Abstract
Purpose
To report use of distal radial artery (dRA) access for carotid artery stenting (CAS) and to discuss procedural setup and technical considerations for a successful intervention.
Methods
A retrospective review of our prospective neurointerventional database of CAS was conducted between May 2019 and March 2020. All CAS cases via dRA in the anatomical snuffbox were identified. Patient demographics, clinical information, procedural and radiographic data was collected.
Results
22 CAS procedures in 20 patients via dRA were identified. Patients’ mean age was 69.4 years (range 53–87 years). 3 patients were female. Mean radial artery diameter was 2.1 mm (range 1.6–2.8 mm). dRA access was achieved in all cases. Conversion to femoral access was required in 2 cases (9.1%) due to persistent radial artery vasospasm resulting in patient discomfort despite multiple additional doses of intraarterial vasodilators and added intravenous sedation as well as tortuous vessel anatomy and limited support of the catheters in a type 3 aortic arch for left CAS.
Conclusion
Our preliminary experience with dRA access for CAS suggests this approach to be feasible and safe for patients. Technical considerations are important and preprocedural planning is necessary for a successful intervention. Catheter systems and devices specifically designed for radial access are needed to enable more interventionalists to safely perform neurointerventional procedures via wrist access.
Keywords: Distal radial artery, transradial access, snuffbox, carotid artery stenting, stenosis
Introduction
Carotid artery stenting (CAS) is traditionally performed via transfemoral access. However, femoral artery access has been shown to be associated with higher complication rates, including groin hematoma, major bleeding, pseudoaneurysm formation or need for surgical access site repair.1,2 The cardiology and more recently also the neurointerventional literature have described a transradial approach as an alternative to femoral access.3–9 While transradial access has overall lower complication rates, there was even a morbidity and mortality benefit of transradial versus transfemoral approach in a systematic review and meta-analysis of randomized interventional cardiology trials.1 The authors reported a 73% reduction of major bleeding as well as a trend for reduction of death, myocardial infarction or stroke.1 Transradial access also reduced a patient’s hospital stay by 0.4 days (P = 0.0001).1
A refinement of the transradial approach is arterial access via the distal radial artery (dRA).10 This approach has recently been shown to be safe and feasible for patients and operators.10–13 There is only limited data available in the cardiology and neurointerventional literature on use of dRA access for endovascular procedures.14 However, available studies suggest dRA access to offer additional advantages over the standard transradial approach, including faster post-procedural hemostasis,15 less ischemic events and decreased rates of RA occlusion,10–13 as well as slightly higher patient satisfaction.15
In this study we describe our initial experience with dRA access for CAS procedures. We also discuss our procedural set-up and important technical considerations for successful CAS interventions via the dRA approach.
Materials and methods
This study was approved by each hospitals’ institutional review board.
Between May 2019 and March 2020, we retrospectively reviewed our prospective neurointerventional database of endovascular procedures and identified all patients who underwent CAS via dRA access in the anatomical snuffbox. Procedural and radiographic data was collected. Information on patient demographic data, including age, gender and comorbidities were also obtained.
Results
Between May 2019 and March 2020, we identified 22 CAS interventions in 20 patients via dRA puncture in the anatomical snuffbox. dRA access was achieved in all cases. However, two cases (9.1%) required conversion to femoral access, due to persistent radial artery vasospasm resulting in patient discomfort despite multiple additional doses of intraarterial vasodilators and added intravenous sedation as well as tortuous anatomy and limited support of the catheters in a type 3 aortic arch for left internal carotid artery stenting in the other case. All other 20 interventions were successfully performed via the dRA approach. We did not observe any peri- or postprocedural access related complications. Cerebral protection devices (Emboshield NAV; Abbott Vascular, Santa Clara, CA, USA or SpiderFX; Medtronic Neurovascular, Irvine, CA, USA) were used in all cases. There was no access site complication or mortality associated with any intervention.
Seventeen patients were male and 3 were female. Mean patient age was 69.4 years. Patient demographics and procedural data is summarized in Table 1. Mean radial artery diameter was 2.1 mm with measurements available in 14/22 cases. In our practice, ultrasound measurements were more often taken during initial transition to dRA access when mostly diagnostic cerebral angiograms were performed. With increased operator experience, vessel size was deemed appropriate by visual inspection with only occasional measurements performed.
Table 1.
