Abstract
Background
Rupture of infectious intracranial aneurysms (IIAs) is associated with a high likelihood of mortality. Endovascular treatment of IIAs via parent artery sacrifice offers good efficacy and outcomes; however, depending on the lesion’s location, neurologic deficit may result.
Case description
We describe a pediatric patient with ruptured IIAs off the left middle cerebral artery (MCA) treated with coil embolization and endovascular flow diversion using the Pipeline Flex Embolization Device (PED) with Shield technology. We chose to place a flow diverter because 1) there was a second, more distal IIA not amenable to direct coil embolization, 2) there was significant potential for aneurysm regrowth and need for retreatment, and 3) we believed the diseased parent MCA needed to be reconstructed.
Conclusions
In the setting of previous hemicraniectomy, PED-Shield gave us the option to discontinue dual antiplatelet therapy should the patient require further neurosurgical intervention. Our case supports a role for PED-Shield to address ruptured pseudoaneurysms.
Keywords: Infectious intracranial aneurysm, flow diversion, Pipeline Embolization Device
Rupture of infectious intracranial aneurysms (IIAs) carries a mortality of up to 80%.1 Use of liquid embolics has generally demonstrated excellent efficacy and good outcomes;2 however, neurologic deficit may result if a parent artery supplying eloquent brain tissue is sacrificed. We present our unusual case of a pediatric patient with ruptured IIAs off the left middle cerebral artery (MCA) who underwent treatment with coil embolization and endovascular flow diversion using a novel flow diverter with phosphocholine surface modification designed to reduce thromboembolic complications.
Case presentation
A 10-year-old girl presented after 7 days of headaches, lethargy, and fever. Magnetic resonance (MR) imaging demonstrated left peri-sylvian and insular intracerebral abscess due to intracranial extension from paranasal sinusitis (Figure 1). The patient was placed on broad-spectrum antibiotics and underwent two endoscopic sinus surgeries and two stereotactic intracerebral abscess aspirations. Cultures revealed growth of Staphylococcus aureus and Streptococcus anginosus. On hospital day 18, she developed a spontaneous left-sided intraparenchymal hemorrhage with transtentorial herniation. The patient underwent emergent left-sided decompressive hemicraniectomy and partial hematoma evacuation.
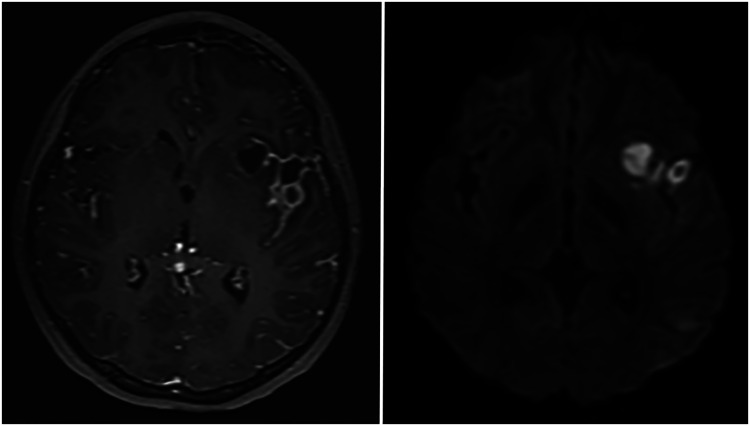
Figure 1.
Axial MRI of the brain with gadolinium demonstrates a contrast-enhancing lesion within the left sylvian fissure, extending into the insula (left) with diffusion restriction (right), representing an intracerebral abscess.
