Abstract
Introduction
Carotid web is increasingly recognized as the cause of ischemic embolic strokes in younger patients. The best way to treat carotid web is debatable and carotid artery stenting (CAS) has been reported as a treatment for the carotid web in only a few case series. In this study we evaluate the safety and feasibility of CAS in symptomatic carotid webs and examined the histopathology of a carotid web.
Materials and methods
At our institution between 2017 and 2019, 10 consecutive patients with symptomatic carotid webs were treated. We retrospectively analyzed the data for patient demographics, clinical presentation, imaging, treatment methodology and follow up.
Results
All the patients had presented with ipsilateral embolic stroke. The mean age at presentation was 50 years (range 37–71) with seven female and three male patients. All patients underwent CAS except one patient who underwent carotid endarterectomy (CEA). In one stented patient, there was significant hypotension in the post-procedural period lasting a week. The patients were followed for a mean of 5.5 months (range one day-12 months). No recurrent stroke or transient ischemic attack (TIA) occurred. Surgical pathological studies confirmed fibromuscular dysplasia in one specimen.
Conclusion
In our experience CAS for carotid web is feasible and safe in patients presenting with ischemic embolic strokes.
Keywords: Stroke, carotid web, histopathology, carotid stent
Introduction
Stroke is a common debilitating illness, and no cause is identified in 10–40% of stroke patients.1,2 Recurrence of stroke occurs in 20–32%3–5 of patients with cryptogenic stroke, suggesting that the underlying cause persists even after the patient has recovered from the first episode. One potential cause for cryptogenic stroke is the presence of a carotid web, especially in younger patients, in the absence of other known risk factors, such as diabetes mellitus, cardiac or lipid abnormalities.6 Carotid web is a shelf-like linear defect in the posterior aspect of the carotid bulb. Among patients with transient ischemic attacks evaluated with CT angiography (CTA) at a major Chinese medical center, 8.9% have carotid webs and 2/3 of these progress to cerebral infarction within three months despite antiplatelet therapy.7 The purpose of this study is to confirm the safety and technical feasibility of carotid artery stenting (CAS) in patients with embolic strokes associated with carotid webs. Additionally, the histopathology and mechanism behind the formation of the carotid web was evaluated in one of the patients.
Materials and methods
The Institutional Review Board approved this study. We retrospectively collected data from medical records during the period from January 2017 to December 2019 of stroke patients with carotid webs who, despite being on dual antiplatelet therapy, developed recurrent stroke on the ipsilateral side as the carotid web. All other causes for stroke were ruled out. Relevant images were reviewed by a single neurointerventional radiologist to confirm the diagnosis of carotid web and stroke. Data on patient demographics, clinical presentation, imaging findings, antiplatelet medication protocol, endovascular treatment, and follow up after each procedure were collected. All patients or their legal representatives signed written informed consent. The neurointerventional team documented any procedural complication or stroke recurrence in the follow-up period for up to 12 months.
All patients scheduled for CAS were placed on a dual antiplatelet regimen of Aspirin 325 mg and Clopidogrel 75 mg daily for five days before the procedure. A femoral approach was used in all patients, and an 80 cm neuron max catheter (Penumbra, Inc., Alameda, CA, USA) was advanced to the common carotid artery. Tapered Acculink stents (Abbott Laboratories, Abbott Park, IL, USA) were deployed in all cases with a distal embolic protection device. The stents were advanced over the microwire of the filter, or an exchange Synchro soft microwire (Stryker, Kalamazoo, MI, USA) placed in the distal cavernous internal carotid artery with the help of an Excelsior SL-10 microcatheter (Stryker, Kalamazoo, MI, USA). The patients were examined in the clinic six weeks after the procedure by carotid ultrasound duplex scan. With the carotid duplex scan showing stent patency, the patient began a single antiplatelet regimen of aspirin 81 mg daily for life.
The carotid endarterectomy (CEA) specimen was fixed overnight in neutral buffered formalin, processed according to the surgical pathology laboratory routine, and embedded longitudinally in paraffin. We stained histologic sections according to standard laboratory procedures with hematoxylin and eosin (H&E), Masson’s trichrome stain, and Verhoeff’s elastic stain.
Results
The study included ten consecutive patients presenting with ipsilateral stroke presumed secondary to a carotid web (Table 1).
Table 1.