Summary of patient demographics and procedural data.
| Number of patients | n = 20 |
| Gender | |
| Male | 17 (85%) |
| Female | 3 (15%) |
| Age (years: mean; range) | 69.4 (53–87) |
| Comorbidities | |
| None | 2 |
| Hypertension | 14 |
| Hyperlipidemia | 16 |
| Smoking | 5 |
| Diabetes mellitus | 9 |
| Coronary artery disease | 4 |
| Chronic kidney failure | 2 |
| Distal transradial access | n = 22 |
| Access side | |
| Right | 22 (100%) |
| Ultrasound guided access | 22 (100%) |
| Vessel size (mm: mean; range) | 2.1 (1.6–2.8) |
| Sheath size | |
| 6F | 17 (77.3%) |
| 7F | 1 (4.5%) |
| Sheathless | 4 (18.2%) |
| CAS interventions | n = 22 |
| Treatment side | |
| Right | 14 (63.6%) |
| Left | 8 (36.4%) |
| Aortic arch type | |
| Type 1 | 8 |
| Type 2 | 5 |
| Type 3 | 5 |
| Bovine | 2 |
| Not determinable | 2 |
| Distal transradial guide catheters | |
| Benchmark | 16 |
| Fubuki | 3 |
| 5 French | 1 |
| 6 French | 1 |
| 7 French | 1 |
| Select Flex 072 Neurovascular Access System | 1 |
| Conversion to Femoral Access | n = 2 |
In 19 cases a Carotid Wallstent (Boston Scientific, Santa Clara, CA, USA) was used. In 2 cases a Viabahn stent graft (Gore, Flagstaff, AZ, USA) was placed and a Precise stent (Cordis, Miami Lakes, FL, USA) was deployed in 1 case. The Viabahn stent grafts (Gore) were placed through a 6 F and 7 F Fubuki Neurovascular sheath (Asahi Intecc, Tokyo, Japan). When we used the large bore long sheaths, we preferred a bareback technique of exchanging the 5/6 F braided sheath for the long sheaths. Given the feasibility of bareback catheterization of these long sheaths with large IDs up to 0.090”, it is possible to use any carotid stent per the operator’s preference. In our institution, the Carotid Wallstent (Boston Scientific) was preferred given its closed cell design and adequate radial force.
Procedural set-up and technical considerations
Snuffbox access
Local anesthesia and ultrasound-guided arterial puncture
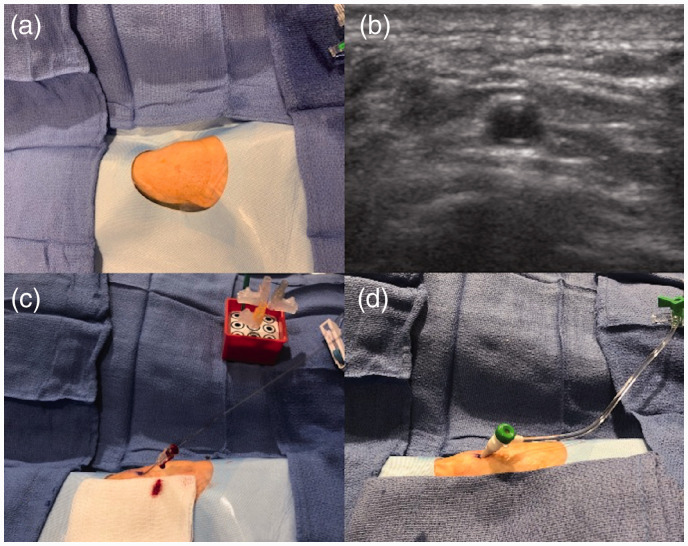
The wrist area at the anatomical snuffbox was prepped and draped in a sterile fashion withe the patient's hand in the neutral semi-prone position (Figure 1(a)). The skin and periarterial soft tissues at the anatomical snuffbox were infiltrated with 1–2 mL of lidocaine for local anesthesia. Nitroglycerine 200–400 µg was infiltrated around the dRA for tumescent anesthesia. Access site pain due to irritation of the periosteum of the scaphoid bone was avoided by pre-access infiltration of vasodilators and local anesthetics. A single wall puncture without the needle touching the underlying bone is also helpful. Aside from prevention of pain during arterial puncture, this combination of anesthetic medications and vasodilators was also used to prevent vasospasm and create a safe window for ultrasound guided micropuncture access. Ultrasound guidance for distal radial artery access in the anatomical snuffbox was used for all CAS interventions (Figure 1(b)). After successful micropuncture access (Figure 1(c)) a radial sheath was advanced over the wire into the dRA (Figure 1(d)). The pulse oximeter probe was attached to the patient’s thumb at the site of dRA access and oxygen saturation levels were monitored throughout the case.
