Abstract
Background and purpose
Early major recurrence (EMR) of cerebral aneurysms treated by coiling has not been investigated. The purpose of this study is to characterize the frequency and risk factors of this phenomenon.
Materials and methods
A retrospective review was performed of consecutive patients who presented with ruptured and unruptured cerebral aneurysms and underwent coiling from July 2009 to June 2019 at a university hospital. We defined EMR as recurrence of the aneurysm greater than its initial size within the first 6 months of an initial satisfactory coil embolization. Patient demographics, clinical information, aneurysm characteristics, angiographic and technical details were reviewed.
Results
From July 2009 to June 2019, 338 aneurysms (190 unruptured aneurysms and 148 ruptured cerebral aneurysms) underwent coiling and satisfied our study criteria. Among these patients, 23 patients (19 ruptured and 4 unruptured aneurysms) were found to have recurrent aneurysm. Of those, 4 were found to have early major aneurysm regrowth occurring within 6 months after coiling (1.2%). The detection of the EMR was as early as 4 weeks and as late as 20 weeks after the initial coil embolization. The average detection time was 10 ± 7.2 weeks (mean ± SD, range:4-20 weeks). In each case, the recurrent aneurysm cavity was more than twice the initial size of presentation. All aneurysms with major recurrence were ruptured with low aspect ratios (dome height to neck ratio) and involved a communicating segment. All patients underwent successful retreatment of the recurrent aneurysm with good outcome.
Conclusions
Early major recurrence of treated aneurysms is a rare but important complication that harbors an impending risk of re-rupture. Early control angiography after endovascular coiling may be warranted for small ruptured aneurysms, even in cases in which the initial result seems technically satisfactory.
Keywords: Recurrence, endovascular coiling, ruptured aneurysm, subarachnoid hemorrhage
Introduction
Despite the demonstrated clinical benefit of endovascular treatment over surgical clipping of cerebral aneurysms, recurrence remains the main shortcoming of the endovascular technique.1,2 Recurrent aneurysm exposes the patient to risk for re-rupture and necessitates imaging follow-up.2–4 Although many studies have addressed the rate and factors associated with recurrence, data about how early an aneurysm may recur is sparse. Typically, the first follow-up is scheduled 6 months to one year after the initial treatment.2,5 However, recurrence may occur within weeks or a few months of initial treatment despite satisfactory initial occlusion with the recurrent aneurysm cavity being even larger than its initial size. This can lead to high risk of aneurysm re-rupture and necessitate urgent intervention.6 We define the phenomenon of early major recurrence as recurrence of the aneurysm greater than its initial aneurysm size within 6 months of technically satisfactory initial coil embolization. To our knowledge, there is no established data about the frequency of early major recurrence, its risk factors and optimal follow up of a coiled aneurysm.
Materials and methods
Patients and techniques
A retrospective review was performed of our prospectively maintained cerebrovascular database of all patients who underwent endovascular coiling of ruptured and unruptured cerebral aneurysms from July 2009 to June 2019. Patients with early major recurrence, defined as recurrence of the aneurysm greater than its initial maximal size within the first 6 months of an initial satisfactory coil embolization, were collected. Inclusion criteria were satisfactory initial coiling of the aneurysm defined by either total (Raymond-Roy Scale 1) or subtotal initial occlusion (minimal residual filling was included, Raymond-Roy Scale 2) and patients with a follow up conventional angiogram, CT or MR angiogram within six months of the initial endovascular treatment for ruptured and one year of unruptured aneurysms. Patients with partial initial coiling, no repeat imaging within 6 or 12 months for ruptured and unruptured aneurysms respectively, or death, were excluded. Patients with dissecting aneurysms, blister-like aneurysms, fusiform aneurysms, and traumatic or mycotic pseudoaneurysms were excluded since their underlying pathophysiology, treatment approach and natural history are different compared to saccular aneurysms. As our study aimed to determine recurrence of coiled aneurysms, all aneurysms that were treated initially by stenting, stent-assisted coiling and flow diversion were also excluded.
Angiographic and endovascular procedures
All endovascular procedures were performed on a monoplane or biplane angiography unit with 3 D rotational angiography capability with patients under general anesthesia. An external ventricular drain was inserted in patients prior to endovascular treatment if it was clinically indicated. Patients were given intravenous heparin during the procedure to keep the activated clotting time above 200-250 seconds. Aneurysms were coiled as densely as possible with coils selected per the operator’s preference.
