Abstract
Background and Objectives:
Transbronchial cryobiopsy (cryo-TBB) is increasingly being used in the diagnosis of diffuse parenchymal lung diseases (DPLD). Varying diagnostic success and complication rates have been reported. Herein we report our experience with cryo-TBB, focusing on diagnostic yield, factors affecting diagnosis, and safety.
Methods:
This retrospective study was conducted in a tertiary referral chest diseases hospital. Data regarding the patients, procedures, complication rates, diagnostic yield, and the final diagnosis made by a multidisciplinary committee at all diagnosis stages were evaluated.
Results:
We recruited 147 patients with suspected DPLD. The definitive diagnosis was made pathologically in 98 of 147 patients (66.6%) and using a multidisciplinary approach in 109 of 147 (74.1%) cases. The number of samples had a significant effect on diagnostic success. Histopathologic diagnostic yield and diagnostic yield with a multidisciplinary committee after a single biopsy were 50%, and histopathological diagnostic yield and diagnostic yield with multidisciplinary committee increased to 71.4% and 85.7%, respectively, with a second biopsy (p = 0.034). The incidence of mild-to-moderate hemorrhage was 31.9%; no severe hemorrhage occurred. Pneumothorax rate was 15.6%, and the mortality rate was 0.68%.
Conclusions:
Cryo-TBB has sufficient diagnostic yield in the context of a multidisciplinary diagnosis with acceptable complication rates. Performing at least 2 biopsies and from at least 2 segments increases diagnostic success.
Keywords: Transbronchial cryobiopsy, bronchoscopy, parenchymal lung diseases, diagnostic yield
Introduction
Diffuse parenchymal lung diseases (DPLDs) are a heterogeneous group of lung parenchymal disorders with varying treatment options and prognoses. Given this heterogeneity, arriving at a differential diagnosis is critical (1, 2). However, arriving at a differential diagnosis could be challenging given the similar and overlapping features between diseases. In most cases, further invasive procedures are required for accurate diagnosis following appropriate radiologic and physiological evaluations (3). Surgical lung biopsy (SLB) continues to be the gold standard in identifying the possible causes of DPLDs and their histopathologic patterns despite the associated mortality and morbidity (4-6). The 60-day mortality rate after SLB has been reported to range from 2% to 4%, signifying the need for less-invasive diagnostic procedures (5, 7). Among these minimally invasive methods, conventional transbronchial forceps biopsy offers a less-invasive approach; however, its diagnostic yield is low owing to small biopsy samples and crush artefacts (8, 9). Transbronchial cryobiopsy (cryo-TBB), which seems to be safe procedure with lower complication and mortality rates compared than SLB in DPLD diagnosis (4). Comparing the results from published cryo-TBB case series is challenging given the differences in technical details of the procedure such as the use of bronchial blockers, non-use of fluoroscopy, and performing the procedure through an intubation tube. These different procedural approaches can also result in differences in diagnostic yield and complication rates (10).
This study aimed to report our experience with the diagnostic yield and complications of cryo-TBB applied at our tertiary referral chest diseases hospital.
Methods
This retrospective study was held in Yedikule Chest Diseases and Thoracic Surgery Hospital interventional pulmonology unit between January 2014 and December 2019. . The total number of patients eligible for cryobiopsy was 155. Among those, 4 patients developed desaturation immediately after the intubation with a rigid bronchoscope, the cryo probe could not be moved distally in 3 patients, and 1 patient developed arrhytmia. Excluding these 8 cases, the study included data from 147 patients. The diagnostic steps are detailed in the scheme (Figure 1) Inclusion criteria were DPLD patients who could not be differentially diagnosed with clinical, laboratory, immunological and high resolution computed tomography (HRCT) data and were indicated for surgical lung biopsy and evaluated with cryo-TBB. All patients had pulmonary function tests (PFTs), complete blood count, HRCT, and echocardiography. The procedure was not applied to patients who had bleeding tendency (INR> 1.5, Platelet count <50.000 / microL), estimated Pulmonary artery pressure > 40 mmHg, uncontrolled cardiac arrhythmia, unstable angina, severe hypoxemia (pO2 <55 mm Hg despite oxygen support), carbon monoxide diffusing capacity (DLCO) <35%, forced expiratory volume (FVC)<50% and whose procedure was terminated without cryo-TBB due to cardiac and respiratory instability detected after intubation.