Figure 1.
Overview of ultrasound guided access and sheath placement in the anatomical snuffbox.
Spasmolytic medication and heparin administration
After insertion of the sheath, all patients received antispasmolytic agents (2.5–5 mg of verapamil, 100–200 µg of nitroglycerin) and heparin 5000 IU via slow intra-arterial infusion through the sheath or guide catheter. Activated clotting time (ACT) was measured at baseline and throughout the procedure with ACT goals of twice baseline. Additional intravenous doses of heparin were administered as needed.
Prior to catheter exchanges, another dose of spasmolytic agents was administered through the radial sheath in order to prevent catheter induced vasospasm.
Sheath size
In our cases, dRA access was mainly obtained with a 6 F Prelude Ideal hydrophilic sheath introducer (Merit Medical, South Jordan, UT, USA). The braided design of this particular sheath is less prone to kinking at the radiocarpal joint when the sheath travels from the snuffbox to the distal forearm and provides the necessary stability for co-axial and triaxial catheter assembly systems. In three cases, a Glidesheath Slender hydrophilic coated introducer sheath (Terumo, Fremont, CA, USA) was used, one of which was to accommodate a 7 F Wahoo Access Catheter (Q’apel Medical, Santa Monica, CA, USA) (see illustrative case 1).
Bareback/sheathless access
In cases where a Fubuki guide catheter (Asahi Intecc) was used (n = 3), the 6 F radial sheath was also removed and the catheter was directly inserted into the dRA over the exchange length wire (sheath-less or bareback catheter use). In one case, a 6 F Benchmark guide catheter (Penumbra, Alameda, CA, USA) was used to gain direct access to the dRA. In patients with taut skin, a small incision with an upwards facing scalpel at the access site was needed to successfully insert the guide catheter over its dilator.
Technical pearls
Radial artery roadmap for catheter navigation
After successful insertion of the radial sheath and administration of the antispasmolytic medication and heparin, we performed a radial artery roadmap in all our cases. Visualization of the radial artery anatomy in our experience facilitates navigation of guide wire and catheter with less generation of radial artery vasospasm. Additionally, any anatomical variations such as radial artery loops or high origin of the radial artery can be visualized easily.
Right versus left dRA access
All procedures are performed via right dRA access as this approach is most familiar to the operator and requires less changes in room set-up. Positioning and accessibility of the right hand is also easier compared to an across-body positioning of the left hand for dRA access from the right side of the table. Some interventional cardiologist will change the entire room set-up for a left dRA approach. They are essentially “mirroring” the room set-up when working from the left side of the table. While most CAS procedures can be performed from a right dRA approach, left dRA access could be considered for right carotid stenting if there is a severe angle between the right subclavian artery and right common carotid artery. Additionally, left dRA access can be used in case of vascular anatomic variations along the right radial artery, an overall too small caliber of the vessel, presence of a right dialysis fistula or refractory vasospasm.
Guide catheter selection and position
The target carotid artery can be either selected directly with an appropriate guide catheter (over a shaped diagnostic catheter) or via catheter exchange after selection of the target carotid with diagnostic Sim2 catheter (Terumo or Merit 5 F Impress catheter).
For the intervention, it is important to pay attention to the length of the catheters, as about 4 to 6 centimeters of catheter length are lost with dRA access. This can be corrected by choosing either longer guide catheters or using the short FLO Hemostasis Valves (Merit Medical).
Exchange wire selection
A stiff Terumo exchange length Glidewire (Terumo) or a Terumo Glidewire Advantage was used for all our catheter exchanges. The Terumo Glidewire Advantage can offer good support across the aortic arch when treating ICA stenosis. Ideally, the exchange length Glidewire is advanced deep into the ECA to offer maximum support at the junction of the right subclavian artery with the brachiocephalic artery and right CCA or, when targeting a left ICA stenosis, from the brachiocephalic artery into the transverse aorta and into the left ECA.
Successful wire exchanges when operating from a radial access are crucial to reduce vessel manipulation and possible associated vasospasm. Once the diagnostic catheter has been removed over-the-wire, we recommend administration of another dose of antispasmolytic medication (5 mg of verapamil, 200 µg of nitroglycerin) to prevent any radial artery vasospasm induced by the larger catheter systems.
“Buddy wire” technique
In some cases, the guide catheter is either not sufficiently distal or may prolapse during the procedure after placement of the distal protection device. We have found that using a buddy wire technique with a stiff 0.035” glidewire is helpful in navigating the guide catheter higher into position. The 0.070” or bigger guide-catheters allow co-navigation of a 0.014” distal protection wire and 0.035” glidewire for “buddy” wire technique.
