Abstract
Diabetes is the leading cause of severe health complications and one of the top 10 causes of death worldwide. To date, diabetes has no cure, and therefore, it is necessary to take precautionary measures to avoid its occurrence. The main aim of this systematic review is to identify the majority of the risk factors for the incidence/prevalence of type 2 diabetes mellitus on one hand, and to give a critical analysis of the cohort/cross-sectional studies which examine the impact of the association of risk factors on diabetes. Consequently, we provide insights on risk factors whose interactions are major players in developing diabetes. We conclude with recommendations to allied health professionals, individuals and government institutions to support better diagnosis and prognosis of the disease.
Keywords: Aging, Cardiovascular disease, Depression, Diabetes mellitus, Dyslipidemia, Ethnicity, Family history of diabetes, Hypertension, Physical inactivity, Prevention, Risk factors, Serum uric acid, Sleep quality, Sleep quantity, Smoking, Type 2 diabetes, Obesity
1. Introduction
Diabetes Mellitus (DM) commonly referred to as diabetes, is a chronic disease that affects how the body turns food into energy [1]. It is one of the top 10 causes of death worldwide causing 4 million deaths in 2017 [2], [3]. According to a report by the International Diabetes Federation (IDF) [3], the total number of adults (20–79 years) with diabetes in 2045 will be 629 million from 425 million in 2017 (48% increase). In 2017, diabetes caused at least 727 billion USD in health expenditure, which is 12% of the total spending on adults [3]. According to the National Diabetes Statistics Report [4], 30.3 million (9.4% of the US population) people have diabetes, and 84.1 million (29.06% of the population) have pre-diabetes. 1 in 2 people (212 million) with diabetes was undiagnosed in 2017 according to IDF [5]. Diabetes if left untreated can cause serious medical issues, such as cardiovascular disease, stroke, chronic kidney disease, foot ulcers, damage to the eyes, and prolonged kidney ailment. To date, there is no permanent cure for diabetes and the patients have to rely on healthy lifestyle and timely medication [6].
There are three main types of diabetes: type 1, type 2, and gestational diabetes (diabetes while pregnant) [1]. Type 1 diabetes mostly occurs in children and adolescents. 1,106,500 children were suffering from type 1 diabetes in 2017 [3]. The symptoms of type 1 diabetes include abnormal thirst and dry mouth, frequent urination, fatigue, constant hunger, sudden weight loss, bed-wetting, and blurred vision. Type 2 diabetes is mostly seen in adults, but it is increasing in children and adolescents due to the rising level of obesity, physical inactivity and unhealthy diet [5]. 372 million adults were at the risk of developing type 2 diabetes in 2019 [3]. In 2017, more than 21 million live births were affected by diabetes during pregnancy [3]. In this paper, we focus on type 2 diabetes due to the alarming numbers.
Type 2 Diabetes is thought to prevail in an individual from an interaction between several lifestyle, medical condition, hereditary, psychosocial and demographic risk factors such as high-level serum uric acid, sleep quality/quantity, smoking, depression, cardiovascular disease, dyslipidemia, hypertension, aging, ethnicity, family history of diabetes, physical inactivity, and obesity [6]. In this paper, we present a systematic review of the literature on the association of these risk factors with the incidence/prevalence of type 2 diabetes. We give insights on the contribution of independent risk factors in the development of type 2 diabetes along with possible solutions towards a preventive approach.
2. Methods
We conduct a systematic literature search using CINAHL, IEEE Xplore, Embase, MEDLINE, PubMed Central, ScienceDirect, Scopus, Springer, and Web of Science databases. Our search criteria does not include a time bound. Its main objective is to retrieve all the studies which examine the association between individual risk factors and the incidence/prevalence of type 2 diabetes. Table A1 shows the search string used for each risk factor. The relevant studies have to meet the following inclusion criteria: 1) published in the English language, 2) prospective cohort or cross-sectional study, 3) type 2 diabetes as a specified risk, 4) one of its risk factors, 5) findings in terms of Odds Ratio (OR), Risk Ratio/Relative Risk (RR), or Hazard Ratio (HR), and the corresponding 95% Confidence Intervals (CIs) for the association between the risk factor and type 2 diabetes. To assess the quality of the studies, we use the National Institutes of Health (NIH) quality assessment tool [7]. The tool consists of 14 questions to evaluate the validity and bias risk of a study. We answered each question by either yes, no, cannot be determined, not applicable, or not reported. The tool then classifies each study as high quality (Good), moderate quality (Fair) and low quality (Poor).
3. Results
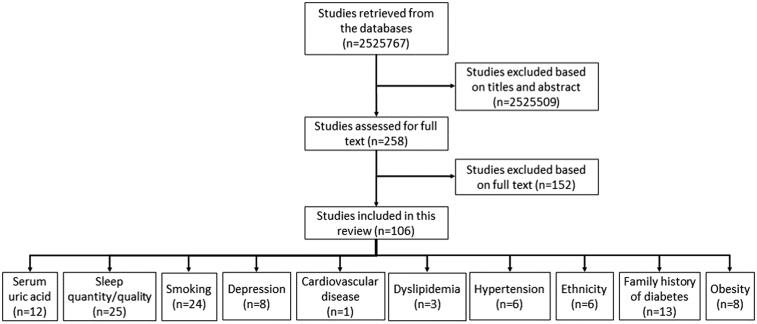
Fig. 1 shows the result of our systematic approach that is used to screen the relevant studies. Irrelevant studies that do not meet the inclusion criteria mentioned in the previous section were excluded after screening titles, abstracts and full texts. At last, 106 papers are considered for this review. These papers are divided into ten categories based on the risk factor under study (Fig. 1). Our review reveals that there is no study that examines the association of age or physical inactivity as an independent risk factor with type 2 diabetes. Table A2 shows the quality assessment results for the studies included in this paper. For smoking, cardiovascular disease and hypertension risk factors, the majority of the studies are of high quality. For serum uric acid, sleep quantity/quality, depression, dyslipidemia, ethnicity, family history of diabetes and obesity, the majority of the studies are of moderate quality.
Fig. 1.
Flowchart of the selection of relevant studies.
3.1. Serum uric acid
Serum uric acid, a common component of urine generated by the metabolic breakdown of purines, have been associated with insulin resistance and type 2 diabetes [8]. High serum uric acid level in an individual leads to: 1) nitric-oxide mediated vasoconstriction (contraction of blood vessels) leading to impaired glucose uptake in the muscles [9], 2) increase in oxidative stress [10] and 3) increase in inflammation leading to a decrease in adiponectin [11], [12]. Consequently, the blood glucose level increases leading to dysfunctional and eventually dead beta-cells [13]. As a result, the individual develops type 2 diabetes. Table 1 shows the characteristics and findings of the work in the literature studying the association between high serum uric acid level and type 2 diabetes.
Table 1.
Characteristics and findings of the studies examining the association between high level serum uric acid and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up duration (Years) | Adjusted variables | Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [14] | 1995 | RS | PCS | 7577 (2.56%) | 100/0 | 40–59 | Britain | 12.8 | Age, BMI, history of heart disease, physical activity, alcohol intake, smoking status, high blood pressure, HDL cholesterol, and heart rate | Uric acid ( mol/l)) 302 412 |
OR 1.0 1.5 (0.9–2.5) |
| [21] | 1998 | RS | PCS | 481 (17.6%) | 53.02/ 46.98 | 30 | Chinese | 3 | Age, sex, BMI, WHR, history of hypertension, HDL cholesterol, fasting insulin, and triglycerides | Uric acid ( mol/l) 420 420 |
OR 1.0 2.581 (1.083–6.149) |
| [22] | 2002 | MONICA [35] | PCS | 6166 (3.45%) | 49.5/ 50.5 | 35–74 | Germany | Mean 7.6 | Age and BMI | Uric acid ( mol/l)) increase by 1000 | OR 2.05 (1.49–1.29) |
| [23] | 2003 | ARIC [36] | PCS | 8574 (9.90%) | 42.6/ 57.4 | 45–65 | Blacks and Whites (USA) | 11 | Age, sex, education, baseline insulin concentration, BMI and blood pressure | Uric acid ( mol/l)) increase by 123.76 | OR 1.3 (1.2–1.4) |
| [19] | 2005 | RS | PCS | 60 | 75/25 | 39–80 | USA | 1 | Age, sex, BMI, baseline insulin concentration, and glomerular filtration rate | Uric acid ( mol/l)) 486 486 (6 months) 486 (12 months) |
OR 1.0 5.47 (1.6–17.7) 3.4 (1.1–10.4) |
| [15] | 2006 | FDPS [37] | – | 475 (21.68%) | 33.68/66.32 | 40–65 | Finland | 3.2 | Age, sex, and baseline fasting | Uric acid ( mol/l)) 99–310 311–380 381–622 |
OR 1.0 1.40 (0.82–2.39) 1.82 (1.07–3.10) |
| [24] | 2008 | CSCCS [38] | PCS | 2960 (20.37%) | 51.7/48.3 | 35–97 | Chinese | Median 9 | Age, sex, BMI, alcohol intake, exercise, marital status, educational level, occupation and family history of diabetes | Uric acid ( mol/l)) 220 280 320 380 460 |
OR 1.0 1.11 (0.82–1.49) 1.29 (0.96–11.73) 1.40 (1.04–1.90) 1.63 (1.20–2.23) |
| [16] | 2008 | Rotterdam [39] | PCS | 4536 (10.18%) | NA | 55 | Netherlands | 10.1 | Age, sex, BMI, waist circumference, systolic and diastolic blood pressure, and HDL cholesterol | Uric acid ( mol/l)) 267 260–310 311–370 370 |
HR 1.0 1.08 (0.78–1.49) 1.12 (0.81–1.53) 1.68 (1.22–2.30) |
| [25] | 2008 | RS | PCS | 4259 (16.81%) | 45.6/ 54.4 | 25–74 | Indians and Creoles | 5 | Ethnicity, serum creatinine, alcohol consumption, family history of diabetes and fasting serum insulin | Uric acid ( mol/l)) Men 363 367 Women 273 287 |
HR 1.0 1.19 (1.07–1.34) 1.0 1.05 (0.95–1.16) |
| [26] | 2008 | MRFIT [40], [41], [42] | PCS | 11351 (10.70%) | 100/0 | 35–57 | Blacks and Whites (USA) | 6 | Smoking status, BMI, hypertension, physical activity, alcohol consumption, total energy intake, cereal fibre, intake of polyunsaturated, mono saturated and saturated fat, coffee intake, high fasting blood glucose, and low HDL cholesterol | Uric acid ( mol/l) 333 464 |
RR 1.0 1.88 (1.52–2.32) |
| [27] | 2009 | RS | PCS | 556 (9.89%) | 41/ 59 | Mean 63.3 8.6 | Brazil | 13 | Age, sex, BMI, diuretic use, and glomerular filtration rate | Uric acid ( mol/l)) increase by 88.4 | OR 1.65 (1.25–2.18) |
| [30] | 2011 | NHANES III [43], [44] | CSS | 14144 | 47.5/ 52.5 | 43–51 | USA | – | Age, sex, race, educational level, smoking, alcohol consumption, BMI, hypertension, and serum total cholesterol | Uric acid ( mol/l)) 380 380–460 460–548 548 |
OR 1.0 0.54 (0.36–0.80) 0.40 (0.29–0.56) 0.48 (0.35–0.66) |
RS-Random Sample, MONICA-Multinational MONItoring of trends and determinants in CArdiovascular disease, ARIC-Atherosclerosis Risk in Communities, FDPS-Finnish Diabetes Prevention Study, CSCCS-Chin Shan Community Cardiovascular study, MRFIT-Multiple Risk Factor Intervention Trial, NHANES-National Health and Nutrition Examination Survey, QFS-Quebec Family Study, M-Men, W-Women, PCS-Prospective Cohort Study, CSS-Cross-Sectional Study.
Perry et al. [14] found that an individual having a uric acid level of more than 411 mol/l is at 1.5 times more risk of developing type 2 diabetes compared to an individual having uric acid level less than 302 mol/l. Niskanen et al. [15] also confirmed that change in uric acid levels is associated with a 2 times increase in the risk of incidence type 2 diabetes. Dehghan et al. [16] in their study showed that individuals having uric acid level 370 mol/l are at high risk of incidence type 2 diabetes (HR 1.68, 95% CI 1.22–2.30) compared to those having uric acid level 267 mol/l. The authors concluded that lowering uric acid level can be a novel approach for diabetes prevention. Xu et al. [17] found that the association between high serum uric acid level and diabetes is the same in both men and women (RR 1.131, 95% CI 1.084–1.179). The association (RR 1.17, 95% CI 1.09–1.25) is also examined by Kodama et al. [18]. Nakagawa et al. [19] showed that uric acid is a significant and independent risk factor in predicting hyperinsulinemia. The authors observed that serum uric acid level 5.5 mg/dl is associated with the development of hyperinsulinemia after 6 months (OR 5.47, 90% CI 1.6–1.77) and 12 months (OR 3.4, 90% CI 1.1–10.4). However, the cohort was controlled for gender and age (60 years). Consequently, it can not be concluded whether uric acid is an independent risk factor or there is an integrated effect of uric acid, gender and age.
Several studies argue that high-level uric acid is not an independent risk factor and it only emphasizes the association between independent risk factors such as age, obesity, hypertension, gender, and dyslipidemia, and type 2 diabetes [20]. Chou et al. show that uric acid has a significant association with type 2 diabetes in old and obese individuals [21]. Another study by Meisinger et al. [22] shows that high-level uric acid is associated with incidence of type 2 diabetes in women only with HR 2.5 per 1 mmol/L increase. Carnethon et al. [23] found that the risk of incidence type 2 diabetes increases (OR 1.3, (1.2–1.4)) with every 1.4 mg/dl increase in uric acid level. However, this is in combination with an increase in waist/hip ratio, smoking and obesity. Chien et al. [24] stated that individuals with a uric acid level of 0.486 mmol/L and having metabolic syndrome have a 3.3 times more risk of incidence type 2 diabetes compared to those with a uric acid level of 0.211 mmol/L and not having metabolic syndrome. Nan et al. [25] examined the impact of ethnicity and gender on the association between uric acid and incidence of type 2 diabetes. The authors found that the high serum uric acid is an independent risk factor for type 2 diabetes in Mauritian Indian men compared to Creole men, and there is a no-to-weak association in women of both ethnicity. Similarly, Choi et al. [26] studied the association between uric acid and type 2 diabetes in men having cardiovascular risk profile. The authors concluded that men with cardiovascular profile having high uric acid level are twice likely to develop type 2 diabetes. The authors also stated that this association between uric acid and diabetes is independent of other risk factors such as obesity, age, family history of diabetes, hypertension, and metabolic syndrome. Kramer et al. [27] analyzed the impact of age and impaired fasting glucose (IFG) on the association and found that high uric acid level can independently predict incidence of type 2 diabetes (OR 1.65, 95% CI 1.25–2.18) in older adults having IFG. Lv et al. [28] found that high serum uric acid level is associated to type 2 diabetes in middle-aged or older people (RR 1.56, 95% CI 1.39–1-76).
In summary, the association between high-level serum uric acid remains obscure. It is debatable whether serum uric acid is an independent risk factor for type 2 diabetes or it only emphasizes the association between other independent risk factors and type 2 diabetes. Some studies reported a positive association between high serum uric acid level and incidence of type 2 diabetes [14], [15], [16], [19], [24], whereas others [25], [29] reported no association. On the contrary, some studies reported an inverse association between uric acid and diabetes [30], [31], [32]. Furthermore, some studies argue that there is a reverse association, i.e., diabetes leads to high uric acid levels [33], [34].
3.2. Sleep quantity/quality
The quality and quantity of sleep are affected by several cultural, social, behavioral, psychological, and environmental factors. The working professionals often experience fatigue, tiredness and daytime napping due to irregular working hours and shifts. Evidence shows that the current average sleep of an individual, i.e., 6.8 h/night, is 1.5 h less than that a century ago [45]. The cause of sleep loss is multi-factorial. For instance 45% of adults report that they sleep fewer hours to get more work done, 43% reported that they watch television or use the Internet, and 22% reported to be suffering from insomnia. The unusual, disturbed and reduced sleep is associated with glucose intolerance [46].
An individual suffering from sleep disorder, known as obstructive sleep apnea (OSA), experiences: 1) deficiency in the amount of oxygen reaching the tissues by total/partial collapse of upper airways while sleeping (hypoxia) and 2) inflammation. Frequent Hypoxia triggers an increase in sympathetic activity [47]. Increased sympathetic activity and inflammation lead to insulin resistance condition [48], [49] and eventually to type 2 diabetes. Table 2 shows the characteristics and findings of the work in the literature studying the association between sleep quantity/quality and type 2 diabetes.
