Summary
Lithium-ion batteries (LIBs) have become one of the main energy storage solutions in modern society. The application fields and market share of LIBs have increased rapidly and continue to show a steady rising trend. The research on LIB materials has scored tremendous achievements. Many innovative materials have been adopted and commercialized by the industry. However, the research on LIB manufacturing falls behind. Many battery researchers may not know exactly how LIBs are being manufactured and how different steps impact the cost, energy consumption, and throughput, which prevents innovations in battery manufacturing. Here in this perspective paper, we introduce state-of-the-art manufacturing technology and analyze the cost, throughput, and energy consumption based on the production processes. We then review the research progress focusing on the high-cost, energy, and time-demand steps of LIB manufacturing. Finally, we share our views of challenges in LIB manufacturing and propose future development directions for manufacturing research in LIBs.
Subject areas: Electrochemical Energy Storage, Industrial Chemistry, Energy Storage, Industrial Processing of Material, Energy Materials
Graphical abstract
Electrochemical Energy Storage ; Industrial Chemistry ; Energy Storage ; Industrial Processing of Material ; Energy Materials
Introduction
Lithium-ion batteries (LIBs) have been widely used in portable electronics, electric vehicles, and grid storage due to their high energy density, high power density, and long cycle life. Since Whittingham discovered the intercalation electrodes in the 1970s, Goodenough et al. developed some key cathode materials (layered, spinel, and polyanion) in the 1980s and the 1990s, and Yoshino created the first safe, production-viable LIB with the combination of LiCoO2 as the cathode and carbon/graphite as the anode, much progress in LIBs have been made in terms of cost, energy density, power density, safety, and cycle life (Whittingham, 1976; Mizushima et al., 1980; Thackeray et al., 1983; Padhi et al., 1997). For example, the cost of LIBs has dropped from over $1,000/kWh in the early 2000 to ∼$200/kWh currently. At the same time, the specific energy density of LIBs has been increased from 150 Wh/kg to ∼300 Wh/kg in the past decades. Although beyond LIBs, solid-state batteries (SSBs), sodium-ion batteries, lithium-sulfur batteries, lithium-air batteries, and multivalent batteries have been proposed and developed, LIBs will most likely still dominate the market at least for the next 10 years.
Currently, most research studies on LIBs have been focused on diverse active electrode materials and suitable electrolytes for high cutoff voltage applications, especially the nickel-rich and/or cobalt-free cathode materials and Si or Li metal anode materials and their associated electrolytes. Progress in LIB manufacturing lags behind and not much progress has been made, although manufacturing contributes about 25% of the cost of LIBs (Kwade et al., 2018). Currently, the manufacturing of LIBs still needs to go through slurry mixing, coating, drying, calendering, slitting, vacuum drying, jelly roll fabrication (stacking for pouch cells and winding for cylindrical and prismatic cells), welding, packaging, electrolyte filling, formation, and aging, a multi-staged process being adopted by industry.
In this perspective paper, we first evaluate each step of the current manufacturing process and analyze their contributions in cost, energy consumption, and throughput impacts for the entire LIB production. Then we summarize the recent progress on the advancement of LIB manufacturing and the challenges and the potential impacts of these new technologies. Finally, we provide our perspectives on future LIB manufacturing. We hope that such a paper helps promote more collaboration between the academia and industry with the ultimate goal to solve some key issues of LIB manufacturing, which may eventually result in increasing the production efficiency and lowering the cost and energy consumption of LIBs.
Current manufacturing processes for LIBs
LIB industry has established the manufacturing method for consumer electronic batteries initially and most of the mature technologies have been transferred to current state-of-the-art battery production. Although LIB manufacturers have different cell designs including cylindrical (e.g., Panasonic designed for Tesla), pouch (e.g., LG Chem, A123 Systems, and SK innovation), and prismatic (e.g., Samsung SDI and CATL), the cell manufacturing processes are very similar.
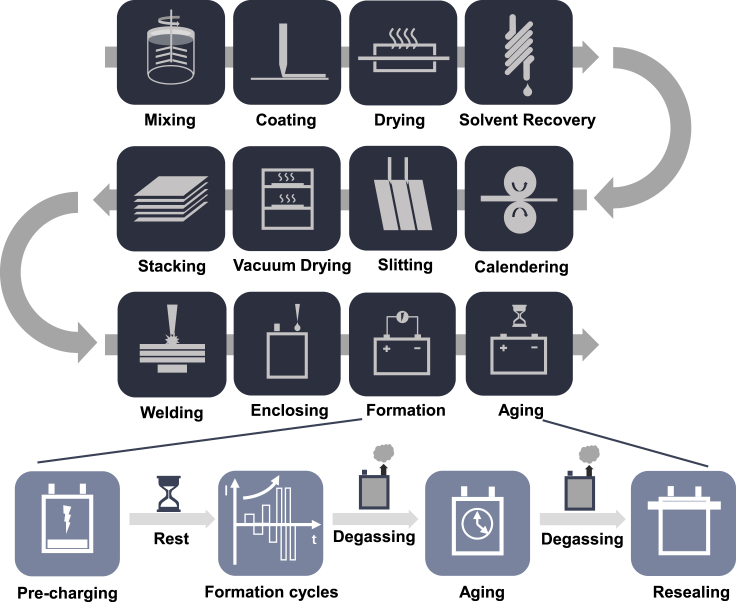
Figure 1 introduces the current state-of-the-art battery manufacturing process, which includes three major parts: electrode preparation, cell assembly, and battery electrochemistry activation. First, the active material (AM), conductive additive, and binder are mixed to form a uniform slurry with the solvent. For the cathode, N-methyl pyrrolidone (NMP) is normally used to dissolve the binder, polyvinylidene fluoride (PVDF), and for the anode, the styrene-butadiene rubber (SBR) binder is dissolved in water with carboxymethyl cellulose (CMC). The slurry is then pumped into a slot die, coated on both sides of the current collector (Al foil for cathode and Cu foil for the anode), and delivered to drying equipment to evaporate the solvent. The common organic solvent (NMP) for cathode slurry is toxic and has strict emission regulations. Thus a solvent recovery process is necessary for the cathode production during drying and the recovered NMP is reused in battery manufacturing with 20%–30% loss (Ahmed et al., 2016). For the water-based anode slurry, the harmless vapor can be exhausted to the ambient environment directly. The following calendering process can help adjust the physical properties (bonding, conductivity, density, porosity, etc.) of the electrodes. After all these processes, the finished electrodes are stamped and slitted to the required dimension to fit the cell design. The electrodes are then sent to the vacuum oven to remove the excess water. The moisture level of the electrodes will be checked after drying to ensure the side reaction and corrosion in the cell are minimized.
Figure 1.
Schematic of LIB manufacturing processes
After the electrodes are well prepared, they are sent to the dry room with dried separators for cell production. The electrodes and separator are winded or stacked layer by layer to form the internal structure of a cell. The aluminum and copper tabs are welded on the cathode and anode current collector, respectively. The most common welding method is ultrasonic welding, and some manufacturers may choose resistance welding for their cell design. The cell stack is then transferred to the designed enclosure, which does not have a consistent standard currently. Each manufacturer has their preference depending on the purpose of the cells. The enclosure is filled with electrolyte before the final sealing and completes the cell production.
Before delivering the cells to the end product manufacturers, the electrochemistry activation steps are applied to these cells to enable operation stability. A stable solid-electrolyte interface (SEI) layer can prevent the irreversible consumption of electrolyte and protect the anode from overpotential during fast charging, which can result in forming Li dendrites (Li et al., 2019). The formation and aging process starts from charging the cells to a relatively low voltage (e.g., 1.5V) to protect the copper current collector from corrosion, followed by a rest session for electrolyte wetting. The cells are charged/discharged under a low rate such as C/20, and then the rate will be gradually increased to ensure a stable SEI layer on the surface of the anode (Wood et al., 2019). The gas generated from the formation process needs to be discharged for safety concerns. After or during formation cycles, the cells are stored on the aging shelves for complete electrolyte wetting and SEI stabilization. Another degassing step is arranged before the cells are finally sealed for future applications. Depending on the formation protocol and aging temperature, this step normally lasts several weeks.
Cost, throughput, and energy consumption
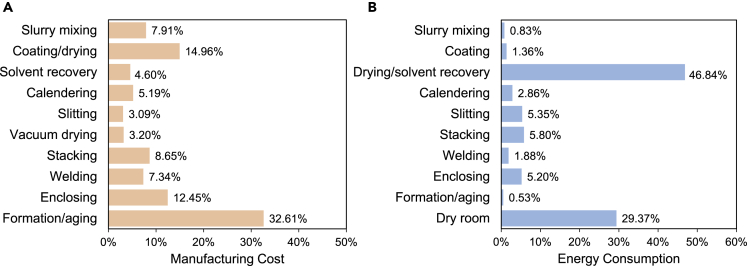
The estimate of the cost, throughput, and energy consumption for these manufacturing steps is critical to help determine the steps that need the most research and innovation. Therefore, more research efforts can be focused on these topics. Table 1 and Figure 2A show the breakdown of manufacturing cost calculated by the BatPac model from Argonne National Laboratory. The model was based on a 67-Ah LiNi0.6Mn0.2Co0.2O2 (NMC622)/graphite cell, 100,000 EV battery packs/year plant (Nelson et al., 2019). The electrode coating, drying, cell formation, and aging contributed to 48% of the entire manufacturing cost. These high capital investments and labor-intense processes are the most urgent fields that need to be studied. The cost saving will be significant if the laboratory innovations can be transferred to these manufacturing processes.
Table 1.