Radiological evaluation
A radiological evaluation was performed on CT angiograms before the procedure, the angiographic images immediately after the procedure, and at follow-up within the first 6 months to one year from the procedure. Aneurysm occlusion was assessed using a 3-point Raymond-Roy Scale RRS (RRS 1, complete obliteration of aneurysm and neck; RRS 2, neck remnant without contrast filling the aneurysm sac; and RRS 3, contrast filling the aneurysm sac).
Statistical analysis
As the design of our project is descriptive and consisted of a small case series, findings were presented as mean, percentage, range, and standard deviation.
Results
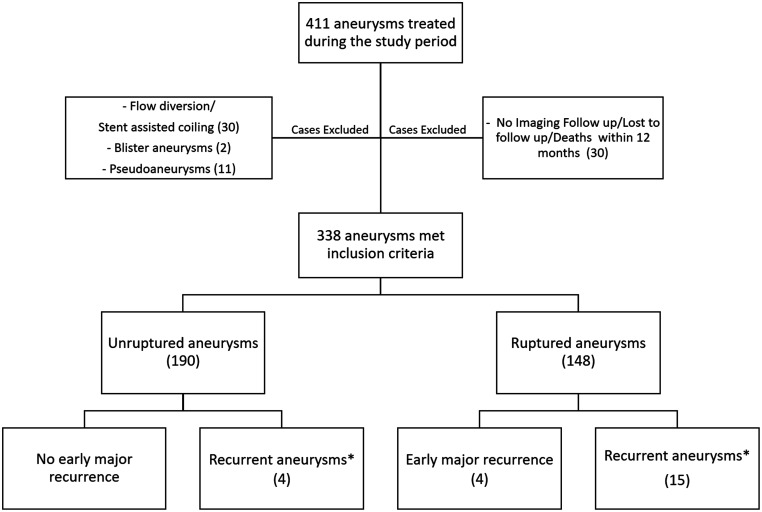
From July 2009 to June 2019, 411 cerebral aneurysms were treated in our institution. Of those, 338 aneurysms (190 unruptured and 148 ruptured aneurysms) were included in our study after excluding patients with partial initial coiling, death, no repeat imaging within 6 or 12 months, dissecting aneurysms, blister-like aneurysms, fusiform aneurysms, and traumatic or mycotic pseudoaneurysms and patients treated by flow diversion or stent assisted coiling. Among the 338 aneurysms, 23 patients (19 ruptured and 4 unruptured aneurysms) were found to have recurrent aneurysm. Of those, four patients (4/338, 1.2%) were found to have early major recurrence as defined by recurrence of the aneurysm greater than its initial size within the first 6 months of an initial satisfactory coil embolization. Patient demographics, aneurysm characteristics, and time of detection are provided in Table 1 for aneurysms complicated by early major recurrence and in Table 2 for recurrent aneurysms that did not meet the criteria of early major recurrence. A flowchart of the study is presented as Figure 1.
Table 1.
Characteristics of patients with early major aneurysm recurrence (Acom: anterior communicating artery, Pcom: Posterior communicating artery, HH: Hunt and Hess Grade, T × H × N: Transverse diameter × Height × Neck diameter, GOS: Glascow Outcome Scale, 5= Complete recovery).
| Age, gender | Aneurysm location | Initial aneurysm size (mm) (T × H × N) | Follow up aneurysm size (mm) | Time to detection of recurrence | Retreat | Outcome (GOS) |
|---|---|---|---|---|---|---|
| 52 F HH2 | Pcom | 8 × 5 × 5 | 17 × 9 | 4 weeks | Coils, flow diversion | 5 |
| 54 F HH3 | Pcom | 3.5 × 2 × 1.5 | 8 × 7 | 6 weeks | Coiling | 5 |
| 60 M HH3 | Acom | 3 × 2 × 2 | 8 × 7 | 12 weeks | Coiling | 5 |
| 81 F HH3 | Pcom | 2 × 2 × 1.6 | 4 × 3 | 20 weeks | Coiling | 5 |
Table 2.