Table 2.
Characteristics and findings of the studies examining the association between sleep quantity/quality and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up duration (Years) | Adjusted variables | Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [79] | 2003 | NHS [80] | PCS | 70026 (2.81%) | 0/100 | 40–65 | United States | 10 | Working hours, hypercholesterolemia, hypertension, smoking, snoring, exercise, alcohol, depression, postmenopausal hormone use, BMI, and family history of diabetes | Sleep (Hours) 5 6 7 8 9 |
OR 1.18 (0.96–1.44) 1.10 (0.97–1.25) 1.02 (0.91–1.16) 1.0 1.29 (1.05–1.59) |
| [56] | 2004 | RS [81] | PCS | 2265 (1.67%) | 100/0 | – | Japanese | 8 | Age, education, occupation, shift work, BMI, leisure time, physical activity, smoking, alcohol consumption and family history of diabetes | Sleep DIS (low frequency) DIS (high frequency) DMS (low frequency) DMS (high frequency) |
HR 1.0 2.98 (1.36–6.53) 1.0 2.23 (1.08–4.61) |
| [57] | 2004 | MPP [82] | PCS | 6599 (4.3%) | 100/0 | Mean 42.6 | Swedish and Caucasians | 15.2 | Age, lifestyle, family history of diabetes, social class, physical activity, BMI, smoking, and alcohol intake | DIS No Yes |
OR 1.0 1.52 (1.05–2.20) |
| [59] | 2005 | MONICA [35] | PCS | 8269 (2.27%) | 50.1/49.9 | 25–75 | Germany | 7.5 | Age,educational level, parental history of diabetes, smoking, alcohol consumption, hypertension, physical activity, history of angina pectoris, BMI, and dyslipidemia | DIS No Yes (M) Yes (W) DMS No Yes (M) No (W) |
OR 1.0 1.10 (0.59–2.03) 1.42 (0.81–2.50) 1.0 1.60 (1.05–2.45) 1.98 (1.20–3.29) |
| [83] | 2005 | SHHS [84] | CSS | 1486 | 48.6/ 51.4 | 53–93 | United States | – | Age, sex, ethnicity, waist girth, and apnea-hypopnea index | Sleep (Hours) 5 6 7–8 9 |
OR 2.51 (1.57–4.02) 1.66 (1.15–2.39) 1.0 1.88 (1.21–2.91) |
| [52] | 2005 | RS | PCS | 1170 (7.52%) | 47/53 | 45–65 | Swedish | 12 | Age, marital status, living conditions, hypertension, obesity, smoking, alcohol use, snoring and depression | Sleep (Hours) 7–8 5 (M) 5 (W) 9 (W) |
RR 1.0 2.8 (1.1–7.3) 1.8 (0.5–6.8) 2.9 (0.6–15.0) |
| [50] | 2005 | RS | PCS | 1462 (8.62%) | 0/100 | 38–60 | Swedish | 32 | Age, subscapular skin-fold thickness, serum lipid values, blood pressure, resting heart rate, physical activity, education and socio-economic status | No association between sleep duration and diabetes. | |
| [85] | 2006 | MMAS [86] | PCS | 1139 (7.90%) | 100/0 | 40–70 | Blacks and Whites (USA) | 17 | Age, hypertension, smoking, self rated health status, waist circumference, education, testosterone, and cortisol | Sleep (Hours) 5 6 7 8 8 |
RR 1.71 (0.81–3.59) 1.95 (1.06–3.58) 1.0 1.40 (0.78–2.54) 3.03 (1.44–6.37) |
| [87] | 2007 | NHANES I [88] | PCS | 8992 (4.78%) | 37.5/ 62.5 | 32–86 | Whites and Non-whites (USA) | 10 | Physical activity, depression, alcohol consumption, ethnicity, education, marital status, age, obesity and hypertension | Sleep (Hours) 5 6 7 8 9 |
OR 1.47 (1.03–2.09) 1.08 (0.80–1.47) 1.0 1.09 (0.83–1.43) 1.52 (1.06–2.17) |
| [89] | 2007 | QFS [90] | CSS | 740 | 43.65/ 56.35 | 21–64 | Europid race | 12 | Age, marital status, employment status, educational level, annual income, physical activity, alcohol intake, coffee intake, hypertension, heart disease and waist circumference | Sleep (Hours) 5–6 7–8 9–10 |
OR 2.09 (1.34–2.98) 1.0 1.58 (1.13–2.31) |
| [58] | 2007 | HIPOP-OHP [91] | PCS | 6509 (3.53%) | 78.4/21.6 | 32–86 | Japanese | 4.2 | Age, sex, BMI, history of smoking, history of hypertension, history of high cholesterol, history of diabetes and physical activity | DIS No Low frequency High frequency |
HR 1.0 1.42 (1.05–1.91) 1.61 (1.00–2.58) |
| [92] | 2008 | FIN-D2D [93] | CSS | 2770 | 48.2/51.8 | 45–74 | Finland | 1 | Age, BMI, medication for sleep, antidepressants, smoking, sleep apnea probability, and physical activity | Subjects with 6 and 8 hours of sleep are more likely rightarrow have type 2 diabetes. | |
| [94] | 2009 | QFS [90] | PCS | 274 | 42.7/ 57.3 | 21–64 | Europid race | 6 | Age, smoking habits, employment status, annual household income, shift working history, resting metabolic rate, coffee intake, waist circumference and physical activity | Sleep (Hours) 6 7–8 9 |
RR 2.42 (1.49–3.33) 1.0 2.31 (1.41–3.15) |
| [55] | 2009 | IRAS [95] | – | 900 (16.22%) | 43.3/56.7 | 40–69 | Non-Hispanic Whites, Hispanics, and African-Americans | 5 | Age, sex, glucose tolerance, hypertension, family history of diabetes, smoking, educational level, BMI, insulin sensitivity, and acute insulin response | Sleep (Hours) 8 NHW/Hispanics 7 9 African-American 7 9 |
OR 1.0 2.36 (1.11–5.99) 2.15 (0.50–9.30) 0.63 (0.14–2.90) 0.39 (0.02–7.19) |
| [96] | 2009 | RS | CSS | 1741 | 42.6/ 57.4 | 20 | Pennsylvania | - | Age, race, sex, BMI, smoking, alcohol consumption, depression and sleep disordered breathing | Sleep (Hours) 5 5–6 6 |
OR 2.95 (1.2–7.0) 2.07 (0.68–6.4) 1.0 |
| [97] | 2009 | RS | – | 515 | 33/67 | 40–64 | Finland | 7 | Age, sex, BMI, study center, smoking, alcohol intake, hypertension medication, leisure time physical activity, and 1 year change in body weight | Sleep (Hours) 6.5 7–8.5 9–9.5 10 | HR 1.68 (0.79–3.59) 1.0 2.29 (1.38–3.80) 2.74 (1.67–4.50) |
| [98] | 2010 | NIH-AARP [99] | PCS | 174344 | 56.8/43.2 | 50–71 | Whites and non-whites (USA) | 8 | Age, race, sex, educational level, marital status, smoking, coffee intake, alcohol intake, calorie intake, BMI, and physical activity | Day napping (Hours) 0 1 1 Sleep (Hours) 5 5–6 7–8 9 |
OR 1.0 1.23 (1.18–1.29) 1.55 (1.45–1.66) 1.46 (1.31–1.63) 1.11 (1.06–1.16) 1.0 1.11 (0.99–1.24) |
| [100] | 2011 | RS | CSS | 3470 (5.2%) | 61.8/ 38.2 | 25 | Taiwan | - | BMI, WHR, family history of diabetes, family history of hypertension, smoking, alcohol consumption and coffee intake | Sleep (Hours) 6 6–8.49 8.5 |
OR 1.55 (1.07–2.24) 1.0 2.83 (1.19–6.73) |
| [51] | 2012 | EPIC-Potsdam [101] | PCS | 23620 (3.6%) | 38.63/ 61.37 | 35–65 | Germany | 7.8 | Age, sex, sleeping disorders, alcohol intake, smoking, walking, cycling, sports, employment status, education, BMI, WHR, hypertension, caffeinated beverages, life satisfaction, health satisfaction, and intake of antidepressants | Sleep (Hours) 6 6-7 7-8 8-9 9 |
HR 1.06 (0.80–1.40) 0.94 (0.78–1.14) 1.0 0.92 (0.77–1.10) 1.05 (0.82–1.33) |
| [102] | 2012 | RS | PCS | 3570 | 78.6/ 21.4 (3.4%) | 35–55 | Japan | 4 | Age, sex, fasting plasma glucose level, education, working hours, shift work, rate of sedentary work, occupational stress, smoking, alcohol intake and physical exercise | Sleep (Hours) 5 5–6 6–7 7–8 |
OR 5.37 (1.38–20.91) 1.38 (0.50–3.79) 1.57 (0.64–3.83) 1.0 |
| [53] | 2012 | NHIS [103] | CSS | 29818 | 53.5/ 46.5 | 18–85 | Blacks and whites (USA) | 10 | Age, sex, income, hypertension, heart disease, depression and obesity | Sleep (Hours) 6–8 5 (Blacks) 5 (Whites) 9 (Blacks) 9 (Whites) |
OR 1.0 1.66 (1.19–2.30) 1.87 (1.57–2.24) 1.68 (1.21–2.33) 2.33 (1.98–2.73) |
| [104] | 2013 | IHHP [105] | CSS | 12514 | 49/ 51 | 19 | – | – | Age, sex, BMI, and waist circumference | Sleep (Hours) 5 6 7–8 9 |
OR 1.62 (1.33–1.99) 0.92 (0.75–1.13) 1.0 1.10 (0.83–1.44) |
| [106] | 2013 | MC [107] | PCS | 47093 (1.85%) | 74.4/ 25.6 | Mean 34.9 | USA | 6 | Age, sex, BMI, education and race | Sleep (Hours) 5 5 6 7 8 8 |
OR 2.04 (1.49–2.8) 1.46 (1.15–1.84) 1.19 (0.99–1.43) 1.0 1.17 (0.95–1.45) 1.30 (0.93–1.81) |
| [54] | 2013 | NHIS [103] | CSS | 130943 (10.12%) | 99.75/ 0.25 | Mean 50.6 | Blacks and whites (USA) | 7 | Age, sex, household income, poverty status, education, occupation, employment status, alcohol consumption, smoking, leisure time physical activity, marital status, heart disease, hypertension, and BMI | Sleep (Hours) 7 6 (Blacks) 6 (Whites) 8 (Blacks) 8 (Whites) |
OR 1.0 1.08 (0.95–1.23) 1.16 (1.07–1.25) 1.01 (0.89–1.15) 1.17 (1.09–1.26) |
| [108] | 2013 | 45 and up [109] | PCS | 156902 | 36/ 64 | 50–82 | Australia | - | Age, sex, education, marital status, residential remoteness, alcohol consumption, smoking status, health insurance status, income, BMI, physical activity and baseline health | Sleep (Hours) 7 6 |
HR 1.0 1.29 (1.08–1.53) |
DIS-Difficulty Initiating Sleep, DMS-Difficulty Maintaining Sleep, EPIC-European Prospective Investigation into Cancer and Nutrition, FIN D2D-Finnish type 2 Diabetes, HIPOP-OHP-High risk and Population Strategy for Occupational Health Promotion, IHHP-Isfahan Healthy Heart Program, IRAS-Insulin Resistance Atherosclerosis Study, M-Men, MC-Millennium Cohort, MMAS-Massachusetts Male Aging Study, MONICA-Multinational MONItoring of trends and determinants in CArdiovascular disease, MPP-Malmo Preventive Project, NHANES-National Health and Nutrition Examination Survey, NHIS-National Health Interview Survey, NHS-Nurse Health Study, NHW-Non Hispanic Whites, NIH AARP-National Institutes of Health American Association of Retired Persons Diet and Health Study, QFS-Quebec Family Study, RS-Random Sample, SHHS-Sleep Heart Health Study, W-Women, PCS-Prospective Cohort Study, CSS-Cross-Sectional Study.
The results in the literature show that compared to a reference sleep duration of 7-8 h, an individual having either short sleep duration (6 h) or long sleep duration (8 h) is at high risk of developing type 2 diabetes. However, [50], [51] concluded that there is no significant association between sleep and incidence of type 2 diabetes. Mallon et al. [52] studied the impact of gender on the association between sleep and diabetes. The authors concluded that short sleep duration increases the risk of incidence diabetes in men, whereas, in women, long sleep duration dominates. The effect of ethnicity on the association is analyzed by [53], [54], [55]. Zizi et al. [53] and Jackson et al. [54] showed that the prevalence of type 2 diabetes is more in whites who sleep less than 5 h or more than 8 -9 h compared to blacks. Beihl [55] showed that the association is more in Hispanics/Non-Hispanic Whites compared to that in African-American. Xu et al. examined the association between day-time napping and type 2 diabetes and showed that an individual taking more than 1 h of day-time nap is at 1.5 times more risk to develop diabetes compared to an individual who does not take a nap during the day. In the context of sleep quality, the risk of incidence type 2 diabetes is more in an individual having difficulty initiating sleep (DIS), and the risk increases with increasing DIS frequency [56], [57], [58]. Furthermore, the association is more in women having DIS compared to men [59].
In summary, there is a strong association between sleep quantity/quality and the incidence of type 2 diabetes. The association is stronger in women sleeping for more duration and in men with short sleep duration. Moreover, this association is affected by ethnicity.
3.3. Smoking
Smoking leads to more than 8 million deaths per year [60]. This is from both active and passive uses, i.e, non-smokers exposed to smokers. Smokers are 30–40% more likely to develop type 2 diabetes compared to non-smokers [61]. When an individual smokes, the level of nicotine increases in his/her body. This leads to a reduction in muscle glucose intake, developing insulin resistance and leading to type 2 diabetes [62]. The characteristics and findings of table:smokingtable:smoking/passive smoking and the incidence of type 2 diabetes are presented in Table 3.
Table 3.
Characteristics and findings of the studies examining the association between smoking and type 2 diabetes.