Advancing catheter during balloon inflation for angioplasty
In some instances when the guide catheter is retracted to deploy the carotid stent in the distal common carotid artery, the guide catheter can be repositioned distally inside the proximal stent by climbing on the inflated angioplasty balloon. This technique can also be used for nascent advancement of the guide catheter if it wasn't possible to advance it with inner slip catheter/glidewire combination or buddy wire technique. Usually these techniques are not frequently needed for transfemoral CAS but might be needed for dRA/RA CAS as the force vector may not allow for advancement of guide-catheters as is common from a trans-femoral approach. Dedicated radial access products will hopefully in the future make these catheterizations reliable without need for crossovers or accessory steps.
Access site closure
The Prelude SYNC DISTAL™ radial compression device (Merit Medical) was used to close the dRA access site upon removal of the catheter systems and sheath. Patent hemostasis was achieved by slow release of air from the initially fully inflated balloon until a small amount of brisk blood flow was visualized from the puncture site. The balloon was then re-inflated with 1-2 mL of air. The pulse oximeter curve and reading were observed for any changes.
Illustrative cases
Case 1
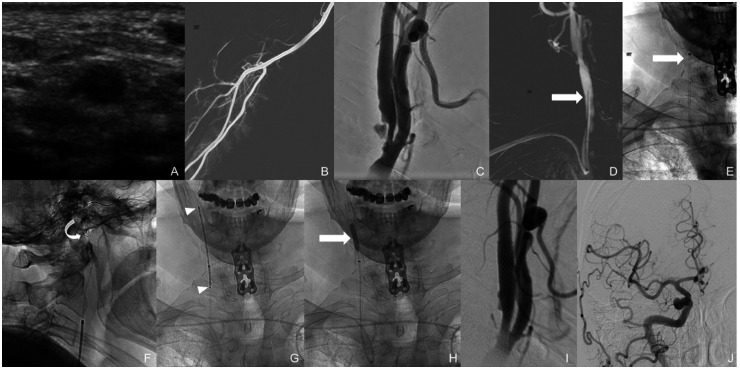
The right dRA was assessed under ultrasound and deemed appropriate in caliber for angiogram access (Figure 2(a)). The right snuff box area was then infiltrated with 1 mL of lidocaine 1% and 200 µg of nitroglycerin subcutaneously. The vessel was successfully accessed under ultrasound guidance and, using modified Seldinger technique, a 7 F Glidesheath Slender hydrophilic coated introducer sheath (Terumo) was placed. A slow intra-arterial infusion of 5.0 mg verapamil, 200 mcg nitroglycerin and 5000 IU heparin was administered through the radial sheath. A right radial artery roadmap was obtained via the side port of the sheath (Figure 2(b)). Then, a 5 F Sim 2 glide catheter (Terumo) was navigated over a Terumo 0.035” Glidewire into the aortic arch and placed into the right common carotid artery. Frontal and lateral angiograms of the neck revealed a short segment 70% stenosis of the proximal right internal carotid artery with calcified and ulcerated plaque (Figure 2(c)). Under roadmap guidance, the 5 F Sim 2 catheter was exchanged for a 7 F Wahoo Access Catheter (Q’apel Medical) over a 5 F MP catheter (Cordis) and a Terumo 0.035” exchange length Glidewire Advantage. The 7 F Wahoo Access Catheter was placed into the right CCA, just proximal to the carotid bifurcation (Figure 2(d) and (e), arrows pointing to tip of the catheters). Prior to the exchange, an additional 5 mg of verapamil and 200 µg of nitroglycerin was administered through the right radial sheath. Next, an Emboshield NAV 7.2 mm cerebral protection device (Abbott) was placed beyond the carotid stenosis within the distal cervical segment of the left ICA (Figure 2(f), curved arrow). Then, an 8×29 mm Carotid Wallstent (Boston Scientific) was placed covering the entire length of the stenotic segment. (Figure 2(g), arrow heads showing proximal and distal markers of the of stent). Follow-up angiograms showed residual waste within the stented segment and, therefore, a 4.0 × 20 mm Sterling balloon (Boston Scientific) was placed within the stent and inflated to 10 atmospheres (Figure 2(h), arrow). Follow-up angiograms of the neck and right anterior circulation were performed which demonstrated satisfactory increase of the luminal diameter across the previously stenotic right ICA segment (Figure 2(i)) with excellent intracranial flow (Figure 2(j)). Subsequently, the cerebral protection device and guide catheter were removed. A radial compression device (inflatable band) was placed and inflated over the right dRA access site. The sheath was withdrawn, and the band was slowly deflated until brisk blood flow was noted through the arteriotomy site. At this point, the band was re-inflated with 2 mL of air to permit patent hemostasis.