A table showing the demographics, radiological characteristics of the initial and the recurrent aneurysms that did not meet the criteria of “early major recurrence” and their management.
| Patient Number | Gender | Age (years) | Location | Rupture status | Maximal diameter(mm) | Height (mm) | Neck (mm) | Height/Neck ration | RRS | Recurrence detection (months) | Maximal diameter (mm) | Height (mm) | Re-treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | Acom | R | 3 | 2 | 2 | 1 | 2 | 6 | 1 | 2 | None |
| 2 | M | 51 | Paraopthalmic | R | 13 | 12 | 7 | 1.7 | 2 | 42* | 16** | 10 | FD |
| 3 | F | 61 | Acom | R | 4.5 | 3 | 2.5 | 1.2 | 1 | 6 | 2 | 2 | BAC |
| 4 | F | 62 | Basilar | U | 11 | 7 | 4 | 1.75 | 1 | 5 | 4 | 4 | SAC |
| 5 | F | 62 | Paraopthalmic | R | 23 | 14 | 4.5 | 3.1 | 2 | 6 | 19 | 9 | FD |
| 6 | F | 62 | Pcom | R | 4 | 3 | 3 | 1 | 2 | 6 | 4 | 3 | SAC |
| 7 | F | 81 | AICA | U | 7 | 5 | 4 | 1.25 | 1 | 96 * | 8** | 6 | Coil |
| 8 | F | 52 | SHA | R | 10 | 8 | 4 | 2 | 2 | 6 | 5 | 4 | SAC |
| 9 | F | 31 | Ophtalmic | R | 6 | 3 | 3 | 1 | 2 | 6 | 3 | 3 | SAC |
| 10 | F | 45 | Ophtalmic | R | 10 | 5 | 3.5 | 1.4 | 1 | 12 | 4.5 | 2.5 | FD |
| 11 | F | 56 | Pcom | R | 9 | 7 | 3 | 2.3 | 1 | 6 | 4 | 3 | Coil |
| 12 | F | 60 | Basilar | U | 14 | 12 | 8 | 1.5 | 1 | 6 | 6 | 4 | Coil |
| 13 | F | 50 | Pcom | U | 4 | 4 | 3 | 1.3 | 1 | 12 | 2 | 2 | None |
| 14 | F | 35 | Pcom | R | 8 | 7 | 3 | 2.3 | 1 | 12 | 3 | 2 | None |
| 15 | F | 55 | Pcom | R | 7.2 | 4.7 | 4 | 1.2 | 2 | 6 | 4 | 3 | BAC |
| 16 | M | 52 | Basilar | R | 8 | 8 | 6 | 1.3 | 1 | 18 | 6 | 4 | SAC |
| 17 | F | 26 | Cavernous | R | 22 | 19 | 10 | 1.9 | 2 | 24* | 8 | 6 | FD |
| 18 | F | 45 | Pcom | R | 5 | 3 | 2.5 | 1.2 | 2 | 6 | 3 | 2 | BAC |
| 19 | F | 70 | Ophtlamic | R | 6 | 5 | 4 | 1.25 | 2 | 6 | 4 | 3 | BAC |
Abbreviations: Acom: Anterior communicating aneurysm; Pcom: posterior communicating aneurysm; AICA: anterior inferior cerebellar artery; SHA: superior hypophyseal artery; R: ruptured; U: unruptured; RRS: Raymond-Roy Scale; FD: flow diversion; SAC: stent-assisted coiling; BAC: balloon assisted coiling; Coil: coiling.
*Recurrence detected at later follow up.
**Recurrence larger than the initial size but detected at a later follow-up.
Figure 1.
A flow chart of the study population (*: Recurrent aneurysms not meeting the criteria of early major recurrence).
Characteristics of aneurysms with early major recurrence
All patients with early major recurrence presented initially in a ruptured state (100%). The mean patient age was 61.8 ± 13 years (mean ± SD, range: 52–81 years). There were three females and one male. At presentation, one patient was Hunt-Hess Grade II and three patients (75%) were Hunt-Hess grade III subarachnoid hemorrhage. The radiological characteristics were the following: anterior communicating artery (ACoA) aneurysm (1/4, 25%); posterior communicating artery (3/4, 75%). Three aneurysms (3/4, 75%) demonstrated a bleb or daughter dome suggestive of the location of rupture. The mean aneurysm maximal diameter, height, neck, and height-to-neck ratio (or aspect ratio) before the initial embolization were 4.1 ± 2.6 mm (mean ± SD, range: 2–8 mm), 2.7 ± 1.5 mm (mean ± SD, range: 2–5 mm), 2.5 ± 1.6 (mean ± SD, range: 1.5–6 mm), and 1.1 ± 0.1 (mean ± SD, range: 1–1.3), respectively. There was no intraluminal thrombus or peri-aneurysmal hematoma in any case.