Figure 1.
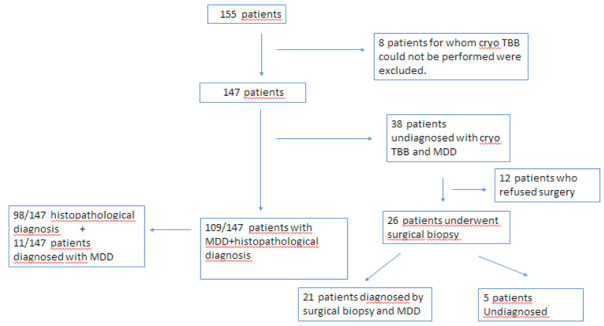
Diagnostic algorithm of the patients
Procedure
Anticoagulant treatments were discontinued before the procedure basis the indications in a patient. HRCT were used to identify the biopsy location before the procedure. Basis the radiological pattern, samples were biopsied from ≥1 distinct lobes. The procedure was performed under anesthesia with intravenous propofol and remifentanil, and intubation was achieved with a rigid bronchoscope. Biopsies were performed under fluoroscopy guidance with a flexible bronchoscope passed through a rigid tube, and the bronchoscope was placed in the determined bronchus. A flexible cryoprobe, 90 cm in length and 1.9 mm in diameter, was used for biopsy (ERBE, Germany) (shown in Figure 2a). The probe was cooled using nitrous oxide (N2O), which caused the probe tip temperature to reach −89°C in a few seconds.
Figure 2.
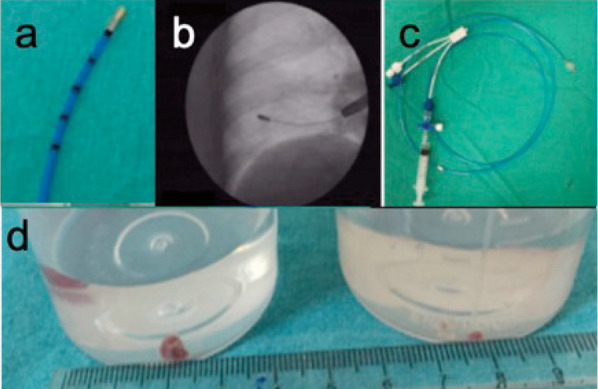
a) The flexible 1.9 mm diameter and 900 mm length cryoprobe, b) Marks on the distal area of the probe (1 cm between each mark) c) Fogarty baloon d) Cryo-transbronchial lung biopsy samples from one of the patients vs conventional biopsy sample comparison
The cryoprobe, which was directed through a flexible bronchoscope in the determined bronchus, was placed perpendicularly to the chest wall up to 10–20 mm from the thoracic wall under fluoroscopy guidance (shown in Figure 2b). When the cryoprobe was placed in the determined position, it was cooled for an average of 5–6 s and withdrawn using the flexible bronchoscope, with the frozen lung tissue attached to the probe tip. The frozen tissue sample was placed in a formalin solution for fixation without damage. The uninflated Fogarty balloon, which was placed in the lobar bronchus closest to the biopsy segment, was inflated after each biopsy and slowly deflated for bleeding control (shown in Figure 2c). Hemorrhages were classified basis the following criteria: grade 1 hemorrhage (mild) if endoscopic aspiration was required; grade 2 (moderate) hemorrhage if additional endoscopic procedures were required (bronchial occlusion and/or cold saline); and grade 3 (severe) hemorrhage if surgical interventions because of hemodynamic or respiratory instability, transfusions, and/or intensive care unit admission were required (4). Patients who could not undergo cryobiopsy in the exclusion criteria were included in the bleeding assessment. Chest x-ray control was planned 2 hours after the procedure to check for an iatrogenic pneumothorax.
Clinical information, radiological features, and biopsy results were then evaluated by a multidisciplinary committee including clinicians, radiologists, and pathologists. This study was approved by the ethics committee of our institution.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Windows 22.0. Continuous variables were presented as means ± standard deviations and medians (min-max) and categorical variables as numbers and percentages. Parametric test assumptions (normality and homogeneity of variances) were checked before comparing the groups. Categorical values were analyzed using the Fisher exact test. The Mann–Whitney U test and paired samples t tests were used to compare continuous variables. A p value < 0.05 was considered statistically significant.
