Abstract
Over 200 in vivo magnetic resonance spectroscopy (MRS) studies of substance use and related disorders (SUD) were published this past decade. The large majority of this work used proton (1H)-MRS to characterize effects of acute and chronic exposures to drugs of abuse on human brain metabolites including N-acetylaspartate, choline-containing metabolites, creatine plus phosphocreatine, glutamate, and GABA. Some studies used phosphorus (31P)-MRS to quantify biomarkers of cerebral metabolism including phosphocreatine and adenosine triphosphate. A few studies used carbon (13C)-MRS to quantify intermediary metabolism. This Mini-review discusses select studies that illustrate how MRS can complement neurocircuitry research including by use of multimodal imaging strategies that combine MRS with functional MRI (fMRI) and/or diffusion tensor imaging (DTI). Additionally, magnetic resonance spectroscopic imaging (MRSI), which enables simultaneous multivoxel MRS acquisitions, can be used to better understand and interpret whole-brain functional or structural connectivity data. The review discusses some limitations in MRS methodology and then highlights important knowledge gaps and areas for potential future investigation, including the use of 1H- and 31P-MRS to quantify cerebral metabolism, oxidative stress, inflammation, and brain temperature, all of which are associated with SUD and all of which can influence neurocircuitry and behavior.
1. Introduction
Magnetic resonance spectroscopy (MRS) is a family of MRI-based methods that provides chemical and physiological information about the brain and other tissues using magnetic resonance imaging (MRI) scanners. Generally, the same scanners are used to acquire in vivo MRS, MRI, and functional MRI (fMRI) scans, although scan protocols differ for each of these modalities and specialized equipment, e.g., transmit and receive coils, are used to acquire some types of MRS data. Because MRS is noninvasive and does not use ionizing radiation, it is well-suited for conducting in vivo studies, including prospective studies. Because the same scan protocols can be used in animals and in humans, MRS, like other MRI modalities, is an excellent translational research tool. MRS studies constitute only a small proportion of the total number of magnetic resonance studies of substance use disorders (SUD) but still comprise a large body of research. Accordingly, since Minireviews are limited to 60 references, we are not able to discuss every SUD MRS paper. Instead, we focus on select in vivo MRS studies conducted since 2010 that illustrate recent progress (Current Landscape), and, to resonate with the theme of this special issue (Pharmacology in the Age of Circuit Neuroscience), we highlight how MRS methods can be used as part of a multimodal imaging approach to complement SUD neurocircuitry research. Then, we discuss some limitations of current methodology, untapped potential of MRS relevant to SUD research, and we end by discussing where the field could head (Summary and Potential Future Directions).
2. Current Landscape
Our literature search of SUD-related in vivo MRS studies between 2010 and July 21, 2020 captured 202 unique publications. To document the breadth and depth of recent work, we stratified these papers by abused substance and we list them in an annotated bibliography in online Supplementary Materials. We provide figures that illustrate study distribution by abused substance, scanner magnetic field strength, and species (Supplementary Figures S1-3). The large majority of these studies (94%) used proton (1H)-MRS, which quantifies metabolites including N-acetylaspartate, glutamate, GABA, glutamine, glutathione, choline-containing metabolites (Cho), and myo-inositol. Ten studies (5%) used phosphorus (31P)-MRS, which can measure bioenergetic metabolites including adenosine triphosphate (ATP) and phosphocreatine (PCr), metabolic flux by measuring activity of creatine kinase (CK), as well as physiological parameters such as pH, magnesium concentration, and the redox ratio, a biomarker of oxidative stress. Three studies (1.4%) used carbon (13C)-MRS, which is capable of quantifying intermediary metabolism including flux through neurotransmitter and bioenergetic pools of glutamate, but technically is very challenging. The MRS signal intensities of 31P and 13C-containing metabolites are only about 7 and 2%, respectively, of the 1H signal intensity, meaning that at any given magnetic field strength, 31P- or 13C-MRS measurements require longer acquisition times or larger voxel sizes than 1H-MRS scans to obtain comparable signal-to-noise ratios. Low sensitivity can be partly overcome by scanning at higher magnetic field strengths, as illustrated in Figure 1.
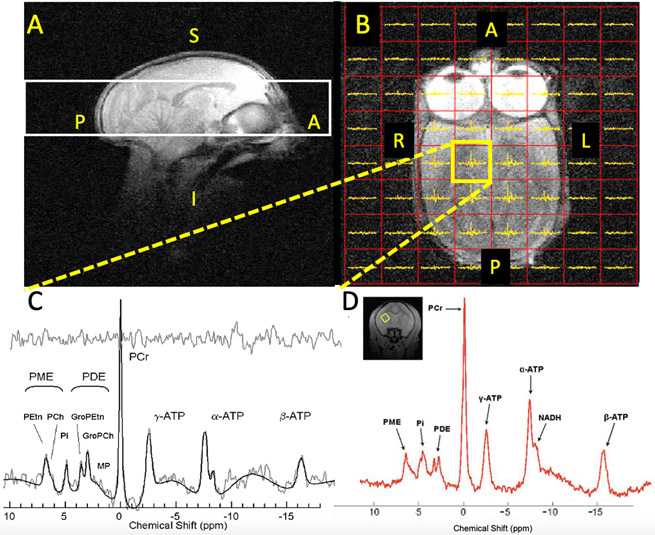
Figure 1:
31P-MRS scans in squirrel monkeys at 4 Tesla (Panels A-C) and 9.4 Tesla (Panel D). A: Sagittal MRI image showing MRSI slab (white box) location covering most of the brain. B: Axial MRI image showing the 8 x 8 MRSI voxel grid (red boxes) within the white MRSI slab in Panel A from which individual spectra can be extracted, including one from the yellow-highlighted box shown in Panel C. Note that voxels completely outside of the brain contain no spectra. C: Midbrain spectrum (2 cm3 volume) from the yellow voxel in Panel B with peak fits and fit residual (top trace). D: Single voxel 9.4 Tesla 31P-MRS putamen spectrum (0.22 cm3 volume, inset: coronal MRI with yellow voxel position) with superior spatial resolution versus 4 Tesla. Legend: A=anterior; P=posterior; L=left; R=right, S=superior, I=inferior; γ-, α-, β-ATP: adenosine-related peaks including ATP (β- resonance); NADH=nicotinamide adenine dinucleotide, reduced and oxidized (NAD+) forms; PCr= phosphocreatine; Pi=inorganic phosphate; PME, PDE and subresonances; phosphomono- and di-esters.