| Work | Year | Study | Design | Sample Size(%DM) | %M/W | Age (Years) | Ethnicity | Follow-up Duration (Years) | Adjusted Variables | Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [110] | 1989 | ZS [111] | PCS | 841 (6.9%) | 100/0 | 40-59 | Dutch | 25 | Age, subscapular skin-fold, resting heart rate, cigarette use, alcohol intake and energy intake | Cigarettes/day 0 20 | HR 1.0 3.3 (1.4-7.9) |
| [67] | 1993 | NHS [80] | PCS | 114247 (2.04%) | 0/100 | 30-55 | USA | 12 | Age, BMI, family history of diabetes, menopause, postmenopausal hormone use, oral contraceptive use, alcohol consumption, and physical activity | Cigarettes/day 0 1-14 15-24 25 Ex-smoker | RR 1.0 0.90 (0.68-1.19) 1.20 (0.96-1.50) 1.49 (1.19-1.87) 1.17 (1.02-1.35) |
| [68] | 1995 | HPFS | PCS | 41810 (1.22%) | 100/0 | 40-75 | USA | 62 | Age, BMI, family history of diabetes, alcohol consumption and physical activity | Cigarettes/day 0 1-14 15-24 25 Ex-smoker | RR 1.0 1.37 (0.77-2.43) 2.38 (1.57-3.59) 1.94 (1.25-3.03) 1.29 (1.05-1.57) |
| [112] | 1997 | RS | PCS | 2312 (1.77%) | 100/0 | - | Japanese | 8 | - | Cigarettes/day 0 1-15 16-25 26 | HR 1.0 1.33 (0.40-4.39) 3.59 (1.32-9.76) 2.68 (0.88-8.05) |
| [73] | 1997 | SOF [113] | CSS | 9435 (7%) | 0/100 | 65 | Non-black (USA) | - | Age, resting heart rate, BMI, education level, alcohol intake, energy expenditure, WHR, and postmenopausal hormone use | Cigarettes/day 0 10 10 Ex-smoker | OR 1.0 0.55 (0.30-0.99) 1.21 (0.87-1.71) 0.99 (0.82-1.19) |
| [114] | 1999 | OHS | PCS | 6250 (7.2%) | 100/0 | 25-60 | Japan | 16 | Age, BMI, alcohol consumption, physical activity, parental history of diabetes, fasting plasma glucose, total cholesterol, and triglycerids | Cigarettes/day 0 1-20 21-30 30 | RR 1.0 1.40 (1.05-1.86) 1.40 (1.02-1.93) 173 (1.20-2.48) |
| [69] | 2000 | PHS [115] | PCS | 21068 (3.65%) | 100/0 | 40-84 | USA | 12.10 | Age, BMI, physical activity, history of hypertension, history of high cholesterol, parental history of myocardial infarction, and alcohol consumption | Cigarettes/day 0 20 20 Ex-smoker | RR 1.0 1.5 (1.0-2.2) 1.7 (1.3-2.3) 1.1 (1.0-1.4) |
| [116] | 2001 | RS | CSS | 3718 | 19.2/ 80.0 | 12-88 | Chinese | - | Age, BMI, alcohol consumption, and family history of diabetes | Smoking No Yes | OR 1.0 1.705 (1.106-2.630) |
| [65] | 2001 | BRHS [117] | PCS | 7124 (4.07%) | 100/0 | 40-59 | UK | 16.8 | Age, BMI, physical activity, alcohol intake, social class, heart disease and antihypertensive treatment | Smoking No Yes Pipe/cigar Ex-smoker (15 yrs.) Ex-smoker (10 yrs.) | RR 1.0 1.61 (1.05-2.46) 2.15 (1.24-3.70) 1.45 (0.95-2.21) 2.03 (1.22-3.37) |
| [63] | 2001 | CPS-I [118] | PCS | 709827 (3.6%) | 38.8/ 61.2 | 30 | Whites and Blacks (USA) | 13 | Age, BMI, alcohol consumption, race, amount of exercise, education level, and intakes of fats and carbohydrates | Cigarettes/day 0 20 (M) 20 (W) 20-39 (M) 20-39 (W) 40 (M) 40 (W) Ex-smoker (M) Ex-smoker (W) | OR 1.0 1.05 (0.98-1.12) 0.98 (0.93-1.03) 1.19 (1.13-1.26) 1.21 (1.14-1.29) 1.45 (1.34-1.57) 1.74 (1.49-2.03) 1.07 (1.02-1.13) 1.07 (0.99-1.15) |
| [119] | 2001 | NHS [80] | PCS | 84941 (3.9%) | 0/100 | 30-55 | USA | 16 | Age, family history of diabetes, menopausal status, postmenopausal hormone use, fat intake, and physical activity | Cigarettes/day 0 1-14 15 | OR 1.0 1.14 (0.85-1.54) 1.40 (1.14-1.71) |
| [120] | 2002 | NCDS [121] | - | 15396 | M/W | - | UK | 33 | Maternal smoking during pregnancy, sex, mother’s age at the time of giving birth, age at which mother left school, family social class at birth, birth weight, own smoking at the age of 16, and BMI at the age of 33 | Cigarettes/week Self 0 1 1-9 10-19 20-29 30 Mother Non-smoker Medium-smoker Medium to heavy-smoker Heavy-smoker | OR 1.0 2.07 (0.25-17.19) 1.92 (0.52-7.10) 2.48 (0.52-11.97) 1.61 (0.20-12.96) 3.62 (1.42-9.24) 1.0 1.01 (0.23-4.53) 3.53 (0.88-14.38) 4.02 (1.14-14.14) |
| [74] | 2004 | RIH | CSS | 27777 | 45/ 55 | 20-69 | France | - | Age, BMI, WHR, and alcohol consumption | Smoking No Yes (M) Yes (W) Ex-smoker (M) Ex-smoker (W) | OR 1.0 1.49 (1.13-1.96) 0.89 (0.54-1.39) 1.31 (1.01-1.70) 1.46 (0.92-2.22) |
| [122] | 2004 | NTHS [123] | PCS | 38805 | 46.9/ 53.1 | 20 | Norwegian | 11 | Age, BMI, and sex | Cigarettes/day 0 20 | RR 1.0 1.64 (1.12-2.39) |
| [70] | 2005 | IRAS [95] | PCS | 906 (25%) | 43.3/ 56.7 | 40-69 | Non-Hispanic Whites, Hispanics, and African-Americans | 5 | Age, sex, ethnicity, BMI, WHR, glucose tolerance status, HDL cholesterol level, triglyceride level and hypertension | Smoking No Ex-smoker Current-smoker | OR 1.0 1.31 (0.82-2.09) 2.66 (1.49-4.77) |
| [77] | 2006 | KMIC [124] | PCS | 27635 | 100/0 | 35-44 | Korea | 8 | Age, baseline fasting serum, glucose, weight change, baseline BMI, family history of diabetes, alcohol consumption, and physical activity | Cigarettes/day No 10 10-19 20 Ex-smoker (8 yrs.) Ex-smoker (7-7.9 yrs.) Ex-smoker (5-6.9 yrs.) | OR 1.0 1.23 (1.86-1.77) 1.60 (1.28-2.00) 1.75 (1.35-2.27) 0.95 (0.72-1.25) 1.44 (0.96-2.15) 2.13 (1.51-3.00) |
| [71] | 2009 | RS | PCS | - | M/W | 40-69 | Ansung and Ansan Korean | 4 | Age, family history of diabetes, rural or urban area, waist, body fat, exercise, alcohol consumption, income, education, WBC, HDL cholesterol, triglyceride, systolic BP, HOMA IR, and HOMA beta | Cigarettes/day No 20 Y20 Ex-smoker | RR 1.0 2.06 (1.35-3.16) 2.41 (1.48-3.93) 1.60 (1.07-2.39) |
| [75] | 2010 | ARIC [36] | PCS | 10892 (11.51%) | 43.3/ 56.7 | 45-64 | Whites and Non-whites (USA) | 9 | Race, sex, level of education, BMI, waist circumference, baseline age, physical activity, HDL cholesterol, triglycerides, and systolic BP | Smoking No Ex-smoker (9 yrs.) Ex-smoker (6-9 yrs.) Ex-smoker (3-6 yrs.) Ex-smoker (3 yrs.) Current-smoker | HR 1.0 1.16 (0.99-1.36) 1.21 (0.89-1.65) 1.54 (1.10-2.14) 1.80 (1.44-2.25) 1.26 (1.08-1.46) |
| [66] | 2010 | KORA S4/F4 [125] | PCS | 885 | 50.4/ 49.6 | 55-74 | Germany | 7 | Age, sex, parental diabetes, socioeconomic status, alcohol intake, physical activity, intake of meat and sausage, intake of salad and vegetables, intake of whole grain bread, coffee consumption, waist circumference, blood pressure, hypertriglyceridemia, HDL cholesterol, log insulin and log adiponectin | Smoking No (passive+active) Passive Passive+prediabetes Active Active+prediabetes | OR 1.0 2.5 (1.1-5.6) 4.4 (1.5-13.4) 2.8 (1.3-6.1) 7.8 (2.4-25.7) |
| [64] | 2010 | KCPS [126] | PCS | 1236443 | 63.7/ 36.3 | 30-95 | Korea | 14 | Age, alcohol drinking, BMI, and physical exercise | Cigarettes/day No 1-9 (M) 1-9 (W) 10-19 (M) 10-19 (W) 20 (M) 20 (W) | HR 1.0 1.30 (1.25-1.32) 1.34 (1.25-1.44) 1.37 (1.34-1.41) 1.26 (1.14-1.38) 1.55 (1.51-1.60) 1.33 (1.15-1.53) |
| [72] | 2011 | NHS [80] | PCS | 100526 (5.36%) | 0/100 | 41-55 | USA | 24 | Age, BMI, physical activity, husband’s education, family history of diabetes, total energy intake, alcohol intake, caffeine, total transa fat, toatl saturated fat, calcium, magnesium and vitamin D | Cigarettes/day No Low passive High passive 1-14 15-24 25 Ex-smoker | RR 1.0 1.10 (0.94-1.23) 1.16 (1-1.35) 1.39 (1.17-1.64) 1.68 (1.43-2.01) 1.98 (1.57-2.36) 1.28 (1.12-1.50) |
| [78] | 2012 | JPHC [127] | PCS | 59834 | 43.24/ 56.76 | Mean 55-57.9 | Japanese | 5 and 10 | Age, BMI, history of hypertension, alcohol intake, family history of diabetes, weight change, study area, and leisure time physical activity | Smoking No Current-smoker (M) Current-smoker (W) Ex-smoker (5 yrs.) (M) Ex-smoker (5 yrs.) (W) | OR 1.0 1.43 (1.16-1.76) 1.42 (1.03-1.94) 1.68 (1.07-2.63) 2.84 (1.53-5.29) |
| [76] | 2012 | RS | PCS | 2070 (11.9%) | 100/0 | 40-69 | Japan | 9.2 | Age, blood glucose, fasting, systolic BP, total cholesterol, log-transformed triglycerides, alcohol consumption, exercise, family history of diabetes, BMI, and change in smoking status during follow-up period | Smoking No Ex-smoker (9 yrs.) Ex-smoker (6-9 yrs.) Ex-smoker (3-5 yrs.) Ex-smoker (3 yrs.) Current-smoker | HR 1.0 2.22 (1.05-4.69) 0,59 (0.13-2.64) 1.95 (0.62-6.17) 1.91 (0.60-6.06) 2.78 (1.43-5.41) |
| [128] | 2013 | WHI [129] | PCS | 11838 | 0/100 | 50-79 | USA | 11 | Age, ethnicity, education, BMI, waist circumference, alcohol consumption, physical activvity, hypertension and medication for high cholesterol | Smoking No Current-smoker (M) Ex-smoker (3 yrs.) | HR 1.0 1.28 (1.20-1.36) 1.43 (1.26-1.63) |
ZS-Zutphen Study, NHS-Nurse Health Study, NHIS-National Health Interview Survey, HPFS-Health Professionals’ Follow-up Study, RS-Random Sample, SOF-Study of Osteoporotic Fractures, OHS-Osaka Health Survey, PHS-Physicians Health Survey, BRHS-British Regional Health Study, CPS-Cancer Prevention Study, NCDS-National Child Development Study, RIH-Regional Institute for Health, NTHS-Nord Trondelag Health Survey, IRAS-Insulin Resistance Atherosclerosis Study, ARIC-Atherosclerosis Risk in Communities, KCPS-Korean Cancer Prevention Study, JPHC-Japan Public Health Center, WHI-Women Health Initiative, KMIC-Korean Medical Insurance Corporation, M-Men, W-Women, PCS-Prospective Cohort Study, CSS-Cross-Sectional Study.
The results in the literature show that the association between smoking and diabetes increases with an increase in the number of cigarettes smoked/day. Will et al. [63] analyzed the impact of gender on this association and showed that the association between cigarette smoking and type 2 diabetes is more in men compared to women. Similar results are obtained by Jee et al. [64]. Wannamethee et al. [65] revealed that an individual smoking pipe/cigar is 2.15 times more likely to develop type 2 diabetes and an individual smoking cigarette is 1.6 times more likely compared to a non-smoker. Kowall et al. [66] showed that the risk of incidence type 2 diabetes is significantly high in active/passive prediabetic smokers compared to active/passive smokers without prediabetes.
The incidence and prevalence of type 2 diabetes in ex-smokers is examined by [67], [68], [69], [70], [71], [72]. and [73] respectively. Results show that ex-smokers are associated with 17–60% increased risk of type 2 diabetes [67], [68], [70], [71], [72]. However, the results obtained by Simon et al. [73] and Manson et al. [69] showed no association between ex-smokers and type 2 diabetes. This discrepancy in the results can be due to the heterogeneous characteristics (sample size, age range, men/women ratio and ethnicity) of the cohorts used in these studies. Beziaud et al. [74] examined gender-based prevalence of type 2 diabetes in ex-smokers and showed that women are at higher risk compared to men. Furthermore, the duration of smoking cessation also impacts the association in ex-smokers [65], [75], [76], [77]. An individual is at high risk of developing type 2 diabetes during first 5–10 years of smoking cessation. The risk then decreases with an increase in cessation duration. The association between smoking cessation and the incidence of type 2 diabetes is more in women than men [78].
In summary, both active and passive smoking are strongly associated with the incidence of type 2 diabetes. The association is more in men compared to women. Moreover, the association remains significant in ex-smokers during first the 5–10 years of smoking. After 10 years of smoking cessation, the risk of incidence type 2 diabetes is the same as that in a non-smoker. Women ex-smokers are at a higher risk of developing diabetes compared to men ex-smokers.
3.4. Depression
Depression is a mood disorder that negatively affects the way a person feels, thinks and acts [130]. It can be due to a family history of depression, early childhood trauma, brain structure, medical conditions, drug use or surrounding environment. Depression is associated with multiple health conditions including diabetes [131]. It elevates the sympathetic nervous system activities and hypothalamic–pituitary–adrenal axis activities [132]. Elevated sympathetic nervous system activities lead to an increase in catecholamines and inflammation, and eventually causing insulin resistance [133]. On the other hand, elevated adrenal axis activities lead to an increase in cortisol and eventually blood sugar level [134]. Both insulin resistance and increased blood sugar levels develop type 2 diabetes. The characteristics and findings of the work in the literature examining the association between depression and the incidence of type 2 diabetes are presented in Table 4.
Table 4.
Characteristics and findings of the studies examining the association between depression and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up Duration (Years) | Adjusted variables | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [137] | 1991 | RS | PCS | 2380 (1.72%) | 100/0 | 18 | Japanese | 8 | Age | Depression (SDS score) 20–39 40–47 48–80 |
HR 1.0 1.07 (0.53–2.13) 2.32 (1.06–5.08) |
|
| [155] | 1996 | ECAPS [156] | - | 1715 (5.2%) | 37.8/ 62.2 | 18 | USA | 13 | Age, sex, race and BMI | Depression No Yes |
OR 1.0 2.23 (0.90–5.55) |
|
| [157] | 2003 | NHANES I [88] | PCS | 6190 | 45.7/ 54.3 | 25–74 | Whites and Non-whites (USA) | 15.6 | Age, sex and race | Depression No Mild Major |
RR 1.0 1.24 (0.91–1.70) 2.52 (1.73–3.67) |
|
| [158] | 2004 | ARIC [36] | PCS | 11615 | 44.85/ 55.15 | 48–67 | Whites and Non-whites (USA) | 6 | Age, sex, race, study site, fasting insulin, fasting glucose, HDL cholesterol, BMI, WHR, systolic BP, physical activity, total calorie intake, smoking status, and education | Depression No Low Mild Major |
HR 1.0 1.12 (0.90–1.39) 1.03 (0.81–1.31) 1.31 (1.04–1.64) |
|
| [136] | 2004 | SWAN [159] | PCS | 2662 (3.64%) | 0/100 | 42–52 | Caucasian, African-American, Hispanic, Japanese-American and Chinese-American | 3 | Age, study site, race, education, and medication use | Depressed African-Americans are 2.56 times more likely rightarrow have diabetes. | ||
| [160] | 2007 | NTHS [123] | PCS | 37291 | 47.2/ 52.8 | 29 | Norwegian | 10 | Age, sex, education, smoking, physical activity, BMI, WHR, waist circumference, and marital status | Depression No Yes |
OR 1.0 1.40 (1.16–1.69) |
|
| [138] | 2007 | CHS [161] | PCS | 4681 | 40.8/ 59.2 | 65 | USA | 8 | Age, race, sex, educational level, marital status, physical activity, smoking, alcohol consumption, BMI, and reactive protein level | Depression (CES-D score) 8 8 |
OR 1.0 1.57 (1.07–2.29) |
|
| [139] | 2014 | RBHCDS | - | 971 | 43/ 57 | 50 | California | 8 | Age, sex, BMI and exercise | Depression (BDI score) 11 11 |
OR 1.0 2.50 (1.29–4.87) |
|
RS-Random Sample, SDS-Self rating Depression Scale, ECAPS-Epidemiologic Catchment Area Program Survey, NHANES-National Health and Nutrition Examination Survey, ARIC-Atherosclerosis Risk in Communities, RNH-RegistratieNet Huisarts Praktijken, SWAN-Study of Womens’ Health Across the Nation, NTHS-Nord Trondelag Health Study, CHS-Cardiovascular Health Study, CESD-Center for Epidemiological Studies Depression Scale, RBHCDS-Rancho Bernardo Heart and Chronic Disease Study, BDI-Beck Depression Inventory, M-Men, W-Women, PCS-Prospective Cohort Study, CSS-Cross-Sectional Study.
The results show that depression is highly associated with the incidence of type 2 diabetes. In the context of gender, depressed men are at higher risk of incidence type 2 diabetes, whereas depression in women is not associated with type 2 diabetes [135]. Moreover, compared to Caucasian, Hispanic, Japanese-American and Chinese-American, depressed African-Americans are at 2.56 times higher risk of incidence type 2 diabetes [136]. Based on self rating depression scale (SDS) score, an individual having a score of 48–80 is at higher risk of developing diabetes compared to an individual having a score of 20–39 [137]. Similarly, an individual having a score 11 using center for epidemiological studies depression scale (CES-D) or a score 8 using beck depression inventory (BDI) is at higher risk of incidence type 2 diabetes [138], [139].