Patient characteristics.
| N | Age | Sex | Race | Side of treatment | Contralateral carotid | Procedure | Filter | Presenting symptom | Follow up | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | F | L | Right | Normal | CAS | Yes | Stroke | 1.5 months | Transient hypotension |
| 2 | 59 | M | AA | Left | Normal | CAS | Tried but not successful | Stroke | 4 days | None |
| 3 | 37 | F | AA | Left | Normal | CEA with bovine pericardial patch | NA | Vertigo | 12 months | None |
| 4 | 56 | F | L | Left | Occluded | CAS | Yes | Stroke | 4 days | None |
| 5 | 60 | F | AA | Left | Occluded | CAS | Yes | Stroke | 3 months | None |
| 6 | 56 | M | AA | Right | Normal | CAS | Tried but not successful | Stroke | 6 months | None |
| 7 | 62 | F | AA | Right | Normal | CAS | Yes | Stroke | 6 months | None |
| 8 | 59 | M | W | Left | Occluded | CAS | Yes | Stroke | 5 months | None |
| 9 | 56 | F | AA | Right | Small web | CAS | Yes | Vertigo | 1 day | None |
| 10 | 64 | F | A | Right | Carotid web | CAS | Yes | Stroke | 30 days | None |
A: Asian; AA: African American; F: female; L: Latino; M: male; W: white; CAS: carotid angioplasty and stenting; CEA: carotid endarterectomy.
The mean age at presentation was 55.7 ± 7.5 years (range 37–64). There were three male and seven female patients. The ethnicity was 7 African Americans, 2 Hispanic, and one white American. A history of hypertension was present in 9 patients, diabetes mellitus in 2 patients, smoking in 2 patients, and hyperlipidemia in 2 patients. All patients underwent CTA of the head and neck (Figure 1) after the diagnosis of ischemic stroke. Each patient had a unilateral carotid web on the same side as the ischemic territory. One patient also had a second small web on the contralateral side without a history of transient ischemic attack or evidence of prior stroke on the side of the small web. Evaluation of the medical records found no evidence of neck trauma. During catheter angiography for CAS, the degree of stenosis was found to be <50%. All patients underwent CAS except for one patient who underwent CEA (Table 1).
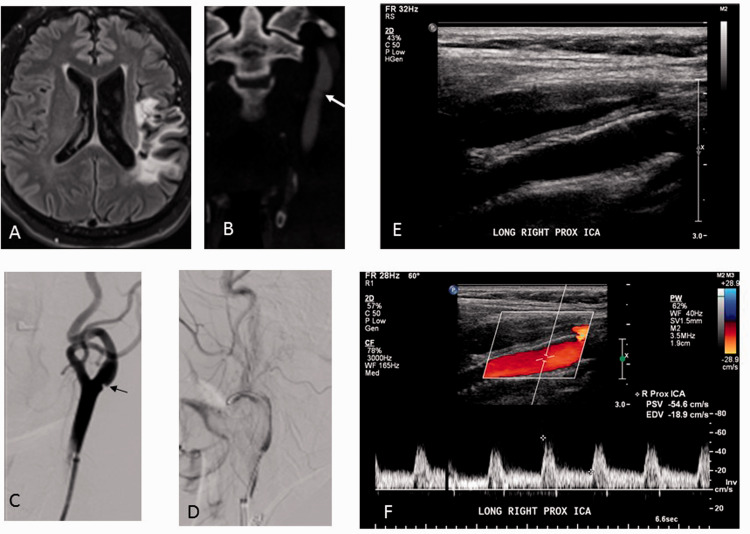
Figure 1.
A patient with a history of 2 episodes of left hemispheric strokes, (a) MRI showing left MCA stroke, (b) CTA showing carotid web (white arrow), (c) DSA showing left ICA carotid web (black arrow), carotid stent deployed (d). Six weeks follow up carotid Doppler (e and f) showing a widely patent stent with normal color flow and Doppler velocity measurements.
There were no intra-operative complications. One stented patient developed significant hypotension in the post-procedural period (BP 74/50 with HR 45–55), which did not respond to Atropine and IV fluids. Therefore, a titrating dose of dopamine was administered for control of hypotension in this patient. We believe the hypotension was related to dampened stimulation of carotid baroreceptors in the stented carotid sinus. The blood pressure returned to normal range after medical treatment and patient was monitored for one week in the hospital. It appears that systemic physiologic adjustments of fluid balance and vasomotor tone may compensate for a change in the carotid baroreceptor firing rate. There were no other peri-procedural complications. The patients were followed up for a mean of 5.5 months (range 1.5–12 months). No recurrent stroke or TIA occurred, and there was no evidence of in-stent stenosis.