Results
147 patients were enrolled to the study. Among those, 4 patients developed desaturation immediately after the intubation with a rigid tracheoscopy, the cryo probe could not be moved distally in 3 patients, and 1 patient developed arrhytmia. Excluding these 8 cases, the study included data from 147 patients.
The average age of the patients analyzed in the study was 56.4 ± 13 and 82 of them were female (55.8%) and 65 were male (44.2%). The baseline characteristics of the patients are provided in Table 1.
Table 1.
Patients’ characteristics at baseline and procedural details
| Patient number (n) | 147 | |
| Mean age (years±SD) | 56.4±13 | |
| Gender n (%) Female Male |
82 (55.8) 65 (44.2) |
|
| Smoking (Pack-years) (n=95, mean±SD) | 12.5±16.8 | |
| CCI (n) 0-2 >3 |
90 57 |
|
| FVC % predicted (Mean±SD) | 82.4±18.5 | |
| DLCO% predicted (Mean) | 59.01±18.1 | |
| Biopsy location | Right lung (n,%) | 134 (91.2) |
| Left lung (n,%) | 13 (8.8) | |
| One segment | 73 | |
| Two different segments | 69 | |
| Three different segments | 5 | |
| Mean biopsy number (n±SD) | 3.3±1 | |
| Freezing duration (seconds, min-max) | 4.8±1.2 s (4-10) | |
| Small axis diameter (mm±SD) | 2.8±0.6 | |
| Large axis diameter (mm±SD) | 6.9±2.3 | |
CCI Charlson Comorbidity index, FVC: Forced vital capacity, DLCO: Carbon monoxide diffusion capacity, cTBB: Transbronchial lung cryobiopsy, SD: standard deviation
The number of biopsies taken from the right and the left lung were 134 (91.2%) and 13 (8.8%), respectively. Biopsies were taken from one segment in 73 (49.6%) patients, from two different segments in 69 (46.9%) patients, and from three different segments in 5 (3.4%) patients. Cryo-TBB was performed on different segments of the same lobe in 61 cases and two different lobes and different segments in 15 cases . The mean biopsy number per patient was 3.3 ± 1 (range 1-6). Mean freezing time during the procedure was 4.8 ± 1.2 seconds (range: 4-10). The shortest and longest diameters of the biopsy materials were 2.8 ± 0.6 mm, 6.9 ± 2.3 mm, respectively (Figure 2d).
Histopathological diagnosis was provided in 98/147 cases (66.6%). The most common diagnosis was nonspecific interstitial pneumonia (NSIP) (n=46, 46.9%). Fourteen cases were diagnosed with granulomatous inflammation (n=14, 14.3%), 12 were malignancy (n=12, 12.2%), 6 with usual interstitial pneumonia (UIP) (n=6, 6.1%), 6 with organizing pneumonia (OP) (n=6, 6.1%), and 6 with hypersensitivity pneumonitis (HP) (n=6, 6.1%). The pathological diagnoses and diagnostic yield are showed in Table 2.
Table 2.