In summary, depression is associated with type 2 diabetes. However, the association is different in men and women. Moreover, the study by Yu et al. [140] show that depression itself is not a risk factor for diabetes, rather the activities related to depression such as physical inactivity, poor diet, and obesity lead to diabetes. In addition, the medical drugs used to treat depression also have an association with the incidence of type 2 diabetes. Consequently, similar to high-level serum uric acid, depression is not an independent risk factor but it emphasizes the impact of other independent risk factors such as gender, ethnicity, physical inactivity, and obesity.
3.5. Cardiovascular disease
Increased heart rate and cardiovascular disease can elevate the blood pressure in the arteries. As a result, the body’s glucose uptake decreases leading to insulin resistance condition. Consequently, a person suffering from heart disease is at a higher risk of developing type 2 diabetes. However, this association is still obscure. Few studies argue that a history of cardiovascular disease leads to the incidence of type 2 diabetes [141], while others claim that type 2 diabetes increases the risk of cardiovascular disease [142], [143], [144]. Yeung et al. [141] examined the association between family history of coronary heart disease (CHD) and type 2 diabetes (Table 5). The authors concluded that a high family CHD score is associated to the incidence of type 2 diabetes in individuals who have a positive history of family diabetes. For the individuals having a negative family history of diabetes, this association was non-significant. In summary, it is debatable whether cardiovascular disease is a risk factor for type 2 diabetes or not.
Table 5.
Characteristics and findings of the studies examining the association between cardiovascular disease and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up duration (Years) | Adjusted variables | Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [141] | 2007 | ARIC [36] | PCS | 11297 (11.52%) | M/W | 45–64 | Blacks and Whites (USA) | 9 | Age, sex, race, smoking, alcohol consumption, educational level, leisure index, BMI, WHR, systolic and diastolic pressure, triglycerides, HDL, glucose, hypertension, WBC count, and fibrinogen | CHD risk score -0.5 −0.5 to 0.49 0.5 |
HR 1.0 1.23 (0.98–1.54) 1.43 (1.03–1.99) |
ARIC-Atherosclerosis Risk in Communities, CDH-Coronary Heart Disease, M-Men, W-Women, PCS-Prospective Cohort Study.
3.6. Dyslipidemia
Dyslipidemia refers to an abnormal level of lipids, such as triglycerides and cholesterol. It is characterized by high triglyceride levels, increased low-density lipoproteins (LDL) levels and decreased high-density lipoproteins (HDL) levels [145]. Elevated LDL and lowered HDL levels lead to beta-cell dysfunction inhibiting insulin secretion and consequently type 2 diabetes [146], [147]. Table 6 shows the characteristics and findings of the work in the literature studying the association between dyslipidemia and type 2 diabetes.
Table 6.
Characteristics and findings of the studies examining the association between dyslipidemia and type 2 diabetes.
| Work | Year | Study | Design | Sample Size(%DM) | %M/W | Age (Years) | Ethnicity | Follow-up Duration (Years) | Adjusted Variables | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [148] | 2001 | LWHS [162] | PCS | 35988 | 0/100 | 55–69 | USA | 11 | Age, total energy, WHR, BMI, physical activity, cigarette smoking, alcohol consumption, education, marital status, residential area and hormone replacement therapy | Median cholesterol intake (mg/day) 185 201 237 281 382 |
RR 1.0 0.87 (0.74–1.03) 1.07 (0.91–1.25) 1.10 (0.94–1.28) 1.24 (1.07–1.43) |
|
| [154] | 2015 | CCHS [163] and CGPS [164] | PCS | 47627 | M/W | 20 | Danish | 36 | Age, sex, study, BMI, hypertension, smoking, alcohol intake, physical inactivity, postmenopausal status and hormonal replacement in women, lipid lowering therapy, and educational level | HDL cholesterol (m mol/L) 2.5 2 1.5 1 |
RR 1.0 1.44 (1.08–1.91) 2.72 (2.09–3.54) 5.74 (4.43–7.43) |
|
| [152] | 2018 | REACTION [165] | PCS | 4882 (14.42%) | 36.5/ 63.5 | 40 | Chinese | 3 | Age, sex, smoking, alcohol, physical activity, family history of diabetes, BMI, and systolic blood pressure | Non-HDL/HDL(m mol/L) 1.4 1.9 2.4 3.1 |
OR 1.0 1.2 (0.9–1.5) 1.2 (0.9–1.5) 1.4 (1.1–1.8) |
|
LWHS-Lowa Women’s Health Study, CCHS-Copenhagen City heart Study, CGPS-Copenhagen General Population Study, MA-Meta Analysis, REACTION-Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study, RS-Random Sample, M-Men, W-Women, PCS-Prospective Cohort Study.
Dietary fats, that raise the total cholesterol and LDL levels, are considered significant in the development of type 2 diabetes [148]. Substituting saturated fatty acid with polyunsaturated fatty acid and animal fat with vegetable fat can help lower blood cholesterol and eventually type 2 diabetes. This is because both polyunsaturated fatty acid and vegetable fat are inversely related to the risk of incidence type 2 diabetes with RR 0.84 (95% CI 0.71–0.98) and RR 0.78 (95% CI 0.67–0.91) respectively for the highest quintile of intake [148]. Tajima et al. [149] also confirmed the association between high cholesterol diet intake (273 mg/day) and type 2 diabetes (RR 1.25, 95% CI 1.16–1.36) compared to low cholesterol intake (185 mg/day).
In order to reduce elevated LDL level, LDL lowering therapy and drugs are suggested. However, these drugs and therapy are found to be associated with a higher risk of type 2 diabetes [150]. Individuals having familial hypercholesterolemia, a genetic disorder that results in high LDL levels, are less likely to have type 2 diabetes compared to individuals having high LDL levels due to dietary patterns [151]. Zhang et al. [152] in their analysis found that the ratio of non-HDL and HDL levels is an independent risk factor for incidence diabetes. They show that an individual having a ratio of 3.1 is at 40% increased risk of incidence diabetes (OR 1.4, 95% CI 1.1–1.8) compared to an individual having a ratio of 1.4. Elevated non-HDL and lowered HDL levels are significantly associated with incidence diabetes [153].
On the contrary to studies confirming the association between low-HDL levels and the incidence of type 2 diabetes, Haase et al. [154] in their study concluded that a life-long reduction in HDL levels are not associated with an increased risk of type 2 diabetes. They found that the association is most likely reverse causation, i.e., type 2 diabetes leads to low HDL levels.
3.7. Hypertension
Hypertension, also known as high blood pressure, is a medical condition in which the blood pressure in the arteries is persistently elevated. Hypertension elevates the sympathetic nervous system activity leading to a decrease in the body’s glucose uptake. This causes the condition of insulin resistance and eventually type 2 diabetes. Hypertension elevates sympathetic nervous system activities leading to impaired vasodilation of skeletal muscles. Consequently, muscle glucose uptake decreases with the eventual development of type 2 diabetes. Table 7 shows the characteristics and findings of the work in the literature studying the association between hypertension and type 2 diabetes.
Table 7.
Characteristics and findings of the studies examining the association between hypertension and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up duration (Years) | Adjusted variables | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [166] | 1999 | RS | PCS | 7594 (7.9%) | 100/0 | 30–65 | Japanese | 16 | Age, BMI, alcohol consumption, smoking habits, leisure time physical activity, and parental history of diabetes | Blood pressure in mmHg 130/85 130–139/85–89 139/89 |
RR 1.0 1.39 (1.14–1.69) 1.76 (1.43–2.16) |
|
| [167] | 2000 | ARIC [36] | PCS | 12550 | 44.39/ 55.61 | 45–64 | Blacks and Whites (USA) | 3 and 6 | Age, sex, race, BMI, WHR, educational level, smoking status, alcohol consumption, physical activity, systolic and diastolic blood pressure, fasting serum insulin concentration, history of hypercholesterolemia, cardiovuscular diseases, pulmonary dieseases, renal insufficiency, and family history of diabetes | Hypertension medication None ACE inhibitor Beta-blocker Calcium-channel antagonist Thiazide diuretic |
HR 1.0 0.98 (0.72–1.34) 1.28 (1.04–1.57) 1.17 (0.83–1.66) 0.91 (0.73–1.13) |
|
| [168] | 2007 | WHS [185] | PCS | 38172 (4.38%) | 0/100 | 45 | USA | 10.2 | Age, ethnicity, smoking, BMI, exercise, alcohol consumption, history of hypercholesterolameia, educational level, family history of diabetes, and randomized treatment assignments | Blood pressure in mmHg 120–129/75–84 130–139/85–89 140/90 |
HR 1.0 1.45 (1.23–1.71) 2.03 (1.77–2.32) |
|
| [170] | 2011 | ARIC [36], CARDIA [186], and FHS [187] | PCS | 10893 (9.45%) | 43/57 | 35–54 | African-American and Whites (USA) | Median 8.9 | Age, sex, BMI, fasting glucose, DL cholesterol and triglycerids | Blood pressure in mmHg 119/ 120–139/80–89 140/90 |
HR 1.0 1.32 (1.09–1.61) 1.25 (1.03–1.53) |
|
| [188] | 2012 | GPPS [189] | PCS | 7494 (12.02%) | 100/0 | 47–55 | Swedish | 35 | Age, BMI, cholesterol level, antihypertensive treatment, smoking, physical activity and occupational class | Blood pressure in mmHg 129 130–159 160 84 85–89 90 |
HR 1.0 1.43 (1.12–1.84) 1.95 (1.55–2.46) 1.0 1.34 (1.12–1.62) 1.08 (1.06–1.11) |
|
| [169] | 2015 | KGES [190] | PCS | 7150 (14.7%) | 47.46/ 52.54 | 40–69 | Korean | 8 | Age, BMI, fasting plasma glucose, total cholesterol, HDL cholesterol, family history of diabetes, education, alcohol consumption and smoking status | Blood pressure in mmHg 120/80 120–139/80–89 (M) 120–139/80–89 (W) 140/90 (M) 140/90 (W) |
HR 1.0 1.24 (1.01–1.52) 1.30 (1.03–1.64) 1.65 (1.34–2.05) 1.34 (1.05–1.70) |
|
RS-Random Sample, ARIC-Atherosclerosis Risk in Communities, WHS, Women’s Health Study, CARDIA-Coronary Artery Risk Development in Young Adults, FHS-Framingham Heart Study, GPPS-Gothenburg Primary Prevention Study, KGES-Korean Genome and Epidemiology Study, M-Men, W-Women, PCS-Prospective Cohort Study.
Hayashi et al. [166] examined the association between high normal blood pressure (130 and 140 mmHg/85 and 90) and hypertension (140 mmHg/90 mmHg), and the incidence of type 2 diabetes in men. The authors concluded that both high normal blood pressure (RR 1.39, 95%1.14–1.69) and hypertension (RR 1.75, 95% CI 1.43–2.16) are associated with an increased risk of type 2 diabetes. This association is dependent on obesity and hypertension medications. Hypertension medications are considered to increase the risk of diabetes depending on the type of medication [167]. For instance, hypertensive individuals taking thiazide diuretics and angiotensin-converting-enzyme medications are at lower risk of diabetes compared to the hypertensive individuals not taking any medication. However, those taking beta-blockers medication are at 28% higher risk of incidence type 2 diabetes (HR 1.28, 95% CI 1.04–1.57) [167]. The association between hypertension and the incidence of type 2 diabetes is significant in women as well [168]. Women having hypertension are at 2 times increased risk of developing diabetes (HR 2.03, 95% CI 1.77–2.32) compared to women having normal blood pressure (120/75) [168]. The association is more in overweight and obese women. Irrespective of gender, prehypertension (HR 1.27, 95%CI 1.09–1.48) and hypertension (HR 1.51, 95% CI 1.29–1.76) are associated with increased risk of incidence type 2 diabetes [169]. In the context of ethnicity, whites individuals having hypertension are at higher risk of developing diabetes (HR 1.25, 95% CI 1.03–1.53), but no such association is seen in African American hypertensive individuals (HR 0.92, 95% CI 0.70–1.21) [170].
In summary, hypertension is associated with the development of type 2 diabetes in both men and women. However, the association is ethnicity-dependent. The selection of hypertensive medications should be made properly as the medication impacts the strength of the association. Furthermore, an obese individual with hypertension is at higher risk compared to a non-obese.
3.8. Aging
The number of elderly people (above 60 years) is increasing worldwide. The 900 million global elderly population in 2015 is expected to rise to 2 billion by 2050 [171]. Aging increases the risk of metabolic syndrome and chronic diseases including type 2 diabetes. Aging increases chronic inflammation in an elderly individual leading to insulin resistance [172]. In addition, lipid metabolism disorder due to aging increases the accumulation of body fat leading to elevated free fatty acids concentration in the blood/plasma and eventually insulin resistance [173]. Consequently, an aged individual is at higher risk of developing type 2 diabetes. However, there is not much work concluding that aging is an independent risk factor for type 2 diabetes. Choi et al. [174] concluded that the risk of diabetes increases with aging only in overweight individuals, and the risk decreases with a moderate level of physical activity. Aging can be considered as triggering the association between independent risk factors and risk of diabetes, but more evidence and studies are required to examine the association between aging as an independent factor and diabetes.
3.9. Ethnicity
Ethnicity is associated with a range of health complications including diabetes because of the heterogeneity in the demographic environmental conditions and lifestyle. It is an independent risk factor which tends to be exacerbated by the social disadvantage and the affluent way of living. Table 8 shows the characteristics and findings of the work in the literature studying the association between ethnicity and type 2 diabetes. Compared to white individuals, type 2 diabetes is more prevalent in Pacific Islanders (OR 3.1, 95% CI 1.4–6.8), followed by Blacks (OR 2.3, 95% CI 2.1–2.6), Native Americans (OR 2.2, 95% CI 1.6–2.9), Hispanics (OR 2.0, 95% CI 1.8–2.3), and Multiracial (OR 1.8, 95% CI 1.5–2.9) [175]. In another study by Shai et al. [176], it was found that compared to whites, Asians (RR 1.94, 95% CI 1.46–2.58), Hispanics (RR 1.70, 95% CI 1.28–2.26), and Blacks (RR 1.36, 95% CI 1.14–1.63) are at higher risk of incidence type 2 diabetes.
Table 8.
Characteristics and findings of the studies examining the association between ethnicity and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up duration (Years) | Adjusted variables | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [177] | 1983 | RS | - | 2638 | 46.81/53.19 | 20 | Melanesians and Indians | - | Age | The prevalence of diabetes in rural Indian men is 7.5 times more than rural Melanesian men, and is 2.93 times more in urban Indian males compared to urban Melanesian men. For women, the prevalence in rural and urban Indians is 12.6 and 1.5 times more compared to rural and urban Melanesians respectively. | ||
| [179] | 1985 | RS | - | 61130 (1.87%) | M/W | All age | Asians and Europeans | - | Age | The prevalence of diabetes in Asians was 3.8 times higher than in Europeans. For the patients age between 40–64, the prevalence was at least 5 times higher in Asians. | ||
| [180] | 1988 | RS | - | 253 | 65.6/ 34.4 | 35–69 | Bangladeshi and Non-Asian | - | Age | The prevalence of diabetes in Bangladeshi men and women is 2.2 and 5.75 times compared to Non-Asian men and women respectively. | ||
| [181] | 1989 | RS | - | 4020 | 48.4/ 51.6 | 20–79 | Asian and White | - | Age | The prevalence of diabetes in Asian men and women are 4 and 2 times compared to White men (11.2% vs 2.8%) and women (8.9% vs 4.3%) respectively. | ||
| [175] | 2003 | BRFSS [191] | - | 163584 | 48.6/51.4 | 30 | Asian, Black, Hispanic, Native American, Pacific Islander, White, Other and Multiracial | - | Age, sex and BMI | Ethnicity White Asian Black Hispanic Native American Pacific Islander Other Multiracial |
OR 1.0 1.0 (10.7–1.4) 2.3 (2.1–2.6) 2.0 (1.8–2.3) 2.2 (1.6–2.9) 3.1 (1.4–6.8) 1.4 (1.0–1.9) 1.9 (1.5–2.9) |
|
| [176] | 2006 | NHS [80] | PCS | 78419 (4.90%) | 0/100 | 30–55 | White, Asian, Hispanic, and Black | 20 | Age, BMI, family history of diabetes, alcohol consumption, physical exercise, and smoking | Ethnicity White Asian Hispanic Black |
RR 1.0 1.94 (1.46–2.58) 1.70(1.28–2.26) 1.36(1.14–1.63) |
|
RS-Random Sample, BRFSS-Behavioral Risk Factor Surveillance System, NHS-Nurses’ Health Study, PCS-Prospective Cohort Study.