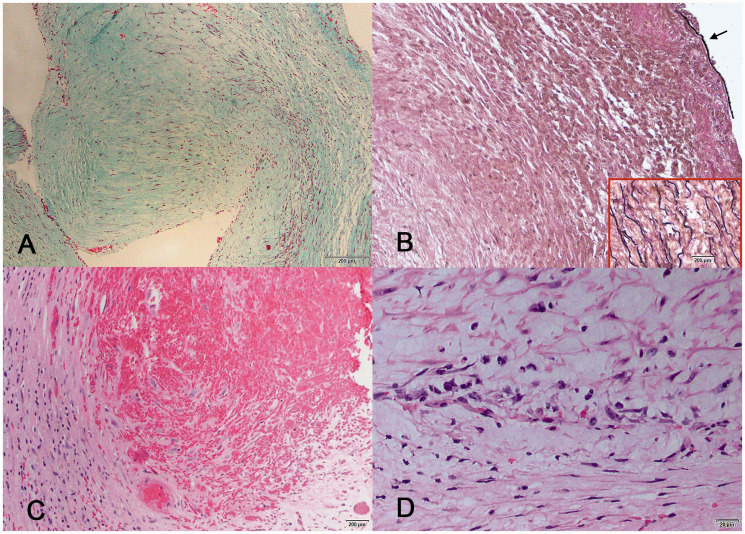
The CEA specimen shows replacement of the entire intima by sparsely cellular fibrous collagenized tissue. There is diffuse intimal thickening and a focal fibrous projection that corresponds to the web seen in the CTA (Figure 2(a)). Adjacent to the fibrous projection is a thrombus with ingrowth of granulation tissue consistent with early organization (Figure 2(c)). Although the surface of this thrombus appears friable, histology shows the base to be firmly adherent with organization of the clot. Trichrome and elastic stains show that the CEA specimen is composed entirely of dense connective tissue with scattered spindle cells (Figure 2(b)). The spindle cells are consistent with myofibroblasts, and there are no smooth muscle cells or elastic fiber elements. The elastic stain shows patches of the internal elastic lamina, revealing that the carotid web consists entirely of intimal fibrosis without vascular media or smooth muscle cell proliferation. Inflammatory cell infiltration is minimal throughout the specimen, with a focal accumulation near the organizing thrombus only (Figure 2(d)).
Figure 2.
Histologic sections of the CEA specimen showing (a) a narrow, sparsely cellular fibrous projection into the internal carotid lumen (trichrome), (b) absence of elastic fibers in the intima, and fragments (arrow) of internal elastic lamina showing the extent of intimal thickening (elastic stain, inset shows a control stain of aorta with abundant elastin between smooth muscle fibers), (c) organizing thrombus adjacent to the fibrous shelf (H&E), and (d) mild lymphohistiocytic inflammatory reaction was present only adjacent to the organizing thrombus.
Discussion
Carotid webs are non-atherosclerotic, non-inflammatory shelf-like intraluminal protrusions in the posterior carotid bulb, sometimes causing a thin crescentic filling defect on CTA. The first description of carotid webs in 1968 observed focal intravascular filling defects causing positional cerebral ischemia. They arise from the proximal bulb of the internal carotid artery,7,8 and may or may not be associated with a thrombus on imaging. The thrombus is thought to arise from the sluggish and turbulent flow secondary to the mural obstruction.8 On histology, they appear as a variant of fibromuscular dysplasia with predominantly intimal hyperplasia and fibrosis, rather than scarring of the muscular vessel wall.9 The shelf-like radiographic appearance is in contrast to typical medial fibromuscular dysplasia, which has a characteristic “string of beads” appearance on imaging and has no direct association with ischemic stroke.10,11 One important differential diagnosis for a carotid web is a small rounded protruding lesion, sometimes seen on CTA. These are less commonly associated with thrombosis than is carotid web, but is thought to represent an early lesion in carotid web evolution.8