Histopathological and multidisciplinary diagnoses and diagnostic yield
| Diagnosis | n (%) | ||
| DIAGNOSIS | HISTOPATHOLOGICAL DIAGNOSIS | Non-Specific Interstitial Pneumonia | 46 (46.9) |
| Hypersensitivity Pneumonia | 6 (6.1) | ||
| Usual Interstitial Pneumonia | 6 (6.1) | ||
| Organizing Pneumonia | 6 (6.1) | ||
| Granulomatous Inflamation | 14 (14.3) | ||
| Malignancy Adenocarcinoma Breast cancer metastas Lymphoma |
12 (12.2) 10 1 1 |
||
| Othersa | 8 (8.2) | ||
| Non-diagnostic | 49 | ||
| Diagnosis | n (%) | ||
| MULTIDISCIPLANARY COUNCIL DIAGNOSIS | Non-Specific Interstitial Pneumonia | 38 (34.8) | |
| Hypersensitivity Pneumonitis | 20 (18.3) | ||
| Idiopathic pulmonary fibrosis | 4 (3.7) | ||
| Sarcoid | 12 (11) | ||
| Organizing Pneumonia | 7 (6.4) | ||
| Malignancy Adenocarsinoma Breast cancer metastas Lymphoma |
12 (12.2) 10 1 1 |
||
| Othersb | 15 (13.8) | ||
| Non-diagnostic | 38 | ||
Othersa: Alveolar microlithiasis (1), Non-spesific inflammation (1), Mosaic pattern (1), Histiocytosis X (1), Folicular bronchiolitis (1), Chronic eosinophilic pneumonia (1), Acute Lung Injury (1), Unclassifiable interstitial lung disease (1)
Othersb: Rheumatoid lung disease (1), Tuberculosis (1), Drug induced lung disease (1), Alveolar microlithiasis (1), Nonresolving pneumonia (1), Mosaic attenuation (1), Histiocytosis X (1), Follicular bronchiolitis (1), Acute interstitial pneumonia (1), Unclassifiable interstitial lung disease (1)
SD: standard deviation
A final diagnosis was made in 109 (74.1%) of 147 cases evaluated by the multidisciplinary committee. The most common diagnosis was NSIP in 38 patients. Other diagnoses are shown in Table 2.
SLB was performed in 26 (17.6%) out of 38 patients who could not be diagnosed after multidisciplinary evaluation, 21 patients were diagnosed while pathological diagnosis could not be made in 5 patients (11 UIP, 5 HP, 2 Adenocarcinoma, 1 NSIP, 1 Emphysema, 1 Anthracosis). The remaining 12 patients were patients who were not suitable for SLB due to their general condition or who did not accept a further examination.
Overall histopathological yield and diagnostic yield with multidisciplinary approach were 66,6% and 74,1%, respectively. Histopathological diagnostic yield and diagnostic yield with multidisciplinary approach after a single biopsy was 50%, and histopathological diagnostic yield and diagnostic yield with multidisciplinary approach increased to 71.4% and 85.7% by a second biopsy (p = 0.034). No further increase in diagnostic yield was observed when more than two samples were taken (Table 3).
Table 3.
Diagnostic yield according to the number of samples
| Overall diagnostic yield (%) | 1-2 biopsy | (diagnostic yield) | (n=31) | 3 biopsy (diagnostic yield) (n=116) | p value* | |
| Pathological diagnosis | 66.6 | 20 (64.51%) | 78 (67.2%) | 0.995 | ||
| 1 biopsy (n=10) 5 (50%) |
2 biopsy (n=21) 15 (71.4%) |
p=0.244* | ||||
| Diagnosis with Multidisciplinary comittee | 74.1 | 23 (74.1%) | 86 (74.1%) | 0.775 | ||
| 1 biopsy (n=10) 5 (50%) |
2 biopsy (n=21) 18 (85.7%) |
p=0.034* | ||||
*p value of the comparison between 1 and 2 biopsies
**p value of the comparison between 1-2 and ≥3 biopsies.
The histopathological diagnostic yield was 61.9% when multiple biopsies were taken from a single segment, and 69.8% with multidisciplinary approach, while the histopathological diagnostic yield was 72.9% when multiple biopsies were taken from different segments of the same lobe and 81.1% with multidisciplinary approach. There is no statistically significant difference in diagnostic yield when biopsy is taken from single or different lobes.
Complications occurred in 77 (49.6%) of 155 patients (patients who were excluded from cryo-TBB and patients who included in the study). 69 (46.9%) patients who developed complications due to the cryo-TBB procedure included in the study. Hemorrhage developed in 47 (31.9%) patients; grade 1 in 28 (19%) patients, grade 2 in 19 (12.9%) patients. Grade 3 hemorrhage was not observed in any patient. Pneumothorax was developed in 23 (15.6%) patients after the procedure. Of those with pneumothorax, 14 (60.9%) had a tube thoracostomy. Respiratory failure occurred in 3 patients (2%), 2 of these patients were discharged with a short-term noninvasive mechanical ventilation application in the pulmonology wards, 1 patient was admitted to an intensive care and administered invasive mechanical ventilation and died on the 15th day (Table 4).
Table 4.