Postoperative computed tomography angiography revealed a left MCA aneurysm involving the M1 segment. The patient underwent catheter angiography, which showed 2 IIAs involving the left M1 segment (Figure 2), a distal aneurysm measuring 1.6 × 0.9 mm in size and a more proximal aneurysm measuring 4.05 × 4.28 mm in size. The patient underwent emergent coil embolization of the proximal aneurysm. Catheters were subsequently placed into the abscess cavity for daily antibiotic irrigation. After removal of the drains, she was started on 325 mg of daily aspirin and 37.5 mg of clopidogrel. Five days later, VerifyNow aspirin and P2Y12 assays revealed an aspirin reactivity unit of 397 and P2Y12 reaction unit of 111. She was subsequently brought to the neurointerventional suite for planned embolization of the IIAs using the Pipeline Flex Embolization Device (PED)-Shield (Medtronic, Minneapolis, MN). General anesthesia was induced, and neurophysiologic monitoring leads were placed for somatosensory evoked potentials and electroencephalography during the case. A 45-cm 6-French Terumo guide sheath (Somerset, NJ) was placed in the left common carotid artery, and a Navien 058 intermediate catheter (Medtronic) was advanced into the cavernous segment of the left internal carotid artery. Intra-arterial verapamil (10 mg) was administered to mitigate any catheter-associated vasospasm as well as any potential intracranial vasospasm. The proximal left MCA just distal to the takeoff of the anterior temporal artery measured approximately 1.51 mm, and the M2 branch distal to the diseased segment measured 1.59 mm. We measured the distance between our proximal and distal target landing zones to be approximately 17.3 mm. Intravenous heparin (3000 units) was administered, resulting in an activated clotting time of 231. Under roadmap guidance, we advanced a Synchro2 microwire (Stryker Neurovascular, Fremont, CA) and Marksman microcatheter (Medtronic) past the diseased M1 segment of the left MCA into the M2 branch. We then placed a 2.5 × 14-mm PED-Shield in the left MCA at our target landing zones, allowing for flow diversion of both aneurysms while avoiding coverage of the ostium of the anterior temporal artery (Figure 3). Postprocedural brain MR imaging and angiography with and without contrast demonstrated no thromboembolic complications and patency of the PED-Shield.
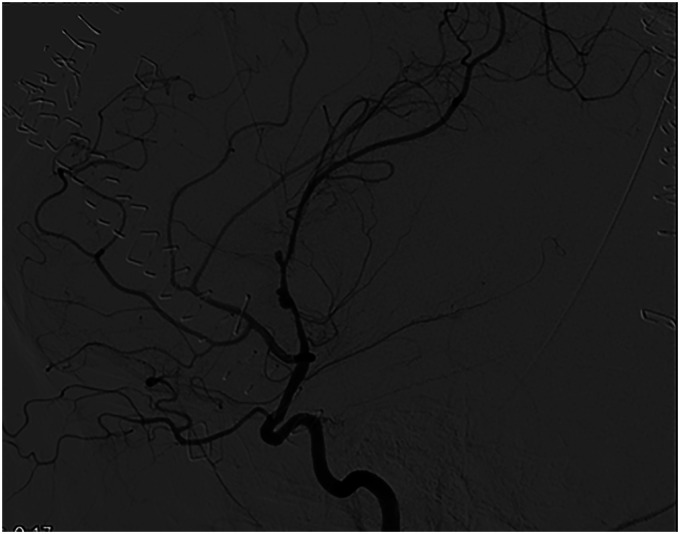
Figure 2.
Digital subtraction angiography, left ICA injection, lateral view, shows the two infectious intracranial aneurysms along the M1 segment of the left middle cerebral artery.
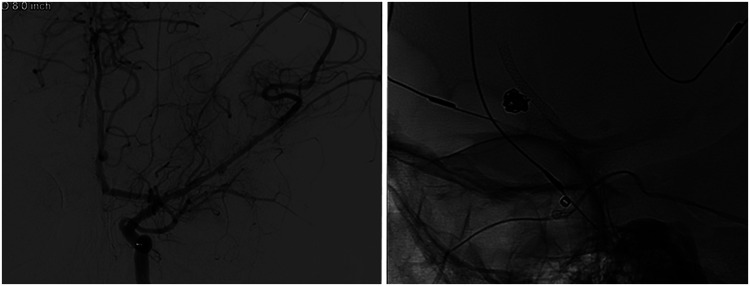
Figure 3.
Digital subtraction angiography, left ICA injection, anteroposterior view, shows the 2.5 × 14-mm PED-Shield deployed in the M1 segment of the left MCA covering the IIAs (left). Unsubtracted, lateral view, image shows the PED-Shield relative to the previously coiled proximal IIA (right).