Cost, throughput, and energy consumption of LIB manufacturing processes
| Manufacturing processes | Cost per year/$∗ (Nelson et al., 2019) | Percentage % | Throughput (Heimes et al., 2019a) | Manufacturing processes | Energy consumption per cell/kWh | Percentage % |
|---|---|---|---|---|---|---|
| Slurry mixing | 7,396,000 | 7.91% | 30 min–5 h | Slurry mixing | 0.11 | 0.83% |
| Coating/drying | 13,984,000 | 14.96% | 35–80 m/min | Coating | 0.18 | 1.36% |
| Solvent recovery | 4,296,000 | 4.60% | NA | Drying/solvent recovery | 6.22 | 46.84% |
| Calendering | 4,849,000 | 5.19% | 60–100 m/min | Calendering | 0.38 | 2.86% |
| Slitting | 2,891,000 | 3.09% | 80-–150 m/min | Slitting | 0.71 | 5.35% |
| Vacuum drying | 2,990,000 | 3.20% | 12–30 h | Stacking | 0.77 | 5.80% |
| Stacking | 8,086,000 | 8.65% | NA | Welding | 0.25 | 1.88% |
| Welding | 6,864,000 | 7.34% | NA | Enclosing | 0.69 | 5.20% |
| Enclosing | 11,636,000 | 12.45% | Depend on the cell design | Formation/aging | 0.07 | 0.53% |
| Formation/aging | 30,482,750 | 32.61% | Up to 1.5–3 weeks | Dry room | 3.9 | 29.37% |
The labor cost was calculated based on the US average factory worker's salary of $15/h (Economic Research Institute, 2020).
The floor space cost was calculated based on $3,000/m2 per year (includes rent, utility, and management) (Nelson et al., 2019).
The depreciation cost was calculated by 16.7% of capital investment and 5% of floor space cost (Nelson et al., 2019).
The manufacturing cost includes equipment depreciation, labor cost, and plant floor space cost.
Figure 2.
Cost and energy consumption breakdown of LIB manufacturing processes
(A and B) (A) Cost breakdown and (B) energy consumption breakdown.
Throughput is highly related to the manufacturing cost. Higher production efficiency can save labor costs and venue rental. The throughput in Table 1 shows the production time distribution (Heimes et al., 2019a). The roll-to-roll manufacturing processes such as coating, calendering, and slitting have a high throughput of over 35 m/min. However, processes like vacuum drying and formation/aging are time-consuming (up to 3 weeks) because of their strict moisture level restriction and sensitive chemical reaction (Heimes et al., 2019a).
The energy consumption of a 32-Ah lithium manganese oxide (LMO)/graphite cell production was measured from the industrial pilot-scale manufacturing facility of Johnson Control Inc. by Yuan et al. (2017) The data in Table 1 and Figure 2B illustrate that the highest energy consumption step is drying and solvent recovery (about 47% of total energy) due to the long-time heating and off-gas cooling. Another energy-consuming part is the dry room, which consumed 29% of total energy, owing to the low moisture requirement during cell assembling processes. These high energy consumption steps can result in a huge amount of greenhouse gas emissions and make LIBs less environment friendly. Therefore, the technology of reducing the amount of solvent usage or even avoid the use of solvent should be considered for battery manufacturing. Meanwhile, it is also important to improve the production efficiency in the dry room to lower the energy consumed by keeping low moisture levels.
Research progresses on LIB manufacturing
The aforementioned analysis clearly shows that some manufacturing steps contribute much to the cost, throughput, and energy consumption during LIB manufacturing. Manufacturing innovation of LIBs is critical to significantly lower the cost and energy consumption and increase the throughput. Here, we will discuss some of the innovative researches on the manufacturing processes for LIB production, mainly focusing on mixing, coating, drying, solvent recovery, calendering, slitting, and formation and aging. Other steps including stacking and enclosing are very mature, and not much research has been done on these steps.
Slurry mixing
The slurry mixing step contributes to 7.9% of the total manufacturing cost, and it takes a relatively long time to get a suitable slurry for the following manufacturing processes. The current industry employs planetary mixers to prepare large-volume slurries for production. The target of modifying mixing technology is to lower the cost by improving the throughput. The electrochemical performance of the electrodes is also highly related to the mixing condition and operation. The mixing uniformity can affect the electrode microstructure and materials distribution (Bockholt et al., 2016).
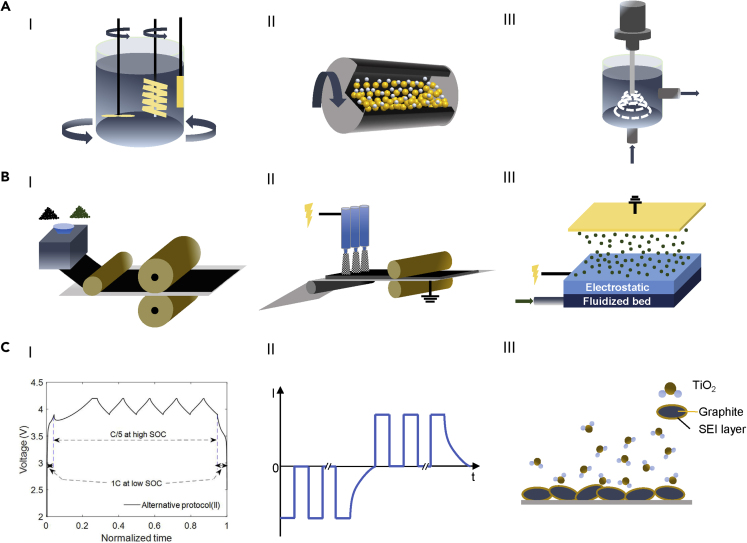
Hydrodynamic shear mixing (HSM) is a mature technology, which is possible to be transferred to the battery industry. It is economical and can be easily scaled up. The HSM mixer usually includes an external stir bar such as Rushton-type turbine. The turbulent stresses are the main mechanism to break the agglomerations during the HSM process (Zhang et al., 2012). To improve the mixing efficiency and uniformity and keep the economic advantage of HSM, Liu et al. proposed a 3D mixing equipment based on the HSM mechanism (Figure 3AI) (Liu et al., 2014). This modified 3D mixer includes a traditional Rushton-type turbine with an additional rotating container for θ direction mixing and an off-centered helical ribbon impeller to provide the mixing in the z direction. A rectangular plate is placed near the wall to push the slow-moving slurry from stuck. The 3D mixer can achieve uniform mixing within 2 h, and the improvement of uniformity results in a 10 mAh/g capacity increase for lithium-nickel-manganese-cobalt oxide (NMC) cathode compared with conventional Rushton turbine mixing.
Figure 3.
Research progresses of LIB manufacturing
(A) The schematics of mixing methods: (I) modified high shear mixing equipment. Reproduced from Liu et al. (Liu et al., 2014); (II) ball milling; (III) ultrasonic mixing.
(B) The schematics of solvent-free coating methods: (I) dry pressing coating technology; (II) dry spray coating technology; (III) electrostatic coating method. Reproduced from Schälicke et al. (Schälicke et al., 2019).
(C) Innovative formation technologies: (I) Fast formation protocol by limiting the voltage window. Reprinted from Wood et al.(Wood et al., 2019). (II) Pulse current formation protocol. (III) The schematic of artificial SEI by atomic layer deposition.
Ball milling is also a common method for dry powder and slurry mixing in battery manufacturing. For the dry powder mixing, the surface energy and work of adhesion of ingredient particles plays an important role in the particle distribution. Ludwig et al. studied these surface properties of lithium cobalt oxide (LCO), conductive carbon C65, and binder PVDF (Ludwig et al., 2017). The work of adhesion between these particles during ball milling can generate C65 and PVDF agglomerates between LCO particles, which can benefit the Li+ exchange between the AMs and the electrolyte. The research for the ball milling slurry mixing of silicon-based anode showed a significant improvement in capacity retention (Chartrel et al., 2019). The 20th cycle capacity retention increased from 65% to 84% compared with conventional stirring mixing. As shown in Figure 3AII, the ball milling can effectively break down the clusters and change the particle surface morphology (Bauer et al., 2015). The modifications may benefit the electrochemistry performance with the risk of harming the structure of some particles (e.g., spherical secondary particles of layered structure cathode materials). Another way to improve the total battery manufacturing efficiency is to increase the concentration of the slurry. The decrease in solvent usage can save both the material cost and the drying time (Schünemann et al., 2016). The ultrasonic mixing allows the high concentration with its unique micro-bubble collapse and micro-turbulence (Figure 3AIII) (Monnier et al., 1999). The external-stirring-free ultrasonic mixing consumes less energy than the conventional HSM method, especially for the high-concentration slurry mixing (Kustersa et al., 1994; Nguyen et al., 2014; Kraytsberg and Ein-Eli, 2016).
The modification of mixing sequences also plays an important role in the mixing uniformity and further affects the electrode properties. Wang et al. explored the effects of different mixing sequences on the electrode structure and rate performance (Wang et al., 2020). They concluded that the distribution of conductive carbon plays the most important role in the property difference. The sequence of adding conductive carbon in the PVDF/NMP solution before the AM can achieve a porous carbon/binder network between the AM particles. On the contrary, the other sequence is to mix the carbon and AM powder first and then add the well-mixed powder in the PVDF/NMP solution and result in a carbon/binder layer on the surface of AM particles after drying. Therefore the better mixing sequence can double the ionic conductivity and 5C rate performance for the LiNi0.33Mn0.33Co0.33O2 (NMC111) electrode.
The mixing uniformity can highly affect the performance of electrodes. The modification based on the current HSM system is the most economical improvement of mixing efficiency and uniformity. Ball milling and ultrasonic mixing can significantly increase the mixing uniformity for dry powder mixing and high-concentration slurry, respectively. However, the cost and reliability of large-scale mixing need to be evaluated before they can be industrialized. Other than the mixing method, the mixing sequence also plays an important role in particle distribution. The researches based on different materials indicated that the mixing sequences could influence the electrode properties (e.g., ionic and electronic conductivity, electrode cohesion, and adhesion) and electrochemistry performance (Lee et al., 2010; Haselrieder et al., 2014; Wang et al., 2020). The basic study of particle surface energy and work of cohesion can help understand the mixing mechanism and further design the mixing technology.