A key limitation of MRS is that it is relatively insensitive when compared to imaging techniques such as positron emission tomography; MRS can only detect metabolites present at relatively high concentrations (high μM range and above) in relatively large tissue volumes (termed voxels). Thus, MRS cannot be used, for example, to quantify synaptic or extracellular glutamate levels, which are tightly maintained below the neurotoxic (low μM) range in generally healthy brain (Moussawi et al., 2011). Also, despite some laudable attempts, it is not yet possible to accurately quantify MRS metabolites in certain brain areas relevant to SUD such as the nucleus accumbens (NAc), due to its small volume in humans (<0.8 cm3 in each hemisphere (Baumann et al., 1999), obviously much smaller in animals) and its irregular shape. These structural features do not conform to cubic MRS voxel shapes or volumes that can be obtained using practical scan times at currently available magnetic field strengths. This means that metabolite signals from adjacent regions contribute to, and in many cases dominate, “nucleus accumbens” MRS metabolite measures. Similar concerns pertain to MRS studies of hippocampus. That being said, glutamate, GABA, glutathione, PCr, and ATP are detectible in many brain areas with MRS and past and present SUD studies report abnormalities for all of these metabolites. Because excellent reviews on MRS methods (e.g., Zhu and Barker, 2011) and findings in SUD and their relevance to neurocircuitry exist, (Licata and Renshaw, 2010, Hellem et al., 2015a, Moeller et al., 2016), we direct readers to such reviews, which discuss technical issues or describe SUD literature published before 2010.
2. A role for MRS in circuit neuroscience
Most MRS research has utilized scan protocols that acquire data from single voxels. Such acquisitions provide snapshot information about the biochemical environment in specific brain regions and have been profitably employed to document metabolite alterations that result from long-term drug exposure, the progression of psychiatric disease states -- including SUD, or the effects of abused substances or medication treatment, etc. (see below; reviewed by Licata and Renshaw, 2010). However, the growing interest in circuit-based analyses of brain processes has encouraged the use of MRS together with other magnetic resonance-based imaging modalities in the same subjects. Such multimodal approaches can provide a more detailed analysis of the relationships between biochemical activity in specific brain regions and functional and/or structural connectivity either locally, or with other brain regions of interest. Further, MRS combined with functional or structural modalities, such as fMRI or diffusion tensor imaging (DTI), yields complementary information about brain function or white matter integrity, respectively, which may be related to neuronal or axonal health or density in specific regions (Hao et al., 2013).
MRS/fMRI.
Of particular interest to the field of SUD has been the application of MRS in combination with fMRI. Current theories of addiction suggest that long-term drug use induces disruptions to the balance of glutamate and GABA within corticostriatal circuits that serve to maintain drug taking behavior and the propensity for relapse (Koob and Volkow, 2010). The observation that glutamatergic alterations reported in drug users, as detected by MRS, overlapped with many of the brain regions identified as having disrupted fMRI functional connectivity, led to the hypothesis that glutamate may be an important mechanism that drives alterations in functional connectivity or neural responses to drug-related stimuli (Moeller et al., 2016). Multimodal MRS/fMRI methods are well-suited for interrogating relationships between changes in glutamate or GABA and functional connectivity of key circuits related to addiction. Recent studies utilizing combined MRS/fMRI methods to investigate various phases of the addiction process, primarily in marijuana or nicotine users, have begun to support this hypothesis. For example, acute THC administration has been shown to increase striatal glutamate concentration and resting-state functional connectivity (rsFC) between striatal and cortical regions (Mason et al, 2019). In heavy marijuana users, glutamate in the dorsal anterior cingulate (dACC) was found to predict resting state connectivity between dACC and NAc (Newman et al., 2019). In tobacco smokers, dACC glutamate appears to be related to changes in dACC rsFC during acute deprivation (Abulseoud et al., 2020), as well as to reactivity to smoking cues (Janes et al., 2016), suggesting that this relationship may predict relapse vulnerability. In line with this suggestion, a previous study found that treatment with the FDA-approved smoking-cessation aid, varenicline, decreased dACC glutamate + glutamine levels and attenuated fMRI changes during performance of a Stroop color-naming task (Wheelock et al., 2014). While the mechanisms that underlie these effects remain unclear, these results suggest that novel medications may be developed that target glutamatergic and connectivity pathways relevant for drug-induced disruptions in behavioral or cognitive processes.
Although the glutamate/fMRI relationship has been most broadly studied in marijuana and nicotine users, both modalities also have been applied to the study of other SUD. For example, the dACC glutamine/glutamate ratio, which may reflect increased glutamate turnover, was found to be elevated in long-term anabolic androgenic steroid (AAS) users relative to matched control subjects (Kaufman et al., 2015). In the same subjects, right amygdala rsFC with the dACC also was found to be disrupted in AAS users, an effect which may be related to observed deficits in their cognitive function (Kaufman et al., 2015). Taken together, the above examples, in which fMRI and MRS were conducted in the same subjects, highlight the potential utility of MRS/fMRI in understanding the progression of the addiction process (initiation, maintenance, relapse) with several substances of abuse and suggest that the information gained by using a multimodal approach may help to identify biomarkers that could be used to predict efficacy of candidate medications.