A study by Zimmet et al. [177] showed that type 2 diabetes is 10 times more prevalent in rural Indians compared to rural Melanesians, and 2 times more prevalent in urban Indians compared to urban Melanesians. They also revealed that the prevalence is 5 times more in urban Melanesians compared to rural Melanesians. One of the reason could be that the rural residents have an increased amount of physical activity compared to the urban ones, leading to decreased risk of diabetes [178]. It should thus important to have a moderate amount of physical activity as a therapy for diabetes prevention. Compared to Europeans, type 2 diabetes is 3.8 times more prevalent in Indians, and the prevalence increases to 5 times for 40–64 years old individuals [179]. In another comparison between Asian and non-Asian ethnicity, it is found that the prevalence of type 2 diabetes in Bangladeshis (Asians) is more [180]. Furthermore, the prevalence is high in women (5.75 times) compared to that in men (2.2 times). However, ethnicity can not be considered as an independent risk factor for this association as Bangladeshis had higher smoking rates and a lower ratio of polyunsaturated fatty acids to saturated fatty acids. Consequently, ethnicity, smoking and dyslipidemia all contributed to the risk of incidence type 2 diabetes. Simmons et al. [181] also confirmed in their study that the prevalence is more in Asians compared to Whites. However, in contrast to the results obtained by [180], Simmons et al. [181] found that the prevalence is more in men compared to women. This inconsistency should be examined further.
In summary, ethnicity is associated with the incidence of type 2 diabetes. However, there is no definite explanation of why individuals of a particular ethnicity are at higher risk of type 2 diabetes compared to the others. One possible explanation can be the ethnicity-dependent relation between BMI and body fat. For instance, Asians have around 3–4 kg/ lower BMI compared to Caucasians for a given percentage of body fat [182]. Another reason could be ethnicity-based insulin sensitivity. Studies show that Asians, Blacks and Mexican Americans are less insulin sensitive compared to non-Hispanic Whites [183], [184].
3.10. Family history of diabetes
Family history information can serve as a useful tool for prognosis/diagnosis and public health. Family history of diabetes reflects both genetic as well as environmental factors and can lead to better prediction of incidence type 2 diabetes than only genetic factors and environmental factors alone [192]. Table 9 shows the characteristics and findings of the work in the literature studying the association between family history of diabetes and type 2 diabetes.
Table 9.
Characteristics and findings of the studies examining the association between family history of diabetes and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up duration (Years) | Adjusted variables | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [204] | 1981 | RS | - | 3177 | - | >5 | Pima Indians | - | Age and BMI | Family History No Mother/father both |
OR 1.0 2.3 3.9 |
|
| [195] | 1993 | SAHS | - | 4914 | 43/ 57 | Mean 42–44.8 | Mexicans, Americans and Non-Hispanics | 9 | Age and ethnicity | Family History No Mother father both Family History No Mother father both |
OR (Men) 1.0 3.44 (2.32–5.12) 3.49 (2.16–5.64) 3.73 (1.72–8.08) OR (Women) 1.0 2.03 (1.47–2.81) 1.35 (0.83–2.19) 2.59 (1.41–4.77) |
|
| [200] | 1993 | MRFIT [40], [41], [42] | - | 5905 | 100/0 | - | Blacks and Whites (USA) | 6 | Age | Family History No Mother/father Family History No Mother/father |
RR (Black) 1.0 3.62 (1.55–8.47) RR (White) 1.0 1.85 (1.38–2.48) |
|
| [205] | 1994 | MA | PCS | 11334 | M/W | 40 | Taiwan | - | - | Family History Age at onset 40–49 No Mother father Family History Age at onset 50–59 No Mother father Family History Age at onset 60 No Mother father |
OR 1.0 4.41 (1.71–10.13) 2.21 (0.25–8.86) RR 1.0 1.57 (0.40–4.41) 2.80 (0.54–9.07) RR 1.0 1.22 (0.38–3.05) 0.56 (0.01–3.31) |
|
| [194] | 1995 | THHP | - | 7210 (12.81%) | 100/0 | 45–68 | Japanese-American | 6 | Age, BMI, subscapular skinfold, triceps ratio, physical activity, glucose, triglycerids, and systolic blood pressure | Family History No yes |
OR 1.0 1.73 (1.29–2.33) |
|
| [201] | 2000 | RS | PCS | 1947 (7.34%) | 100/0 | Mean 49.5–50.3 | Norway | 22.5 | Age, BMI, fasting glucose, fitness and triglycerids | Family History No Mother Father Both |
OR 1.0 2.51 (1.55–4.07) 1.41 (0.657–3.05) 3.96 (1.22–12.9) |
|
| [198] | 2000 | EPIC [101] | CSS | 6473 | 45.54/ 54.46 | 45–74 | USA | 22.5 | Age and sex | Family History No BMI 22.5–24.9 BMI 27.5–29.9 BMI 30–34.9 BMI 35 Yes BMI 22.4 BMI 22.5–24.9 BMI 25–27.4 BMI 27.5–29.9 BMI 30–34.9 BMI 35 |
OR 1.0 2.0 (1.2–3.1) 2.5 (1.6–4.0) 6.1 (3.4–11.2) 1.1 (0.2–5.1) 2.6 (1.3–5.3) 2.8 (1.5–5.3) 2.2(1.1–4.6) 6.4 (3.6–11.3) 26.7 (14.4–49.4) |
|
| [202] | 2000 | FHS [187] | - | 2527 | M/W | 26–82 | African-American and White (USA) | 40 | Age | Family History No Mother Father Both |
OR 1.0 3.4 (2.3–4.9) 3.5 (2.3–5.2) 6.1 (2.9–13.0) |
|
| [203] | 2001 | MONICA [35] | CSS | 12751 | 49.6/ 50.4 | - | Germany | - | Age and sex | Family History No Mother Father |
OR 1.0 2.9 (2.3–3.6) 2.8 (2.1–3.8) |
|
| [197] | 2007 | NHANES [88] | - | 16388 | 49.3/ 50.7 | 18 | USA | 6 | sex, race/ethnicity, age, BMI, hypertension, and household income | Family History Average risk Moderate risk High risk |
OR 1.0 2.3 5.5 |
|
| [199] | 2009 | NHANES [88] | CSS | 10899 | 48/ 52 | Mean 51.3–61 | Blacks, Whites and Hispanics | 5 | Age and sex | Black Average risk, BMI24.9 High risk, BMI24.9 High risk, 25BMI29.9 High risk, BMI30 Hispanic Average risk, BMI24.9 High risk, BMI24.9 High risk, 25BMI29.9 High risk, BMI30 |
OR 1.0 20.4 (6.5–64.5) 5.2 (2.2–12.3) 5.0 (2.5–10.3) 1.0 14.0 (3.4–58.0) 5.6 (1.8–17.3) 8.5 (3.8–19.4) |
|
| [196] | 2011 | RS | CSS | 3723 | 49.1/ 50.9 | 7–15 | Mexican | 2 | Age, sex, and BMI | Family History No vYes |
OR 1.0 11.7 (9.5–21.2) |
|
| [193] | 2016 | MIDUS 1 and 2 [212] | - | 978 | 45/ 55 | 34–84 | Black and White (USA) | - | Age, sex, and socioeconomic status | Family History No Yes |
OR 1.0 2.77 (2.03–3.78) |
|
RS-Random Sample, SAHS-San Antonio Heart Study, MRFIT-Multiple Risk Factor Intervention Trial, MA-Meta Analysis, THHP-The Honolulu Heart Program, EPIC-European Prospective Investigation into Cancer, FHS-Framingham Heart Study, MONICA-Multinational MONItoring of trends and determinants in CArdiovascular disease, NHANES-National Health and Nutrition Examination Survey, PD-Prediabetes, IFG-Impaired Fasting Glucose, IGT-Impaired Glucose Tolerance, M-Men, W-Women, PCS-Prospective Cohort Study, CSS-Cross-Sectional Study.
A study by Tsenkova et al. [193] revealed that a family history of diabetes is strongly associated with incidence diabetes (OR 2.77, 95% CI 2.03–3.78). Another study also shows that parental history of diabetes is an independent risk factor for diabetes (OR 1.73, 95% CI 1.29–2.33) [194]. However, the association becomes weaker in men free of cardiovascular disease (OR 1.63, 95% CI 1.18-.2.24). Moreover, the association is much higher in 45–54 years old men (OR 1.99, 95% CI 1.38–2.89) compared to 55–68 years old men (OR 1.33, 95% CI 0.70–2.52). Furthermore, the prevalence of type 2 diabetes is stronger in men compared to women [195]. This indicates that parental history of diabetes in combination with other risk factors such as aging, gender and cardiovascular diseases, increases the risk of incidence type 2 diabetes.
Rodríguez-Moran et al. [196] showed that a family history of diabetes in first degree of relative (parents, offspring and siblings) is a strong and independent risk factor for the prevalence of impaired fasting glucose (prediabetes) (OR 11.7, 95% 9.5–21.2) in children and adolescents. This is in the absence of obesity. The results reveal that is it important to consider the parental history of diabetes while screening for diabetes children and adolescents. This is because only obesity-based screening could lead to underestimation. Valdez et al. [197] also showed that the family history of diabetes in at least two first-degree relatives or one first-degree and at least two second-degree relatives is significant for prevalence of type 2 diabetes. However, it can not be denied that the presence of a family history of diabetes can make the association between obesity and diabetes stronger [198]. Given a BMI35, an individual with a family history of diabetes is at a higher risk of incidence diabetes (OR 26.7, 95% CI 14.4–49.4) compared to the one without a family history of diabetes (OR 6.1, 95% CI3.4–11.2). Furthermore, ethnicity is also considered an important factor in an obese individual with a family history of diabetes [199], [200].
An individual having a family history of diabetes can have an early onset of diabetes compared to the ones without a family history. However, it is hard to conclude that which among the maternal, paternal and both maternal and paternal family history of diabetes is more significant for incidence/prevalence of type 2 diabetes as the results in the literature are inconsistent [195], [201], [202], [203], [204], [205].
3.11. Obesity
Obesity is a complex health condition that involves an excessive amount of body fat. It is defined by the BMI and further evaluated in terms of fat distribution via the waist-hip ratio. Abdominal fat in the body increases inflammation which decreases insulin sensitivity by disrupting the function of beta-cells. The insulin resistance condition then leads to the prevalence of type 2 diabetes. Table 10 shows the characteristics and findings of the work in the literature studying the association between obesity and type 2 diabetes.
Table 10.
Characteristics and findings of the studies examining the association between obesity and type 2 diabetes.
| Work | Year | Study | Design | Sample size (%DM) | %M/W | Age (Years) | Ethnicity | Follow-up duration (Years) | Adjusted variables | Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [206] | 2002 | - | PCS | 4737 | 100/0 | 45–64 | Japanese | 4 | Age, smoking status, alcohol intake, family history, and baseline value of fasting blood glucose. | BMI (kg/) 18.49 29 30 and 35 |
RR 1.0 5.16 (1.92–13.80) 5.25 (1.96–14.04) |
| [213] | 2007 | BWHS [214] | - | 49766 (4.96%) | 0/100 | 21–69 | African-American (USA) | 8 | Age, physical activity, family history of diabetes, cigarette smoking, years of education, and time period of data collection | BMI (kg/) 23 45 |
IRR 1.0 23 (17–31) |
| [119] | 2001 | - | PCS | 84941 (3.88%) | 0/100 | 30–55 | - | 16 | Age (in five-year categories), time (eight periods), presence or absence of a family history of diabetes, menopausal status, and use or nonuse of postmenopausal hormone therapy | BMI (kg/) 23 23–24.9 25–29.9 30–34.9 35 |
RR 1.0 2.67 (2.13–3.34) 7.59 (6.27–9.19) 20.1 (16.6–24.4) 38.8 (31.9–47.2) |
| [207] | 2006 | 27 cohorts | PCS + CSS | 154989 (0.20%) | 54/ 46 | Mean 51 | - | Mean 8 | Age, sex, cohort, and smoking habit | 5.3 cmEach 2 kg/ lower BMI is associated with a 23% (15–30%) lower risk of total DM in men and 27% (23–31%) lower risk in women. In the Asian cohort, each 2 kg/ lower BMI was associated with a 37% (26–46%) lower risk and in Australasian cohorts the same reduction in BMI was associated with 25% (21–29%) lower risk. | |
| [208] | 2006 | RS | - | 827 (7.86%) | - | - | Japanese | 10 | Age, sex, total cholesterol, systolic pressure, smoking and overall obesity | WC (cm) 85 (M) 90 (W) |
RR 2.07 (1.03–4.16) |
| [209] | 2006 | TLGS | PCS | 4479 (3.70%) | 41.34/ 58.66 | 3 | Tehran | 3.6 (mean) | Age, smoking, family history of diabetes, HTN, TG, HDL and other anthropometric variables | 5.3 cmCentral obesity is defined as WC 102 cm in men and WC 88 cm in women. The central obese individuals 60 years old are at higher risk of incidence type 2 diabetes (OR 3.8, 95% CI 1.8–7.7). | |
| [210] | 2009 | RS | - | 5071 | 37.80/ 62.2 | 40 | Chinese | - | Educational level, age group, smoking and alcohol drinking | WC (cm) 90(M) 90(M) 80(W) 80(W) |
OR 1.0 2.308 (1.473–3.615) 1.0 2.875(1.987–4.160) |
| [211] | 2001 | MAHES | - | 835 | 39.16/ 60.84 | 60–92 | Hispanics and Non-Hispanics | - | Age, physical activity and smoking | WC (cm) 102(M)(H) 102(M)(NH) 88(W)(H) 88(W)(NH) |
OR 2.1(1.2–3.9) 0.9 (0.3–3.1) 1.6 (1.0–2.8) 15.1(1.9–117.6) |
SWHS-Shanghai Women’s Health Study, BWHS- Black Women’s Health Study, RS-Random Sample, WC-Waist Circumference, TLGS-Tehran Lipid and Glucose Study, MAHES-Massachusetts Hispanic Elderly Study, H-Hispanics, NH-Non Hispanics, M-Men, W-Women, PCS-Prospective Cohort Study, CSS-Cross-Sectional Study.
Ishikawa-Takata et al. [206] found that the risk of diabetes increases significantly for an individual having a BMI greater than 29 kg/. The relative risk of diabetes increases up to 38.8 (95% CI 31.9–47.2) for an individual having a BMI greater than 34.9 kg/ [119]. Furthermore, study shows that the association between obesity and incidence diabetes is gender-dependent [207]. For each 2 kg/ lower BMI, men are at 23% (15–30%) lower risk of diabetes, whereas women are at 27% (23–32%) lower risk. Further, the association between obesity and diabetes is also dependent on ethnicity [207]. For each 2 kg/ lower BMI, Asians are at 37% (26–46%) lower risk of diabetes, whereas Australians are at 25% (21–29%) lower risk.
Ohnishi et al. [208] found that compared to overall obesity, central obesity is highly associated with the risk of type 2 diabetes (RR 2.07, 95% CI 1.03–4.16). This association is more in elderly people (60 years) (OR 3.8, 95% CI 1.8–7.7) [209]. The association between central obesity and the incidence of type 2 diabetes is found significant in both men and women. However, centrally obese women are at higher risk (OR 2.875, 95% CI 1.987–4.160) compared to centrally obese men (OR 2.308, 95% CI 1.473–3.615) [210]. The prevalence of type 2 diabetes in obese individual is ethnicity dependent [211]. Non-Hispanics centrally obese women are at higher risk of developing type 2 diabetes (OR 15.1, 95% CI 1.9–117.6) compared to centrally obese Hispanic women (OR 1.6, 95% CI 1.0–2.8). The centrally Hispanic men are also at risk of developing type 2 diabetes (OR 2.1, 95% CI 1.2–3.9). No such association is found in centrally obese Non-Hispanic men. However, all these studies examining the association between central obesity and the incidence of type 2 diabetes consider different definitions of central obesity. For instance, [208] defines central obesity as waist circumference (WC) 85 cm in men and 90 cm in women, whereas [211] defines it as WC102 cm in men and 88 cm in women. Consequently, it is difficult to conclude the association between central obesity and the incidence of type 2 diabetes.
