Scholar]
- 107.Roland J. Are the “seeds” of spatial variation in cyclic dynamics apparent in spatially-replicated short time-series? An example from the forest tent caterpillar. Ann Zool Fenn. 2005;42:397–407. [Google Scholar]
- 108.Roland J, Taylor PD. Insect parasitoid species respond to forest structure at different spatial scales. Nature. 1997;386:710–713. doi: 10.1038/386710a0. [DOI] [Google Scholar]
- 109.Rothman LD, Roland J. Forest fragmentation and colony performance of forest tent caterpillar. Ecography. 1998;21(4):383–391. doi: 10.1111/j.1600-0587.1998.tb00403.x. [DOI] [Google Scholar]
- 110.Roth D, Roland J, Roslin T. Parasitoids on the loose - experimental lack of support of the parasitoid movement hypothesis. Oikos. 2006;115(2):277–285. doi: 10.1111/j.2006.0030-1299.15252. [DOI] [Google Scholar]
- 111.Wood DM, Parry D, Yanai RD, Pitel NE. Forest fragmentation and duration of forest tent caterpillar (Malacosoma disstria Hübner) outbreaks in northern hardwood forests. For Ecol Manag. 2010;260(7):1193–1197. doi: 10.1016/j.foreco.2010.07.011. [DOI] [Google Scholar]
- 112.Wildemeersch M, Franklin O, Seidl R, Rogelj J, Moorthy I, Thurner S. Modelling the multi-scaled nature of pest outbreaks. Ecol Model. 2019;409:108745. doi: 10.1016/j.ecolmodel.2019.108745. [DOI] [Google Scholar]
- 113.Seidl R, Albrich K, Thom D, Rammer W. Harnessing landscape heterogeneity for managing future disturbance risks in forest ecosystems. J Environ Manag. 2018;209:46–56. doi: 10.1016/j.jenvman.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnstone JF, Chapin FS. Non-equilibrium succession dynamics indicate continued northern migration of lodgepole pine. Glob Chang Biol. 2003;9:1401–1409. doi: 10.1046/j.1365-2486.2003.00661.x. [DOI] [Google Scholar]
- 115.Ayres MP, Lombardero MJ. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci Total Environ. 2000;262:263–286. doi: 10.1016/S0048-9697(00)00528-3. [DOI] [PubMed] [Google Scholar]
- 116.Jactel H, Koricheva J, Castagneyrol B. Responses of forest insect pests to climate change: not so simple. Curr Opin Insect Sci. 2019;35:103–108. doi: 10.1016/j.cois.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 117.Büntgen U, Liebhold A, Nievergelt D, Wermelinger B, Roques A, Reinig F, Krusic PJ, Piermattei A, Egli S, Cherubini P, Esper J. Return of the moth: rethinking the effect of climate on insect outbreaks. Oecologia. 2020;192(2):543–552. doi: 10.1007/s00442-019-04585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carroll AL, Régnière J, Logan JA, Taylor SW, Bentz BJ, Powell JA. Impacts of climate change on range expansion by the mountain pine beetle. In: Mountain Pine Beetle Working Paper 2006-14, Canadian Forest Service. Victoria, BC20 p; 2006.
- 119.Bentz BJ, Duncan JP, Powell JA. Elevational shifts in thermal suitability for mountain pine beetle population growth in a changing climate. Forestry. 2016;89:271–283. doi: 10.1093/forestry/cpv054. [DOI] [Google Scholar]
- 120.Safranyik L, Carroll AL, Régnière J, Langor DW, Riel WG, Shore TL, Peter B, Cooke BJ, Nealis VG, Taylor SW. Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can Entomol. 2010;142:415–442. doi: 10.4039/n08-CPA01. [DOI] [Google Scholar]
- 121.Showalter DN, Raffa KF, Sniezko RA, Herms DA, Liebhold AM, Smith JA, Bonello P. Strategic development of tree resistance against forest pathogen and insect invasions in defense-free space. Front Ecol Evol. 2018;6:124. doi: 10.3389/fevo.2018.00124. [DOI] [Google Scholar]