Initial treatment and recurrence characteristics of aneurysms with early major recurrence
Three of the four aneurysms with early major recurrence were initially completely occluded angiographically (RS 1) (3/4, 75%) and one aneurysm had minimal residual filling at the aneurysm neck that was left intentionally to not compromise the parent artery (1/4, 25%). The detection of the recurrence was as early as 4 weeks and as late as 20 weeks after the initial coil embolization. The average detection time was 10 ± 7.2 weeks (mean ± SD, range: 4–20 weeks). Recurrence was detected during work up of new neurological symptoms (2 patients, 50%) or incidentally on cross-sectional or angiographic imaging follow up (two patients, 50%). The maximal diameter and the maximal height of the recurrent aneurysmal cavity were 9.2 ± 5.5 mm (range: 4–17 mm) and 6.5 ± 2.5 mm (range: 3–9 mm), respectively.
Re-treatment outcomes and complications
All four patients with major recurrence were re-treated endovascularly without procedural related complications (3/4 or 75% with balloon-assisted coiling, and 1/4 or 25% with flow diversion and coiling). All patients recovered completely (Glasgow Outcome Scale 5, 100%, 4/4). Control angiograms of all patients at 1 year from the second embolization showed stable good and complete occlusion of all recurrent aneurysms. Representative cases are presented as Figures 2 to 5.
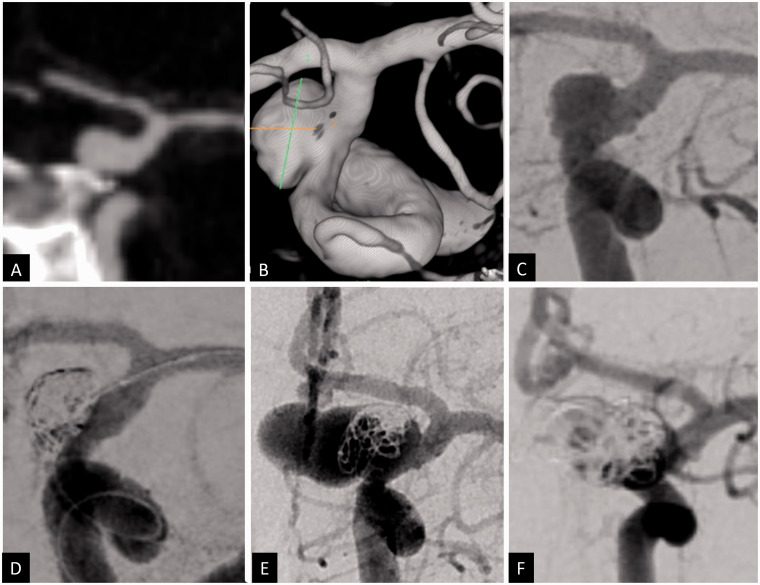
Figure 2.
Coronal CT angiogram (a), 3D volume-rendered angiogram reconstruction (b) and 2D anteroposterior angiogram (c) showing a ruptured 8 × 5 mm posterior communicating artery aneurysm with 5 mm neck. The aneurysm was treated with balloon assisted coiling. A small remnant was left at the inferior neck due to difficulty of recannulating this pocket (d). The plan was for elective flow diversion after the patient’s acute hospitalization phase. While recovering in the rehabilitation center, the patient developed transient right face and arm weakness 1 month after her initial hemorrhage. CT angiogram revealed major recurrence of the coiled aneurysm, measuring approximately 17 × 9 mm with multiple lobulations that was confirmed by angiography (e). The patient was retreated with coils and flow diversion across the aneurysm (d).