In summary, although obesity is a significant predictor, the association between obesity and diabetes is a factor of gender and ethnicity. Women with high BMI are at greater risk of diabetes compared to men. Moreover, the association is stronger in Asians compared to Australians. The association between central obesity is also found to be significant for the prevalence of type 2 diabetes. This association is the strongest in Non-Hispanics women. However, more studies are required to examine the association between central obesity and type 2 diabetes following one standard criterion defining central obesity.
3.12. Physical inactivity
An individual is considered physically inactive if he/she does not get the recommended 30–60 min of exercise three to four times a week. Physical inactivity decreases insulin sensitivity with progressive loss of beta-cells. This leads to impaired glucose tolerance and eventually type 2 diabetes. However, no work examines the association between physical inactivity as an independent factor and the prevalence of diabetes. One of the reasons that physical inactivity leads to type 2 diabetes can be that physical inactivity can cause obesity which in turn is a significant risk factor for type 2 diabetes.
4. Conclusion
Diabetes is a global crisis that is primarily driven by rapid urbanization, changing lifestyles, and uneven dietary patterns [215], [216]. It is crucial to predict the prevalence of diabetes in an individual to reduce the risk of diabetes development and save lives. Diabetes is thought to prevail due to several risk factors such as high-level serum uric acid, sleep quality/quantity, smoking, depression, cardiovascular disease, dyslipidemia, hypertension, aging, ethnicity, family history of diabetes, physical inactivity, and obesity. Studies in the literature have examined the association between each of these risk factors and the risk of developing type 2 diabetes. In this review, we provide an analysis of the studies in the literature to deduce inferences on the relationship between the risk factors and incidence/prevalence of type 2 diabetes.
In conclusion, it can be observed that sleep quantity/quality, smoking, dyslipidemia, hypertension, ethnicity, family history of diabetes, obesity and physical inactivity are strongly associated with the development of type 2 diabetes. Both sleep quantity and quality are found to be strongly associated with the development of type 2 diabetes. The association is stronger in women sleeping for more hours and in men sleeping for fewer hours. However, the sleeping quantity and quality data in these studies are self-reported by the participants, and therefore, prone to errors. More studies are required that use measurement techniques for data collection to validate the association between sleep quantity/quality and type 2 diabetes. Smoking is also found to be a significant risk factor for type 2 diabetes. Both active and passive smokers are at higher risk of developing type 2 diabetes. Moreover, the risk for developing type 2 diabetes remains high in ex-smokers for the first 5–10 years of smoking cessation. Dyslipidemia is associated with the development of type 2 diabetes. Increased non-HDL and decreased HDL levels are strongly associated with type 2 diabetes. However, in the majority of these studies, the incidence or prevalence of type 2 diabetes is self-reported. Consequently, further studies are needed to validate this association between dyslipidemia and type 2 diabetes using standardized measurement techniques, such as A1C test [217]. Hypertension is a significant risk factor for type 2 diabetes and this is further elevated in obese individuals. Ethnicity strongly associates with the development of type 2 diabetes. This could be due to the fact that insulin sensitivity varies among individuals of different ethnicity. Family history of diabetes in first degree of relatives is strongly associated with the development of type 2 diabetes. In addition, family history of diabetes also signifies the association between obesity and type 2 diabetes. Obesity is found to a significant risk factor for incidence of type 2 diabetes and the association is stronger in women compared to men.
The association between serum uric acid and type 2 diabetes remains obscure. It can not be concluded that serum uric acid is an independent risk factor for type 2 diabetes or it only elevates the association between other independent risk factors such as obesity, hypertension, and dyslipidemia, and type 2 diabetes. Moreover, our analysis shows that there might be no association between serum uric acid and the development of type 2 diabetes, but rather there might be a reverse association, i.e., diabetes leads to elevated serum uric acid level. Similarly, based on the evidence in the literature, aging can not be considered as an independent risk factor for type 2 diabetes. Aging only emphasizes the association between obesity and type 2 diabetes. Depression as well is not found to an independent risk factor contributing to the development of type 2 diabetes. Rather, the activities related to depression such as physical inactivity, poor diet, and obesity leads to diabetes. There is no sufficient evidence to conclude the association between cardiovascular disease and type 2 diabetes. It is debatable whether cardiovascular disease leads to the development of type 2 diabetes. Consequently, more studies are required to study the direct association between these risk factors, i.e., serum uric acid, aging, depression, and cardiovascular disease, and incidence of type 2 diabetes.
Based on this study, we devise recommendations to different stakeholders leading to better patient care. In particular, we provide recommendations for allied healthcare professionals, individuals, and government institutions as follows:
-
•
Allied healthcare professionals:The hypertensive medications and the LDL lowering therapy and drugs should be carefully prescribed as they are associated with increased risk of type 2 diabetes. In addition, overweight and obese adults should be screened for diabetes.
-
•
Individuals:A healthy lifestyle, which involves intake of polyunsaturated fatty acids and vegetable fats, regular exercise, a healthy diet and proper sleep, is crucial. Individuals should avoid both active and passive smoking.
-
•
Government:Physical activity in the nation should be promoted for a healthy nation. Law policies should be implemented to restrict public smoking as passive smoking significantly increases the risk of type 2 diabetes. For instance, designated smoking areas can be established to eliminate the risk of developing passive smokers. It would be beneficial to have periodic surveys that include the demographic and lifestyle features of the citizens and the surveys’ results can be then used to develop a nation-wide diabetes prevention plan, in coordination with the allied health professionals.
CRediT authorship contribution statement
Leila Ismail: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing. Huned Materwala: Investigation, Writing - original draft. Juma Al Kaabi: Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is funded by the National Water and Energy Center of the United Arab Emirates University (Grant No. 31R215). We thank the anonymous reviewers for their valuable comments which helped us improve the paper.
Appendix A.
Table A1.
Search string used to retrieve the studies on the association between risk factor and type 2 diabetes.
| Risk factor | Search string |
|---|---|
| Serum uric acid | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“uric acid” OR uric-acid OR hyperuricemia OR “serum uric acid” OR gout) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Sleep quantity/quality | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“sleep hour” OR “sleeping hour” OR “hours of sleep” OR “sleep duration” OR “sleep time” OR “sleep length” OR “sleep period” OR “sleeping time” OR “sleep span” OR nap OR napping OR “daytime sleep” OR vsleep quality” OR “sleep disturbance” OR “sleep apnea” OR insomnia OR “sleep deprivation”) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Smoking | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (smoking OR “smoking cessation” OR cigarette OR “cigarette smoking” OR “passive smoking” OR “secondhand tobacco smoke”) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Depression | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“depressive disorder” OR depression OR “dysthymic disorders”) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Cardiovascular disease | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“cardiovascular disease” OR stroke OR “heart disease”) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| dyslipidemia | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (cholesterol OR “cholesterol intake” OR “cholesterol consumption” OR diet* OR fat OR “density lipoprotein” OR density-lipoprotein OR dyslipidemia) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Hypertension | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“high blood pressure” OR “blood pressure” OR hypertensi* OR “Hypertension-*”) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Aging | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (age OR aging OR old OR elderly) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Ethnicity | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (ethnicity OR race OR *rac* OR community) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Family history of diabetes | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“family history” OR “parental history” OR “parental diabetes” OR “parental transmission” OR paternal OR maternal) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Physical inactivity | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“physical inactivity”) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
| Obesity | (risk OR “risk factor” OR etiology OR association OR development OR progression OR incidence) AND (“body mass index” OR BMI OR “body fat distribution” OR “over weight” OR overweight OR obesity OR “weight change” OR “weight gain” OR “central obesity”) AND (diabetes OR “diabetes mellitus” OR “type 2 diabetes” OR “type II diabetes” OR “non-insulin dependent diabetes” OR “non insulin dependent diabetes” OR “noninsulun dependent diabetes”) |
Table A2.
Quality assessment of the included studies according to the Quality assessment tool for observational cohort and cross-sectional studies.
| Work | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Quality | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-level serum uric acid | ||||||||||||||||
| [14] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes* | NR | NR | Yes | Yes | Good | |
| [21] | Yes | Yes | NR | Yes | No | CD | No | No | NR | No | CD | NR | No | Yes | Poor | |
| [22] | Yes | Yes | Yes | CD | No | Yes | Yes | CD | Yes | Yes | No | NR | No | Yes | Fair | |
| [23] | No | Yes | NR | Yes | No | Yes | Yes | CD | Yes | Yes | Yes | NR | CD | Yes | Fair | |
| [19] | No | No | NR | NR | No | Yes | CD | No | NR | Yes | NR | NR | NR | No | Poor | |
| [15] | No | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes | Good | |
| [24] | No | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes | Good | |
| [16] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NR | Yes | NR | NR | NR | Yes | Fair | |
| [25] | Yes | Yes | NR | No | No | Yes | Yes | No | Yes | Yes | Yes | NR | NR | Yes | Fair | |
| [26] | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | Yes | Yes | NR | Yes | Yes | Fair | |
| [27] | Yes | No | NR | CD | No | Yes | Yes | No | Yes | Yes | Yes | NR | NR | Yes | Fair | |
| [30] | No | Yes | NR | Yes | No | No | No | Yes | Yes | NR | Yes | NR | NR | Yes | Fair | |
| Sleep quantity/quality | ||||||||||||||||
| [79] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | Yes | Yes | NR | NR | Yes | Fair | |
| [56] | No | No | NR | Yes | No | Yes | Yes | NA | No | Yes | Yes | NR | Yes | Yes | Fair | |
| [57] | Yes | Yes | NR | Yes | No | Yes | Yes | NA | No | No | No | NR | NR | Yes | Fair | |
| [59] | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | No | No | Yes | NR | NR | Yes | Good | |
| [83] | No | Yes | NR | Yes | No | No | No | Yes | No | NR | Yes | NR | NR | Yes | Fair | |
| [52] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | No | NR | Yes | Yes | Fair | |
| [50] | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | NR | No | Yes | Fair | |
| [85] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | Yes | NR | NR | No | Yes | Fair | |
| [87] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | Yes | No | NR | Yes | Yes | Fair | |
| [89] | Yes | Yes | NR | Yes | No | No | No | Yes | No | No | Yes | NR | NR | Yes | Fair | |
| [58] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | CD | Yes | NR | NR | Yes | Fair | |
| [92] | Yes | Yes | NR | Yes | No | No | No | Yes | No | NR | Yes | NR | NR | Yes | Fair | |
| [94] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | NR | Yes | NR | NR | Yes | Fair | |
| [55] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | No | Yes | NR | Yes | Yes | Fair | |
| [96] | CD | Yes | Yes | No | No | No | No | Yes | No | NR | Yes | NR | CD | Yes | Fair | |
| [97] | Yes | No | No | Yes | No | Yes | Yes | Yes | No | NR | Yes | NR | NR | Yes | Fair | |
| [98] | Yes | Yes | CD | Yes | No | Yes | Yes | Yes | No | No | No | NR | NR | Yes | Fair | |
| [100] | Yes | Yes | NR | CD | No | No | No | Yes | No | NR | Yes | NR | NR | Yes | Fair | |
| [51] | No | Yes | CD | Yes | No | Yes | No | Yes | No | Yes | No | NR | Yes | Yes | Fair | |
| [102] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | NR | No | NR | Yes | Yes | Fair | |
| [53] | Yes | Yes | Yes | No | No | No | No | Yes | No | NR | No | NR | NR | Yes | Fair | |
| [104] | Yes | Yes | NR | CD | No | No | No | Yes | No | NR | Yes | NR | NR | Yes | Fair | |
| [106] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | NR | No | Yes | Good | |
| [54] | Yes | Yes | Yes | No | No | No | No | Yes | No | Yes | No | NR | NR | Yes | Fair | |
| [108] | Yes | Yes | No | Yes | No | Yes | NR | No | No | NR | No | NR | NR | Yes | Poor | |
| Smoking | ||||||||||||||||
| [110] | No | Yes | NR | Yes | No | Yes | Yes | No | NA | Yes | No | NR | NR | Yes | Fair | |
| [67] | No | Yes | NR | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | Yes | NR | Yes | Good | |
| [68] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | No | Yes | Yes | Good | |
| [112] | No | No | Yes | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | Yes | Yes | Good | |
| [73] | CD | Yes | NR | Yes | No | No | No | Yes | NA | NR | No | NR | NR | Yes | Poor | |
| [114] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | Yes | Yes | Good | |
| [69] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NA | Yes | No | Yes | NR | Yes | Good | |
| [116] | No | Yes | NR | Yes | No | No | No | No | NA | NR | Yes | NR | NR | Yes | Poor | |
| [65] | Yes | Yes | NR | Yes | No | Yes | Yes | No | NA | Yes | Yes | NR | Yes | Yes | Fair | |
| [63] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NA | NR | CD | NR | NR | Yes | Fair | |
| [119] | No | Yes | No | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | Yes | Yes | Good | |
| [120] | No | CD | NR | CD | No | Yes | Yes | Yes | NA | Yes | NR | NR | NR | Yes | Fair | |
| [74] | Yes | Yes | Yes | CD | No | No | No | No | NA | No | Yes | NR | NR | Yes | Fair | |
| [122] | Yes | Yes | Yes | Yes | No | Yes | Yes | No | NA | No | Yes | No | No | Yes | Good | |
| [70] | Yes | Yes | No | Yes | No | Yes | Yes | No | NA | No | Yes | NR | NR | Yes | Fair | |
| [77] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | CD | Yes | Good | |
| [71] | Yes | CD | Yes | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | NR | Yes | Good | |
| [75] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | NR | Yes | Good | |
| [66] | No | Yes | Yes | Yes | No | Yes | Yes | No | NA | No | Yes | NR | No | Yes | Good | |
| [64] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NA | Yes | CD | NR | NR | Yes | Fair | |
| [72] | Yes | No | NR | CD | No | Yes | Yes | Yes | NA | Yes | Yes | NR | Yes | Yes | Good | |
| [78] | Yes | Yes | Yes | Yes | No | Yes | Yes | No | NA | Yes | Yes | NR | Yes | Yes | Good | |
| [76] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | Yes | Yes | Good | |
| [128] | No | Yes | NR | Yes | No | Yes | Yes | No | NA | NR | No | NR | NR | Yes | Fair | |
| Depression | ||||||||||||||||
| [137] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | NR | Yes | Yes | Good | |
| [155] | No | Yes | NR | Yes | No | Yes | Yes | No | Yes | No | No | NR | NR | Yes | Fair | |
| [157] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | NR | No | NR | NR | Yes | Fair | |
| [158] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | NR | Yes | NR | NR | Yes | Fair | |
| [136] | Yes | Yes | NR | Yes | No | Yes | CD | No | Yes | Yes | Yes | NR | CD | Yes | Fair | |
| [160] | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | NR | NR | Yes | Good | |
| [138] | No | Yes | NR | Yes | No | Yes | Yes | No | Yes | No | CD | NR | NR | Yes | Fair | |
| [139] | No | Yes | NR | Yes | No | Yes | Yes | No | Yes | No | Yes | NR | NR | Yes | Fair | |
| Cardiovascular disease | ||||||||||||||||
| [141] | No | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes | Good | |
| Dyslipidemia | ||||||||||||||||
| [148] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | CD | CD | No | NR | No | Yes | Fair | |
| [154] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | NR | NR | NR | NR | Yes | Yes | Fair | |
| [152] | No | Yes | NR | Yes | No | Yes | CD | Yes | Yes | No | Yes | NR | Yes | Yes | Fair | |
| Hypertension | ||||||||||||||||
| [166] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | NR | Yes | NR | Yes | Yes | Good | |
| [167] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | No | Yes | NR | NR | Yes | Good | |
| [168] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | No | No | NR | CD | Yes | Fair | |
| [170] | Yes | Yes | NR | No | No | Yes | Yes | Yes | Yes | No | Yes | NR | NR | Yes | Good | |
| [188] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | NR | CD | Yes | Good | |
| [169] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | NR | NR | Yes | Good | |
| Ethnicity | ||||||||||||||||
| [177] | No | Yes | Yes | No | No | No | No | NA | NA | NR | Yes | NR | NR | Yes | Fair | |
| [179] | No | Yes | Yes | No | No | No | No | NA | NA | NR | NR | NR | NR | Yes | Poor | |
| [180] | No | Yes | Yes | No | No | No | No | NA | NA | No | Yes | NR | NR | Yes | Fair | |
| [181] | Yes | Yes | Yes | CD | No | No | No | NA | NA | No | Yes | NR | NR | Yes | Fair | |
| [175] | Yes | Yes | Yes | Yes | No | No | No | NA | NA | No | No | NR | NR | Yes | Poor | |
| [176] | Yes | Yes | NR | Yes | No | Yes | Yes | NA | NA | Yes | Yes | NR | NR | Yes | Good | |
| Family history of diabetes | ||||||||||||||||
| [204] | No | No | NR | CD | No | Yes | NR | No | Yes | NA | Yes | NR | NR | Yes | Fair | |
| [195] | Yes | Yes | Yes | Yes | No | No | No | Yes | No | NA | Yes | NR | NR | Yes | Fair | |
| [200] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | No | NA | Yes | NR | NR | Yes | Fair | |
| [205] | No | Yes | NR | No | No | Yes | CD | Yes | NR | NA | NR | NR | NR | No | Poor | |
| [194] | No | Yes | NR | Yes | No | Yes | Yes | No | NR | NA | Yes | NR | NR | Yes | Fair | |
| [201] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | CD | NA | Yes | NR | Yes | Yes | Good | |
| [198] | Yes | Yes | NR | Yes | No | No | No | No | No | NA | Yes | NR | NR | Yes | Poor | |
| [202] | No | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | NA | Yes | NR | NR | Yes | Good | |
| [203] | Yes | Yes | Yes | No | No | No | No | Yes | No | NA | No | NR | NR | Yes | Fair | |
| [197] | Yes | Yes | NR | Yes | No | No | No | Yes | No | NA | Yes | NR | NR | Yes | Fair | |
| [199] | Yes | Yes | No | No | No | No | No | Yes | Yes | NA | Yes | NR | NR | Yes | Fair | |
| [196] | Yes | Yes | Yes | Yes | No | No | No | No | Yes | NA | Yes | NR | NR | Yes | Fair | |
| [193] | Yes | Yes | Yes | Yes | No | CD | NR | No | No | NA | Yes | NR | NR | Yes | Fair | |
| Obesity | ||||||||||||||||
| [206] | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes | Good | |
| [213] | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | Yes | Yes | NR | Yes | Yes | Fair | |
| [119] | No | Yes | No | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | NR | Yes | Yes | Good | |
| [207] | No | No | NR | CD | No | Yes | Yes | Yes | NR | NR | Yes | NR | NR | Yes | Fair | |
| [208] | No | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | Fair | |
| [209] | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | No | Yes | Good | |
| [210] | Yes | Yes | Yes | Yes | No | CD | CD | No | Yes | NR | Yes | NR | NR | Yes | Fair | |
| [211] | Yes | Yes | NR | Yes | No | Yes | CD | No | Yes | NR | Yes | NR | NR | Yes | Fair | |
Q1. Was the research question or objective in this paper clearly stated?.