- 122.Ward NL, Masters GJ. Linking climate change and species invasion: an illustration using insect herbivores. Glob Chang Biol. 2007;13(8):1605–1615. doi: 10.1111/j.1365-2486.2007.01399.x. [DOI] [Google Scholar]
- 123.Allen CD. Climate-induced forest dieback: an escalating global phenomenon? Unasylva. 2009;231(232):43–49. [Google Scholar]
- 124.Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH(T), Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim JH, Allard G, Running SW, Semerci A, Cobb N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag. 2010;259:660–684. doi: 10.1016/j.foreco.2009.09.001. [DOI] [Google Scholar]
- 125.Anderegg WRL, Anderegg LDL, Kerr KL, Trugman AT. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Glob Chang Biol. 2019;25:3793–3802. doi: 10.1111/gcb.14771. [DOI] [PubMed] [Google Scholar]
- 126.McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol. 2011;26:523–532. doi: 10.1016/j.tree.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 127.McDowell NG, Michaletz ST, Bennett KE, Solander KC, Xu C, Maxwell RM, Middleton RS. Predicting chronic climate-driven disturbances and their mitigation. Trends Ecol Evol. 2018;33:15–27. doi: 10.1016/j.tree.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 128.Panayotov M, Georgiev D. Dynamics in the Ips typhographus outbreak following the 2001 windthrow in Bistrishko braniste reserve, Bulgaria. Silva Balc. 2012;13:38–48. [Google Scholar]
- 129.Hogg EH, Brandt JP, Kochtubajda B. Growth and dieback of aspen forests in northwestern Alberta, Canada, in relation to climate and insects. Can J For Res. 2002;32:823–832. doi: 10.1139/x01-152. [DOI] [Google Scholar]
- 130.Kolb TE, Fettig CJ, Ayres MP, Bentz BJ, Hicke JA, Mathiasen R, Stewart JE, Weed AS. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For Ecol Manag. 2016;380:321–334. doi: 10.1016/j.foreco.2016.04.051. [DOI] [Google Scholar]
- 131.Itter MS, D’Orangeville L, Dawson A, Kneeshaw D, Duchesne L, Finley AO. Boreal tree growth exhibits decadal-scale ecological memory to drought and insect defoliation, but no negative response to their interaction. J Ecol. 2019;107:1288–1301. doi: 10.1111/1365-2745.13087. [DOI] [Google Scholar]
- 132.De Grandpré L, Kneeshaw DD, Perigon S, Boucher D, Marchand M, Pureswaran D, Girardin MP. Adverse climatic periods precede and amplify defoliator-induced tree mortality in eastern boreal North America. J Ecol. 2019;107(1):452–467. doi: 10.1111/1365-2745.13012. [DOI] [Google Scholar]
- 133.Balducci L, Fierravanti A, Rossi S, Delzon S, De Grandpré L, Kneeshaw DD, Deslauriers A. The paradox of defoliation: declining tree water status with increasing soil water content. Agric For Meteorol. 2020;290:108025. doi: 10.1016/j.agrformet.2020.108025. [DOI] [Google Scholar]
- 134.Andrew NR, Hill SJ. Effect of climate change on insect pest management. In: Environmental Pest Management: Challenges for Agronomists, Ecologists, Economists and Policymakers; 2017. p. 195–223.
- 135.Eveleigh ES, McCann KS, McCarthy PC, et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc Natl Acad Sci U S A. 2007;104:16976–16981. doi: 10.1073/pnas.0704301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Régnière J, Delisle J, Pureswaran DS, Trudel R. Mate-finding Allee effect in spruce budworm population dynamics. Entomol Exp Appl. 2013;146(1):112–122. doi: 10.1111/eea.12019. [DOI] [Google Scholar]
- 137.Battisti A, Larsson S. Climate change and insect pest distribution range. In: Climate Change and Insect Pests, CABI; 2015. p. 1–15. 10.1079/9781780643786.0001.