Another necessary exclusion is atherosclerotic narrowing of the distal common carotid artery. Soft atherosclerotic plaque shows circumferential thickening of the carotid wall with segmental stenosis and cholesterol deposition underlying the plaque. Carotid webs also must be differentiated from dissection flaps, which extend beyond the carotid bulb and may are associated with pseudoaneurysm or intramural hematoma. Another differential inflammatory carotid irregularity from lesions such as Takayasu arteritis, which usually is excluded radiographically and clinically without a biopsy.12
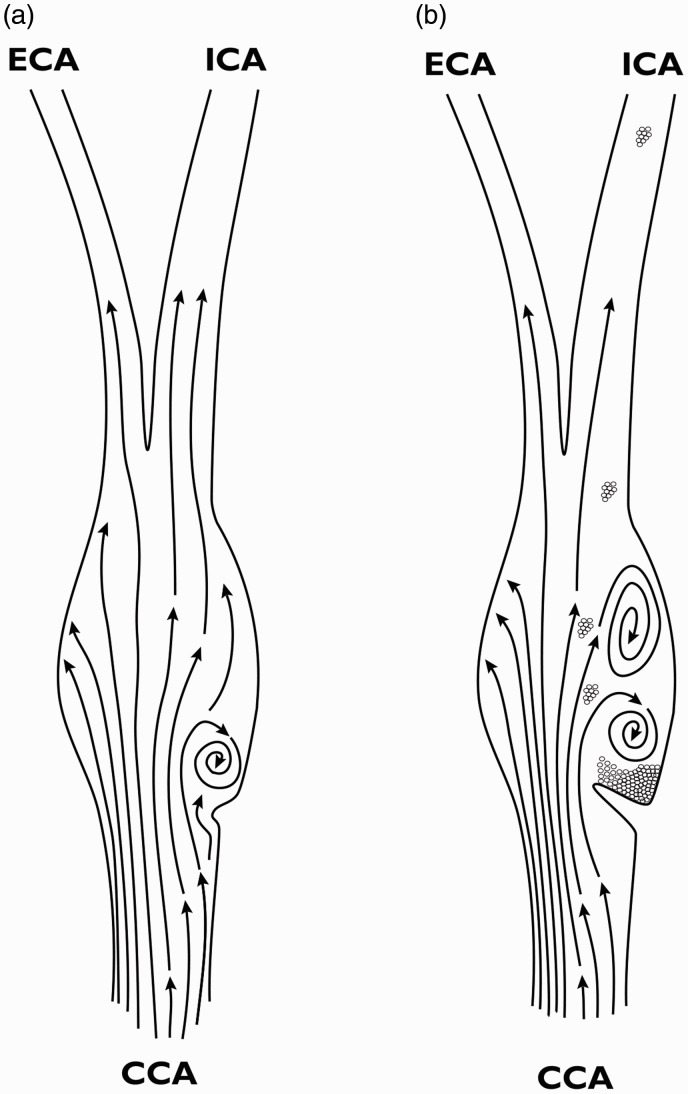
Carotid web is considered a type of fibromuscular dysplasia (FMD), a systemic disease.5 Atherosclerosis, on the other hand, is a disease caused by vascular inflammation and lipid accumulation in zones of turbulent flow. FMD, on the other hand, has only myofibroblast proliferation and collagen deposition, without inflammation or significant cholesterol deposition. When traumatic forces distort vessels, myocytes in the media transform into myofibroblasts with the ability to migrate into the area of greatest injury, whether intimal or medial, and begin synthesizing collagen to reinforce the vessel. One intriguing observation suggesting an underlying cause of the carotid web is an association with hypermobility of the carotid artery, although the study contained no control group or objective measurement.13 Perhaps kinking at the junction of the common carotid artery (CCA) and carotid sinus (CS) leads to pulsatile turbulence in the posterior CS. Thus, the posterior location of carotid web is determined by the fact that about 2/3 of the CCA blood flow distributes to the internal carotid artery (ICA). Because of this differential pulsation and tethering of the external carotid artery (ECA) by its proximal branches, the CCA-CS junction protrudes in an anteromedial direction, causing turbulence mainly in the posterior CS (Figure 3(a)).
Figure 3.
Carotid web etiologic hypothesis. (a) Kinking of the thin carotid sinus at the junction of CCA and ICA causes focal pulsatile oscillatory recirculation inducing endothelial-to-myoepithelial transdifferentiation. (b) As focal endothelial thickening by extracellular matrix deposition from myofibroblasts progresses, the zone of recirculation widens, high ESS at the point of maximal occlusion activates platelets, and increased endothelial thrombogenicity of the downstream surface of the web lead to friable clot accumulation and embolization.