During the remainder of her inpatient hospitalization, the patient had no untoward events and was discharged to rehabilitation. She remained on intravenous ceftriaxone for six weeks and on dual antiplatelet medications, after which repeat brain MR imaging and angiography were performed; these showed improvement in the appearance of the intracranial abscess with no evidence of aneurysm and no new areas of infarct. Final MR imaging four months after treatment showed complete regression of the intracranial abscess with no further enhancement. A follow-up angiogram at 6 months showed complete vessel remodeling with no flow into the treated IIAs and no new aneurysms (Figure 4). The clopidogrel was stopped, and the patient underwent cranioplasty. Clinically, she regained the ability to speak and improved to nearly full strength in her right side.
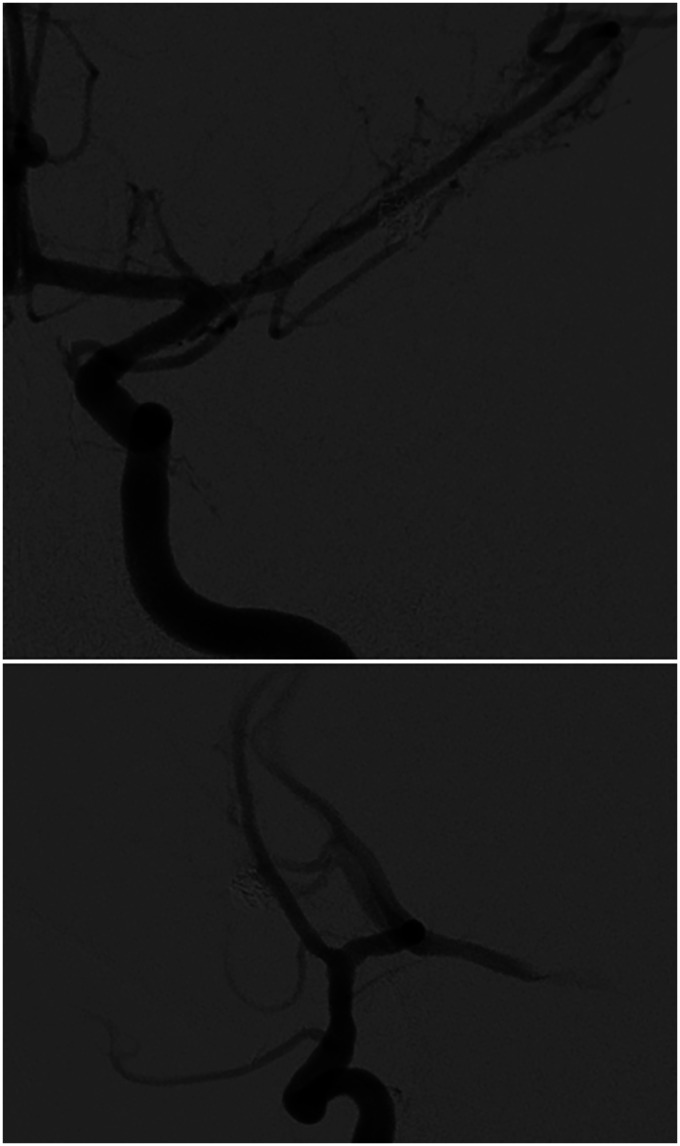
Figure 4.
Digital subtraction angiography, left ICA injection, anteroposterior (top) and lateral (bottom) views, at 6 months follow-up after PED-Shield placement demonstrates normal filling of left anterior circulation with no evidence of filling of the IIAs and remodeling of the diseased M1 segment of the left MCA.
Discussion
Other than early initiation of antibiotic therapy, management of IIAs is not standardized.1 Conservative treatment with antibiotic therapy can be considered for small, unruptured lesions if paired with close angiographic follow-up, with intervention reserved for lesions that fail to regress. Rupture status, associated intracranial hemorrhage, and eloquence of supplied tissue are important considerations when considering surgical or endovascular intervention.3 Surgical treatment of IIAs, as is typical with pseudoaneurysms, is challenging because of their irregular morphology, friable nature, and disruption of the parent vessel microarchitecture. For our patient, open surgical options were considered, given that eloquent cortex was involved, but there were concerns for catastrophic hemorrhage associated with aneurysm and vessel manipulation. We also had significant reservations because the patient had undergone numerous surgical interventions, which heightened our concern for scarring of the arachnoid and increased tissue friability.