MRS/DTI.
Several lines of evidence point to alterations in brain structure that increase the propensity for developing problems with substance abuse or are the result of neurotoxicity associated with long-term drug use (Acheson et al., 2014; Ersche et al., 2012; Battistella et al., 2014; Kaufman et al., 2015). Such anatomical abnormalities reported using DTI or volumetric measurements may be related to cognitive deficits often reported in SUD patients. The DTI parameter fractional anisotropy (FA), for example, is often used as an index of white matter integrity. FA can provide important information about whether changes in functional connectivity between two (or more) regions is related to changes in microstructural connectivity, e.g., axonal health or myelination, within a specific tract. When coupled with MRS, metabolites thought to reflect cellular and axonal health, cellular membrane breakdown, and energy metabolism such as NAA, Cho, creatine plus phosphocreatine, ATP, or PCr, can be quantified within the same tract (or voxel). The combination of DTI and MRS then can provide unique insights about the cellular mechanisms that may be responsible for the development or breakdown of neural circuitry. Such combined analyses can be particularly useful in the context of longitudinal designs to track the progression of disease states such as neurodegeneration in methamphetamine or MDMA users (Liu et al., 2011a; Lin et al., 2015), to assess the impact of drug use during sensitive periods on subsequent development (Acheson et al., 2014), or to determine the effects of polydrug use on brain macrostructure and neuronal integrity (Durazzo et al., 2013).
MRS as a circuit-based approach.
The above sections focused on employing MRS with other magnetic resonance imaging modalities to enhance our understanding of neural circuitry related to SUD. A less widely-used MRS technique termed magnetic resonance spectroscopic imaging (MRSI), also known as chemical shift imaging (CSI), simultaneously acquires scans from multiple voxels in two or three dimensions, yielding data that are well-suited for complementing whole-brain fMRI neurocircuitry research. MRSI was used in only 12% of SUD studies published this past decade. Importantly, MRSI methods enable retrospective spatial shifting of voxels after scans are completed so voxels can be optimally positioned with respect to anatomical regions of interest, a feature not available with standard single-voxel MRS. Figure 1 (left panel) shows an example of 31P-MRS MRSI data we acquired from a squirrel monkey at 4 Tesla (unpublished data). Although the spatial resolution of this acquisition is low (2 cm3), better spatial (and spectral) resolution can be obtained at higher magnetic field strengths, for example in the 31P-MRS single voxel spectrum (0.22 cm3) also obtained from a squirrel monkey at 9.4 Tesla (Figure 1, right panel). MRSI, particularly when acquired on higher magnetic field systems that enable use of voxels approaching the size of key brain nuclei, offers additional opportunities to complement and extend fMRI and DTI data by simultaneously assessing the neurochemical environment in multiple regions and neural circuits.
3. Current Limitations of MRS: Glutamate and GABA: what is detected and what does it reflect?
Given the seminal roles played by glutamate and GABA in brain function, it is important to appreciate what information MRS measurements of these substances can, and can’t provide, and which MRS methods are optimal for quantifying these substances. This is important because MRS methods used to date in SUD research often have been inadequate for quantifying glutamate. The most widely-used protocol, Point-resolved Spectroscopy (PRESS), is unable to fully separate glutamate from glutamine, GABA, or other overlapping metabolite resonances at magnetic field strengths of less than 7 Tesla (Godlewska et al., 2017). This problem is amplified by the fact that glutamate, glutamine, and GABA are present in interdependent biochemical pathways (Laake et al., 1992; Walls et al., 2015; Chen et al., 2019). Thus, changes in any one of these metabolites could affect levels or changes of other metabolites, confounding their quantification. This means that the combined resonance of glutamate, GABA, and glutamine (often termed Glx) detected with standard Point Resolved Spectroscopy (PRESS) MRS at 3 Tesla, the most widely-used magnetic field strength (Supplement Figure S3), only indirectly reflects glutamate. Glx measurements have been widely reported in SUD studies this past decade, sometimes have been misrepresented as glutamate, and make up more than two-thirds of SUD MRS studies reporting on glutamate. MRS methods that better separate glutamate from overlapping resonances include a multi-echo time-averaged variation of the PRESS MRS sequence (Hurd et al., 2004) and 2-dimensional J-resolved PRESS (e.g., Janes et al, 2016), and are increasingly being used. MEGA-PRESS now is widely used to quantify GABA (Mikkelsen et al., 2017). Use of higher magnetic field scanners also helps reduce the resonance overlap problem by increasing spectral (chemical shift) resolution (Godlewska et al., 2017).
Even when Glu or GABA are quantified with 1H-MRS, their signals are derived from multiple tissue compartments (intracellular (neuronal and glial), extracellular, synaptic) and pathways (neurotransmission, metabolism), meaning that glutamate and GABA signals do not simply represent neurotransmitter available for neurotransmission. In this regard, neuronal glutamate compartmentalized within synaptic vesicles is highly concentrated (~50-100 mM) and constitutes up to 30% of total brain glutamate (Laake et al., 1992), but vesicular glutamate is not optimally-detected with standard 1H-MRS. This is because vesicular glutamate is spatially-confined and diffuses minimally within ~35-40 nm-diameter synaptic vesicles (Qu et al., 2009), resulting in an attenuated 1H-MRS signal. Diffusion-weighted 1H-MRS can detect glutamate and other metabolites in small tissue compartments (Chen et al., 2019) and this methodology may ultimately help to better quantify glutamate and other metabolites involved in neurotransmission.