Q2. Was the study population clearly specified and defined?.
Q3. Was the participation rate of eligible persons at least 50%?
Q4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants?.
Q5. Was a sample size justification, power description, or variance and effect estimates provided?.
Q6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured?.
Q7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed?.
Q8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)?.
Q9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?.
Q10. Was the exposure(s) assessed more than once over time?.
Q11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?.
Q12. Were the outcome assessors blinded to the exposure status of participants?.
Q13. Was loss to follow-up after baseline 20% or less?.
Q14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?.
CD-Cannot be Determined; NA-Not Applicable; NR-Not Reported.
References
- 1.Basics —diabetes— cdc. URL:https://www.cdc.gov/diabetes/basics/diabetes.html [Accessed on 03/05/2019].
- 2.The top 10 causes of death. URL:https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [Accessed on 03/05/2019].
- 3.International diabetes federation – home. URL:https://idf.org/52-about-diabetes.html [Accessed on 03/06/2019].
- 4.C. for Disease Control, Prevention, et al. National diabetes statistics report: estimates of diabetes and its burden in the united states, 2014, Atlanta, GA: US Department of Health and Human Services; 2014.
- 5.8940_idf_atlas_2017_english_interactive, URL:http://diabetesatlas.org/IDF_Diabetes_Atlas_8e_interactive_EN/, (Accessed on 03/06/2019).
- 6.Complications of diabetes. URL:https://www.mayoclinic.org/diseases-conditions/diabetes/symptoms-causes/syc-20371444 [Accessed on 10/20/2020].
- 7.N.I. of Health. Study quality assessment tools, URL:https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Accessed on 02/27/2021].
- 8.Modan M., Halkin H., Karasik A., Lusky A. Elevated serum uric acid–a facet of hyperinsulinaemia. Diabetologia. 1987;30(9):713–718. doi: 10.1007/BF00296994. [DOI] [PubMed] [Google Scholar]
- 9.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: Nadph oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. [DOI] [PubMed]
- 10.Corry D.B., Eslami P., Yamamoto K., Nyby M.D., Makino H., Tuck M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin–angiotensin system. J Hypertension. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 11.Kanellis J, Kang D-H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. In: Seminars in nephrology, vol. 25, Elsevier; 2005. p. 39–42. [DOI] [PubMed]
- 12.Johnson R.J., Nakagawa T., Sanchez-Lozada L.G., Shafiu M., Sundaram S., Le M., Ishimoto T., Sautin Y.Y., Lanaspa M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY. Glucose induces -cell apoptosis via upregulation of the fas receptor in human islets. Diabetes 50 (8): 2001; 1683–1690. [DOI] [PubMed]
- 14.Perry I.J., Wannamethee S.G., Walker M.K., Thomson A., Whincup P.H., Shaper A.G. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged british men. BMJ. 1995;310(6979):560–564. doi: 10.1136/bmj.310.6979.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niskanen L., Laaksonen D.E., Lindström J., Eriksson J.G., Keinänen-Kiukaanniemi S., Ilanne-Parikka P., Aunola S., Hämäläinen H., Tuomilehto J., Uusitupa M. Serum uric acid as a harbinger of metabolic outcome in subjects with impaired glucose tolerance: the finnish diabetes prevention study. Diabetes Care. 2006;29(3):709–711. doi: 10.2337/diacare.29.03.06.dc05-1465. [DOI] [PubMed] [Google Scholar]
- 16.Dehghan A., Van Hoek M., Sijbrands E.J., Hofman A., Witteman J.C. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31(2):361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y.-L., Xu K.-F., Bai J.-L., Liu Y., Yu R.-B., Liu C.-L., Shen C., Wu X.-H. Elevation of serum uric acid and incidence of type 2 diabetes: a systematic review and meta-analysis. Chronic Diseases Transl Med. 2016;2(2):81–91. doi: 10.1016/j.cdtm.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodama S., Saito K., Yachi Y., Asumi M., Sugawara A., Totsuka K., Saito A., Sone H. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa T., Tuttle K.R., Short R.A., Johnson R.J. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Rev Nephrol. 2005;1(2):80. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 20.Herman J., Medalie J., Goldbourt U. Diabetes, prediabetes and uricaemia. Diabetologia. 1976;12(1):47–52. doi: 10.1007/BF01221964. [DOI] [PubMed] [Google Scholar]
- 21.Chou P., Li C.-L., Wu G.-S., Tsai S.-T. Progression to type 2 diabetes among hign-risk groups in kin-chen, kinmen: exploring the natural history of type 2 diabetes. Diabetes Care. 1998;21(7):1183–1187. doi: 10.2337/diacare.21.7.1183. [DOI] [PubMed] [Google Scholar]
- 22.Meisinger C., Thorand B., Schneider A., Stieber J., Döring A., Löwel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the monica augsburg cohort study. Arch Internal Med. 2002;162(1):82–89. doi: 10.1001/archinte.162.1.82. [DOI] [PubMed] [Google Scholar]
- 23.Carnethon M.R., Fortmann S.P., Palaniappan L., Duncan B.B., Schmidt M.I., Chambless L.E. Risk factors for progression to incident hyperinsulinemia: the atherosclerosis risk in communities study, 1987–1998. Am J Epidemiol. 2003;158(11):1058–1067. doi: 10.1093/aje/kwg260. [DOI] [PubMed] [Google Scholar]
- 24.Chien K.-L., Chen M.-F., Hsu H.-C., Chang W.-T., Su T.-C., Lee Y.-T., Hu F.B. Plasma uric acid and the risk of type 2 diabetes in a chinese community. Clin Chem. 2008;54(2):310–316. doi: 10.1373/clinchem.2007.095190. [DOI] [PubMed] [Google Scholar]
- 25.Nan H., Qiao Q., Söderberg S., Pitkäniemi J., Zimmet P., Shaw J., Alberti G., Uusitalo U., Pauvaday V., Chitson P. Serum uric acid and incident diabetes in mauritian indian and creole populations. Diabetes Res Clin Pract. 2008;80(2):321–327. doi: 10.1016/j.diabres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Choi H., De Vera M., Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology. 2008;47(10):1567–1570. doi: 10.1093/rheumatology/ken305. [DOI] [PubMed] [Google Scholar]
- 27.Kramer C.K., Von Mühlen D., Jassal S.K., Barrett-Connor E. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose: the rancho bernardo study. Diabetes Care. 2009;32(7):1272–1273. doi: 10.2337/dc09-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv Q., Meng X.-F., He F.-F., Chen S., Su H., Xiong J., Gao P., Tian X.-J., Liu J.-S., Zhu Z.-H. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0056864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi Y., Hayashi T., Tsumura K., Endo G., Fujii S., Okada K. Serum uric acid and the risk for hypertension and type 2 diabetes in japanese men: the osaka health survey. J Hypertension. 2001;19(7):1209–1215. doi: 10.1097/00004872-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Bandaru P., Shankar A. Association between serum uric acid levels and diabetes mellitus. Int J Endocrinol. 2011 doi: 10.1155/2011/604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oda E., Kawai R., Sukumaran V., Watanabe K. Uric acid is positively associated with metabolic syndrome but negatively associated with diabetes in japanese men. Internal Med. 2009;48(20):1785–1791. doi: 10.2169/internalmedicine.48.2426. [DOI] [PubMed] [Google Scholar]
- 32.Nan H., Dong Y., Gao W., Tuomilehto J., Qiao Q. Diabetes associated with a low serum uric acid level in a general chinese population. Diabetes Res Clin Pract. 2007;76(1):68–74. doi: 10.1016/j.diabres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Facchini F., Chen Y.-D.I., Hollenbeck C.B., Reaven G.M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. Jama. 1991;266(21):3008–3011. [PubMed] [Google Scholar]
- 34.Takada T., Kikuchi K., Hasegawa T., Komura H., Suzuki S., Satoh N., Ohotomo T., Nanba M., Marusaki S., Iimura O. The role of hyperinsulinemia on the renal mechanism of hyperuricemia in overweight patients with essential hypertension. Nihon Naibunpi Gakkai zasshi. 1991;67(8):861. doi: 10.1507/endocrine1927.67.8_861. [DOI] [PubMed] [Google Scholar]
- 35.Investigators W.M.P.P. The world health organization monica project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41(2):105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 36.Investigators A. The atherosclerosis risk in communit (aric) study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 37.Eriksson J., Lindström J., Valle T., Aunola S., Hämäläinen H., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Laakso M., Lauhkonen M., Lehto P. Prevention of type ii diabetes in subjects with impaired glucose tolerance: the diabetes prevention study (dps) in finland study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia. 1999;42(7):793–801. doi: 10.1007/s001250051229. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y.-T., Lin R.S., Sung F.C., Yang C.-Y., Chien K.-L., Chen W.-J., Su T.-C., Hsu H.-C., Huang Y.-C. Chin-shan community cardiovascular cohort in taiwan–baseline data and five-year follow-up morbidity and mortality. J Clin Epidemiol. 2000;53(8):838–846. doi: 10.1016/s0895-4356(00)00198-0. [DOI] [PubMed] [Google Scholar]
- 39.Hofman A., Grobbee D., De Jong P., Van den Ouweland F. Determinants of disease and disability in the elderly: the rotterdam elderly study. Eur J Epidemiol. 1991;7(4):403–422. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 40.Group MRFIT, et al. Statistical design considerations in the nhli multiple risk factor intervention trial (mrfit). J Chronic Diseases 30 (5): 1977; 261–275. [DOI] [PubMed]
- 41.Group MRFITR, et al. Multiple risk factor intervention trial: risk factor changes and mortality results. Jama 248: 1982; 1465–1477. [PubMed]
- 42.Sherwin R., Kaelber C.T., Kezdi P., Kjelsberg M.O., Thomas H.E., Jr The multiple risk factor intervention trial (mrfit).: Ii. The development of the protocol. Prevent Med. 1981;10(4):402–425. doi: 10.1016/0091-7435(81)90058-x. [DOI] [PubMed] [Google Scholar]
- 43.Nchs – analytic and reporting guidelines: Nhanes iii. URL: https://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf [Accessed on 02/26/2019].
- 44.Nhanes – nhanes iii – manuals and reports. URL: https://www.cdc.gov/nchs/nhanes/nh3rrm.htm [Accessed on 02/26/2019].
- 45.Bonnet M.H., Arand D.L. We are chronically sleep deprived. Sleep. 1995;18(10):908–911. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 46.Zizi F., Jean-Louis G., Brown C.D., Ogedegbe G., Boutin-Foster C., McFarlane S.I. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diabetes Rep. 2010;10(1):43–47. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fletcher E.C. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol. 2000;119(2–3):189–197. doi: 10.1016/s0034-5687(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 48.Punjabi N.M., Shahar E., Redline S., Gottlieb D.J., Givelber R., Resnick H.E. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the sleep heart health study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 49.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J Clin Investig. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Björkelund C., Bondyr-Carlsson D., Lapidus L., Lissner L., Månsson J., Skoog I., Bengtsson C. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in gothenburg. Diabetes Care. 2005;28(11):2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 51.Von Ruesten A., Weikert C., Fietze I., Boeing H. Association of sleep duration with chronic diseases in the european prospective investigation into cancer and nutrition (epic)-potsdam study. PloS One. 2012;7(1) doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mallon L., Broman J.-E., Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28(11):2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 53.Zizi F., Pandey A., Murrray-Bachmann R., Vincent M., McFarlane S., Ogedegbe G., Jean-Louis G. Race/ethnicity, sleep duration, and diabetes mellitus: analysis of the national health interview survey. Am J Med. 2012;125(2):162–167. doi: 10.1016/j.amjmed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson C.L., Redline S., Kawachi I., Hu F.B. Association between sleep duration and diabetes in black and white adults. Diabetes Care. 2013;36(11):3557–3565. doi: 10.2337/dc13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beihl D.A., Liese A.D., Haffner S.M. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19(5):351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Kawakami N., Takatsuka N., Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27(1):282–283. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson P.M., Rööst M., Engström G., Hedblad B., Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27(10):2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 58.Hayashino Y., Fukuhara S., Suzukamo Y., Okamura T., Tanaka T., Ueshima H. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in japan: the high-risk and population strategy for occupational health promotion (hipop-ohp) study. BMC Public Health. 2007;7(1):129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meisinger C., Heier M., Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48(2):235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 60.Tobacco. URL: https://www.who.int/news-room/fact-sheets/detail/tobacco [Accessed on 09/28/2020].
- 61.Smoking and diabetes — overviews of diseases/conditions. URL: https://www.cdc.gov/tobacco/campaign/tips/diseases/diabetes.html [Accessed on 10/20/2020].