Figure 2.
Successful 7 F right dRA access for treatment of a right carotid stenosis using a Wahoo Access Catheter (Q’apel Medical).
Case 2
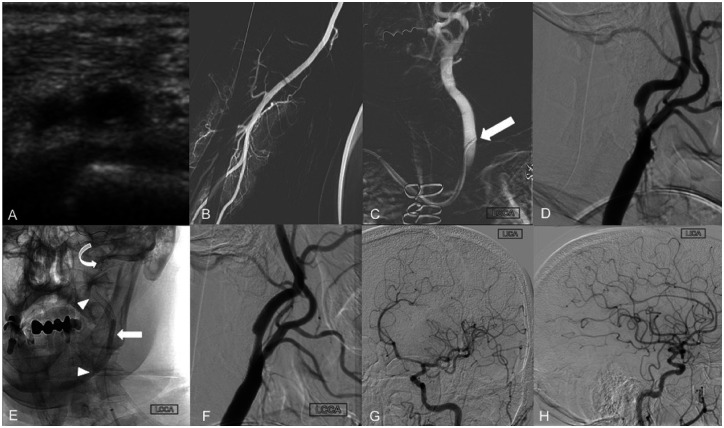
The right dRA was assessed under ultrasound and deemed appropriate in caliber for angiogram access (Figure 3(a)). The right snuff box area was then infiltrated with 1 mL of lidocaine 1% and 200 µg of nitroglycerin subcutaneously. The vessel was successfully accessed under ultrasound guidance and, using modified Seldinger technique, a 6 F Prelude Ideal hydrophilic sheath introducer (Merit Medical) was placed. A slow intra-arterial infusion of 5.0 mg verapamil, 200 mcg nitroglycerin and 5000 IU heparin was administered through the radial sheath. A right radial artery roadmap was obtained via the side port of the sheath (Figure 3(b)). Then, a 5 F Sim 2 glide catheter (Terumo) was navigated over a Terumo 0.035” Glidewire into the aortic arch and placed into the left CCA (Figure 3(c), arrow). Angiogram of the neck revealed atherosclerotic changes at the left carotid bifurcation with ulcerating plaque resulting in approximately 70% stenosis of the proximal left ICA (Figure 3(d)). The 5 F Sim2 glide catheter (Terumo) was exchanged for a 6 F Benchmark (Penumbra) over a Terumo 0.035” exchange length Glidewire Advantage (Terumo) and placed into the left ICA, just proximal to the carotid bifurcation. Prior to the exchange, an additional 5 mg of verapamil and 200 mcg of nitroglycerin were administered through the right radial sheath. Following frontal and lateral angiograms of the left neck, a 5 mm NAV Emboshield (Abbott) cerebral protection device was placed beyond the carotid stenosis within the distal cervical segment of the left ICA. Then, an 8 × 36 mm Carotid Wallstent was placed covering the entire length of the stenotic segment. Due to residual waste within the stented segment, angioplasty was performed using a 3.5 × 20 mm Sterling balloon (Figure 3(e), arrow heads showing the proximal and distal aspects of the stent, curved arrow indicating the position of the cerebral protection device and block arrow pointing to the angioplasty balloon). Follow-up angiograms showed satisfactory increase of the luminal diameter across the previously stenotic segment (Figure 3(f)). Subsequently, the cerebral protection device was recaptured and removed through the guide catheter. Frontal oblique and lateral view angiograms of the left anterior circulation demonstrated excellent intracranial flow (Figure 3(g) and 3(h)). The guide catheter was then withdrawn. A radial compression device (inflatable band) was placed and inflated over the right radial access site. The vascular sheath was withdrawn and the band was slowly deflated until brisk blood flow was noted through the arteriotomy site. The band was then re-inflated with 2 mL of air to permit patent hemostasis.
Figure 3.
Successful 6 F right dRA access for treatment of a left carotid stenosis using a Benchmark guide catheter (Penumbra).