Safety outcomes and length of hospital stay (n=147)
| Complications | n (%) |
| Pneumothorax Oxygen therapy Tube Thoracostomy |
23/147 (total) 9 (39.1) 14 (60.9) |
| Hemorrhage | Grade 1: 28/147 (19) Grade 2: 19/147 (12.9) Grade 3: 0/147 |
| Respiratory failure | 3/147 (2.0) |
| Mortality within 30 days | 1/147 (0.7) |
| Length of Hospital stay Discharged same day Discharged next day 1-3 days >4 days Hospital stay (mean days±SD) |
114 (77.5) 33/147 (22.4) 15/33 (45.5) 18/33 (54.5) 4.3±2.8 |
When the factors affecting the development of pneumothorax and hemorrhage were evaluated, there was no significant difference in pneumothorax rates between patients who underwent 1 and 2 biopsies and those who had more than 3 biopsies (Table 3). While pneumothorax was detected in 13.7% of patients who underwent single-segment biopsy, and in 17.6% of patients who underwent 2 or more segment biopsies, but it was not statistically significant. There was no significant difference between pneumothorax rates in patients who underwent a biopsy from the same and different lobes. The pneumothorax rate was 6.3% when a biopsy was taken from the upper lobes, and 18.3% when a biopsy was taken from the lower lobes. No statistically significant difference was found in terms of hemorrhage rates in biopsies taken from the same or different lobes and biopsies taken from the upper and lower lobes.
Discussion
Cryo-TBB is used for the diagnosis of DPLD as an alternative to SLB at many centers. Our study presents real-world data from patients who underwent cryo-TBB at our tertiary referral hospital with experience in interventional bronchoscopy. We found a sufficient histopathologic diagnostic yield of 66.6%, and when these histopathologic diagnoses were combined with clinical and radiographic information using a multidisciplinary approach, the diagnostic yield reached 74.1% for specific diagnoses in most cases. In addition, cryo-TBB was associated with a lower pneumothorax rate and controllable hemorrhagic complications.
Reports from various centers indicate the diagnostic yield in DPLD with cryo-TBB to range from 50% to 100% and the complication rates (e.g., pneumothorax) to range from 1% and 30% (4, 11, 12). These varying results are probably because of the lack of standardization in patient selection and cryo-TBB techniques. A 2019 review assessed the literature on cryo-TBB and furnished an evidence-based expert panel report (14). However, the cryo-TBB technique remains unstandardized. In our series of 147 cases, histopathologic diagnostic yield with cryo-TBB was 66.6%. When these histopathologic diagnoses were combined with a multidisciplinary approach, the diagnostic yield was 74.1%. The most frequently made histopathologic diagnoses and histopathologic diagnosis rates differ among the published reports by centers where the procedures were performed. Several studies have reported diagnostic yields between 44% and 91% (13, 15-17). This wide distribution in histopathologic diagnosis rates could be attributed to the different equipment used at the centers, differing experience, and the differing perspectives of pathologists.
The higher diagnostic yield with cryo-TBB over conventional TBB has been attributed to the larger biopsy size (18-20). The sample size may affect the diagnostic yield. Although the optimal sample size for cryo-TBB materials remains unestablished in the literature, some pathologists contend that samples of 5 mm diameter (equivalent to the size of the full area seen with a 4X microscope objective lens) are sufficient (21). In our study, the shortest mean diameter was 2.8 ± 0.6 mm and the longest was 6.9 ± 2.3 mm. Wälscher et al reported the average diameter of the biopsy sample in their study to be 5 mm (22). However, our diagnostic success rate is similar to that in the study by Wälscher et al (22). In the study by Ravaglia (10) that included 699 patients, the shortest diameter was 4.6 ± 1.2 mm, and the longest diameter was 6.3 ± 1.9 mm; the histopathologic diagnostic yield was 87.8%. In comparison, the short diameter in our study was smaller. We suppose that the differences in diagnostic yield could be because of the differences in the biopsy diameter. Considering the effect of the heterogeneity of the disease and the distribution of parenchymal pathology on the diagnosis, usually more than 1 biopsy samples (mean number of biopsies per patient, 3.3 ± 1) were collected in our study. The optimal number of biopsies for cryo-TBB remains undetermined in the literature. Similar to those in the 2 studies by Ravaglia, the diagnostic yield in our study significantly increased in patients with 2 biopsies instead of 1 (10, 23). No significant difference was observed in the diagnostic yield with ≥2 biopsies.