This local turbulence reduces tangential endothelial shear stress, and induces endothelial-to-mesenchymal transdifferentiation of intimal cells into myofibroblasts.14 In the complex anatomy of the carotid pressure-sensing organ, not only diminished ESS but also, more significantly, oscillatory and pulsatile recirculation contributes to fibroproliferative vascular lesions, especially at the entrance to the CS.15 In the carotid artery, the elevated intima (Figure 3(a)) not only further increases the proximal local laminar flow velocity, but also increases downstream turbulence stimulating further elevation of the intima, expanding the intimal nodule into a flap (Figure 3(b)). The flap bending in the direction of flow creates an area of stasis where platelets aggregate, casting off emboli that go toward the internal carotid because of the higher flow and posterior position of the thrombus on that side of the vessel (Figure 2(b)).7
Histopathology of the CEA specimen from one of our patients confirms the diagnosis of the carotid web. Previously published photomicrographs of carotid webs show identical features.8 Our specimen consisted entirely of fibrotic intima, so we were unable to comment on myxoid degeneration of smooth muscle or increased elastic fiber deposition observed in the smooth muscle wall of other published specimens.16 Our specimen does, however, confirm the fibrous intimal proliferation, focal intraluminal projection of intima, and mural thrombus formation on one surface of the fibrous shelf seen in previous specimens.
CTA is the most commonly used method to diagnose a carotid web because the image can be reconstructed in multiple planes, and the contrast image identifies any thrombus associated with the carotid web17 (Figure 1). Additionally, CTA provides high spatial density resolution images to evaluate for underlying atherosclerotic plaques or cholesterol deposits. In the venous phase of the angiography in our study, contrast pooling due to sluggish flow was observed downstream from the carotid web. This intra-arterial delayed washout may indicate turbulence, oscillatory recirculation, and stasis in that zone. MRI and Doppler have also been found to be useful in the evaluation of a carotid web.18,19
Various studies have reported the prevalence of carotid webs in cryptogenic stroke for patients below 60 years of age.4,16,20 One study reported an incidence as low as 9.4% in a general Canadian population,21 whereas Joux et al. have reported an incidence as high as 37% in the initial study of Afro-Caribbean patients with cerebral ischemia symptoms.6 The US Registry for FMD, including both cerebrovascular and systemic vascular involvement, contains 91% women, and an international consensus conference on FMD found that 80%–90% of patients worldwide are women.22,23
There are several treatment options for carotid webs. Medical treatments alone have resulted in an ischemic recurrence rate as high as 30%.6 There are several studies and case reports which have advocated CAS for the management of embolic stroke associated with a carotid web.5,20,24 Haussen et al. evaluated 24 patients with embolic stroke associated with a carotid web, of which 61% were females and 75% were black. All webs caused <50% stenosis and 29% of patients had a superimposed thrombus. They demonstrated a 29% recurrence rate following medical management. Sixteen patients underwent CAS with no recurrence during follow up.5 In smaller case series Brinjikji et al. and Elmokadem et al. also have shown similar results with CAS. Surgical endarterectomy for a carotid web has a success rate similar to stenting, but with greater cost, longer recovery time, and more risk of neck hematoma or anesthetic complication than endovascular procedures.6,25–27 Joux et al. in a 5 year study on Afro-Caribbean patients identified 25 patients with embolic stroke associated with a carotid web. 7 of these patients underwent surgical excision of the carotid web and did not have any recurrence of symptoms. On histopathologic examination the specimen showed fibromuscular dysplasia.6 Kawahara et al. also in a smaller series of patients treated the carotid web patients with CEA with no recurrence of stroke.25 Catheter angiography with stent placement is a treatment option for patients with embolic stroke associated with a carotid web, and was found to be both feasible and safe.
Limitations
Our study is limited by the small number of patients, and its retrospective nature. Additionally, the patients were followed up only for a short period. As a result, the true benefit of stenting may not be reflected in this study. In the long term, reactive hyperplasia in response to the presence of a stent may lead to stent stenosis.