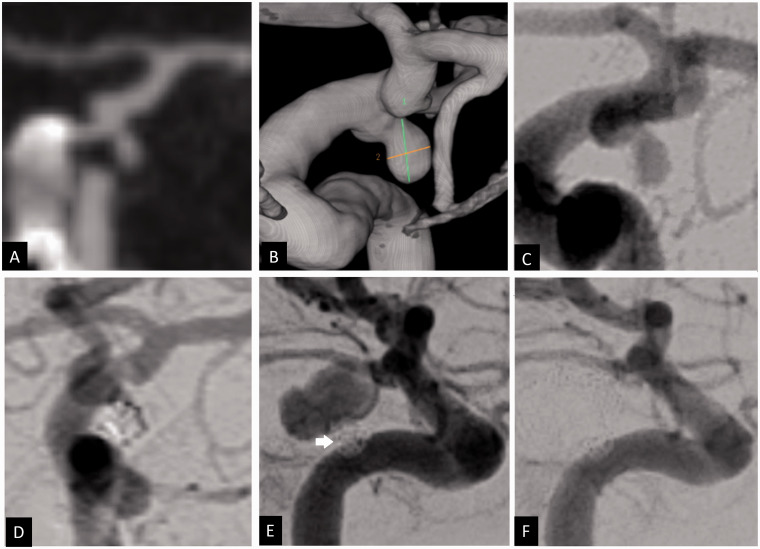
Figure 3.
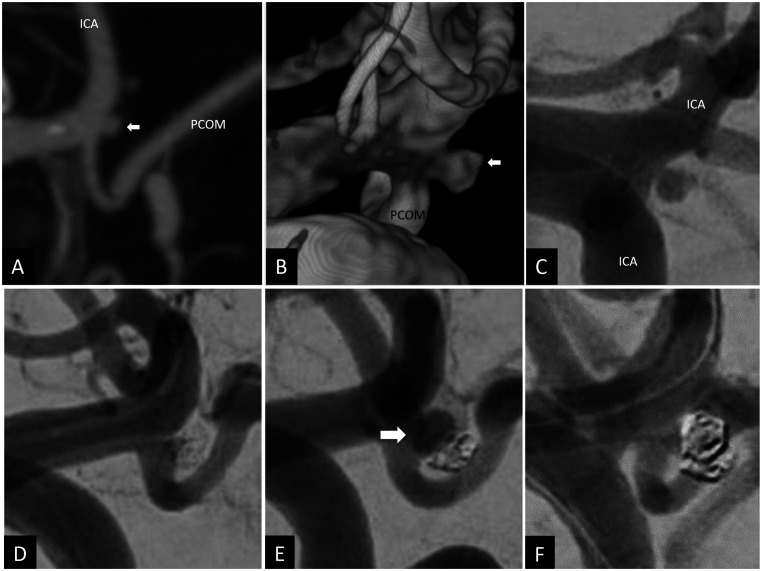
Coronal CT angiogram (a), 3 D volume-rendered angiogram reconstruction (b) and 2 D anteroposterior angiogram (c) showing a ruptured left posterior communicating artery aneurysm of 3.5 × 2 mm and 1.5 mm neck. Using balloon assisted coiling, the aneurysm was coiled with good occlusion (d). Repeat angiogram 6 weeks after the embolization in the setting of oculomotor nerve palsy showed major recurrence of the treated aneurysm, measuring 8 × 7 mm with a neck of 1.5 mm. The initial coil mass is noted displace postero-inferiorly by the coil mass (white arrow) (e). The recurrent aneurysm was treated using balloon assisted coiling with complete occlusion (f).