Discussion
The dRA approach in the anatomical snuffbox requires ultrasound guidance for safe access and is overall a little more difficult than the transradial approach given the added imaging-guided puncture and overall smaller caliber of the vessel. Nevertheless, interventional cardiology literature proved that dRA access has a similar safety and efficacy profile than the conventional transradial approach.16 The current literature even suggests dRA access to have some additional advantages over transradial access including decreased risk of hand ischemia, decreased time to hemostasis, greater patient and operator comfort.10–13,15 dRA also offers the option for repeat dRA access and/or future radial artery access. A recent meta-analysis of 4 observational studies and 1 randomized controlled trial showed a statistically significant lower rate of radial artery occlusions with dRA versus transradial access (2.3% versus 4.86%, P = 0.004).16 In his 2017 paper on left dRA access for coronary angiography and interventions Kiemeneij17 mentions a 2014 EuroPCR talk by A. Kaledin who treated 656 patients via dRA and reported 1.5% of dRA occlusions. Other likely access related complications in this cohort included arm edema in 0.2%, arteriovenous and pseudoaneurysm formation in 0.2% each, radial artery dissection in 0.3%, transitory finger numbness in 0.6% and wrist and/or forearm hematoma in 0.8%.18 No “conventional” radial artery occlusions were seen.18
Operators have to commit to a learning curve when transitioning from transfemoral to transradial and also from transradial to dRA access. In our institutions, we have gained initial experience and confidence in using the dRA approach during diagnostic cerebral angiograms which allowed the operator some additional time to focus on safely obtaining vascular access and to practice catheter navigation.19 In a next step, after the operator has gained more experience with the dRA access, the first CAS cases were carefully selected. CT angiogram images were reviewed to understand individual patient anatomy. Patient selection played a crucial role, as patients needed to be able to remain cooperative throughout a more extended period of time and willing to undergo dRA access while knowing of the possibility of conversion to femoral access. Access conversion rates in the cardiology literature requiring cross over from dRA to transfemoral access are reported to lie between 0.3% to 11%.10–13,15,20 For diagnostic cerebral angiograms, Brunet et al.14 and Patel et al.21 reported their dRA failure rate to be 8% and 11.8%, respectively. We observed an 9.1% (n = 2) cross over rate from dRA to transfemoral access for our CAS procedures which was due to radial artery vasospasm in one case and a type 3 aortic arch anatomy which did not offer sufficient catheter support for a left carotid intervention in the other case. While stenting of the ipsilateral right ICA and left ICA in the setting of a bovine-type aortic arch is considered uncomplicated22 and likely also associated with reduced catheter-induced embolizations from the transverse aortic arch,23 left carotid artery stenting can be challenging with a non-bovine arch anatomy.23,24 Folmar et al.22 described successful CAS procedures in 97% of interventions on the right, 80% of interventions with bovine-type aortic arch anatomy but only in 54% of left carotid stenting procedures. Our first CAS cases via dRA access were mostly on the ipsilateral, right side. While brachiocephalic anatomy and angle of right common carotid artery takeoff needed to be evaluated (unfavorable anatomy; namely, acute angles of <50°,22 predict technical difficulties), ipsilateral interventions appeal to the familiarity and ease of the interventionalist. With more experience and confidence, operators will be able to tackle more difficult anatomy and are also more flexible in adjusting their approach to the anatomy at hand.
Despite operators experiencing some initial challenges with this novel approach, the benefits for their patients are indisputable. The interventional cardiology literature supports the safety and efficacy of wrist over transfemoral access.1,2,25,26 A 2016 meta-analysis20 of 777,841 from 3 randomized controlled and 13 observational studies concluded that transradial access for coronary angiography and intervention is associated lower rate of vascular complications overall, a reduced risk of stroke and a mortality benefit for patients with ST elevation myocardial infarction. Another study evaluated the incidences of in-hospital major and minor puncture-related hemorrhages between transradial and transfemoral access and showed that femoral access was an independent predictor of major bleeding.27
Despite the vastly available interventional cardiology and emerging neurointerventional literature in favor of wrist over transfemoral access, there are still concerns with the dRA approach including lack of familiarity and uncertainty of complications associated with placing larger access catheters into the radial artery. Our initial experience with dRA access for CAS is promising but given our small sample size the advantages of this approach will need to be confirmed in larger studies. In addition, and despite reports of successfully performed neurointerventions via dRA,17,28,29 interventionalists lack catheters and devices specifically designed for wrist access. Currently used catheter systems are originally aimed for transfemoral interventions and are converted for dRA. Understandably, some operators do not wish to use catheters and devices with dimensions and specifications not specifically tailored to radial access. Industry is now taking notice and dedicated catheter systems are developed/being developed that will facilitate radial access and permit navigating standard as well as difficult anatomy.