Endovascular obliteration of IIAs is generally favored, with an approach involving parent artery sacrifice rather than direct aneurysm embolization.2 A crucial consideration in parent artery sacrifice for treatment of IIAs is the eloquence of the cortex supplied by the involved artery.4 Reuschel et al.5 described a 58-year-old man with a ruptured left M3 aneurysm initially treated with stent-assisted coiling who required a left ventricular assist device and was discovered to have a rehemorrhage and morphologically atypical recurrence of the aneurysm 21 months later; he was treated with parent vessel occlusion with a good outcome. Given the location of the IIAs along the left M1 segment in our patient, endovascular parent artery sacrifice was prohibitive. Despite the risks involved with direct embolization, we chose to first coil embolize the proximal aneurysm because of its size and risk for re-rupture.
Despite the limited evidence for stenting, approaches involving stent-assisted coiling and stent monotherapy have been used effectively for management of IIAs;6 however, arguably better options for endovascular management have emerged. The use of flow diverters for treatment of saccular intracranial aneurysms is well documented, but there are few reports for the use of flow diverters in IIAs.7–11 Appleboom et al.7 first reported the use of a flow diverter to embolize an unruptured, near-giant IIA involving the cavernous segment of the right internal carotid artery (ICA) using a SILK device (Balt Extrusion, Montmorency, France) after an increase in aneurysm size despite antibiotic therapy. Kobets et al.11 placed telescoping PEDs without complication to treat an unruptured large right cavernous ICA IIA in an adult who similarly presented with cavernous sinus thrombosis. The patient had clinical improvement and angiographic aneurysm occlusion at follow-up. Hartmann et al.9 reported a case of a 70-year-old man initially treated for a saccular posterior circulation aneurysm in the setting of bacteremia and atypical morphology that recurred as a mycotic aneurysm; the recurrence was treated with flow diversion into a stent-bearing segment of the basilar artery.
Parent artery preservation and endothelialization with formation of a “neo-intima” within the stent represent key advantages of flow-diversion therapy. Although device-associated infection has not been reported to date, this is an important consideration in using an implantable technology to treat an infectious condition. In addition, the need for dual antiplatelet therapy, potential for delayed rupture of the lesion, and thromboembolic complications are important caveats. Attention to the inherent thrombogenicity of stents has led to the creation of PED-Shield, which wields a phosphocholine surface modification that offers reduced thrombogenicity and faster endothelial overgrowth.12 Short-term safety was demonstrated in one prospective multicenter trial for treatment of unruptured intracranial aneurysms,13 and several cases have demonstrated successful embolization of saccular aneurysms14,15 and fusiform aneurysms16 with implementation of single antiplatelet therapy. Girdhar et al.17 studied the thrombogenicity of the Pipeline Flex, Pipeline Shield, and FRED flow diverters in an in-vitro human flow loop model and demonstrated the Pipeline Shield had lower thrombin–antithrombin complex formation and lower beta-thromboglobulin than the other devices and was comparable with a negative control. Additional study has involved the use of hydrophilic substances; Lenz-Habijan et al.18 studied two hydrophilic polymer coatings and determined that there was significant decrease in platelet adherence on nickel titanium surfaces versus uncoated surfaces. Martinez Moreno et al.19 demonstrated that antithrombogenic hydrophilic coating was biocompatible without acute inflammation in canine models.
The combined use of PED-Shield with monotherapy also carries a significant risk of complication; in fact, the initial report of use of in the United States by Hanel et al.14 described occlusion of the device just 10 days after placement because of inadequate platelet reactivity while the patient was on aspirin monotherapy.