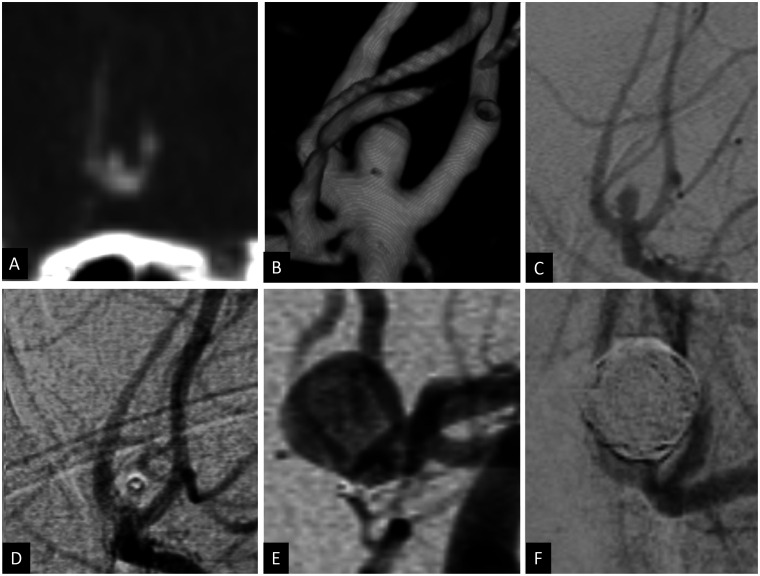
Figure 4.
Coronal CT angiogram (a), 3D volume rendered angiogram reconstruction (b) and 2D anteroposterior angiogram (c) showing a ruptured anterior communicating artery aneurysm measuring 3 × 2 mm with a 2 mm neck. Using balloon assisted coiling, the aneurysm was coiled with complete occlusion (d). Attempts to deploy further coils were unsuccessful because of coil herniation as well as dislodgment of the microcatheter from the aneurysm. Angiogram 3 months after embolization showed major recurrence of the previously treated aneurysm measuring 8 × 7 mm (e). The patient was retreated with balloon assisted coiling with complete occlusion of the aneurysm (f).
Figure 5.
Sagittal CT angiogram (a), 3D volume-rendered angiogram reconstruction (b) and 2D oblique angiogram (c) showing a ruptured 2 × 2 mm left posterior communicating artery aneurysm (white arrow in (a) and (b)). The aneurysm was coiled using balloon assisted coiling with satisfactory occlusion (d). Routine cerebral angiogram done at 20 weeks showed recurrent 4 × 3 mm posterior communicating artery aneurysm (white arrow) with a 1.2 mm neck (e) that was retreated with balloon assisted coiling (f).
Characteristics of aneurysms with recurrence not meeting the criteria of early major recurrence
The recurrent aneurysms that did not fit the inclusion criteria of early major recurrence included 19 aneurysms (15 ruptured and 4 unruptured). Nine aneurysms (9/19, 47%) were initially completely occluded angiographically (RRS 1) and ten aneurysms (10/19, 53%) were partially treated initially (RRS2). The aneurysms locations were posterior communicating artery (6/19, 32%), anterior communicating artery (2/19, 10%), basilar artery (3/19, 16%), ophthalmic (3/19, 16%), paraopthalmic (2/19, 10%), superior hypophyseal artery (1/19, 5%), cavernous carotid artery (1/19, 5%), anterior inferior cerebellar artery (1/19, 5%). The mean patients' age was 52.4 ± 13.5 years (mean ± SD, range: 26–81 years). There were 17 females (17/19, 89%) and 2 males (2/19, 11%). The mean aneurysm maximal diameter, height, neck, and height-to-neck ratio (or aspect ratio) before the initial embolization were 9 ± 5.4 mm (mean ± SD, range: 3-23 mm), 7 ± 4.4 mm (mean ± SD, range: 2–19 mm), 4 ± 2 (mean ± SD, range: 2–10 mm), and 2 ± 0.6 (mean ± SD, range: 1–3.1), respectively. The detection of the recurrence was at follow up performed at 6 months for ruptured aneurysms and at one year for unruptured aneurysms. The recurrent cavity was detected as late as 96 months weeks after the initial coil embolization noting that there was no recurrence of these aneurysms between 6 and 12 months follow up. Sixteen recurrent aneurysms (16/19, 84%) were treated endovascularly (coiling (3/16, 19%), balloon-assisted coiling (4/16, 25%), stent assisted coiling (5/16, 31%) and flow diversion (4/16, 25%)). Three aneurysms (3/19, 16%) were not treated because of their small size.