4. Untapped Potential of MRS in SUD
Bioenergetics and oxidative stress
The primary means to produce energy required for brain function is mitochondrial oxidative phosphorylation and mitochondria are estimated to be the source of 90% of reactive oxygen species (ROS) that induce oxidative stress (Balaban et al., 2005). Thus, brain energy production and oxidative stress go hand in hand. ROS must be buffered by endogenous antioxidant systems for normal mitochondrial and brain function (Cobley et al., 2018). Yet, most abused substances including alcohol, nicotine, marijuana, stimulants, opioids, and anabolic steroids, and their combinations, induce excess ROS and oxidative stress (Kaufman et al., 2019), which may contribute to metabolic abnormalities (Volkow et al., 2003). Excess ROS not only impair mitochondrial function but also alter activities of proteins relevant to SUDs including glutamate and dopamine transporters (Trotti et al., 1997; Park et al., 2002), which contain redox-sensitive molecular elements including thiol groups that maintain these proteins in optimal functional conformations. Excess oxidative stress also impairs function of creatine kinase (CK), a key metabolic enzyme (Eliuk et al., 2007). CK synthesizes the high energy phosphate storage metabolite phosphocreatine (PCr) when energy supply is high and catabolizes PCr to form ATP when energy demand is high (Schlattner et al., 2006). CK activity is very important in energy-demanding periods (e.g., during complex cognitive tasks) because CK synthesizes ATP at a rate that is nearly 6 times faster than de novo ATP synthesis via mitochondrial oxidative phosphorylation (Du et al., 2008). Excess oxidative stress also exerts broader effects that are just beginning to be appreciated, and it has been estimated that nearly 30% of the human proteome is redox-sensitive (Erdős et al., 2019). ROS can exert beneficial effects, e.g., by modifying N-methyl-D-aspartate (NMDA) receptor function to facilitate synaptic plasticity (Hidalgo et al., 2016) as well as deleterious effects when excess ROS are present (Go and Jones, 2013), including by impairing synaptic plasticity (Hidalgo et al., 2016). Accordingly, the ability to quantify brain bioenergetics and oxidative stress in SUD are critical and to date, understudied areas. Illustrative examples include studies reporting low frontal cortex PCr levels in adult methamphetamine-dependent subjects, whose PCr levels were inversely correlated with lifetime methamphetamine use (Sung et al., 2013). This same research group subsequently demonstrated that dietary creatine loading for 8 weeks increased frontal lobe PCr levels in methamphetamine-dependent women with comorbid major depression, an effect that was associated with lower anxiety and depression levels (Hellem et al., 2015b). A 31P-MRS study of obese individuals with eating-disorders found lower steady-state brain ATP and PCr levels and smaller brain ATP and PCr declines in response to transcranial direct current stimulation, indicative of both static and dynamic bioenergetic abnormalities (Jauch-Chara et al., 2015).
31P-MRS also can be used in vivo to quantify oxidized nicotinamide adenine dinucleotide (NAD+) and its reduced form (NADH) and to determine the NAD+/NADH (redox) ratio, a biomarker of oxidative stress (Kim et al., 2017). To date, the redox ratio has not been reported in SUD studies. However, several in vivo 1H-MRS studies have quantified another biomarker of oxidative stress, glutathione (GSH, in its reduced form), the most abundant endogenous small molecule antioxidant (Forman et al., 2009). Alcohol drinking and tobacco smoking were associated with GSH abnormalities in adolescents with bipolar disorder (Chitty et al., 2015). N-acetylcysteine (NAC) is a GSH precursor that when infused increases human brain GSH as measured with 1H-MRS (Holmay et al., 2013). A recent 1H-MRS study in opioid-dependent neonatal rats reported that NAC pretreatment prevented GSH depletion after naloxone-precipitated opioid withdrawal and attenuated withdrawal symptoms, suggesting an association between GSH depletion and withdrawal symptoms (Ward et al., 2020).
MRS studies of inflammation
Inflammation currently is a hot topic in psychiatry and neuroscience and 1H-MRS can be used to quantify several tissue metabolites linked to inflammation including myo-inositol, choline-containing compounds, creatine plus phosphocreatine, or lactate. However, these metabolites do not always reflect inflammation and thus are considered suboptimal biomarkers (Woodcock et al., 2019). GSH, which as noted above is a well-established biomarker of oxidative stress (Forman et al., 2009), also is depleted by inflammatory mediators including by lipopolysaccharide, tumor necrosis factor-1α (TNFα), and Interleukin-1β (IL-1β) (Noble et al., 2007; Gavillet et al., 2008). Involvement of GSH both in inflammation and oxidative stress is not surprising because both processes are interdependent, each catalyzes the other, and the inflammatory tissue response involves ROS production (Biswas, 2016). Accordingly, GSH measurements can reflect both inflammation and oxidative stress and its quantification (along with redox ratio measurements) could be very informative in SUD involving inflammation and/or oxidative stress, such as in opioid use disorder (Eisenstein, 2019; Kaufman et al., 2019; Ward et al., 2020).
MRS thermometry
The ability to quantify brain temperature in vivo in SUD research may be important because many psychoactive drugs, including cocaine, opioids, marijuana, methamphetamine, and MDMA, induce body and brain hyperthermia (Kiyatkin, 2019), which can have profound functional consequences. For example, hyperthermia depletes ATP and alters the function of glutamate transporters (Madl and Allen, 1995). An MRI contrast agent sensitive to temperature has been used in combination with 1H-MRS to show that MDMA increases brain temperature by 2-3°C in rats (Coman et al., 2015).