The “Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases: CHEST Guideline and Expert Panel Report” published in 2019 recommends collecting biopsy samples from at least 2 different regions (14). We performed cryo-TBB on different lobes in patients with radiographic interlobar heterogeneity. In patients with diffuse radiographic patterns in both the upper and lower lobes, cryo-TBB was generally performed on different segments of the same lobe. In our study, we found that collecting 2 biopsy samples from different parts of the same lobe or from different lobes significantly increased the diagnostic yield. In line with our observation, Ravaglia, in 2 distinct studies, reported that collecting biopsy samples from 2 different locations significantly increased diagnostic yield (8, 23).
Pneumothorax is one of the most common complications reported to be associated with cryo-TBB. However, the incidence of pneumothorax varies significantly across publications, ranging from 1% to 30% (4, 8, 11, 18-20, 24-26). Deep sedation and jet ventilation have been reported to increase the incidence of pneumothorax (4). In fact, in the study by Alvarez et al, the incidence of pneumothorax on performing cryo-TBB with local anesthesia and under conscious sedation was 4.7% (12). In our study, the incidence of pneumothorax was 15.6%, and all procedures were performed under deep sedation by intubation with a rigid bronchoscope. The incidence of pneumothorax could also be affected by the sample size and technical. In fact, in the study by Ravaglia et al. (10), the incidence of pneumothorax was higher in case of biopsy from >1 and/or upper lobes. In our study, while the incidence of pneumothorax due to cryo-TBB was unaffected by the number of biopsies, we observed that it increased in cases of biopsies performed on the lower lobes and on multiple regions.
Hemorrhage is another common complication associated with cryo-TBB, with reported incidence between 2.5% and 87% (8, 12, 18-20, 22, 24, 25, 27, 28). In our study, in accordance with the literature, grade 1 and grade 2 hemorrhages occurred in 31.9% of the patients. Hemorrhages were easily controlled with adrenaline and cold saline in addition to the use of the Fogarty balloon placed in the bronchus in each patient. Six patients needed absorbable hemostat use. None of the patients had a life-threatening bleeding requiring transfusion, intensive care unit follow-up, or surgical intervention. Although hemorrhage has been reported to be more common in biopsies on the lower lobes (10), the incidence of bleeding was not associated with the number of samples or sampling strategy (≥1 regions, lower or upper lobes) in our study.
While 77.5% of our patients were discharged on the same day, the average hospital stay among our hospitalized patients was 4.3 ± 2.8 days. In the study by Ravaglia et al (4), the average hospital staying among patients who underwent SLB was 6.1 days. In terms of hospital stay, cryo-TBB could be considered as a cost-effective method.
Studies have reported mortality rates associated with cryo-TBB to range between 0% and 4.1% (4, 24, 33, 34). The mortality rate among our patients was 0.7%: 3 patients needed non-invasive mechanical ventilation after the procedure, and 2 of these patients recovered in a short time (1 exitus). However, 1 patient was taken to the intensive care unit and invasive mechanical ventilation was needed on the 5th day; the patient subsequently died because of diffuse alveolar damage and respiratory failure. In a retrospective study by Ravaglia et al, patients who underwent cryo-TBB and SLB were compared (0.3% vs 2.7%), and the mortality rate in the SLB group was higher than that in our study (4). In the study by Hutchinson that included 12,000 patients who were examined using SLB, mortality rate was reported to be 1.7% among elective cases, respectively. This result is higher than the mortality rates in patients who underwent cryo-TBB in our study (35).
Our study has some limitations. First, it is a retrospective study. Second, we could not compare results with FOB or SBL without general anesthesia and jet ventilation. Furthermore, this study did not include a control group, and cryo-TBB was not compared with surgical lung biopsy in the same population.
Conclusion
Our results show that cryo-TBB has sufficient diagnostic success in most DPLD cases. The diagnostic yield increases with at least 2 biopsies and biopsies from at least 2 segments. Cryo-TBB has a higher diagnostic success, controllable complication and lower mortality rates; it can hence be considered as a first step in the diagnostic strategy for DPLD in patients requiring invasive diagnosis with a multidisciplinary committee at a center with expertise in these procedure.
Statement of Ethics:
This research comply with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All authors state that subjects (or their parents or guardians) have given their written informed consent and that the study protocol was approved by the Yedikule Chest Disease and Thoracic Surgery Education and Research Hospital ethical committee on human research.