Conclusion
Limited data on use of distal radial artery access for neurointerventional procedures is currently available in the literature. Our initial experience with distal radial artery access for carotid artery stenting using currently available guide catheters appears to be feasible and without complications. Technical and anatomical considerations are important and proper pre-procedural planning is necessary for a successful intervention. However, catheter systems and devices specifically designed for radial access are needed and will likely enable more interventionalists to safely perform such procedures via wrist access.
Authors’ contribution: Study Conception and Design: ALK, ASP; Data Acquisition: all authors; Literature Search: ALK, VMM, KdMR, FM, AM; Data Analysis and Interpretation: ALK, JS, VMM, SRS, ASP; Manuscript Preparation: ALK, JS, ASP; Revision of manuscript for important intellectual content: VMM, SRS, KdMR, FM, MJG, AM; Approval of final version of manuscript: all authors.
Ethical approval: The Institutional Review Board at the University of Massachusetts (H00001860_10) and Christiana Health System (CCC number: 34154 and DDD number 602798) have approved the study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: ASP is a consultant for Stryker, Cerenovus, CereVasc, Merit and Medtronic; research grant from Stryker Neurovascular and Medtronic. Stocks in InNeuroCo.
SRS is a consultant for Balt, Stryker Neurovascular, Cerenovus, Medtronic, Microvention and Terumo as well as a proctor for Cerenovus and Medtronic.
ALK, JS, VMM, KdMR, FM and AM have no competing interests.
MJG declares he has been a consultant on a fee-per-hour basis for Cerenovus, Imperative Care, Phenox, Medtronic Neurovascular, Route 92 Medical, Stryker Neurovascular; holds stock in Imperative Care, InNeuroCo and Neurogami; and has received research support from the National Institutes of Health (NIH), the United States – Israel Binational Science Foundation, Anaconda, ApicBio, Axovant, Cerenovus, Cook Medical, Gentuity, Imperative Care, InNeuroCo, Magneto, Microvention, Medtronic Neurovascular, MIVI Neurosciences, Neuravi, Neurogami, Philips Healthcare, Rapid Medical, Route 92 Medical, Stryker Neurovascular, Syntheon, and the Wyss Institute.
ORCID iDs
Anna Luisa Kühn https://orcid.org/0000-0001-9512-9461
Sudhakar R Satti https://orcid.org/0000-0001-6671-7918
Archie McGowan https://orcid.org/0000-0002-5710-1895
References
- 1.Jolly SS, Amlani S, Hamon M, et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and Meta-analysis of randomized trials. Am Heart J 2009; 157: 132–140. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol 2004; 44: 349–356. [DOI] [PubMed] [Google Scholar]
- 3.Khanna O, Sweid A, Mouchtouris N, et al. Radial artery catheterization for neuroendovascular procedures. Stroke 2019; 50: 2587–2590. [DOI] [PubMed] [Google Scholar]
- 4.Zussman BM, Tonetti DA, Stone J, et al. A prospective study of the transradial approach for diagnostic cerebral arteriography. J NeuroIntervent Surg 2019; 11: 1045–1049. [DOI] [PubMed] [Google Scholar]
- 5.Zussman BM, Tonetti DA, Stone J, et al. Maturing institutional experience with the transradial approach for diagnostic cerebral arteriography: overcoming the learning curve. J Neurointerv Surg 2019; 11: 1235–1238. [DOI] [PubMed] [Google Scholar]
- 6.Almallouhi E, Leary J, Wessell J, et al. Fast-track incorporation of the transradial approach in endovascular neurointervention. J Neurointerv Surg 2020; 12: 176–180. [DOI] [PubMed] [Google Scholar]
- 7.Snelling BM, Sur S, Shah SS, et al. Transradial cerebral angiography: techniques and outcomes. J NeuroIntervent Surg 2018; 10: 874–881. [DOI] [PubMed] [Google Scholar]
- 8.Snelling BM, Sur S, Shah SS, et al. Transradial approach for complex anterior and posterior circulation interventions: Technical nuances and feasibility of using current devices. Oper Neurosurg (Hagerstown) 2019; 17: 293–302. [DOI] [PubMed] [Google Scholar]
- 9.Stone JG, Zussman BM, Tonetti DA, et al. Transradial versus transfemoral approaches for diagnostic cerebral angiography: a prospective, single-center, non-inferiority comparative effectiveness study. J Neurointerv Surg. Epub ahead of print 23 January 2020. DOI: 10.1136/neurintsurg-2019-015642. [DOI] [PubMed]