Coating, drying, and solvent recovery
The coating and drying (including the solvent recovery) make up about 20% of the total manufacturing cost. The conventional coating and drying processes are connected by a roll-to-roll system. The well-mixed slurry was pumped to a slot die and coated on the surface of the current collector with a certain thickness. The coated wet electrode was then delivered to the long dryer to evaporate the solvent. The toxic and expensive NMP solvent was recovered by a condenser and then followed by a distillation process.
The drying and solvent recovery processes have the highest energy consumption (46.8%). The organic solvent NMP in cathode production (boiling point: 202°C) is the main reason for the high energy and time demand, which makes replacing or avoiding the organic solvent the most effective way to lower the energy and time consumption. The modification of drying is highly related to the coating method. Therefore, new inventions of drying technology usually come with different coating methods.
PPG Inc. replaced the organic system by applying a water-based cathode binder (Jansen et al., 2016). The aqueous slurry can be sprayed on the current collector and skip the solvent recovery process. Other water-based binders such as cellulose and lignin-based polymers are also low-cost choices, which could be easily processed from natural products (Nirmale et al., 2017). However, most of the cathode materials are sensitive to water, especially the layered oxide cathode (Park et al., 2016). Immersing the cathode materials in water can cause severe degradation because of the surface structure change and the formation of alkaline lithium compounds (Li2CO3, LiOH), which will corrode the aluminum collector. Bichon et al. controlled the pH of the slurry by adding phosphoric acid to avoid corrosion and thus alleviated the capacity fading. However, the capacity is not comparable with the organic solvent-based electrode (Bichon et al., 2019).
Reducing the amount of toxic organic solvent can be a compromised choice. The high-concentration slurry has a higher viscosity and cannot be cast by the conventional slot die. Schünemann et al. adopted the extrusion method to provide extra pressure to deposit the high-viscosity slurry on the current collector (Schünemann et al., 2016). Although this method cannot fully avoid the organic solvent, the increase of the solid content from 55% to 75% can increase the drying speed up to 80% and further improve the throughput (Haarmann et al., 2019).
Beyond these compromised ameliorations based on the conventional slurry cast technology, eliminating the toxic organic solvent can be an ultimate solution for electrode production. Maxwell Technologies Inc. (acquired by Tesla) developed a solvent-free electrode coating technology by calendering the well-mixed dry powder to form a continuous self-supporting electrode film (Duong et al., 2018). The film was then laminated onto the current collector and became the finished electrode (Figure 3BI). The 4 mAh/cm2 dry coated NMC111/graphite full cell can achieve 90% of capacity retention after 1,500 cycles, and the maximum area loading of dry coating electrode can reach 36 mg/cm2. Ludwig et al. applied electrostatic spraying and hot pressing technologies to produce solvent-free electrodes (Ludwig et al., 2016). The as-sprayed electrode was delivered to a hot roller, which can thermally activate the binder to provide enough bonding strength between the particles and the current collector (Figure 3BII). The work of cohesion/adhesion between the dry particles results in a special microstructure to allow more access surface on AM particles to contact with electrolyte. The cross-linked-like network formed by the binder and conductive carbon has been proved to increase the conductivity, lower the overall resistance, and improve the electrochemistry performance (Mayer et al., 2019). Liu et al. further developed the dry printed anode with a discontinuous PVDF interlayer to enhance the bonding strength between the electrode and collector (Liu et al., 2019). Schälicke et al. combined a fluidized bed and electrostatic system to achieve a solvent-free coating method for the graphite anode and compared the effect of different binders (tetrafluoroethylene, hexafluoropropylene vinylidene fluoride [THV], and fluorinated ethylene propylene [FEP]) (Figure 3BIII) (Schälicke et al., 2019).
Other than the innovation of the new coating and drying system, modifying the current drying method is another efficient way to lower the cost and shorten the time. Jaiser et al. invented a three-stage drying strategy, which can reduce 40% of the drying time (Jaiser et al., 2017). The initial and final high drying rate stage can save the drying time, whereas the intermediate low drying rate stage can prevent the binder from migration. To overcome the slow drying rate of the conventional air convection drying method, extra heating sources such as infrared ray and laser beam can improve the drying efficiency significantly (Pfleging, 2018). By applying a 450-W/1,070-nm fiber laser, the drying energy consumption can be halved.
Coating and drying are the key processes of electrode fabrication. The organic solvent in the slurry could increase the drying time and recovery cost significantly. The modification of the current drying method cannot directly solve the problem. However, the feasibility and low investment can be advantages. Although the aqueous-based cathode slurry is easy to be transferred to the current coating technology without extra cost, the sacrifice of capacity and cycle stability is not acceptable for battery production. Solvent-free manufacturing emerges as an effective method to skip the drying process and avoid the organic solvent. Another benefit of solvent-free manufacturing is the potential of making thick electrodes, which are challenging for the traditional slurry cast method due to the structure distortion after the solvent evaporation. Based on the calculation from Sigh et al., the cell energy density increases from 337 to 412Wh/L when the electrode thickness increased from 70 to 320 μm (Singh et al., 2015). Despite the fact that most of the solvent-free manufacturing methods face uniformity and scale-up challenges, the benefits of huge cost saving and the high efficiency still attract the industry. Tesla acquired Maxwell Technologies Inc. in 2019 and made the dry electrode manufacturing technology part of its future battery production plan (Tesla Inc, 2019). This acquisition proved the confidence in the solvent-free coating technologies from the industrial community.
Calendering
Calendering is a simple process to define the electrode's physical properties and increase the bonding strength between the electrode and the current collector. Although few studies have been done on the new calendering methods due to the low manufacturing cost (5.19% of the total cost) and mature state-of-the-art technology, the significance of calendering parameters should not be ignored. The studies showed that both calendered LiFePO4 and organic dilithium benzenediacrylate cathode had better electrochemistry performance and cycle stability than the cathodes without calendering (Oladimeji et al., 2016; Oltean et al., 2016). The swelling behavior after calendering can increase the pressure inside the cell and may cause further safety concerns. Park et al. adopted a two-step roll pressing method on graphite anode to reduce the swelling rate from 5% to 4.47% after 25 cycles (Park et al., 2019). They first used soft roll pressing to guarantee the uniform pore distribution, and the second roll pressing could adjust the electrode to target thickness and density. This two-step calendering method also improved the capacity retention from 82.77% to 85.83% for LCO/graphite full cell after 200 cycles. Meyer et al. investigated the relationship between the calendering temperature and the mechanical properties (Meyer et al., 2019a). The research showed that by applying higher rolling temperature, the electrodes could achieve lower porosity and compaction resistance and higher adhesion strength. These improvements can be explained as the thermal treatment increased the elastic deformability (decrease of the shear modulus) of the thermoplastic binder, such as PVDF (Billot et al., 2019). Similar superior mechanical properties also occurred in the solvent-free coated electrodes, which were hot calendered or hot pressed after dry deposition (Ludwig et al., 2016; Schälicke et al., 2019).
The calendering defects are easy to occur by applying incorrect parameters (Günther et al., 2019). For a better understanding of how the calendering parameters affect the electrode properties, Giménez et al. applied numerical simulation combined with experimental data to analyze the behavior of electrodes during the calendering process (Sangrós Giménez et al., 2019a). The discrete element method (DEM) proposed by them revealed the relationship of the calendering stress and the contact area between the AM and the current collector. The simulation also captured the viscoelastic response of the electrode after calendering and predicted that the elastic recovery could be up to 17% after the pressure was removed. Further than the numerical simulation, introducing artificial intelligence (AI) to modify the manufacturing parameters can be more efficient. Duquesnoy et al. transferred the experimental and characterization results to a 3D physics-based model and calculated the electrode properties (Duquesnoy et al., 2020). These calculation results worked as the developed database to train and validate the machine learning algorithm, which identifies the interdependencies between the calendering parameters and the electrode properties. For example, the tortuosity has a strong relationship with initial porosity and calendering pressure, whereas it has an inverse relationship with the amount of AM. Sangrós Giménez et al. also proposed that the ionic conductivity is related to the free surface area and the directionality of particle contacts through DEM simulations (Sangrós Giménez et al., 2019b). These studies can provide valuable references for the industry to optimize the calendering parameters to avoid the incorrect setup.
Slitting
Slitting is a step of the roll-to-roll operation to prescribe electrode width after calendering. It is a low-cost (3.09% of total cost) and high throughput (80–150 m/min) process with established techniques. The conventional slitting machine usually uses a blade or chisel depending on the electrode type and shape (Nagano Automation, 2020). Although the cost and throughput do not have much room to improve, the edge defects such as burr and dross may penetrate the separator and cause a short circuit (Lee, 2018).
Laser cutting is a wildly applied shaping technology with high flexibility. Lee et al. studied the cutting edge and width on laser cut cathode and anode with different parameters (Lee and Ahn, 2017; Lee, 2018). The laser cutting can achieve a clean edge with less deformation, and the cutting width and efficiency can be controlled by the laser power and scanning speed. Demir et al. compared the cutting efficiency and clearance width with different types of laser sources (Demir and Previtali, 2014). The result showed that the infrared fiber laser could reach 30 m/min cutting speed with only 54 W power on both the cathode and anode. The green laser can restrict the clearance width under 20 μm, which can lower the possibility of a short circuit. However, the maximum cutting speed of the precise green laser was only 4.5 m/min. Besides the edge quality, the metal spatters on the electrode surface caused by the laser cutting can be a potential concern for the uneven current density, which could lead to the growth of Li dendrite (Jansen et al., 2019).