Brain and other tissue temperatures also can be quantified with 1H-MRS without using exogenous contrast agents, because as temperature changes, the 1H-MRS water resonance position (chemical shift) changes while metabolite resonances do not (Zhu et al., 2008). Thus, relative positions (chemical shifts) between water and metabolite peaks can be quantified and used to determine tissue temperature with high resolution of about 0.1°C (Zhu et al., 2008). Importantly, water and metabolite peaks of relatively low signal-to-noise ratios are adequate for temperature quantification. This means that MRSI methods using smaller voxel sizes and/or shorter acquisition times could be applied for multivoxel temperature mapping at spatial resolutions approaching those used for functional connectivity assessments. This type of multimodal experiment has the potential to be very informative because hyperthermia alters local and long range connectivity of frontal lobe regions, as well as cognition (Han et al., 2018). Accordingly, temperature quantification may aid in the interpretation of functional connectivity assessments. Further, MRS thermometry data can be extracted retrospectively from existing MRS or MRSI datasets (e.g., Mintzopoulos et al., 2019) as long as water and metabolite data are available. Thus, published MRS studies relevant to SUD could be re-analyzed to quantify brain temperature effects of acute and chronic drug use. We are not aware of any studies to date using contrast-free 1H-MRS thermometry methods in SUD research, making this area an open, and potentially-impactful area of future investigation, especially when paired with other types of MRI assessments.
5. Summary and Potential Future Directions
The past decade of SUD research using in vivo MRS methods has been very productive, yielding more than 200 publications. The vast majority of reports used single-voxel 1H-MRS methods to quantify metabolites including N-acetylaspartate, choline-containing compounds, myo-inositol, creatine, glutamate, glutamine, and GABA in adults. Only a small proportion of studies (9%) involved human infants, children, or adolescents although that percentage is likely to increase over the next few years as the Adolescent Brain and Cognitive Development (ABCD) study advances. This decade witnessed increased application of MRS as part of multimodal studies combining fMRI, DTI, and/or structural MRI assessments, but only 7% of all SUD MRS studies involved multimodal approaches. Yet, most of those studies illustrate that MRS integration with other MRI types produces valuable data that can deepen our understanding of SUD pathophysiology. Use of spectroscopic imaging (MRSI) methods and small voxel volumes on the order of brain regions and circuits could further complement and extend fMRI, DTI, and structural MRI findings. While few examples exist within the SUD literature pairing MRS with targeted brain stimulation techniques such as transcranial direct current stimulation (Jauch-Chara et al., 2015), MRS, when combined with brain stimulation types that can be directed at particular regions, circuits, or cell types (e.g., via optogenetic or chemogenetic stimulation), could be very informative. 1H-MRS methods such as contrast-free MRS thermometry, which have not yet been applied in SUD research, could be used in combination with MRS or MRSI to help interpret functional and structural connectivity studies, especially those involving substances that induce hyperthermia that could disrupt neural circuitry (Han et al., 2018).
MRS methods evolution and dissemination, as well as the availability of higher magnetic field scanners, have improved quantification of glutamate and GABA. Broader use of advanced 1H-MRS methods should help to clarify roles that glutamate and GABA play in SUD and its treatment, although the interpretation of glutamate and GABA findings will be limited by the inability of current 1H-MRS methods to selectively quantify these metabolites in different functional (e.g., neurotransmission or metabolic) pools.
Given that individuals with SUD experience brain metabolic abnormalities (Volkow et al., 2003) and oxidative stress (Kaufman et al., 2019), 31P-MRS studies of static (e.g., ATP and PCr levels) and dynamic (e.g., CK flux) metabolism, as well as of the redox ratio, will help broaden and deepen our understanding of SUD pathophysiology. It is plausible that cognitive deficits commonly reported in SUDs result in part from impaired CK activity, which could substantially limit ATP availability during high energy-demand periods. Thus, MRS studies characterizing the effects of abused drugs on CK, which are completely lacking in human SUD research, have the potential to suggest novel strategies for medications development to attenuate cognitive deficits associated with drug use. Scanner manufacturers now are routinely offering the necessary hardware (headcoils) and software to acquire and analyze 31P-MRS data, which should enable more groups with MRI scanners to implement 31P-MRS research programs. GSH quantification with 1H-MRS also is evolving (e.g., Mlynárik et al., 2006) and this could facilitate SUD research on oxidative stress and inflammation, both of which affect GSH levels.
As with other imaging specialties, there is increased interest in standardizing MRS protocols to reduce site-to-site variability, which can be considerable. For example, a multicenter 1H-MRS study of the MEGA-PRESS scan sequence for GABA quantification reported that nearly 30% of the variance in GABA concentration was attributable to site and scanner (hardware and MRS pulse sequence) differences (Mikkelsen et al., 2017). This variability is large enough to reduce experimental rigor and reproducibility. Accordingly, MRS methods standardization efforts in the future are needed to help reduce potential confounds associated with methodologic variability.
The majority of MRS studies conducted during this period were on 3 Tesla MRI scanners. While use of this magnetic field strength is advantageous when multimodal MRS includes fMRI, higher magnetic field systems enable scans with better spatial and spectral resolution, which can improve MRS metabolite quantification. That being said, magnetic susceptibility artifacts in fMRI scans increase at higher magnetic field strengths and advances are necessary to enable multimodal MRS and fMRI studies at 7 Tesla and above. Fewer than 17% of the published MRS studies in this period involved animals. Preclinical research offers a number of advantages for evaluating relationships between drug exposure and biochemistry, functional or structural neural circuitry, and behavior without the potential confounds of previous drug history and psychiatric comorbidities often associated with human research (e.g., Liu et al., 2011b). While preclinical MRS technically is challenging due to smaller brain sizes of laboratory animals, the growing availability of high magnetic field scanners presents exciting opportunities for preclinical researchers to implement MRS in SUD investigations. Accordingly, investments in higher magnetic field systems, while expensive, could substantially benefit human and nonhuman SUD research, and could help to advance early and later treatment development phases. In sum, there are abundant opportunities to fill knowledge gaps on the neurochemical and physiological consequence of SUD using MRS methods. We anticipate a very exciting and informative next decade of MRS research.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants DA039044, DA041866, DA039306, RR019356, and by the Counter-Drug Technology Assessment Center (CTAC), an office within the Office of National Drug Control Policy (ONDCP), via Contract Number DBK39-03-C-0075 awarded by the Army Contracting Agency. The content of the information does not necessarily reflect the position or the policy of the Government and no official endorsement should be inferred.