- 10.Valsecchi O, Vassileva A, Cereda AF, et al. Early clinical experience with right and left distal transradial access in the anatomical snuffbox in 52 consecutive patients. J Invasive Cardiol 2018; 30: 218–223. [PubMed] [Google Scholar]
- 11.Babunashvili A. TCT-810 novel distal transradial approach for coronary and peripheral interventions. J Am Coll Cardiol 2018; 72: B323. [Google Scholar]
- 12.Al-Azizi KM, Lotfi AS. The distal left radial artery access for coronary angiography and intervention: a new era. Cardiovasc Revasc Med 2018; 19: 35–40. [DOI] [PubMed] [Google Scholar]
- 13.Soydan E, Akın M. Coronary angiography using the left distal radial approach – an alternative site to conventional radial coronary angiography. Anatol J Cardiol 2018; 19: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunet M-C, Chen SH, Sur S, et al. Distal transradial access in the anatomical snuffbox for diagnostic cerebral angiography. J Neurointerv Surg 2019; 11: 710–713. [DOI] [PubMed] [Google Scholar]
- 15.Koutouzis M, Kontopodis E, Tassopoulos A, et al. Distal versus traditional radial approach for coronary angiography. Cardiovasc Revasc Med 2019; 20: 678–680. [DOI] [PubMed] [Google Scholar]
- 16.Hamandi M, Saad M, Hasan R, et al. Distal versus conventional transradial artery access for coronary angiography and intervention: a meta-analysis. Cardiovasc Revasc Med. Epub ahead of print 14 March 2020. DOI: 10.1016/j.carrev.2020.03.020. [DOI] [PubMed]
- 17.Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (IdTRA) and interventions (IdTRI). Eurointervention 2017; 13: 851–857. [DOI] [PubMed] [Google Scholar]
- 18.Kaledin AL, Kochanov IN, Seletskiy SS, et al. Peculiarities of arterial access in endovascular surgery in elderly patients [article in Russian]. Adv Gerontol 2014; 27: 115–119. [PubMed] [Google Scholar]
- 19.Kühn AL, de Macedo Rodrigues K, Singh J, et al. Distal radial access in the anatomical snuffbox for neurointerventions: a feasibility, safety, and proof-of-concept study. J Neurointerv Surg 2020; 12: 798--801. [DOI] [PubMed]
- 20.Alnasser SM, Bagai A, Jolly SS, et al. Transradial approach for coronary angiography and intervention in the elderly: a Meta-analysis of 777,841 patients. Int J Cardiol 2017; 228: 45–51. [DOI] [PubMed] [Google Scholar]
- 21.Patel P, Majmundar N, Bach I, et al. Distal transradial access in the anatomic snuffbox for diagnostic cerebral angiography. AJNR Am J Neuroradiol 2019; 40: 1526–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folmar J, Sachar R, Mann T. Transradial approach for carotid artery stenting: a feasibility study. Catheter Cardiovasc Interv 2007; 69: 355–361. [DOI] [PubMed] [Google Scholar]
- 23.Faggioli G, Ferri M, Rapezzi C, et al. Atherosclerotic aortic lesions increase the risk of cerebral embolism during carotid stenting in patients with complex aortic arch anatomy. J Vasc Surg 2009; 49: 80–85. [DOI] [PubMed] [Google Scholar]
- 24.Patel T, Shah S, Ranjan A, et al. Contralateral transradial approach for carotid artery stenting: a feasibility study. Catheter Cardiovasc Interv 2010; 75: 268–275. [DOI] [PubMed] [Google Scholar]
- 25.Ferrante G, Rao SV, Jüni P, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a Meta-analysis of randomized trials. JACC Cardiovasc Interv 2016; 9: 1419–1434. [DOI] [PubMed] [Google Scholar]
- 26.Feldman DN, Swaminathan RV, Kaltenbach LA, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007–2012). Circulation 2013; 127: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 27.Pristipino C, Pelliccia F, Granatelli A, et al. Comparison of access-related bleeding complications in women versus men undergoing percutaneous coronary catheterization using the radial versus femoral artery. Am J Cardiol 2007; 99: 1216–1221. [DOI] [PubMed] [Google Scholar]
- 28.Al Saiegh F, Mouchtouris N, Sweid A, et al. Placement of the woven EndoBridge (WEB) device via distal transradial access in the anatomical snuffbox: a technical note. J Clin Neurosci 2019; 69: 261–264. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy DJ, Chen SH, Brunet MC, et al. Distal radial artery access in the anatomical snuffbox for neurointerventions: case report. World Neurosurg 2019; 122: 355–359. [DOI] [PubMed] [Google Scholar]