The various battery design by the manufacturer results in the different shape and size requirement of electrodes. Laser cutting can be easily adapted to different designs without additional cost. The high initial investment may hinder the application of laser cutting from large-scale applications in the battery industry. Also, the risk for laser current is the melted metal spatters, which can be the source of internal shorting. With the development of a cheap laser source and solving the internal shorting by adjusting the process parameters, the laser cutting system for battery manufacturing has a high chance to be utilized in the future.
Vacuum drying
Vacuum drying is a necessary process with intense energy and time consumption. The reaction between the residual moisture and commercial Li salt LiPF6 can generate hydrogen fluoride (HF) gas, which can damage the AMs and cause a safety concern. These side reactions also lead to inferior electrochemistry performance (Stich et al., 2017). According to the investigations on the state-of-the-art electrodes, the graphite anode contains a much higher amount of moisture than the cathode because of the residual moisture in the aqueous CMC binder (Stich et al., 2017; Eser et al., 2019). Current drying technology usually places the electrodes under a low-pressure environment with 60°C–150°C heating for over 12 h with the option of inert gas supply. However, the lower moisture level may not always lead to better electrochemistry and mechanical properties. Huttner et al. compared the influence of different drying times and proposed a fast argon-purging post-drying method at room temperature (Huttner et al., 2019). Their results showed that although the long-term vacuum drying under high temperature (120°C) resulted in the lowest moisture level, the electrochemistry performance of the electrode was the worst. On the contrary, the electrode treated with quick argon purging (20 min at 20°C) showed the highest capacity for both rate and cycling tests. The analysis indicated that the long-term high-temperature drying could impair the bonding strength of the binder PVDF and CMC/SBR and damage the structure of the electrode. Thus the argon-purging method has the potential to replace vacuum drying with high throughput and low energy consumption advantages. The research also indicated that a relatively low moisture level (326 ppm with argon purging) could already achieve a good electrochemistry performance, and the small amount of water may improve the formation and stability of the SEI layer (Langklotz et al., 2013).
Welding
Welding only makes up 7.34% of the total manufacturing cost and consumes less than 2% of the total energy. The state-of-art welding technologies are highly automated. However, the challenges for cell tab joining have not been solved and the potential failure can cause serious safety issues. The high operating temperature (up to 80°C) of LIB especially the power battery for automotive can result in an increase of connection resistance and temperature variation, which will cause thermal expansion or even thermal fatigue and damage the tab joint (Brand et al., 2013; Zhao et al., 2014). The Electric vehicles (EVs) are usually charged under high current (250 kW for Tesla V3 supercharger) to shorten the charging time (The Tesla Team, 2019). Therefore, contact resistance plays an important role in welding quality. The high resistance not only causes energy loss but also generates much heat, which will result in cell degradation or even thermal runaway (Yang et al., 2016).
The application of welding technology depends on the cell packing method. Ultrasonic welding is widely used for pouch cell tab joining and applied for cylindrical and prismatic cells on some occasions (Zwicker et al., 2020). The resistance spot welding is also a choice for cylindrical and prismatic cells. Although ultrasonic welding has the advantages of low energy consumption and the ability to join dissimilar materials, the limitation on the joint thickness and potential high heat generation can restrict the application situation (Das et al., 2018; Zhao et al., 2013). Resistance welding has low cost and thermal input. However, the disadvantages include the difficulty to join highly conductive and dissimilar materials (Das et al., 2018).
To evaluate the potential choice of battery welding, Brand et al. compared laser welding with ultrasonic welding and resistance spot welding (Brand et al., 2015). The result showed that laser welding had the lowest contact resistance and highest tensile strength. However, the challenges for joining dissimilar and high reflective materials restrict the application of laser welding. Heinemann et al. proposed to overcome these challenges by reducing the wavelength and shortening the pulse width to the nanoscale (Heinemann et al., 2018). Tesla applied a wire bonding method to connect the cylindrical cells to the bus bar on Model S (Kohngene et al., 2011). The aluminum wire was joined to the cell by ultrasonic wedge welding with low energy consumption. The ultrasonic wedge welding is only suitable for thin wires, which results in low joint strength. Recently Tesla released its new 4,680 cells with “tabless” technology (Tsuruta et al., 2020). Other than using the welded electrode tab to connect the electrodes and the cells, the uncoated bottom edge of the electrode work as the “tab” to connect with the specially designed multi-contact area case bottom. The increased contact area can lower the cell impedance and the heat generated by charge/discharge current significantly. The saving from the electrode tabs and the larger cell size also results in a 16% increase in energy density. This technology jumps out of the traditional rules of tab welding and has the potential to be a game-changing invention for fast-charging batteries due to the high market share of Tesla.
Because of the low cost and energy consumption of welding in the total manufacturing process, the current research on battery welding technology mainly focuses on evaluating the existing welding method rather than developing anything new. Although advanced technology like laser welding showed better performance compared with conventional welding methods, it currently only has the potential for high-end products due to cost concerns. The welding technology is highly related to the cell packaging design, which changes quickly with the requirements of customers. This situation makes the development of welding technology more difficult. A unified industry standard for battery packaging design can significantly help the research on the welding technology.
Formation and aging
In the state-of-the-art battery, the intercalation potential for anode material graphite (0–0.25 V versus Li+/Li) is lower than the reduction potential of commercial electrolyte (about 1 V versus Li+/Li) (An et al., 2016). Therefore during the formation and aging process, the electrolyte will decompose and form the SEI layer on the surface of the anode. If the formation current or temperature is too high, the porous SEI layer cannot prevent contact between the electrolyte and anode surface (Bhattacharya and Alpas, 2012). The continuous electrolyte decomposition will consume limited lithium from electrolyte and cathode and cause capacity degradation. The dense and stable SEI layer usually requires multiple low rate charge and discharge formation cycles. These slow formation steps can significantly increase the cost of capital investment and consume more labor and space resources. The formation and aging process makes up 32% of the total cost and can take up to 3 weeks to finish. The acceleration of formation will be eagerly embraced by the battery industry. However, the accelerated formation step cannot sacrifice battery performance. The most direct way to reduce the formation time is to increase the formation C rate. The formation current has a high impact on the microstructure and morphology of the SEI layer and simply increasing the current will result in an ununiformed SEI layer (Bhattacharya and Alpas, 2012). The high current formation can also result in Li plating on the surface of graphite due to the high polarization and cause a safety concern (Mao et al., 2018).
From the consideration of cost saving, decreasing the formation voltage window and increasing the formation current could be the most feasible way. Lee et al. proposed a formation formula that decreasing the upper cutoff voltage from 4.2 to 3.7 V can halve the formation time of LCO/graphite cell (Lee et al., 2004). They claimed that the formation of SEI was mostly completed before 3.7 V by comparing the cycling performance of different cutoff voltage cells. The electrochemistry performance showed no significant difference in the cycling stability and coulombic efficiency between the cells with fast formation (2.7–3.7 V) and the control group (2.7–4.2 V). On the contrary, Wood et al. found that the major formation reactions of the SEI layer happened between the high state of charge (SOC) ranges (high-voltage charge and discharge), and the SEI formed at high SOC is more compact and stable (An et al., 2017). Therefore repeating the high SOC charge and discharge between 3.9 and 4.2 V can achieve a stable SEI with shorter formation time without compromising the electrochemistry performance, as shown in Figure 3CI. Their fast formation protocol for NMC/graphite pouch cells can make the formation process 8.5 times faster than the conventional 3-cycle 0.05 C charge and discharge and only takes about 14 h.
Besides the formation strategies with direct current, the pulse current charging with a specific frequency provides a short rest time between the reactions and can eliminate the concentration polarization at the electrolyte-anode interface (Figure 3CII) (Li et al., 2001). The application for pulse current charging allows a higher charging rate in the formation process and thus reduces the formation time (Mckinley et al., 2010). The pulse current charging strategy can also alleviate the capacity fading and restrain the heat generation (Lv et al., 2020). However, the pulse current and frequency need to be customized for different cells. An unsuitable pulse current parameter can cause capacity degradation or even damage the battery (Majid et al., 2017).
Jumping out of the complex compositions and unclear forming mechanism of the SEI layer, the inert artificial SEI layer is a potential solution to accelerate the formation process. The dense and stable post-generated SEI layer can replace the electrochemistry-formed SEI and run the formation cycles at a higher rate. Wang et al. used atomic layer deposition (ALD) to deposit TiO2 thin film on the surface of graphite (Figure 3CIII) (Wang and Wang, 2013). The artificial SEI layer showed better electrochemistry and thermal stability than that of the SEI formed during electrochemistry formation. However, the artificial SEI layer usually introduces additional costs and is hard to be applied to high-volume-driven situation, which makes it hard to be applied on LIB manufacturing.
For the industrial level of cell production, practical challenges such as electrolyte wetting can be amplified due to the lower electrolyte/materials ratio. For a better understanding of electrolyte wetting conditions, precise characterizations are eagerly required. With the help of neutron radiography, Günter et al. visualized the electrolyte distribution in pouch cells and proposed a pressure cycle wetting procedure to accelerate the electrolyte wetting (Günter et al., 2019). To achieve a lower cost and better visualization, Deng et al. applied ultrasonic scanning to observe the electrolyte distribution during the formation and aging process (Deng et al., 2020). Depending on the characterization ability of gas generation by the ultrasonic scanning, they discovered that higher formation and aging temperature could exacerbate the electrolyte decomposition and lead to excessive SEI growth. A similar conclusion was drawn by Heimes et al. that the elevated ambient temperature during formation could only provide a limited reduction in the formation duration time (Heimes et al., 2019b). Based on their investigation, the mechanical load on the LIB cells showed more improvement in duration reduction (about 20%). In addition, the combination of high mechanical loading and temperature could prevent exothermic reactions.