References
- Abulseoud OA, Ross TJ, Nam HW, Caparelli EC, Tennekoon M, Schleyer B, Castillo J, Fedota J, Gu H, Yang Y, Stein E. Short-term nicotine deprivation alters dorsal anterior cingulate glutamate concentration and concomitant cingulate-cortical functional connectivity. Neuropsychopharmacology 2020. June 19. doi: 10.1038/s41386-020-0741-9. PMID: 32559759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Wijtenburg SA, Rowland LM, Bray BC, Gaston F, Mathias CW, Fox PT, Lovallo WR, Wright SN, Hong LE, McGuire S, Kochunov P, Dougherty DM. Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Hum Brain Mapp. 2014. December;35(12):5877–87. doi: 10.1002/hbm.22591. PMID: 25044331; PMC4219410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, Favrat B, Mall JF, Maeder P, Giroud C. Long-term effects of cannabis on brain structure. Neuropsychopharmacology 2014. August;39(9):2041–8. doi: 10.1038/npp.2014.67. PMID: 24633558; PMC4104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005. February 25;120(4):483–95. doi: 10.1016/j.cell.2005.02.001. PMID: 15734681. [DOI] [PubMed] [Google Scholar]
- Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci. 1999. Winter;11(1):71–8. doi: 10.1176/jnp.11.1.71. PMID: 9990559. [DOI] [PubMed] [Google Scholar]
- Biswas SK. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. PMID: 26881031; PMC4736408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tamang SM, Du F, Ongur D. Glutamate diffusion in the rat brain in vivo under light and deep anesthesia conditions. Magn Reson Med. 2019. July;82(1):84–94. doi: 10.1002/mrm.27722. PMID: 30860289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitty KM, Lagopoulos J, Hickie IB, Hermens DF. A longitudinal proton magnetic resonance spectroscopy study investigating oxidative stress as a result of alcohol and tobacco use in youth with bipolar disorder. J Affect Disord. 2015. April 1;175:481–7. doi: 10.1016/j.jad.2015.01.021. PMID: 25679204. [DOI] [PubMed] [Google Scholar]
- Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018. May;15:490–503. doi: 10.1016/j.redox.2018.01.008. PMID: 29413961; PMC5881419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman D, Sanganahalli BG, Jiang L, Hyder F, Behar KL. Distribution of temperature changes and neurovascular coupling in rat brain following 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) exposure. NMR Biomed. 2015. October;28(10):1257–66. doi: 10.1002/nbm.3375. PMID: 26286889; PMC4573923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A 2008. April 29;105(17):6409–14. doi: 10.1073/pnas.0710766105. PMID: 18443293; PMC2359810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict Biol. 2013. March;18(2):379–91. doi: 10.1111/j.1369-1600.2011.00407.x. PMID: 22070867; PMC4157587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK. The Role of Opioid Receptors in Immune System Function. Front Immunol. 2019. December 20;10:2904. doi: 10.3389/fimmu.2019.02904. PMID: 31921165; PMC6934131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliuk SM, Renfrow MB, Shonsey EM, Barnes S, Kim H. active site modifications of the brain isoform of creatine kinase by 4-hydroxy-2-nonenal correlate with reduced enzyme activity: mapping of modified sites by Fourier transform-ion cyclotron resonance mass spectrometry. Chem Res Toxicol. 2007. September;20(9):1260–8. doi: 10.1021/tx7000948. PMID: 17696488. [DOI] [PubMed] [Google Scholar]
- Erdős G, Mészáros B, Reichmann D, Dosztányi Z. Large-Scale Analysis of Redox-Sensitive Conditionally Disordered Protein Regions Reveals Their Widespread Nature and Key Roles in High-Level Eukaryotic Processes. Proteomics 2019. March;19(6):e1800070. doi: 10.1002/pmic.201800070. PMID: 30628183. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science 2012. February 3;335(6068):601–4. doi: 10.1126/science.1214463. PMID: 22301321. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009. Feb-Apr;30(1-2):1–12. doi: 10.1016/j.mam.2008.08.006. PMID: 18796312; PMC2696075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavillet M, Allaman I, Magistretti PJ. Modulation of astrocytic metabolic phenotype by proinflammatory cytokines. Glia. 2008. July;56(9):975–89. doi: 10.1002/glia.20671. PMID: 18383346. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. The redox proteome. J Biol Chem. 2013. September 13;288(37):26512–20. doi: 10.1074/jbc.R113.464131. PMID: 23861437; PMC3772199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Clare S, Cowen PJ, Emir UE. Ultra-High-Field Magnetic Resonance Spectroscopy in Psychiatry. Front Psychiatry 2017. July 11;8:123. doi: 10.3389/fpsyt.2017.00123. PMID: 28744229; PMC5504194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Qian S, Jiang Q, Liu K, Li B, Sun G. Regional and long-range neural synchronization abnormality during passive hyperthermia. Behav Brain Res. 2018. April 2;341:9–15. doi: 10.1016/j.bbr.2017.12.011. PMID: 29247749. [DOI] [PubMed] [Google Scholar]