Conflict of Interest:
The authors have no conflicts of interest to declare.
Authors’ Contributions:
All authors participated in the study design and/or or implementation, analysis, and interpretation of the data. All authors had full access to the data, participated in manuscript development, and gave final approval before submission.
References
- 1.Mikolasch TA, Garthwaite HS, Porter JC. Up-date in diagnosis and management of intersti-tial lung disease. Clin Med (Lond) 2016 Dec;;16(Suppl 6):s71–8. doi: 10.7861/clinmedicine.16-6s-s71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poletti V, Ravaglia C, Gurioli C, et al. Invasive diagnostic techniques in idiopathic interstitial pneumonias. Respirology. 2016 Jan;21(1):44–50. doi: 10.1111/resp.12694. [DOI] [PubMed] [Google Scholar]
- 3.Nead MA, Morris DG. InterstitialLungDisease: A Clinical Over view and General Approach. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI, editors. Fishman’s Pulmonary Diseases and Disorders. 4th edition. McGrawHill; 2008. pp. 1105–24. [Google Scholar]
- 4.Ravaglia C, Bonifazi M, Wells AU, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration. 2016;91(3):215–27. doi: 10.1159/000444089. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoraco-scopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007 Mar;83(3):1140–4. doi: 10.1016/j.athoracsur.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology. 2014;19:900–6. doi: 10.1111/resp.12322. [DOI] [PubMed] [Google Scholar]
- 9.Tomasetti S, Cavazza A, Colby TV, et al. Transbronchial biopsy is useful in predicting UIP pattern. Raspir Res. 2012 Oct 29;13:96. doi: 10.1186/1465-9921-13-96. doi: 10.1186/1465-9921-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravaglia C, Wells AU, Tomassetti S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med. 2019;19:16. doi: 10.1186/s12890-019-0780-3. doi: 10.1186/s12890-019-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iftikhar IH, Alghothani L, Sardi A, Berkowits D, Musani AI. Transbronchial lung cryobiopsy and video-assisted thoracoscopic lung biopsy in the diagnosis of diffuse parenchymal lung disease: a meta-analysis of diagnostic test accuracy. Ann Am Thorac Soc. 2017;14:1197–211. doi: 10.1513/AnnalsATS.201701-086SR. [DOI] [PubMed] [Google Scholar]
- 12.Bango-Alvarez A, Ariza-Prota M, Torres-Rivas H, et al. Transbronchial cryobiopsy in interstitial lung disease: experience in 106 cases – how to do it. ERJ Open Res. 2017;3:00148–2016. doi: 10.1183/23120541.00148-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial Cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the Cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95:188–200. doi: 10.1159/000484055. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado F, Danoff SK, Wells AU, et al. Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases. CHEST Guideline and Expert Panel Report CHEST. 2019 doi: 10.1016/j.chest.2019.10.048. doi: https://doi.org/10.1016/j.chest.2019.10.048. [DOI] [PubMed] [Google Scholar]
- 15.DiBardino DM, Haas AR, Lanfranco AR, Litzky LA, Sterman D, Bessich JL. High complication rate after introduction of transbronchial cryobiopsy into clinical practice at an Academic Medical Center. Ann Thorac Soc. 2017;14:851–7. doi: 10.1513/AnnalsATS.201610-829OC. [DOI] [PubMed] [Google Scholar]
- 16.Lentz RJ, Taylor TM, Kropski JA, et al. Utility of flexible bronchoscopic cryobiopsy for diagnosis of diffuse parenchymal lung diseases. J Bronchology Interv Pulmonol. 2018;25:88–96. doi: 10.1097/LBR.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ussavarungsi K, Kern RM, Roden AC, Ryu JH, Edeli ES. Transbronchial cryobiopsy in diffuse parenchymal lung disease: retrospective analysis of 74 cases. Chest. 2017;151:400–408. doi: 10.1016/j.chest.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration. 2009;78(2):203–8. doi: 10.1159/000203987. [DOI] [PubMed] [Google Scholar]