The formation and aging process is important for battery manufacturing because of not only the high cost and time demand but also the tight relationship with battery degradation and safety issues. The complex composites and formation mechanism of SEI are the biggest challenges for the development of new formation and aging technology. With a better understanding of the SEI formation mechanism, formation between narrow voltage ranges can be more reliable and suitable for different AMs and electrolyte. The artificial SEI coating can avoid the uncertainty caused by the SEI layer effectively. However, the current coating method such as ALD is difficult to be scaled up and will introduce an extra cost. The innovative characterization methods have the potential to increase the quality control (QC) efficiency significantly and can verify the influence of ambient parameters. Adjusting the ambient parameters such as thermal and mechanical loads (especially for the pouch cells without metal can) can help to reduce the formation time, although the reduction is limited.
Conclusion and perspectives
It is certain that LIBs will be widely used in electronics, EVs, and grid storage. Both academia and industries are pushing hard to further lower the cost and increase the energy density for LIBs. Compared with the very dynamic research on different materials in the LIB field, the research and development of manufacturing technologies lack impactful progress. From the analysis of different manufacturing steps, it is clearly shown that the steps of formation and aging (32.16%), coating and drying (14.96%), and enclosing (12.45%) are the top three contributors to the manufacturing cost of LIBs; formation and aging (1.5–3 weeks), vacuum drying (12–30 h), and slurry mixing (30 min–5 h) contribute the most in the production time; drying and solvent recovery (46.84%) and dry room (29.37%) contribute the most in energy consumption. Innovations of these steps make great impacts on LIB manufacturing, although other manufacturing steps are also important.
Table 2 is the summary of different manufacturing processes with associated methods, significance, and challenges. However, most manufacturing innovations have been reported with very limited adoption by the industry. The most notable case is that Tesla acquired Maxwell and announced to use the dry manufacturing technology in its battery fabrication. We hope that such a perspective can spark the manufacturing innovations that will be applied in the LIB industry. We see the following research is especially needed, which could make significant impacts on the future manufacturing of LIBs.
-
1
Research on different mixing technology from other manufacturing fields is needed. These studies provide the industry with more options to prepare the slurry based on their materials recipe. Besides, the fundamental research on the mixing sequences and particle behavior can provide important references to improving the mixing uniformity and efficiency. We recommend the manufacturers establish the proper mixing sequence for their electrode formula because it is a cost-effective way to improve electrode uniformity, yield, and performance.
-
2
Coating and drying processes have been researched the most. The concept of the solvent-free coating can eliminate the drying step, which is an energy- and time-consuming process. Unlike most laboratory research, either dry spraying or dry calendering technology has the potential to be scaled up and transferred to a roll-to-roll production system. The dry coating technology can currently reach pilot-scale production. With the help of industrial expertise, it has the potential to be industrialized.
-
3
Contrary to the high capital investment and time demand, the research on the formation and aging is limited. Thus, the improvement of the formation and aging technology is exigent. Narrowing the voltage range of the formation cycle can reduce the formation time effectively. However, the research results on the formation voltage window remain controversial (An et al., 2017; Lee et al., 2004). The unclear forming mechanism and composition of SEI are the most challenging obstacles for the study of formation and aging. With the development of characterization techniques, recent research analyzed the formation of the SEI layer on the surface of Li metal and Si nanoparticles by cryogenic electron microscopy (Fang et al., 2019; Huang et al., 2019). These advanced characterization methods could also be applied to the commercial battery system and contribute to the development and verification of formation technology. However, the artificial SEI coating technology remains at the laboratory-scale level and has difficulty moving to the next level due to the cost. The decrease of ALD cost and the industrialization of Si anode (with nearly 10 times of the capacity than graphite) provides the potential for low-cost artificial SEI products. As the main contents of SEI are the decomposition products of electrolyte, the study of the electrolyte system can help form a stable SEI layer in a shorter formation time. The high-concentration electrolyte (>3 M salt) can enable a stable anion-derived SEI layer (Yamada et al., 2019). However, the high cost and viscosity prevent it from practical application. Recently, Yao et al. invented a special weakly solvating electrolyte (WSE), which leads to an anion-derived, inorganic-rich SEI layer (Yao et al., 2020). The limited voltage window and the high melting point of 1,4-dioxane solvent may restrict the compatibility of the WSE electrolyte. Therefore the research on the new electrolyte system has the potential to reduce the formation time and increase the electrochemistry performance at the same time.
-
4
Besides the cell manufacturing, “macro”-level manufacturing from cell to battery system could affect the final energy density and the total cost, especially for the EV battery system. The energy density of the EV battery system increased from less than 100 to ∼200 Wh/kg during the past decade (Löbberding et al., 2020). However, the potential for battery integration technology has not been depleted. Increasing the size and capacity of the cells could promote the energy density of the battery system, such as Tesla 4680 cylindrical cells and BMW 120 Ah prismatic cells. Looking forward to the future EV requirement, new strategies like the “cell to pack” design proposed by CATL and BYD's blade battery set are also following the trend to further reduce the space of packing materials (Byd Co Ltd, 2020; Contemporary Amperex Technology Co. Limited, 2020). These innovations are based on the progress of higher electrode uniformity and better battery management system. Although the battery manufacturers are rolling out the new designs, the “macro”-level manufacturing research in the academic field is not common. The limited resources and space in the laboratory restrict the research activity on the battery system. Therefore, more collaboration between academic researchers and battery manufacturers could help the development of battery systems.
-
5
Recycling becomes an inevitable topic with the surging of LIB manufacturing capacity. Battery recycling technology has been widely studied in recent years, which mainly focuses on material recovery (Chen et al., 2019; Ma et al., 2019). The manufacturing processes could play a big role in recycling and need to be studied. For example, the manufacturing scrap could be integrated by the “short-loop” recycling, which recycles the useful materials directly and puts them back to the manufacturing stream. The physical powder separation method demonstrated by Jafari et al. has the potential to improve the recovery efficiency, especially for the electrode scrap abandoned during slitting (Jafari et al., 2020). However, there is damage to the cathode powder, which needs to be overcome. As the materials are not cycled, solid-state treatment could be the best option to recover the performance with minimum time and cost (Li et al., 2017; Song et al., 2017; Shi et al., 2018). To recycle the end-of-life batteries, disassembling the battery packs and cells is a labor- and energy-intensive process. Although the researchers have studied different automatic disassembly systems and even introduce robots to increase the disassembly efficiency, the various battery, pack, and module designs are still hindering the development of high-efficiency recycling (Herrmann et al., 2014; Wegener et al., 2015; Waldmann et al., 2016). The recycling convenience should be considered when the manufacturer designs the battery shell, pack, and module.
-
6
Quality control is an important step run through almost all the LIB manufacturing steps. The characterization methods can help to detect the defects early and prevent waste in the following steps (Deng et al., 2020). However, it is hard to estimate the QC fail rate for the manufacturing innovations. The novel manufacturing concepts are usually in an early stage that can only operate on a small scale. Therefore, estimating the production quality with the help of modeling and other statistical methods could solve this problem to a certain extent (Meyer et al., 2019b). The participation of industrial experience can also support the scale-up process for the laboratory-born manufacturing technology to lower the QC fail rate. Moreover, it is meaningful to consider the QC fail rate at the beginning of the research for the novel manufacturing technology, which is usually practical oriented.
-
7
AI technology on battery manufacturing needs more research. The application of AI technology has been spotlighted in battery research (Aykol et al., 2020). With the help of machine learning technology, screening materials such as solid electrolyte candidates no longer need complex experimental attempts (Ahmad et al., 2018; Sendek et al., 2018). The prediction of battery life and degradation is possible with the involvement of the AI, and when combined with a Bayesian optimization algorithm, the AI can explore the parameters for fast charging efficiently (Pan et al., 2018; Severson et al., 2019; Attia et al., 2020; Bhowmik and Vegge, 2020). The AI technology also expanded to the manufacturing field. The data-driven methods can help evaluate the manufacturing processes and optimize quality control (Meyer et al., 2019b; Turetskyy et al., 2019). The analysis of manufacturing energy efficiency by the machine learning approach provided the improvement potentials for the battery industry, and the perspective on the inverse design of the SEI layer by deep learning may help the development of formation technology (Bhowmik et al., 2019; Thiede et al., 2020). However, compared with the rapidly growing trend of AI application on the materials innovation and battery state of health and life prediction fields, the AI study on the manufacturing processes and commercialized battery materials is lacking. As a high efficiency and precision tool, AI technology could be the key factor in developing the next generation of battery technology and accelerate smart manufacturing.
-
8
The collaboration between the industry and academia has led to many exciting achievements. The evolution of the cathode materials in the industry especially in EVs is being processed at a surprising speed. We can see the ambition of Tesla to develop its own materials through a close partnership with Prof. Jeff Dahn from the University of Dalhousie. There are similar efforts between EV companies and national laboratories or universities. However, most of the collaborations between the industry and academia focus on materials development and beyond LIB technology. Although different battery manufacturing innovations have been proposed and developed in academia, very few can be adopted by the industry due to various reasons (e.g., cost, reliability, scalability, etc.). It is understandable that the risks of adopting new manufacturing technologies with low technology readiness levels may be high. Therefore, instead of adopting the new manufacturing technologies directly, more effective collaborations on the scale-up process could help both research and industrial ends.