- Hao X, Xu D, Bansal R, Dong Z, Liu J, Wang Z, Kangarlu A, Liu F, Duan Y, Shova S, Gerber AJ, Peterson BS. Multimodal magnetic resonance imaging: The coordinated use of multiple, mutually informative probes to understand brain structure and function. Hum Brain Mapp. 2013. February;34(2):253–71. doi: 10.1002/hbm.21440. PMID: 22076792; PMC4284056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellem T, Shi X, Latendresse G, Renshaw PF. The Utility of Magnetic Resonance Spectroscopy for Understanding Substance Use Disorders: A Systematic Review of the Literature. J Am Psychiatr Nurses Assoc. 2015a. Jul-Aug;21(4):244–75. doi: 10.1177/1078390315598606. PMID: 26282670; PMC5495546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellem TL, Sung YH, Shi XF, Pett MA, Latendresse G, Morgan J, Huber RS, Kuykendall D, Lundberg KJ, Renshaw PF. Creatine as a Novel Treatment for Depression in Females Using Methamphetamine: A Pilot Study. J Dual Diagn. 2015b;11(3–4):189–202. doi: 10.1080/15504263.2015.1100471. PMID: 26457568; PMC4684979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Arias-Cavieres A. Calcium, Reactive Oxygen Species, and Synaptic Plasticity. Physiology (Bethesda) 2016. May;31(3):201–15. doi: 10.1152/physiol.00038.2015. PMID: 27053734. [DOI] [PubMed] [Google Scholar]
- Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Öz G, Cloyd JC, Tuite PJ. N-Acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013. Jul-Aug;36(4):103–6. doi: 10.1097/WNF.0b013e31829ae713. PMID: 23860343; PMC3934795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004. March;51(3):435–40. doi: 10.1002/mrm.20007. PMID: 15004781. [DOI] [PubMed] [Google Scholar]
- Janes AC, Betts J, Jensen JE, Lukas SE. Dorsal anterior cingulate glutamate is associated with engagement of the default mode network during exposure to smoking cues. Drug Alcohol Depend. 2016. October 1;167:75–81. doi: 10.1016/j.drugalcdep.2016.07.021. PMID: 27522872; PMC5037039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch-Chara K, Binkofski F, Loebig M, Reetz K, Jahn G, Melchert UH, Schweiger U, Oltmanns KM. Blunted brain energy consumption relates to insula atrophy and impaired glucose tolerance in obesity. Diabetes 2015. June;64(6):2082–91. doi: 10.2337/db14-0421. PMID: 25576052. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, Jensen JE, Pope HG Jr. Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015. July 1;152:47–56. doi: 10.1016/j.drugalcdep.2015.04.023. PMID: 25986964; PMC4458166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MJ, Kanayama G, Hudson JI, Pope HG Jr. Supraphysiologic-dose anabolic-androgenic steroid use: A risk factor for dementia? Neurosci Biobehav Rev. 2019. May;100:180–207. doi: 10.1016/j.neubiorev.2019.02.014. PMID: 30817935; PMC6451684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, Li T, Du F, Öngür D. Redox Dysregulation in Schizophrenia Revealed by in vivo NAD+/NADH Measurement. Schizophr Bull. 2017. January;43(1):197–204. doi: 10.1093/schbul/sbw129. PMID: 27665001; PMC5216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature and its role in physiology and pathophysiology: Lessons from 20 years of thermorecording. Temperature (Austin) 2019. December 3;6(4):271–333. doi: 10.1080/23328940.2019.1691896. PMID: 31934603; PMC6949027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010. January;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum in: Neuropsychopharmacology 2010. March;35(4):1051. PMID: 19710631; PMC2805560. [Google Scholar]
- Laake JH, Torp R, Ottersen OP. Ultrastructural immunocytochemical studies as a means of distinguishing between transmitter and non-transmitter glutamate. Biochem Soc Trans. 1993. February;21(1):45–9. doi: 10.1042/bst0210045. PMID: 8095471. [DOI] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci. 2010. February;1187:148–71. doi: 10.1111/j.1749-6632.2009.05143.x. PMID: 20201852; PMC3040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Jan RK, Kydd RR, Russell BR. Investigating the microstructural and neurochemical environment within the basal ganglia of current methamphetamine abusers. Drug Alcohol Depend. 2015. April 1;149:122–7. doi: 10.1016/j.drugalcdep.2015.01.026. PMID: 25700612. [DOI] [PubMed] [Google Scholar]
- Liu HS, Chou MC, Chung HW, Cho NY, Chiang SW, Wang CY, Kao HW, Huang GS, Chen CY. Potential long-term effects of MDMA on the basal ganglia-thalamocortical circuit: a proton MR spectroscopy and diffusion-tensor imaging study. Radiology 2011a. August;260(2):531–40. doi: 10.1148/radiol.11101918. PMID: 21633053. [DOI] [PubMed] [Google Scholar]
- Liu X, Jensen JE, Gillis TE, Zuo CS, Prescot AP, Brimson M, Cayetano K, Renshaw PF, Kaufman MJ. Chronic cocaine exposure induces putamen glutamate and glutamine metabolite abnormalities in squirrel monkeys. Psychopharmacology (Berl). 2011b. October;217(3):367–75. doi: 10.1007/s00213-011-2292-6. PMID: 21494788; PMC3169716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl JE, Allen DL. Hyperthermia depletes adenosine triphosphate and decreases glutamate uptake in rat hippocampal slices. Neuroscience. 1995. November;69(2):395–405. doi: 10.1016/0306-4522(95)00247-g. PMID: 8552237. [DOI] [PubMed] [Google Scholar]