Despite our consideration of monotherapy with aspirin only to mitigate additional hemorrhagic risk if further neurosurgical intervention was needed, we believed that the risk-benefit profile favored placing the patient on dual antiplatelet medication. In an emergency setting, administration of temporary dual antiplatelet therapy is the safest course of action with immediate testing for responsiveness followed by monotherapy; the choice of agent should be based on functional assays.
Although the use of flow diversion has been demonstrated in children,20–22 our case represents the first instance of use of PED-Shield in a pediatric patient with an IIA. Our reasons for placing a flow diverter were 1) the presence of a second, more distal IIA along the left MCA that was not amenable to direct coil embolization, 2) the significant potential for aneurysm regrowth and need for further treatment, and 3) our belief that the parent MCA vessel was diseased and needed to be reconstructed. Given that our patient had undergone hemicraniectomy, PED-Shield gave us the option to discontinue dual antiplatelet therapy should the patient require urgent neurosurgical intervention. Our case supports a role for PED-Shield in the endovascular armamentarium available to address ruptured pseudoaneurysms. The use of antiplatelet therapy in children is commonplace23 and believed to be safe in the setting of blunt cerebrovascular injury,24,25 but there is little evidence regarding the lifelong implications of these medications in children, which certainly requires further inquiry.
Footnotes
Authors’ contributions: Samples: Writing original draft, investigation, review and revision.
Ravindra: Writing original draft, review and revision.
Thoms: investigation, review and revision.
Tarasiewicz: Conceptualization, review and revision.
Grandhi: Review and revision, supervision.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Grandhi is a consultant for Medtronic Neurovascular, Cerenovus, and BALT Neurovascular.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ramesh Grandhi https://orcid.org/0000-0001-9000-6083
References
- 1.Chun JY, Smith W, Halbach VV, et al. Current multimodality management of infectious intracranial aneurysms. Neurosurgery 2001; 48: 1203–1213; discussion 1213–1214. [DOI] [PubMed] [Google Scholar]
- 2.Grandhi R, Zwagerman NT, Linares G, et al. Onyx embolization of infectious intracranial aneurysms. J Neurointerv Surg 2014; 6: 353–356. [DOI] [PubMed] [Google Scholar]
- 3.Flores BC, Patel AR, Braga BP, et al. Management of infectious intracranial aneurysms in the pediatric population. Childs Nerv Syst 2016; 32: 1205–1217. [DOI] [PubMed] [Google Scholar]
- 4.Eddleman CS, Surdell D, DiPatri A, et al. Infectious intracranial aneurysms in the pediatric population: endovascular treatment with onyx. Childs Nerv Syst 2008; 24: 909–915. [DOI] [PubMed] [Google Scholar]
- 5.Reuschel V, Groll M, Quäschling U, et al. Middle cerebral artery (M3) aneurysm: atypical primary morphology and early recurrence after stent-assisted coil occlusion during the long-term left ventricular assist device treatment, accompanied by temporary septicemia; parent vessel occlusion as the final treatment with good clinical outcome. In: Henkes H, Lylyk P, Ganslandt O. (eds) The aneurysm casebook: a guide to treatment selection and technique. Cham: Springer International Publishing, 2018. DOI: 10.1007/978-3-319-70267-4_76-1 [Google Scholar]
- 6.Han DK, Tadros RO, Chung C, et al. Endovascular treatment of 2 synchronous extracranial carotid artery aneurysms using stent-assisted coil embolization and double bare-metal stenting. Vasc Endovascular Surg 2016; 50: 102–106. [DOI] [PubMed] [Google Scholar]
- 7.Appelboom G, Kadri K, Hassan F, et al. Infectious aneurysm of the cavernous carotid artery in a child treated with a new-generation of flow-diverting stent graft: case report. Neurosurgery 2010; 66: E623–624; discussion E624. [DOI] [PubMed] [Google Scholar]