-
9
Manufacturing research for SSBs is needed. The risks of shorting and fire caused by flammable organic electrolyte are the major concerns from the customers, especially in the EV market. Although the compromise of electrochemistry performance hinders the commercialization of SSBs, the advantages of safety and energy density make it one of the most promising beyond lithium-ion technologies. For most of SSB (sulfides and oxides) manufacturing, the solvent is not included but reactive thin Li foil becomes part of the bill of materials, which means the state-of-the-art LIBs electrode manufacturing technologies are no longer compatible with the SSBs. Specific manufacturing methods are required to be developed despite the SSB system being not currently mature. Researchers such as Laue et al. started to develop specific manufacturing methods like the mixing strategies for SSBs through modeling (Laue et al., 2019). However, some of the innovative manufacturing technologies we present in this perspective such as solvent-free coating methods have the potential to be transferred to the SSB manufacturing. Therefore, when evaluating the new manufacturing technologies, transferability to beyond LIB manufacturing should be considered.
Table 2.
Summary of different manufacturing processes with methods, significance, and challenges
| Manufacturing processes | Developed method | Significance | Challenge |
|---|---|---|---|
| Mixing | 3D hydrodynamic shear mixing | Low cost, easy to scale-up | Limited improvement |
| Ball milling | High efficiency, good uniformity | The risk of damaging the structure of active materials particles | |
| Ultrasonic mixing | High efficiency especially for high concentration slurry | Instrumental cost and hard to scale-up | |
| Different mixing sequence | Improve the uniformity without marginal modifications | Need more study for different materials | |
| Coating/drying | Low solvent content extrusion | Lower the drying time and energy consumption | Cannot avoid the toxic organic solvent and potential instrumental investment |
| Dry calendering | Save the drying time and energy | Potential instrumental investment; scale-up ability | |
| Dry printing | Potential instrumental investment, scale-up ability, and spray uniformity | ||
| Electrostatic coating | Potential instrumental investment, scale-up ability, and coating uniformity | ||
| Three-stage drying | Lower the drying time without extra instrument cost | Limited improvement | |
| Laser annealing | Lower the drying time and energy consumption | ||
| Infrared heating | |||
| Slitting | Laser cutting | Improve the cutting quality and flexibility for different shape design | Potential instrumental investment and relatively low throughput |
| Vacuum drying | Argon purging | High throughput and room-temperature operation | The efficiency after scale-up needs to be verified |
| Welding | Laser welding | Low contact resistance and high tensile force | Difficulty to join dissimilar and high-reflective materials |
| Wire bonding | Low energy consumption | Only feasible for cylindrical cell | |
| Formation | Narrow the voltage window | Save the formation time without extra cost | Mechanism still unclear |
| Pulse current charging | Save the formation time, low cost | Specific frequency needs to be discovered for different types of cells | |
| Artificial SEI layer | Save the formation time | Potential instrumental investment and scale-up ability |
Although the invention of new battery materials leads to a significant decrease in the battery cost, the US DOE ultimate target of $80/kWh is still a challenge (U.S. Department Of Energy, 2020). The new manufacturing technologies such as high-efficiency mixing, solvent-free deposition, and fast formation could be the key to achieve this target. Besides the upgrading of battery materials, the potential of increasing the energy density from the manufacturing end starts to make an impact. The thick electrodes, larger cell design, compact modules, and other manufacturing innovations provide a practical way to build a higher energy battery system with limited volume and weight. Besides these positive trends, a stronger collaboration between academia and industry is pivotal to make EV more affordable and increase market penetration. The policymakers and investors can also play an important role to transform the battery industry and encourage innovation in manufacturing technology. In conclusion, to achieve a low-cost and high-efficiency LIB manufacturing system, the research on the new manufacturing technology and the study on the fundamental problems during manufacturing are indispensable and more efforts from the multiple communities are needed.
Limitations of the study
In Figure 2, the numbers were calculated based on the references, which obtained the data from simulation or pilot-scale manufacturing facility (may not represent the industrial benchmarks).
Acknowledgments
Author contributions
Writing – original draft, Y.L.; writing – review & editing, R.Z., J.W., and Y.W.; supervision, Y.W.
Declaration of interests
The authors declare no competing interests.
The views expressed in the paper are those of the authors, not their organizations.
References
- Ahmad Z., Xie T., Maheshwari C., Grossman J.C., Viswanathan V. Machine learning enabled computational screening of inorganic solid electrolytes for suppression of dendrite formation in lithium metal anodes. ACS Cent. Sci. 2018;4:996–1006. doi: 10.1021/acscentsci.8b00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Nelson P.A., Gallagher K.G., Dees D.W. Energy impact of cathode drying and solvent recovery during lithium-ion battery manufacturing. J. Power Sources. 2016;322:169–178. [Google Scholar]
- An S.J., Li J., Daniel C., Mohanty D., Nagpure S., Wood D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon. 2016;105:52–76. [Google Scholar]
- An S.J., Li J., Du Z., Daniel C., Wood D.L. Fast formation cycling for lithium ion batteries. J. Power Sources. 2017;342:846–852. [Google Scholar]
- Attia P.M., Grover A., Jin N., Severson K.A., Markov T.M., Liao Y.H., Chen M.H., Cheong B., Perkins N., Yang Z. Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature. 2020;578:397–402. doi: 10.1038/s41586-020-1994-5. [DOI] [PubMed] [Google Scholar]
- Aykol M., Herring P., Anapolsky A. Machine learning for continuous innovation in battery technologies. Nat. Rev. Mater. 2020;5:725–727. [Google Scholar]
- Bauer W., Nötzel D., Wenzel V., Nirschl H. Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries. J. Power Sources. 2015;288:359–367. [Google Scholar]
- Bhattacharya S., Alpas A.T. Micromechanisms of solid electrolyte interphase formation on electrochemically cycled graphite electrodes in lithium-ion cells. Carbon. 2012;50:5359–5371. [Google Scholar]
- Bhowmik A., Vegge T. AI fast track to battery fast charge. Joule. 2020;4:717–719. [Google Scholar]
- Bhowmik A., Castelli I.E., Garcia-Lastra J.M., Jørgensen P.B., Winther O., Vegge T. A perspective on inverse design of battery interphases using multi-scale modelling, experiments and generative deep learning. Energy Storage Mater. 2019;21:446–456. [Google Scholar]
- Bichon M., Sotta D., Dupre N., De Vito E., Boulineau A., Porcher W., Lestriez B. Study of immersion of LiNi0.5Mn0.3Co0.2O2 material in water for aqueous processing of positive electrode for Li-ion batteries. ACS Appl. Mater. Interfaces. 2019;11:18331–18341. doi: 10.1021/acsami.9b00999. [DOI] [PubMed] [Google Scholar]
- Billot N., Günther T., Schreiner D., Stahl R., Kranner J., Beyer M., Reinhart G. Investigation of the adhesion strength along the electrode manufacturing process for improved lithium-ion anodes. Energy Technol. 2019;8:1801136. [Google Scholar]
- Bockholt H., Indrikova M., Netz A., Golks F., Kwade A. The interaction of consecutive process steps in the manufacturing of lithium-ion battery electrodes with regard to structural and electrochemical properties. J. Power Sources. 2016;325:140–151. [Google Scholar]
- Brand M., Gläser S., Geder J., Menacher S., Obpacher S., Jossen A., Quinger D. Electrical safety of commercial Li-ion cells based on NMC and NCA technology compared to LFP technology. World Electric Vehicle J. 2013;6:572–580. [Google Scholar]
- Brand M.J., Schmidt P.A., Zaeh M.F., Jossen A. Welding techniques for battery cells and resulting electrical contact resistances. J. Energy Storage. 2015;1:7–14. [Google Scholar]
- Byd Co Ltd Byd's new blade battery set to redefine ev safety standards. 2020. https://www.byd.com/en/news/2020-03-30/BYD%27s-New-Blade-Battery-Set-to-Redefine-EV-Safety-Standards
- Chartrel T., Ndour M., Bonnet V., Cavalaglio S., Aymard L., Dolhem F., Monconduit L., Bonnet J.-P. Revisiting and improving the preparation of silicon-based electrodes for lithium-ion batteries: ball milling impact on poly(acrylic acid) polymer binders. Mater. Chem. Front. 2019;3:881–891. [Google Scholar]
- Chen M., Ma X., Chen B., Arsenault R., Karlson P., Simon N., Wang Y. Recycling end-of-life electric vehicle lithium-ion batteries. Joule. 2019;3:2622–2646. [Google Scholar]
- Contemporary Amperex Technology Co. Limited CTP Module-free. 2020. https://www.catl.com/en/research/technology/ 2.
- Das A., Li D., Williams D., Greenwood D. Joining technologies for automotive battery systems manufacturing. World Electric Vehicle J. 2018;9:22. [Google Scholar]
- Demir A.G., Previtali B. Remote cutting of Li-ion battery electrodes with infrared and green ns-pulsed fibre lasers. Int. J. Adv. Manufacturing Technol. 2014;75:1557–1568. [Google Scholar]
- Deng Z., Huang Z., Shen Y., Huang Y., Ding H., Luscombe A., Johnson M., Harlow J.E., Gauthier R., Dahn J.R. Ultrasonic scanning to observe wetting and “Unwetting” in Li-ion pouch cells. Joule. 2020;4:2017–2029. [Google Scholar]
- Duong, H., Shin, J. & Yudi, Y. 2018. Dry electrode coating technology. 48th Power Sources Conference.