Conclusion
Our study revealed that carotid stent placement is a safe and feasible treatment for symptomatic carotid webs with low risk of periprocedural complications and low incidence of recurrent strokes.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: J Tejada https://orcid.org/0000-0002-1374-1903
References
- 1.Yaghi S, Bernstein RA, Passman R, et al. Cryptogenic stroke: research and practice. Circ Res 2017; 120: 527–540. [DOI] [PubMed] [Google Scholar]
- 2.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke 2009; 40: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 3.Rutten-Jacobs LC, Maaijwee NA, Arntz RM, et al. Long-term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol 2013; 74: 592–601. [DOI] [PubMed] [Google Scholar]
- 4.Haussen DC, Grossberg JA, Bouslama M, et al. Carotid web (intimal fibromuscular dysplasia) has high stroke recurrence risk and is amenable to stenting. Stroke 2017; 48: 3134–3137. [DOI] [PubMed] [Google Scholar]
- 5.Haussen DC, Grossberg JA, Koch S, et al. Multicenter experience with stenting for symptomatic carotid web. Interv Neurol 2018; 7: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joux J, Chausson N, Jeannin S, et al. Carotid-bulb atypical fibromuscular dysplasia in young Afro-Caribbean patients with stroke. Stroke 2014; 45: 3711–3713. [DOI] [PubMed] [Google Scholar]
- 7.Hu H, Zhang X, Zhao J, et al. Transient ischemic attack and carotid web. AJNR Am J Neuroradiol 2019; 40: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi PM, Singh D, Trivedi A, et al. Carotid webs and recurrent ischemic strokes in the era of CT angiography. AJNR Am J Neuroradiol 2015; 36: 2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenck S, Labeyrie MA, Saint-Maurice JP, et al. Diaphragms of the carotid and vertebral arteries: an under-diagnosed cause of ischaemic stroke. Eur J Neurol 2014; 21: 586–593. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe S, Tanaka K, Nakayama T, et al. Fibromuscular dysplasia at the internal carotid origin: a case of carotid web. No Shinkei Geka Neurological Geka 1993; 21: 449–452. [PubMed] [Google Scholar]
- 11.Touze E, Oppenheim C, Trystram D, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke 2010; 5: 296–305. [DOI] [PubMed] [Google Scholar]
- 12.Abdel Razek AA, Alvarez H, Bagg S, et al. Imaging spectrum of CNS vasculitis. Radiographics 2014; 34: 873–894. [DOI] [PubMed] [Google Scholar]
- 13.Miller DJ, Marin H, Aho T, et al. Fibromuscular dysplasia unraveled: the pulsation-induced microtrauma and reactive hyperplasia theory. Med Hypotheses 2014; 83: 21–24. [DOI] [PubMed] [Google Scholar]
- 14.Moonen JR, Lee ES, Schmidt M, et al. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 2015; 108: 377–386. [DOI] [PubMed] [Google Scholar]
- 15.Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007; 49: 2379–2393. [DOI] [PubMed] [Google Scholar]
- 16.Sajedi P, Chelala L, Nunez-Gonalez J, et al. Carotid webs and ischemic stroke: experiences in a comprehensive stroke center. J Neuroradiol 2019; 46: 136–140. [DOI] [PubMed] [Google Scholar]
- 17.Wojcik K, Milburn J, Vidal G, et al. Survey of current management practices for carotid webs. Ochsner J 2019; 19: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boesen ME, Eswaradass PV, Singh D, et al. MR imaging of carotid webs. Neuroradiology 2017; 59: 361–365. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Li Z. Ultrasonic risk stratification of carotid web. Echocardiography 2019; 36: 2103–2107. [DOI] [PubMed] [Google Scholar]
- 20.Mac Grory B, Cheng D, Doberstein C, et al. Ischemic stroke and internal carotid artery web. Stroke 2019; 50: e31–e4. [DOI] [PubMed] [Google Scholar]
- 21.Coutinho JM, Derkatch S, Potvin AR, et al. Carotid artery web and ischemic stroke: a case-control study. Neurology 2017; 88: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gornik HL, Persu A, Adlam D, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med 2019; 24: 164–189. [DOI] [PubMed] [Google Scholar]
- 23.Sharma AM, Kline B. The United States registry for fibromuscular dysplasia: new findings and breaking myths. Tech Vasc Interv Radiol 2014; 17: 258–263. [DOI] [PubMed] [Google Scholar]
- 24.Gouveia EE, Mathkour M, Bennett G, et al. Carotid web stenting. Ochsner J 2019; 19: 63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawahara I, Hiu T, Ono T, et al. Reconsideration of carotid web and the therapeutic strategy. No Shinkei Geka Neurological Geka 2019; 47: 659–666. [DOI] [PubMed] [Google Scholar]
- 26.Brinjikji W, Agid R, Pereira VM. Carotid stenting for treatment of symptomatic carotid webs: a single-center case series. Interv Neurol 2018; 7: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmokadem AH, Ansari SA, Sangha R, et al. Neurointerventional management of carotid webs associated with recurrent and acute cerebral ischemic syndromes. Interv Neuroradiol 2016; 22: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]