- 8.Ding D, Raper DM, Carswell AJ, et al. Endovascular stenting for treatment of mycotic intracranial aneurysms. J Clin Neurosci 2014; 21: 1163–1168. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann A, Hoffmann K-T, Sander C, et al. Superior cerebellar artery aneurysm: spontaneous subarachnoid hemorrhage and stent-assisted coil occlusion of a ruptured aneurysm at the superior cerebellar artery origin and of an unruptured small basilar artery bifurcation aneurysm, bacterial endocarditis, early major aneurysm recurrence with oculomotor palsy, and treatment of the “mycotic” aneurysm by flow diverter implantation, with resolution of the aneurysm and good clinical recovery. In: Henkes H, Lylyk P, Ganslandt O. (eds) The aneurysm casebook: a guide to treatment selection and technique. Cham: Springer International Publishing, 2018. DOI: 10.1007/978-3-319-70267-4_56-1 [Google Scholar]
- 10.Imamura H, Sakai N, Alexander MJ. Flow-diverter stenting of intracavernous internal carotid artery mycotic aneurysm. J Stroke Cerebrovasc Dis 2019; 28: e81–e82. [DOI] [PubMed] [Google Scholar]
- 11.Kobets AJ, Scoco A, Nakhla J, et al. Flow-diverting stents for the obliteration of symptomatic, infectious cavernous carotid artery aneurysms. Oper Neurosurg 2018; 14: 681–685. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda Y, Jang DK, Chung J, et al. Preliminary outcomes of single antiplatelet therapy for surface-modified flow diverters in an animal model: analysis of neointimal development and thrombus formation using OCT. J NeuroIntervent Surg 2019; 11: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Galdamez M, Lamin SM, Lagios KG, et al. Periprocedural outcomes and early safety with the use of the pipeline flex embolization device with shield technology for unruptured intracranial aneurysms: preliminary results from a prospective clinical study. J NeuroIntervent Surg 2017; 9: 772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanel RA, Aguilar-Salinas P, Brasiliense LB, et al. First US experience with pipeline flex with shield technology using aspirin as antiplatelet monotherapy. BMJ Case Rep 2017; 2017: bcr219406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning NW, Cheung A, Phillips TJ, et al. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J NeuroIntervent Surg 2019; 11: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agnoletto GJ, Aguilar-Salinas P, Santos R, et al. PED flex with shield technology: a feasible alternative for fusiform MCA aneurysms. Stroke Vasc Neurol 2018; 3: 185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girdhar G, Andersen A, Pangerl E, et al. Thrombogenicity assessment of pipeline flex, pipeline shield, and FRED flow diverters in an in vitro human blood physiological flow loop model. J Biomed Mater Res A 2018; 106: 3195–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz-Habijan T, Bhogal P, Peters M, et al. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: initial results in vitro. Cardiovasc Intervent Radiol 2018; 41: 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez Moreno R, Bhogal P, Lenz-Habijan T, et al. In vivo canine study of three different coatings applied to p64 flow-diverter stents: initial biocompatibility study. Eur Radiol Exp 2019; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhogal P, Perez MA, Wendl C, et al. Paediatric aneurysms – review of endovascular treatment strategies. J Clin Neurosci 2017; 45: 54–59. [DOI] [PubMed] [Google Scholar]
- 21.Navarro R, Brown BL, Beier A, et al. Flow diversion for complex intracranial aneurysms in young children. J Neurosurg Pediatr 2015; 15: 276–281. [DOI] [PubMed] [Google Scholar]
- 22.Vargas SA, Diaz C, Herrera DA, et al. Intracranial aneurysms in children: the role of stenting and flow-diversion. J Neuroimaging 2016; 26: 41–45. [DOI] [PubMed] [Google Scholar]
- 23.Israels SJ, Michelson AD. Antiplatelet therapy in children. Thromb Res 2006; 118: 75–83. [DOI] [PubMed] [Google Scholar]
- 24.Dewan MC, Ravindra VM, Gannon S, et al. Treatment practices and outcomes after blunt cerebrovascular injury in children. Neurosurgery 2016; 79: 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravindra VM, Bollo RJ, Dewan MC, et al. Comparison of anticoagulation and antiplatelet therapy for treatment of blunt cerebrovascular injury in children <10 years of age: a multicenter retrospective cohort study. Childs Nerv Syst 2021; 37: 47–54. [DOI] [PubMed] [Google Scholar]