- Duquesnoy M., Lombardo T., Chouchane M., Primo E.N., Franco A.A. Data-driven assessment of electrode calendering process by combining experimental results, in silico mesostructures generation and machine learning. J. Power Sources. 2020;480:229103. [Google Scholar]
- Economic Research Institute Factory worker salary. 2020. https://www.erieri.com/salary/job/factory-worker/united-states
- Eser J.C., Wirsching T., Weidler P.G., Altvater A., Börnhorst T., Kumberg J., Schöne G., Müller M., Scharfer P., Schabel W. Moisture adsorption behavior in anodes for Li-ion batteries. Energy Technol. 2019;8:1801162. [Google Scholar]
- Fang C., Li J., Zhang M., Zhang Y., Yang F., Lee J.Z., Lee M.H., Alvarado J., Schroeder M.A., Yang Y. Quantifying inactive lithium in lithium metal batteries. Nature. 2019;572:511–515. doi: 10.1038/s41586-019-1481-z. [DOI] [PubMed] [Google Scholar]
- Günter F.J., Rössler S., Schulz M., Braunwarth W., Gilles R., Reinhart G. Influence of the cell format on the electrolyte filling process of lithium-ion cells. Energy Technol. 2019;8:1801108. [Google Scholar]
- Günther T., Schreiner D., Metkar A., Meyer C., Kwade A., Reinhart G. Classification of calendering-induced electrode defects and their influence on subsequent processes of lithium-ion battery production. Energy Technol. 2019;8:1900026. [Google Scholar]
- Haarmann M., Haselrieder W., Kwade A. Extrusion-based processing of cathodes: influence of solid content on suspension and electrode properties. Energy Technol. 2019;8:1801169. [Google Scholar]
- Haselrieder W., Ivanov S., Tran H.Y., Theil S., Froböse L., Westphal B., Wohlfahrt-Mehrens M., Kwade A. Influence of formulation method and related processes on structural, electrical and electrochemical properties of LMS/NCA-blend electrodes. Prog. Solid State Chem. 2014;42:157–174. [Google Scholar]
- Heimes H., Kampker A., Lienemann C., Locke M., Offermanns C. VDMA Battery Production; 2019. Lithium-ion Battery Cell Production Process. ISBN: 978-3-947920-03-7, https://www.researchgate.net/publication/330902286_Lithium-ion_Battery_Cell_Production_Process. [Google Scholar]
- Heimes H.H., Offermanns C., Mohsseni A., Laufen H., Westerhoff U., Hoffmann L., Niehoff P., Kurrat M., Winter M., Kampker A. The effects of mechanical and thermal loads during lithium-ion pouch cell formation and their impacts on process time. Energy Technol. 2019;8:1900118. [Google Scholar]
- Heinemann S.W., Kaierle S., Dold E.-M., Faisst B., Kirchhoff M., Hesse T., Kaiser E., Gabzdyl J., Pantsar H. High-Power Laser Materials Processing: Applications, Diagnostics, and Systems VII. Vol. 10525. International Society for Optics and Photonics; 2018. New welding techniques and laser sources for battery welding. [Google Scholar]
- Herrmann C., Raatz A., Andrew S., Schmitt J. Scenario-based development of disassembly systems for automotive lithium ion battery systems. Adv. Mater. Res. 2014;907:391–401. [Google Scholar]
- Huang W., Wang J., Braun M.R., Zhang Z., Li Y., Boyle D.T., Mcintyre P.C., Cui Y. Dynamic structure and chemistry of the silicon solid-electrolyte interphase visualized by cryogenic electron microscopy. Matter. 2019;1:1232–1245. [Google Scholar]
- Huttner F., Haselrieder W., Kwade A. The influence of different post-drying procedures on remaining water content and physical and electrochemical properties of lithium-ion batteries. Energy Technol. 2019;8:1900245. [Google Scholar]
- Jafari M., Torabian M.M., Bazargan A. A facile chemical-free cathode powder separation method for lithium ion battery resource recovery. J. Energy Storage. 2020;31:101564. [Google Scholar]
- Jaiser S., Friske A., Baunach M., Scharfer P., Schabel W. Development of a three-stage drying profile based on characteristic drying stages for lithium-ion battery anodes. Drying Technol. 2017;35:1266–1275. [Google Scholar]
- Jansen T., Kandula M.W., Blass D., Hartwig S., Haselrieder W., Dilger K. Evaluation of the separation process for the production of electrode sheets. Energy Technol. 2019;8:1900519. [Google Scholar]
- Jansen A.N., Trask S.E., Polzin B.J., Lu W., Feridun O.K., Krumdick G.K., Hellring S.D., Stewart M., Kornish B. New aqueous binders for lithium-ion batteries. In: U. S. D. O. E, editor. DOE. Argonne National Lab.(ANL); 2016. pp. 7–20. [Google Scholar]
- Kohngene S., Berdichevskybrian, Hewett C. 2011. Tunable Frangible Battery Pack System. US patent number: US20080241667A1, https://patents.google.com/patent/US20080241667A1/en. [Google Scholar]
- Kraytsberg A., Ein-Eli Y. Conveying advanced Li-ion battery materials into practice the impact of electrode slurry preparation skills. Adv. Energy Mater. 2016;6:1600655. [Google Scholar]
- Kustersa K.A., Pratsinisa S.E., Thomab S.G., Smith D.M. Energy—size reduction laws for ultrasonic fragmentation. Powder Technol. 1994;80:253–263. [Google Scholar]
- Kwade A., Haselrieder W., Leithoff R., Modlinger A., Dietrich F., Droeder K. Current status and challenges for automotive battery production technologies. Nat. Energy. 2018;3:290–300. [Google Scholar]
- Langklotz U., Schneider M., Michaelis A. Water uptake of tape-cast cathodes for lithium ion batteries. J. Ceram. Sci. Technol. 2013;4:69–76. [Google Scholar]
- Laue V., Wolff N., Röder F., Krewer U. Modeling the influence of mixing strategies on microstructural properties of all-solid-state electrodes. Energy Technol. 2019;8:1801049. [Google Scholar]
- Lee D. Investigation of physical phenomena and cutting efficiency for laser cutting on anode for Li-ion batteries. Appl. Sci. 2018;8:266. [Google Scholar]
- Lee D., Ahn S. Investigation of laser cutting width of LiCoO2 coated aluminum for lithium-ion batteries. Appl. Sci. 2017;7:914. [Google Scholar]
- Lee H.-H., Wang Y.-Y., Wan C.-C., Yang M.-H., Wu H.-C., Shieh D.-T. A fast formation process for lithium batteries. J. Power Sourc. 2004;134:118–123. [Google Scholar]
- Lee G.-W., Ryu J.H., Han W., Ahn K.H., Oh S.M. Effect of slurry preparation process on electrochemical performances of LiCoO2 composite electrode. J. Power Sourc. 2010;195:6049–6054. [Google Scholar]
- Li J., Murphy E., Winnick J., Kohl P.A. The effects of pulse charging on cycling characteristics of commercial lithium-ion batteries. J. Power Sourc. 2001;102:302–309. [Google Scholar]
- Li X., Zhang J., Song D., Song J., Zhang L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO 4 batteries. J. Power Sourc. 2017;345:78–84. [Google Scholar]
- Li T., Zhang X.-Q., Shi P., Zhang Q. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule. 2019;3:2647–2661. [Google Scholar]
- Liu D., Chen L.-C., Liu T.-J., Fan T., Tsou E.-Y., Tiu C. An effective mixing for lithium ion battery slurries. Adv. Chem. Eng. Sci. 2014;04:515–528. [Google Scholar]
- Liu J., Ludwig B., Liu Y., Pan H., Wang Y. Strengthening the electrodes for Li-ion batteries with a porous adhesive interlayer through dry-spraying manufacturing. ACS Appl. Mater. Interfaces. 2019;11:25081–25089. doi: 10.1021/acsami.9b03020. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Zheng Z., Shou W., Wang Y., Pan H. Solvent-free manufacturing of electrodes for lithium-ion batteries. Sci. Rep. 2016;6:23150. doi: 10.1038/srep23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbberding H., Wessel S., Offermanns C., Kehrer M., Rother J., Heimes H., Kampker A. From cell to battery system in BEVs: analysis of system packing efficiency and cell types. World Electric Vehicle J. 2020;11:77. [Google Scholar]
- Ludwig B., Liu J., Chen I.-M., Liu Y., Shou W., Wang Y., Pan A.H. Understanding interfacial-energy-driven dry powder mixing for solvent-free additive manufacturing of li-ion battery electrodes. Adv. Mater. Interfaces. 2017;4:1700570. [Google Scholar]
- Lv H., Huang X., Liu Y. Analysis on pulse charging–discharging strategies for improving capacity retention rates of lithium-ion batteries. Ionics. 2020;26:1749–1770. [Google Scholar]
- Ma X., Chen M., Chen B., Meng Z., Wang Y. High-performance graphite recovered from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2019;7:19732–19738. [Google Scholar]
- Majid N., Hafiz S., Arianto S., Yuono R.Y., Astuti E.T., Prihandoko B. Analysis of effective pulse current charging method for lithium ion battery. J. Phys. Conf. Ser. 2017;817:012008. [Google Scholar]
- Mao C., An S.J., Meyer H.M., Li J., Wood M., Ruther R.E., Wood D.L. Balancing formation time and electrochemical performance of high energy lithium-ion batteries. J. Power Sourc. 2018;402:107–115. [Google Scholar]
- Mayer J.K., Almar L., Asylbekov E., Haselrieder W., Kwade A., Weber A., Nirschl H. Influence of the carbon black dispersing process on the microstructure and performance of Li-ion battery cathodes. Energy Technol. 2019;8:1900161. [Google Scholar]
- Mckinley, J.P., Sellers, S.A. & Colclazier, K.R. 2010. Battery formation and charging system and method. 12/604,234. US patent number: US20100164437A1, https://patents.google.com/patent/US20100164437A1/en.