- 138.Raffa KF, Powell EN, Townsend PA. Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proc Natl Acad Sci U S A. 2013;110:2193–2198. doi: 10.1073/pnas.1216666110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DeRose RJ, Long JN. Resistance and resilience: a conceptual framework for silviculture. For Sci. 2014;60:1205–1212. doi: 10.5849/forsci.13-507. [DOI] [Google Scholar]
- 140.Budde KB, Nielsen LR, Ravn HP, Kjær ED. The natural evolutionary potential of tree populations to cope with newly introduced pests and pathogens—lessons learned from forest health catastrophes in recent decades. Curr For Reports. 2016;2:18–29. doi: 10.1007/s40725-016-0029-9. [DOI] [Google Scholar]
- 141.Jõgiste K, Korjus H, Stanturf JA, Frelich LE, Baders E, Donis J, Jansons A, Kangur A, Köster K, Laarmann D, Maaten T, Marozas V, Metslaid M, Nigul K, Polyachenko O, Randveer T, Vodde F. Hemiboreal forest: natural disturbances and the importance of ecosystem legacies to management. Ecosphere. 2017;8(2):e01706. doi: 10.1002/ecs2.1706. [DOI] [Google Scholar]
- 142.Cohen WB, Spies TA, Alig RJ, Oetter DR, Maiersperger TK, Fiorella M. Characterizing 23 years (1972-95) of stand replacement disturbance in western Oregon forests with Landsat imagery. Ecosystems. 2002;5:122–137. doi: 10.1007/s10021-001-0060-X. [DOI] [Google Scholar]
- 143.Perring MP, Bernhardt-Römermann M, Baeten L, Midolo G, Blondeel H, Depauw L, Landuyt D, Maes SL, de Lombaerde E, Carón MM, Vellend M, Brunet J, Chudomelová M, Decocq G, Diekmann M, Dirnböck T, Dörfler I, Durak T, de Frenne P, Gilliam FS, Hédl R, Heinken T, Hommel P, Jaroszewicz B, Kirby KJ, Kopecký M, Lenoir J, Li D, Máliš F, Mitchell FJG, Naaf T, Newman M, Petřík P, Reczyńska K, Schmidt W, Standovár T, Świerkosz K, van Calster H, Vild O, Wagner ER, Wulf M, Verheyen K. Global environmental change effects on plant community composition trajectories depend upon management legacies. Glob Chang Biol. 2018;24:1722–1740. doi: 10.1111/gcb.14030. [DOI] [PubMed] [Google Scholar]
- 144.Taylor SW, Carroll AL. Disturbance, forest age, and mountain pine beetle outbreak dynamics in BC: a historical perspective. In: Mountain Pine Beetle Symposium: Challenges and Solutions, Canadian Forest Service; 2003. p. 41–51.
- 145.Bouchard M, Pothier D. Simulations of the effects of changes in mean fire return intervals on balsam fir abundance, and implications for spruce budworm outbreaks. Ecol Model. 2008;218(3-4):207–218. doi: 10.1016/j.ecolmodel.2008.07.001. [DOI] [Google Scholar]
- 146.Klimo E, Hager H, Kulhavý J, editors. Spruce monocultures in central Europe: problems and prospects. 2000. [Google Scholar]
- 147.Prach J, Kopecký M. Landscape-scale vegetation homogenization in Central European sub-montane forests over the past 50 years. Appl Veg Sci. 2018;21(3):373–384. doi: 10.1111/avsc.12372. [DOI] [Google Scholar]
- 148.Cholewińska O, Adamowski W, Jaroszewicz B. Homogenization of temperate mixed deciduous forests in Białowieza forest: similar communities are becoming more similar. Forests. 2020;11(5):545. doi: 10.3390/F11050545. [DOI] [Google Scholar]
- 149.Frelich LE, Reich PB. Spatial patterns and succession in a Minnesota southern-boreal forest. Ecol Monogr. 1995;65:325–346. doi: 10.2307/2937063. [DOI] [Google Scholar]
- 150.Fahrig L. Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst. 2003;34:487–515. doi: 10.1146/annurev.ecolsys.34.011802.132419. [DOI] [Google Scholar]
- 151.Lindenmayer DB, Fischer J. Habitat fragmentation and landscape change. Washington, DC: Island Press; 2006. [Google Scholar]
- 152.Thompson ID. Forest vegetation of Ontario: factors influencing landscape change. In: Ecology of a Managed Terrestrial Landscape: Patterns and Processes of Forest Landscapes in Ontario; 2000. p. 30–53.
- 153.Friedman SK, Reich PB. Regional legacies of logging: departure from presettlement forest conditions in northern Minnesota. Ecol Appl. 2005;15(2):726–744. doi: 10.1890/04-0748. [DOI] [Google Scholar]
- 154.Etheridge DA, MacLean DA, Wagner RG, Wilson JS. Effects of intensive forest management on stand and landscape characteristics in northern New Brunswick, Canada (1945-2027) Landsc Ecol. 2006;21:509–524. doi: 10.1007/s10980-005-2378-9. [DOI] [Google Scholar]
- 155.Laquerre S, Harvey BD, Leduc A. Spatial analysis of response of trembling aspen patches to clearcutting in black spruce-dominated stands. For Chron. 2011;87:77–85. doi: 10.5558/tfc87077-1. [DOI] [Google Scholar]
- 156.Boucher D, De Grandpré L, Kneeshaw D, St-Onge B, Ruel JC, Waldron K, Lussier JM. Effects of 80 years of forest management on landscape structure and pattern in the eastern Canadian boreal forest. Landsc Ecol. 2015;30(10):1913–1929. doi: 10.1007/s10980-015-0220-6. [DOI] [Google Scholar]
- 157.Kusters K, Buck L, de Graaf M, Minang P, van Oosten C, Zagt R. Participatory planning, monitoring and evaluation of multi-stakeholder platforms in integrated landscape initiatives. Environ Manag. 2018;62:170–181. doi: 10.1007/s00267-017-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Estreguil C, Caudullo G, de Rigo D, San Miguel J. Forest landscape in Europe: pattern, fragmentation and connectivity -- executive report. In: JRC Sci. Policy Reports, European Union; 2012.