- 19.Yarmus L, Akulian J, Gilbert C, et al. Cryoprobe transbronchial lung biopsy in patients after lung transplanta-tion: a pilot safety study. Chest. 2013 Mar;143(3):621–6. doi: 10.1378/chest.12-2290. [DOI] [PubMed] [Google Scholar]
- 20.Kropski JA, Pritchett JM, Mason WR, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One. 2013 Nov;8(11):e78674. doi: 10.1371/journal.pone.0078674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colby TV, Tomassetti S, Cavazza A, Dubini A, Poletti V. Transbronchial cryobiopsy in diffuse lung disease: update for the pathologist. Arch Pathol Lab Med. 2017;141:891–900. doi: 10.5858/arpa.2016-0233-RA. [DOI] [PubMed] [Google Scholar]
- 22.Wälscher J, Groß B, Eberhardt R, et al. Transbronchial Cryobiopsies for Diagnosing Interstitial Lung Disease: Real-Life Experience from a Tertiary Referral Center for Interstitial Lung Disease. Respiration. 2018 doi: 10.1159/000493428. DOI: 10.1159/000493428. [DOI] [PubMed] [Google Scholar]
- 23.Ravaglia C, Wells AU, Tomassetti S, et al. Transbronchial lung cryobiopsy in diffuse parenchymal lung disease: comparison between biopsy from 1 segment and biopsy from 2 segments - diagnostic yield and complications. Respiration. 2017;93:285–92. doi: 10.1159/000456671. [DOI] [PubMed] [Google Scholar]
- 24.Hagmeyer L, Theegarten D, Wohlschlager J, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J. 2016;10:589–95. doi: 10.1111/crj.12261. [DOI] [PubMed] [Google Scholar]
- 25.Sharp C, McCabe M, Adamali H, Medford AR. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease-a systematic review and cost analysis. QJM. 2017;110:207–14. doi: 10.1093/qjmed/hcw142. [DOI] [PubMed] [Google Scholar]
- 26.Gershman E, Fruchter O, Benjamin F, et al. Safety of cryo-transbronchial biopsy in diffuse lung diseases: analysis of three hundred cases. Respiration. 2015;90:40–6. doi: 10.1159/000381921. [DOI] [PubMed] [Google Scholar]
- 27.Fruchter O, Fridel L, Rosengarten D, Raviv Y, Rosanov V, Kramer MR. Transbronchial cryo-biopsy in lung transplantation patients: First report. Respiralogy. 2013;18:669–673. doi: 10.1111/resp.12037. doi: 10.1111/resp.12037. [DOI] [PubMed] [Google Scholar]
- 28.Griff S, Ammenwerth W, Schonfeld N, et al. Morphometrical analysis of transbronchial cryobiopsies. Diagn Pathol. 2011;6:53. doi: 10.1186/1746-1596-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Echevarria-Uraga JJ, Perez-Izquierdo J, Garcia-Garai N, et al. Usefulness of an angioplasty balloon as selective bronchial blockade device after transbronchial cryobiopsy. Respirology. 2016;21:1094–9. doi: 10.1111/resp.12827. [DOI] [PubMed] [Google Scholar]
- 31.Linhas R, Marcoa R, Oliveira A, Almedida J, Neves S, Campaninha S. Transbronchial lung cryobiopsy: associated complications. Rev Port Pneumol. 2017;23:331–7. doi: 10.1016/j.rppnen.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Tomic R, Cortes-Puentes GA, Murugan P, Joo Kim H, Amin K, Dincer HA. Acute exacerbation of interstitial lung disease after cryobiopsy. J Bronchology Interv Pulmonol. 2017;24:319–22. doi: 10.1097/LBR.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 33.Cascante JA, Cebollero P, Herrero S, et al. Transbronchial Cryobiopsy in Interstitial Lung Disease: Are We on the Right Path. Journal of bronchology & interventional pulmonology. 2016;23(3):204–209. doi: 10.1097/LBR.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 34.Sriprasart T, Aragaki A, Baughman R, et al. A Single US Center Experience of Transbronchial Lung Cryobiopsy for Diagnosing Interstitial Lung Disease With a 2-Scope Technique. Journal of bronchology & interventional pulmonology. 2017;24(2):131–135. doi: 10.1097/LBR.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchinson JP, Fogarty AW, McKeever TM, Hubbard R. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000–2011. Am J Respir Crit Care Med. 2016;193:1161–7. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]