- Meyer C., Weyhe M., Haselrieder W., Kwade A. Heated calendering of cathodes for lithium-ion batteries with varied carbon black and binder contents. Energy Technol. 2019;8:1900175. [Google Scholar]
- Meyer O., Weihs C., Mähr S., Tran H.-Y., Kirchhof M., Schnackenberg S., Neuhaus-Stern J., Rößler S., Braunwarth W. Development and implementation of statistical methods for quality optimization in the large-format lithium-ion cells production. Energy Technol. 2019;8:1900244. [Google Scholar]
- Mizushima K., Jones P.C., Wiseman P.J., Goodenough J.B. LixCoO2 (0<x<-1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 1980;15:783–789. [Google Scholar]
- Monnier H., Wilhelm A.M., Delmas H. The influence of ultrasound on micromixing in a semi-batch reactor. Chem. Eng. Sci. 1999;54:2953–2961. [Google Scholar]
- Nagano Automation Electrode cutting machine for lithium-ion secondary batteries. 2020. https://www.nagano-automation.co.jp/en/implementation/1705/https://www.nagano-automation.co.jp/en/implementation/1705/
- Nelson P.A., Ahmed S., Gallagher K.G., Dees D.W. Modeling the performance and cost of lithium-ion batteries for electric-drive vehicles. In: U. S. D. O, editor. Energy. Third Edition. Argonne National Lab.(ANL); 2019. pp. 82–110. [Google Scholar]
- Nguyen V.S., Rouxel D., Vincent B. Dispersion of nanoparticles: from organic solvents to polymer solutions. Ultrason. Sonochem. 2014;21:149–153. doi: 10.1016/j.ultsonch.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Nirmale T.C., Kale B.B., Varma A.J. A review on cellulose and lignin based binders and electrodes: small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017;103:1032–1043. doi: 10.1016/j.ijbiomac.2017.05.155. [DOI] [PubMed] [Google Scholar]
- Oladimeji C.F., Moss P.L., Weatherspoon M.H. Analyses of the calendaring process for performance optimization of Li-ion battery cathode. Adv. Chem. 2016;2016:1–7. [Google Scholar]
- Oltean V.A., Renault S., Brandell D. Enhanced performance of organic materials for lithium-ion batteries using facile electrode calendaring techniques. Electrochem. Commun. 2016;68:45–48. [Google Scholar]
- Padhi A.K., Nanjundaswamy K.S., Goodenough J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997;144:1188–1194. [Google Scholar]
- Pan H., Lü Z., Wang H., Wei H., Chen L. Novel battery state-of-health online estimation method using multiple health indicators and an extreme learning machine. Energy. 2018;160:466–477. [Google Scholar]
- Park J.-H., Park J.-K., Lee J.-W. Stability of LiNi0.6Mn0.2Co0.2O2as a cathode material for lithium-ion batteries against air and moisture. Bull. Korean Chem. Soc. 2016;37:344–348. [Google Scholar]
- Park K., Myeong S., Shin D., Cho C.-W., Kim S.C., Song T. Improved swelling behavior of Li ion batteries by microstructural engineering of anode. J. Ind. Eng. Chem. 2019;71:270–276. [Google Scholar]
- Pfleging W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics. 2018;7:549–573. [Google Scholar]
- Sangrós Giménez C., Finke B., Schilde C., Froböse L., Kwade A. Numerical simulation of the behavior of lithium-ion battery electrodes during the calendaring process via the discrete element method. Powder Technol. 2019;349:1–11. [Google Scholar]
- Sangrós Giménez C., Schilde C., Froböse L., Ivanov S., Kwade A. Mechanical, electrical, and ionic behavior of lithium-ion battery electrodes via discrete element method simulations. Energy Technol. 2019;8:1900180. [Google Scholar]
- Schälicke G., Landwehr I., Dinter A., Pettinger K.-H., Haselrieder W., Kwade A. Solvent-free manufacturing of electrodes for lithium-ion batteries via electrostatic coating. Energy Technol. 2019;8:1900309. [Google Scholar]
- Schünemann J.-H., Dreger H., Bockholt H., Kwade A. Smart electrode processing for battery cost reduction. ECS Trans. 2016;73:153. [Google Scholar]
- Sendek A.D., Cubuk E.D., Antoniuk E.R., Cheon G., Cui Y., Reed E.J. Machine learning-assisted discovery of solid Li-ion conducting materials. Chem. Mater. 2018;31:342–352. [Google Scholar]
- Severson K.A., Attia P.M., Jin N., Perkins N., Jiang B., Yang Z., Chen M.H., Aykol M., Herring P.K., Fraggedakis D. Data-driven prediction of battery cycle life before capacity degradation. Nat. Energy. 2019;4:383–391. [Google Scholar]
- Shi Y., Chen G., Liu F., Yue X., Chen Z. Resolving the compositional and structural defects of degraded LiNixCoyMnzO2 particles to directly regenerate high-performance lithium-ion battery cathodes. ACS Energy Lett. 2018;3:1683–1692. [Google Scholar]
- Singh M., Kaiser J., Hahn H. Thick electrodes for high energy lithium ion batteries. J. Electrochem. Soc. 2015;162:A1196–A1201. [Google Scholar]
- Song X., Hu T., Liang C., Long H.L., Zhou L., Song W., You L., Wu Z.S., Liu J.W. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017;7:4783–4790. [Google Scholar]
- Stich M., Pandey N., Bund A. Drying and moisture resorption behaviour of various electrode materials and separators for lithium-ion batteries. J. Power Sources. 2017;364:84–91. [Google Scholar]
- Tesla Inc Tesla completes acquisition of Maxwell technologies. 2019. https://ir.tesla.com/press-release/tesla-completes-acquisition-maxwell-technologies
- Thackeray M.M., David W.I.F., Bruce P.G., Goodenough J.B. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983;18:461–472. [Google Scholar]
- The Tesla Team Introducing V3 supercharging. 2019. https://www.tesla.com/blog/introducing-v3-supercharging
- Thiede S., Turetskyy A., Loellhoeffel T., Kwade A., Kara S., Herrmann C. Machine learning approach for systematic analysis of energy efficiency potentials in manufacturing processes: a case of battery production. CIRP Ann. 2020;69:21–24. [Google Scholar]
- Tsuruta K., Dermer M.E., Dhiman R. 2020. Cell with a Tabless Electrode. US patent number: US20200144676A1, https://patents.google.com/patent/US20200144676A1/en. [Google Scholar]
- Turetskyy A., Thiede S., Thomitzek M., Von Drachenfels N., Pape T., Herrmann C. Toward data-driven applications in lithium-ion battery cell manufacturing. Energy Technol. 2019;8:1900136. [Google Scholar]
- U.S. Department Of Energy . 2020. Energy Storage Grand Challenge Roadmap.https://www.energy.gov/energy-storage-grand-challenge/downloads/energy-storage-grand-challenge-roadmap [Google Scholar]
- Waldmann T., Iturrondobeitia A., Kasper M., Ghanbari N., Aguesse F., Bekaert E., Daniel L., Genies S., Gordon I.J., Löble M.W. Review—post-mortem analysis of aged lithium-ion batteries: disassembly methodology and physico-chemical analysis techniques. J. Electrochem. Soc. 2016;163:A2149–A2164. [Google Scholar]
- Wang H.-Y., Wang F.-M. Electrochemical investigation of an artificial solid electrolyte interface for improving the cycle-ability of lithium ion batteries using an atomic layer deposition on a graphite electrode. J. Power Sources. 2013;233:1–5. [Google Scholar]
- Wang M., Dang D., Meyer A., Arsenault R., Cheng Y.-T. Effects of the mixing sequence on making lithium ion battery electrodes. J. Electrochem. Soc. 2020;167:100518. [Google Scholar]
- Wegener K., Chen W.H., Dietrich F., Dröder K., Kara S. Robot assisted disassembly for the recycling of electric vehicle batteries. Proced. CIRP. 2015;29:716–721. [Google Scholar]
- Whittingham M.S. Electrical energy storage and intercalation chemistry. Science. 1976;192:1126–1127. doi: 10.1126/science.192.4244.1126. [DOI] [PubMed] [Google Scholar]
- Wood D.L., Li J., An S.J. Formation challenges of lithium-ion battery manufacturing. Joule. 2019;3:2884–2888. [Google Scholar]
- Yamada Y., Wang J., Ko S., Watanabe E., Yamada A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy. 2019;4:269–280. [Google Scholar]
- Yang N., Zhang X., Shang B., Li G. Unbalanced discharging and aging due to temperature differences among the cells in a lithium-ion battery pack with parallel combination. J. Power Sourc. 2016;306:733–741. [Google Scholar]
- Yao Y.X., Chen X., Yan C., Zhang X.Q., Cai W.L., Huang J.Q., Zhang Q. Regulating interfacial chemistry in lithium-ion batteries by a weakly-solvating electrolyte. Angew. Chem. Int. Ed. 2020;60:4090–4097. doi: 10.1002/anie.202011482. [DOI] [PubMed] [Google Scholar]
- Yuan C., Deng Y., Li T., Yang F. Manufacturing energy analysis of lithium ion battery pack for electric vehicles. CIRP Ann. 2017;66:53–56. [Google Scholar]
- Zhang J., Xu S., Li W. High shear mixers: a review of typical applications and studies on power draw, flow pattern, energy dissipation and transfer properties. Chem. Eng. Process. Process Intensification. 2012;57-58:25–41. [Google Scholar]
- Zhao J., Li H., Choi H., Cai W., Abell J.A., Li X. Insertable thin film thermocouples for in situ transient temperature monitoring in ultrasonic metal welding of battery tabs. J. Manufacturing Process. 2013;15:136–140. [Google Scholar]
- Zhao N., Zhao D., Xu L.-P., Chen L., Wen Y., Zhang X. A multimode responsive aptasensor for adenosine detection. J. Nanomater. 2014;2014:1–7. [Google Scholar]
- Zwicker M.F.R., Moghadam M., Zhang W., Nielsen C.V. Automotive battery pack manufacturing – a review of battery to tab joining. J. Adv. Joining Process. 2020;1:100017. [Google Scholar]