- 159.European Union . Forests, forestry and logging: statistics explained. 2020. [Google Scholar]
- 160.(2008) European Parliament legislative resolution of 19 November 2008 on the proposal for a Council decision amending Decision 2006/144/EC on the Community strategic guidelines for rural development (programming period 2007 to 2013)(COM(2008)0306 — C6-0239/2008 .
- 161.European Commission. Overview of CAP Reform 2014-2020. In: Agricultural Policy Perspectives Brief 5, European Commission; 2013.
- 162.Weetman GF. Declining forest industrial forest management research in Canada. For Chron. 1989;65:2. doi: 10.5558/tfc65002-1. [DOI] [Google Scholar]
- 163.McKenney D. What’s the economics of intensive silviculture? For Chron. 2000;76:275–281. doi: 10.5558/tfc76275-2. [DOI] [Google Scholar]
- 164.Martell DL. The impact of fire on timber supply in Ontario. For Chron. 1994;70(2):164–173. doi: 10.5558/tfc70164-2. [DOI] [Google Scholar]
- 165.Maclean DA. Impact of forest pests and fire on stand growth and timber yield: implications for forest management planning. Can J For Res. 1990;20:391–404. doi: 10.1139/x90-057. [DOI] [Google Scholar]
- 166.Hlásny T, Trombik J, Bošeľa M, Merganič J, Marušák R, Šebeň V, Štěpánek P, Kubišta J, Trnka M. Climatic drivers of forest productivity in Central Europe. Agric For Meteorol. 2017;234(235):258–273. doi: 10.1016/j.agrformet.2016.12.024. [DOI] [Google Scholar]
- 167.Sonntag P. Attack of the budworms: the current infestation threatens Canadian forests. 2016. https://thewalrus.ca/attack-of-the-budworms/.
- 168.Spiecker H. Silvicultural management in maintaining biodiversity and resistance of forests in Europe - Temperate zone. J Environ Manag. 2003;67:55–65. doi: 10.1016/S0301-4797(02)00188-3. [DOI] [PubMed] [Google Scholar]
- 169.Pretzsch H, del Río M, Ammer C, Avdagic A, Barbeito I, Bielak K, Brazaitis G, Coll L, Dirnberger G, Drössler L, Fabrika M, Forrester DI, Godvod K, Heym M, Hurt V, Kurylyak V, Löf M, Lombardi F, Matović B, Mohren F, Motta R, den Ouden J, Pach M, Ponette Q, Schütze G, Schweig J, Skrzyszewski J, Sramek V, Sterba H, Stojanović D, Svoboda M, Vanhellemont M, Verheyen K, Wellhausen K, Zlatanov T, Bravo-Oviedo A. Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. Eur J For Res. 2015;134:927–947. doi: 10.1007/s10342-015-0900-4. [DOI] [Google Scholar]
- 170.Knoke T, Ammer C, Stimm B, Mosandl R. Admixing broadleaved to coniferous tree species: a review on yield, ecological stability and economics. Eur J For Res. 2008;127:89–101. doi: 10.1007/s10342-007-0186-2. [DOI] [Google Scholar]
- 171.Sainte-Marie GB, Kneeshaw DD, MacLean DA, Hennigar CR. Estimating forest vulnerability to the next spruce budworm outbreak: will past silvicultural efforts pay dividends? Can J For Res. 2015;45:314–324. doi: 10.1139/cjfr-2014-0344. [DOI] [Google Scholar]
- 172.Kerr G. The use of silvicultural systems to enhance the biological diversity of plantation forests in Britain. Forestry. 1999;72:191–205. doi: 10.1093/forestry/72.3.191. [DOI] [Google Scholar]
- 173.Armstrong GW. Considerations for boreal mixedwood silviculture: a view from the dismal science. For Chron. 2014;90:44–49. doi: 10.5558/tfc2014-009. [DOI] [Google Scholar]
- 174.Fan V, Jamison D, Summers L. The inclusive cost of pandemic influenza risk. Natl Bur Econ Res. 2016. 10.3386/w22137.
- 175.Burki T. CEPI: preparing for the worst. Lancet Infect Dis. 2017;17:265–266. doi: 10.1016/S1473-3099(17)30062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fernandes N. Economic effects of coronavirus outbreak (COVID-19) on the world economy. SSRN Electron J. 2020. 10.2139/ssrn.3557504.