Discussion
There are no published guidelines defining when to follow-up a coiled intracranial aneurysm. In most studies, the first follow-up imaging is 6 months to one year after initial treatment.2,5,7,8 In our study, we noted major recurrence may occur within weeks or within a few months of initial treatment of ruptured aneurysms despite satisfactory initial occlusion and the recurrent aneurysm cavity may become even larger than its initial size harboring high risk of re-rupture and necessitate urgent treatment.6 We define this phenomenon as early major recurrence characterized by recurrence of the aneurysm greater than the initial aneurysm size within the first 6 months of initial satisfactory endovascular treatment. Although many studies have addressed the rate and factors associated with long term recurrence, data about how early an aneurysm may recur is sparse. Only one case report was found in the literature documenting early major recurrence.6 In addition, the magnitude and clinical relevance of this phenomenon are still poorly documented.
Many technical, morphological, and anatomical factors have been described in association with higher rates of aneurysm recurrence.2,9,10 Incomplete initial occlusion secondary to residual neck or low coil packing density (less than 25%),11–13 ruptured aneurysms,2,9 large aneurysms (fundus equal or more than 10 mm) and/or wide neck (equal to or more than 4 mm),2 location of the aneurysm (pericallosal artery,14 basilar15 or internal carotid artery bifurcations16). The aforementioned factors are usually described in association with ‘long term’ recurrence but may have contributed to the early dramatic regrowth in our series. For instance, all patients with major recurrence in our series presented initially with ruptured status. In addition, there was minimal residual filling at the neck of majority of the aneurysms. Rapid growth may be due to shear stress and turbulence created by blood flow on this part of the aneurysmal wall that is not sufficiently covered by coils especially at the level of the inflow zone.17,18 However, in contrast to what is commonly known that larger aneurysms, wide necks and bifurcations aneurysms have higher risk of recurrence,16 most aneurysms with early major recurrence in our series were small and had small necks and were located at a communicating segment. These factors are generally associated with greater technical difficulty to treat and higher complication rates19–21 (Figures 2 to 5).
Other potential causes of rapidly changing/growing aneurysms include coil compaction or coil migration through the aneurysm wall which may also lead to apparent growth or recurrence after initial coiling.11,22 Development of an acute thrombus within the aneurysm cavity, which may not be seen on initial CT angiogram or cerebral angiography, may obliterate visualization of the true lumen of the aneurysm. The true aneurysm lumen size may subsequently be revealed on follow-up as the clot retracts. Presence of vasospasm may also make the aneurysmal cavity appear smaller than its true size. Other potential causes of rapidly changing/growing aneurysms include dissecting aneurysms, traumatic or mycotic pseudoaneurysms, and blister aneurysms. These factors/mechanisms were excluded by detailed assessment of the clinical, laboratory and radiological findings in our series.
The decision to choose endovascular re-treatment over surgical treatment was made based on favorable endovascular anatomical factors, as well as the reported low risk of endovascular treatment of recurrent aneurysms.23,24
Given the unexpected development of early major recurrence within 6 months of the initial treatment in this series of patients, we suggest short term neuroimaging follow up for patients with significant risk factors for regrowth or recurrence (e.g., small, ruptured, incompletely coiled aneurysms, communicating segment, or posterior circulation aneurysm). A suggested follow-up regimen could involve an early angiographic imaging study before discharge or a serial non-invasive imaging. This protocol may be modified based on the clinician’s suspicion for recurrence and should be balanced against patient safety, aneurysm characteristics, method of follow-up, and cost. Future research should involve prospective serial non-invasive imaging over regular controlled time periods in high risk patients to answer the question as to when should follow up imaging be occurring.
Our study has a number of limitations, including its small size, retrospective nature and being performed at a single institution. The small sample size affects the power and significance of the findings and limits the ability to perform detailed statistical analysis. Another limiting factor is the selection bias that may have occurred.