- Mason NL, Theunissen EL, Hutten NRPW, Tse DHY, Toennes SW, Stiers P, Ramaekers JG. Cannabis induced increase in striatal glutamate associated with loss of functional corticostriatal connectivity. Eur Neuropsychopharmacol. 2019. December;29(2):247–256. doi: 10.1016/j.euroneuro.2018.12.003. PMID: 30553697. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Barker PB, Bhattacharyya PK, Brix MK, Buur PF, Cecil KM, Chan KL, Chen DY, Craven AR, Cuypers K, Dacko M, Duncan NW, Dydak U, Edmondson DA, Ende G, Ersland L, Gao F, Greenhouse I, Harris AD, He N, Heba S, Hoggard N, Hsu TW, Jansen JFA, Kangarlu A, Lange T, Lebel RM, Li Y, Lin CE, Liou JK, Lirng JF, Liu F, Ma R, Maes C, Moreno-Ortega M, Murray SO, Noah S, Noeske R, Noseworthy MD, Oeltzschner G, Prisciandaro JJ, Puts NAJ, Roberts TPL, Sack M, Sailasuta N, Saleh MG, Schallmo MP, Simard N, Swinnen SP, Tegenthoff M, Truong P, Wang G, Wilkinson ID, Wittsack HJ, Xu H, Yan F, Zhang C, Zipunnikov V, Zöllner HJ, Edden RAE. Big GABA: Edited MR spectroscopy at 24 research sites. Neuroimage 2017. October 1;159:32–45. doi: 10.1016/j.neuroimage.2017.07.021. PMID: 28716717; PMC5700835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzopoulos D, Ratai EM, He J, Gonzalez RG, Kaufman MJ. Simian immunodeficiency virus transiently increases brain temperature in rhesus monkeys: detection with magnetic resonance spectroscopy thermometry. Magn Reson Med. 2019. May;81(5):2896–2904. doi: 10.1002/mrm.27635. PMID: 30652349; PMC6414245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006. November;56(5):965–70. doi: 10.1002/mrm.21043. PMID: 16991116. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, London ED, Northoff G. Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: Relationships to resting-state functional connectivity. Neurosci Biobehav Rev. 2016. February;61:35–52. doi: 10.1016/j.neubiorev.2015.11.010. PMID: 26657968; PMC4731270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, Kalivas PW. Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci. 2011. November 24;5:94. doi: 10.3389/fnsys.2011.00094. PMID: 22275885; PMC3254064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Cheng H, Kim DJ, Schnakenberg-Martin A, Dydak U, Dharmadhikari S, Hetrick W, O'Donnell B. An investigation of the relationship between glutamate and resting state connectivity in chronic cannabis users. Brain Imaging Behav. 2019. July 13: 10.1007/s11682-019-00165-w. doi: 10.1007/s11682-019-00165-w. PMID: 31302844; PMC6955389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble F, Rubira E, Boulanouar M, Palmier B, Plotkine M, Warnet JM, Marchand-Leroux C, Massicot F. Acute systemic inflammation induces central mitochondrial damage and mnesic deficit in adult Swiss mice. Neurosci Lett. 2007. September 7;424(2):106–10. doi: 10.1016/j.neulet.2007.07.005. PMID: 17716817. [DOI] [PubMed] [Google Scholar]
- Park SU, Ferrer JV, Javitch JA, Kuhn DM. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. J Neurosci. 2002. June 1;22(11):4399–405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. PMID: 12040046; PMC6758823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Akbergenova Y, Hu Y, Schikorski T. Synapse-to-synapse variation in mean synaptic vesicle size and its relationship with synaptic morphology and function. J Comp Neurol. 2009. June 1;514(4):343–52. doi: 10.1002/cne.22007. PMID: 19330815. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 2006. February;1762(2):164–80. doi: 10.1016/j.bbadis.2005.09.004. PMID: 16236486. [DOI] [PubMed] [Google Scholar]
- Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, Hellem TL, Huber RS, Fiedler KK, Harrell RE, Nickerson BR, Kim SE, Jeong EK, Renshaw PF. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Depend. 2013. April 1;129(1-2):102–9. doi: 10.1016/j.drugalcdep.2012.09.015. PMID: 23084413; PMC3572261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci. 1997. June;9(6):1236–43. doi: 10.1111/j.1460-9568.1997.tb01478.x. PMID: 9215707. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003. May;111(10):1444–51. doi: 10.1172/JCI18533. PMID: 12750391; PMC155054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res. 2015. February;40(2):402–9. doi: 10.1007/s11064-014-1473-1. PMID: 25380696. [DOI] [PubMed] [Google Scholar]
- Ward P, Moss HG, Brown TR, Kalivas P, Jenkins DD. N-acetylcysteine mitigates acute opioid withdrawal behaviors and CNS oxidative stress in neonatal rats. Pediatr Res. 2020. July;88(1):77–84. doi: 10.1038/s41390-019-0728-6. PMID: 31935745; PMC7326708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Reid MA, To H, White DM, Cropsey KL, Lahti AC. Open label smoking cessation with varenicline is associated with decreased glutamate levels and functional changes in anterior cingulate cortex: preliminary findings. Front Pharmacol. 2014. July 8;5:158. doi: 10.3389/fphar.2014.00158. PMID: 25071576; PMC4085720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Hillmer AT, Mason GF, Cosgrove KP. Imaging Biomarkers of the Neuroimmune System among Substance Use Disorders: A Systematic Review. Mol Neuropsychiatry 2019. June;5(3):125–146. doi: 10.1159/000499621. PMID: 31312635; PMC6597912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Barker PB. MR spectroscopy and spectroscopic imaging of the brain. Methods Mol Biol. 2011;711:203–26. doi: 10.1007/978-1-61737-992-5_9. PMID: 21279603; PMC3416028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Bashir A, Ackerman JJ, Yablonskiy DA. Improved calibration technique for in vivo proton MRS thermometry for brain temperature measurement. Magn Reson Med. 2008. September;60(3):536–41. doi: 10.1002/mrm.21699. PMID: 18727039; PMC2913520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.