- 177.Dzau VJ, Sands P. Beyond the Ebola battle - winning the war against future epidemics. N Engl J Med. 2016;375(3):203–204. doi: 10.1056/NEJMp1605847. [DOI] [PubMed] [Google Scholar]
- 178.Björklund N, Lindgren BS. Diameter of lodgepole pine and mortality caused by the mountain pine beetle: factors that influence their relationship and applicability for susceptibility rating. Can J For Res. 2009;39(5):908–916. doi: 10.1139/X09-020. [DOI] [Google Scholar]
- 179.Creeden EP, Hicke JA, Buotte PC. Climate, weather, and recent mountain pine beetle outbreaks in the western United States. For Ecol Manag. 2014;312:239–251. doi: 10.1016/j.foreco.2013.09.051. [DOI] [Google Scholar]
- 180.Gottschalk KW. ilvicultural guidelines for forest stands threatened by the gypsy moth. Stand. 1993.
- 181.Gottschalk KW, Colbert JJ, Feicht DL. Tree mortality risk of oak due to gypsy moth. Eur J For Pathol. 1998;28:121–132. doi: 10.1111/j.1439-0329.1998.tb01173.x. [DOI] [Google Scholar]
- 182.Hrinkevich KH, Progar RA, Shaw DC. History of the balsam woolly adelgid, Adelges piceae (Ratzeburg), in British Columbia with notes on a recent range expansion. J Entomol Soc Brit Columbia. 2017;113:21–38. [Google Scholar]
- 183.Hrinkevich KH, Progar RA, Shaw DC. Climate risk modelling of balsam woolly adelgid damage severity in subalpine fir stands of Western North America. PLoS One. 2016;11:e0165094. doi: 10.1371/journal.pone.0165094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jenkins MJ, Hebertson E, Page W, Jorgensen CA. Bark beetles, fuels, fires and implications for forest management in the Intermountain West. For Ecol Manag. 2008;254:16–34. doi: 10.1016/j.foreco.2007.09.045. [DOI] [Google Scholar]
- 185.Langor DW, Cameron EK, MacQuarrie CJK, McBeath A, McClay A, Peter B, Pybus M, Ramsfield T, Ryall K, Scarr T, Yemshanov D, DeMerchant I, Foottit R, Pohl GR. Non-native species in Canada’s boreal zone: diversity, impacts, and risk1. Environ Rev. 2014;22:372–420. doi: 10.1139/er-2013-0083. [DOI] [Google Scholar]
- 186.Man R, Kayahara GJ, Rice JA, MacDonald GB. Response of trembling aspen to partial cutting and subsequent forest tent caterpillar defoliation in a boreal mixedwood stand in northeastern Ontario, Canada. Can J For Res. 2008;38:1349–1356. doi: 10.1139/X08-005. [DOI] [Google Scholar]
- 187.Sharov AA, Liebhold AM. Model of slowing the spread of gypsy moth (Lepidoptera: Lymantriidae) with a barrier zone. Ecol Appl. 1998;8:1170–1179. doi: 10.1890/1051-0761(1998)008[1170:MOSTSO]2.0.CO;2. [DOI] [Google Scholar]
- 188.Tabakovic-Tosic M, et al. Invasion species Coleophora laricella - one of the main limiting factor of Larix decidua during the forest aforestation and recultivation. Afr J Agric Res. 2011. 10.5897/AJAR10.246.
- 189.Taylor SW, Carroll AL, Alfaro RI, S. L. Forest, climate and mountain pine beetle outbreak dynamics in Western Canada. In: Safranyik L, Wilson B, editors. The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; 2006. p. 67–94.
- 190.Uelmen JA, et al. Effects of winter temperatures, spring degree-day accumulation, and insect population source on phenological synchrony between forest tent caterpillar and host trees. For Ecol Manag. 2016;362:241–250. doi: 10.1016/j.foreco.2015.11.045. [DOI] [Google Scholar]
- 191.Ward SF, Aukema BH. Anomalous outbreaks of an invasive defoliator and native bark beetle facilitated by warm temperatures, changes in precipitation and interspecific interactions. Ecography. 2019;42:1068–1078. doi: 10.1111/ecog.04239. [DOI] [Google Scholar]
- 192.Safranyik L, Carroll AL. The biology and epidemiology of the mountain pine beetle in lodgepole pine forests. Mount Pine Beetle – A Synthes Biol Manag Impacts Lodgepole Pine. 2006;11:1–9. doi: 10.1673/031.011.12701. [DOI] [Google Scholar]