Conclusion
Early major recurrence of treated aneurysms is rare but harbors an impending risk of re-rupture and poor outcome. Early neuroimaging after endovascular coiling may be warranted for small or incompletely-treated ruptured aneurysms, even in cases in which the initial result seems technically satisfactory. Future research should involve prospective serial non-invasive imaging over regular controlled time periods in high risk patients to answer the question as to when should follow up imaging be occurring.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mohamad Abdalkader https://orcid.org/0000-0002-9528-301X
References
- 1.Lecler A, Raymond J, Rodriguez-Régent C, et al. Intracranial aneurysms: recurrences more than 10 years after endovascular treatment – a prospective cohort study, systematic review, and meta-analysis. Radiology 2015; 277: 173–180. [DOI] [PubMed] [Google Scholar]
- 2.Raymond J, Guilbert Fois, Weill Alain, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003; 34: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 3.The CARAT Investigators. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke 2006; 37: 1437–1442. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux AJ, Birks J, Clarke A, et al. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the international subarachnoid aneurysm trial (ISAT). Lancet 2015; 385: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the international subarachnoid aneurysm trial (ISAT). Stroke 2007; 38: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 6.Makoui AS, Smith DA, Evans AJ, et al. Early aneurysm recurrence after technically satisfactory Guglielmi detachable coil therapy: is early surveillance needed? Case report. J Neurosurg 2000; 92: 355–358. [DOI] [PubMed] [Google Scholar]
- 7.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 1997; 41: 1235–1245. [DOI] [PubMed] [Google Scholar]
- 8.Eskey CJ, Meyers PM, Nguyen TN, et al. ; American Heart Association Council on Cardiovascular Radiology and Intervention and Stroke Council. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation 2018; 137: e661–e689. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TN, Hoh BL, Amin-Hanjani S, et al. Comparison of ruptured vs unruptured aneurysms in recanalization after coil embolization. Surg Neurol 2007; 68: 19–23. [DOI] [PubMed] [Google Scholar]
- 10.van Rooij WJ, Sluzewski M. Opinion: imaging follow-up after coiling of intracranial aneurysms. Am J Neuroradiol 2009; 30: 1646–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chueh J-Y, Vedantham S, Wakhloo AK, et al. Aneurysm permeability following coil embolization: packing density and coil distribution. J Neurointervent Surg 2015; 7: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawanabe Y, Sadato A, Taki W, et al. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: Correlation between coil packing density and coil compaction. Acta Neurochirurgica 2001; 143: 451–455. [DOI] [PubMed] [Google Scholar]
- 13.Raymond J, Ghostine J, van Adel BA, et al. Does increasing packing density using larger caliber coils improve angiographic results of embolization of intracranial aneurysms at 1 year: a randomized trial. AJNR Am J Neuroradiol 2020; 41: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen TN, Raymond J, Roy D, et al. Endovascular treatment of pericallosal aneurysms. J Neurosurg 2007; 107: 973–976. [DOI] [PubMed] [Google Scholar]
- 15.Ferns SP, Sprengers MES, van Rooij WJ, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 2009; 40: e523–e529. [DOI] [PubMed] [Google Scholar]
- 16.Hope JK, Byrne JV, Molyneux AJ. Factors influencing successful angiographic occlusion of aneurysms treated by coil embolization. AJNR Am J Neuroradiol 1999; 20: 391–399. [PMC free article] [PubMed] [Google Scholar]
- 17.Graves VB, Strother CM, Duff TA, et al. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995; 37: 640–648. [DOI] [PubMed] [Google Scholar]
- 18.Futami K, Sano H, Misaki K, et al. Identification of the inflow zone of unruptured cerebral aneurysms: comparison of 4D flow MRI and 3D TOF MRA data. AJNR Am J Neuroradiol 2014; 35: 1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TN, Raymond J, Guilbert F, et al. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg 2008; 108: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 20.Brinjikji W, Lanzino G, Cloft HJ, et al. Endovascular treatment of very small (3 mm or smaller) intracranial aneurysms: report of a consecutive series and a meta-analysis. Stroke 2010; 41: 116–121. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TN, Masoud H, Tarlov N, et al. Expanding endovascular therapy of very small ruptured aneurysms with the 1.5-mm coil. Interv Neurol 2015; 4: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdalkader M, Piotin M, Chen M, et al. Coil migration during or after endovascular coiling of cerebral aneurysms. J NeuroIntervent Surg 2020; 12: 505–511. [DOI] [PubMed] [Google Scholar]
- 23.Henkes H, Fischer S, Liebig T, et al. Repeated endovascular coil occlusion in 350 of 2759 intracranial aneurysms: safety and effectiveness aspects. Neurosurgery 2006; 58: 224–232. [DOI] [PubMed] [Google Scholar]
- 24.Kang H-S, Han MH, Kwon BJ, et al. Repeat endovascular treatment in post-embolization recurrent intracranial aneurysms. Neurosurgery 2006; 58: 60–70. [DOI] [PubMed] [Google Scholar]