- 62.Bajaj M. Nicotine and insulin resistance: when the smoke clears. Diabetes. 2012;61(12):3078–3080. doi: 10.2337/db12-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Will J.C., Galuska D.A., Ford E.S., Mokdad A., Calle E.E. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30(3):540–546. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- 64.Jee S.H., Foong A.W., Hur N.W., Samet J.M. Smoking and risk for diabetes incidence and mortality in korean men and women. Diabetes Care. 2010;33(12):2567–2572. doi: 10.2337/dc10-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wannamethee S.G., Shaper A.G., Perry I.J. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes CCare. 2001;24(9):1590–1595. doi: 10.2337/diacare.24.9.1590. [DOI] [PubMed] [Google Scholar]
- 66.Kowall B., Rathmann W., Strassburger K., Heier M., Holle R., Thorand B., Giani G., Peters A., Meisinger C. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the kora s4/f4 cohort study. Eur J Epidemiol. 2010;25(6):393–402. doi: 10.1007/s10654-010-9452-6. [DOI] [PubMed] [Google Scholar]
- 67.Rimm E.B., Manson J.E., Stampfer M.J., Colditz G.A., Willett W.C., Rosner B., Hennekens C.H., Speizer F.E. Cigarette smoking and the risk of diabetes in women. Am J Public Health. 1993;83(2):211–214. doi: 10.2105/ajph.83.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rimm E.B., Chan J., Stampfer M.J., Colditz G.A., Willett W.C. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310(6979):555–559. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manson J.E., Ajani U.A., Liu S., Nathan D.M., Hennekens C.H. A prospective study of cigarette smoking and the incidence of diabetes mellitus among us male physicians. Am J Med. 2000;109(7):538–542. doi: 10.1016/s0002-9343(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 70.Foy C.G., Bell R.A., Farmer D.F., Goff D.C., Wagenknecht L.E. Smoking and incidence of diabetes among us adults: findings from the insulin resistance atherosclerosis study. Diabetes Care. 2005;28(10):2501–2507. doi: 10.2337/diacare.28.10.2501. [DOI] [PubMed] [Google Scholar]
- 71.Cho N.H., Chan J.C., Jang H.C., Lim S., Kim H.L., Choi S.H. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin Endocrinol. 2009;71(5):679–685. doi: 10.1111/j.1365-2265.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L., Curhan G.C., Hu F.B., Rimm E.B., Forman J.P. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care. 2011;34(4):892–897. doi: 10.2337/dc10-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon J.A., Seeley D.G., Lipschutz R.C., Vittinghoff E., Browner W.S. The relation of smoking to waist-to-hip ratio and diabetes mellitus among elderly women. Prevent Med. 1997;26(5):639–644. doi: 10.1006/pmed.1997.0230. [DOI] [PubMed] [Google Scholar]
- 74.Beziaud F., Halimi J., Lecomte P., Vol S., Tichet J. Cigarette smoking and diabetes mellitus. Diabetes Metab. 2004;30(2):161–166. doi: 10.1016/s1262-3636(07)70102-7. [DOI] [PubMed] [Google Scholar]
- 75.Yeh H.-C., Duncan B.B., Schmidt M.I., Wang N.-Y., Brancati F.L. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Internal Med. 2010;152(1):10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morimoto A., Ohno Y., Tatsumi Y., Nishigaki Y., Maejima F., Mizuno S., Watanabe S. Impact of smoking cessation on incidence of diabetes mellitus among overweight or normal-weight japanese men. Diabetes Res Clin Pract. 2012;96(3):407–413. doi: 10.1016/j.diabres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Hur N.W., Kim H.C., Mo Nam C., Ha Jee S., Lee H.C., Suh I. Smoking cessation and risk of type 2 diabetes mellitus: Korea medical insurance corporation study. Eur J Cardiovasc Prevent Rehab. 2007;14(2):244–249. doi: 10.1097/01.hjr.0000239474.41379.79. [DOI] [PubMed] [Google Scholar]
- 78.Oba S., Noda M., Waki K., Nanri A., Kato M., Takahashi Y., Poudel-Tandukar K., Matsushita Y., Inoue M., Mizoue T. Smoking cessation increases short-term risk of type 2 diabetes irrespective of weight gain: the japan public health center-based prospective study. PloS One. 2012;7(2) doi: 10.1371/journal.pone.0017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayas N.T., White D.P., Al-Delaimy W.K., Manson J.E., Stampfer M.J., Speizer F.E., Patel S., Hu F.B. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 80.Colditz GA, Manson JE, Hankinson SE. The nurses’ health study: 20-year contribution to the understanding of health among women. J Women’s Health 6 (1): 1997; 49–62. [DOI] [PubMed]
- 81.Kawakami N., Takatsuka N., Shimizu H., Ishibashi H. Depressive symptoms and occurrence of type 2 diabetes among japanese men. Diabetes Care. 1999;22(7):1071–1076. doi: 10.2337/diacare.22.7.1071. [DOI] [PubMed] [Google Scholar]
- 82.Nilsson P., Berglund G. Prevention of cardiovascular disease and diabetes: lessons from the malmö preventive project. J Internal Med. 2000;248(6):455–462. doi: 10.1046/j.1365-2796.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 83.Gottlieb D.J., Punjabi N.M., Newman A.B., Resnick H.E., Redline S., Baldwin C.M., Nieto F.J. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Internal Med. 2005;165(8):863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 84.Quan S.F., Howard B.V., Iber C., Kiley J.P., Nieto F.J., O’Connor G.T., Rapoport D.M., Redline S., Robbins J., Samet J.M. The sleep heart health study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 85.Yaggi H.K., Araujo A.B., McKinlay J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 86.O’donnell AB, Araujo AB, McKinlay JB. The health of normally aging men: the massachusetts male aging study (1987–2004). Exp Gerontol 39 (7): 2004; 975–984. [DOI] [PubMed]
- 87.Gangwisch J.E., Heymsfield S.B., Boden-Albala B., Buijs R.M., Kreier F., Pickering T.G., Rundle A.G., Zammit G.K., Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large us sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nhanes – national health and nutrition examination survey homepage, URL:https://www.cdc.gov/nchs/nhanes/index.htm [Accessed on 02/27/2019].
- 89.Chaput J.-P., Després J.-P., Bouchard C., Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50(11):2298–2304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- 90.Bouchard C. Genetics of human obesity: recent results from linkage studies. J Nutr. 1997;127(9):1887S–1890S. doi: 10.1093/jn/127.9.1887S. [DOI] [PubMed] [Google Scholar]
- 91.Okamura T., Tanaka T., Babazono A., Yoshita K., Chiba N., Takebayashi T., Nakagawa H., Yamato H., Miura K., Tamaki J. The high-risk and population strategy for occupational health promotion (hipop-ohp) study: study design and cardiovascular risk factors at the baseline survey. J Human Hypertension. 2004;18(7):475. doi: 10.1038/sj.jhh.1001680. [DOI] [PubMed] [Google Scholar]
- 92.Tuomilehto H., Peltonen M., Partinen M., Seppä J., Saaristo T., Korpi-Hyövälti E., Oksa H., Puolijoki H., Saltevo J., Vanhala M. Sleep duration is associated with an increased risk for the prevalence of type 2 diabetes in middle-aged women–the fin-d2d survey. Sleep Med. 2008;9(3):221–227. doi: 10.1016/j.sleep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 93.Oy KH. Finnish diabetes association: Implementation of type 2 diabetes prevention plan, Helsinki 33.
- 94.Chaput J.-P., Després J.-P., Bouchard C., Astrup A., Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the quebec family study. Sleep Med. 2009;10(8):919–924. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 95.Wagenknecht L.E., Mayer E.J., Rewers M., Haffner S., Selby J., Borok G.M., Henkin L., Howard G., Savage P.J., Saad M.F. The insulin resistance atherosclerosis study (iras): objectives, design, and recruitment results. Ann Epidemiol. 1995;5(6):464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 96.Vgontzas A.N., Liao D., Bixler E.O., Chrousos G.P., Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tuomilehto H., Peltonen M., Partinen M., Lavigne G., Eriksson J.G., Herder C., Aunola S., Keinänen-Kiukaanniemi S., Ilanne-Parikka P., Uusitupa M. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: the finnish diabetes prevention study. Diabetes Care. 2009;32(11):1965–1971. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu Q., Song Y., Hollenbeck A., Blair A., Schatzkin A., Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33(1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schatzkin A., Subar A.F., Thompson F.E., Harlan L.C., Tangrea J., Hollenbeck A.R., Hurwitz P.E., Coyle L., Schussler N., Michaud D.S. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the national institutes of health–american association of retired persons diet and health study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 100.Chao C.-Y., Wu J.-S., Yang Y.-C., Shih C.-C., Wang R.-H., Lu F.-H., Chang C.-J. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism. 2011;60(6):799–804. doi: 10.1016/j.metabol.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 101.Boeing H., Wahrendorf J., Becker N. Epic-germany–a source for studies into diet and risk of chronic diseases. Ann Nutr Metab. 1999;43(4):195–204. doi: 10.1159/000012786. [DOI] [PubMed] [Google Scholar]
- 102.Kita T., Yoshioka E., Satoh H., Saijo Y., Kawaharada M., Okada E., Kishi R. Short sleep duration and poor sleep quality increase the risk of diabetes in japanese workers with no family history of diabetes. Diabetes Care. 2012;35(2):313–318. doi: 10.2337/dc11-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Massey JT, Moore TF, Tadros W, Parsons V. Design and estimation for the national health interview survey 1985–94. Vital Health Stat Series 2 Data Eval Methods Res (110): 1989; 1–33. [PubMed]
- 104.Najafian J., Mohamadifard N., Sadri G., Rahmati M. Association between sleep duration and diabetes mellitus: Isfahan healthy heart program. Nigerian J Clin Pract. 2013;16(1):59–62. doi: 10.4103/1119-3077.106756. [DOI] [PubMed] [Google Scholar]
- 105.Sarraf-Zadegan N., Sadri G., Malek-Afzali H., Baghaei M., Mohammadi-Fard N., Shahrokhi S., Tolooie H., Poormoghaddas M., Sadeghi M., Tavassoli A. Isfahan healthy heart programme: a comprehensive integrated community-based programme for cardiovascular disease prevention and control. design, methods and initial experience. Acta Cardiol. 2003;58(4):309–320. doi: 10.2143/AC.58.4.2005288. [DOI] [PubMed] [Google Scholar]
- 106.Boyko EJ, Seelig AD, Jacobson IG, Hooper TI, Smith B, Smith TC, Crum-Cianflone NF, MCS Team, et al. Sleep characteristics, mental health, and diabetes risk: a prospective study of us military service members in the millennium cohort study. Diabetes Care 36 (10): 2013; 3154–3161. [DOI] [PMC free article] [PubMed]
- 107.Ryan M.A., Smith T.C., Smith B., Amoroso P., Boyko E.J., Gray G.C., Gackstetter G.D., Riddle J.R., Wells T.S., Gumbs G. Millennium cohort: enrollment begins a 21-year contribution to understanding the impact of military service. J Clin Epidemiol. 2007;60(2):181–191. doi: 10.1016/j.jclinepi.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 108.Holliday E.G., Magee C.A., Kritharides L., Banks E., Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Banks E., Jorm L., Lujic S., Rogers K. Health, ageing and private health insurance: baseline results from the 45 and up study cohort. Aust New Zealand Health Policy. 2009;6(1):16. doi: 10.1186/1743-8462-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feskens E.J., Kromhout D. Cardiovascular risk factors and the 25-year incidence of diabetes mellitus in middle-aged men. Am J Epidemiol. 1989;130(6):1101–1108. doi: 10.1093/oxfordjournals.aje.a115437. [DOI] [PubMed] [Google Scholar]
- 111.Feinleib M. Seven countries: a multivariate analysis of death and coronary heart disease. Jama. 1981;245(5):511–512. [Google Scholar]
- 112.Kawakami N., Takatsuka N., Shimizu H., Ishibashi H. Effects of smoking on the incidence of non-lnsulin-dependent diabetes mellitus: replication and extension in a japanese cohort of male employees. Am J Epidemiol. 1997;145(2):103–109. doi: 10.1093/oxfordjournals.aje.a009080. [DOI] [PubMed] [Google Scholar]
- 113.Cummings S.R., Black D.M., Nevitt M.C., Browner W.S., Cauley J.A., Genant H.K., Mascioli S.R., Scott J.C., Seeley D.G., Steiger P. Appendicular bone density and age predict hip fracture in women. Jama. 1990;263(5):665–668. [PubMed] [Google Scholar]
- 114.Uchimoto S., Tsumura K., Hayashi T., Suematsu C., Endo G., Fujii S., Okada K. Impact of cigarette smoking on the incidence of type 2 diabetes mellitus in middle-aged japanese men: the osaka health survey. Diabet Med. 1999;16(11):951–955. doi: 10.1046/j.1464-5491.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 115.F.R. on the Aspirin Component of the Ongoing Physicians’ Health Study. Steering committee of the physicians’ health study research group. N Engl J Med 321 (3): 1989; 129–135. [DOI] [PubMed]
- 116.Ko G., Chan J., Tsang L., Critchley J., Cockram C. Smoking and diabetes in chinese men. Postgr Med J. 2001;77(906):240–243. doi: 10.1136/pmj.77.906.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shaper A., Pocock S., Walker M., Cohen N., Wale C., Thomson A. British regional heart study: cardiovascular risk factors in middle-aged men in 24 towns. Br Med J (Clin Res Ed) 1981;283(6285):179–186. doi: 10.1136/bmj.283.6285.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garfinkel L. Selection, follow-up, and analysis in the american cancer society prospective studies. Nat Cancer Inst Monograph. 1985;67:49–52. [PubMed] [Google Scholar]
- 119.Hu F.B., Manson J.E., Stampfer M.J., Colditz G., Liu S., Solomon C.G., Willett W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. New England J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 120.Montgomery S.M., Ekbom A. Smoking during pregnancy and diabetes mellitus in a british longitudinal birth cohort. BMJ. 2002;324(7328):26–27. doi: 10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ekinsmyth C, Bynner J, Montgomery S, Shepherd P. An integrated approach to the design and analysis of the 1970 british cohort study (bcs70) and the national child development study (ncds). London: Centre for Longitudinal Studies, University of London
- 122.Carlsson S., Midthjell K., Grill V. Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: an 11-year follow-up of incidence of diabetes in the nord-trøndelag study. Diabetologia. 2004;47(11):1953–1956. doi: 10.1007/s00125-004-1554-9. [DOI] [PubMed] [Google Scholar]
- 123.Midthjell K., Bjørndal A., Holmen J., Krüger Ø., Bjartveit K. Prevalence of known and previously unknown diabetes mellitus and impaired glucose tolerance in an adult norwegian population. indications of an increasing diabetes prevalence. the nord-trøndelag diabetes study. Scand J Primary Health Care. 1995;13(3):229–235. doi: 10.3109/02813439508996766. [DOI] [PubMed] [Google Scholar]
- 124.Hyeon C.K., Chung M.N., Sun H.J., Kwang H.H., Oh D.K., Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328(7446):983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rathmann W, Haastert B, Icks AA, Löwel H, Meisinger C, Holle R, Giani G. High prevalence of undiagnosed diabetes mellitus in southern germany: target populations for efficient screening. The kora survey 2000. Diabetologia 46 (2): 2003; 182–189. [DOI] [PubMed]
- 126.Jee S.H., Ohrr H., Sull J.W., Yun J.E., Ji M., Samet J.M. Fasting serum glucose level and cancer risk in korean men and women. Jama. 2005;293(2):194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 127.Tsugane S., Sobue T. Baseline survey of jphc study design and participation rate. J Epidemiol. 2001;11(6sup):24–29. doi: 10.2188/jea.11.6sup_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luo J., Rossouw J., Tong E., Giovino G.A., Lee C.C., Chen C., Ockene J.K., Qi L., Margolis K.L. Smoking and diabetes: does the increased risk ever go away? Am J Epidemiol. 2013;178(6):937–945. doi: 10.1093/aje/kwt071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Study TWHI, et al. Design of the women’s health initiative clinical trial and observational study, Controlled Clin Trials 19 (1): 1998; 61–109. [DOI] [PubMed]
- 130.What is depression? URL:https://www.psychiatry.org/patients-families/depression/what-is-depression [Accessed on 09/29/2020].
- 131.Jacobson A.M. Depression and diabetes. Diabetes Care. 1993;16(12):1621–1623. doi: 10.2337/diacare.16.12.1621. [DOI] [PubMed] [Google Scholar]
- 132.Plotsky P.M., Owens M.J., Nemeroff C.B. Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21(2):293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- 133.Mannelli M, Parenti G, Zampetti B, Canu L, Mannucci E. Diabetes from catecholamine excess. In: Diabetes secondary to endocrine and pancreatic disorders, vol. 22, Karger Publishers; 2014. p. 44–51.
- 134.Vreeburg S.A., Hoogendijk W.J., van Pelt J., DeRijk R.H., Verhagen J.C., van Dyck R., Smit J.H., Zitman F.G., Penninx B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 135.van den Akker M, Schuurman A, Metsemakers J, Buntinx F. Is depression related to subsequent diabetes mellitus? Acta Psychiatr Scand 110 (3): 2004; 178–183. arXiv:https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1600-0447.2004.00333.x, doi:10.1111/j.1600-0447.2004.00333.x. URL:https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.2004.00333.x. [DOI] [PubMed]
- 136.Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torréns JI, Kravitz HM, Bromberger JT, Matthews KA. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care 27 (12): 2004; 2856–2862. arXiv: http://care.diabetesjournals.org/content/27/12/2856.full.pdf, doi: 10.2337/diacare.27.12.2856. URL: http://care.diabetesjournals.org/content/27/12/2856. [DOI] [PubMed]
- 137.Kawakami N., Takatsuka N., Shimizu H., Ishibashi H. Depressive symptoms and occurrence of type 2 diabetes among japanese men. Diabetes Care. 1999;22(7):1071–1076. doi: 10.2337/diacare.22.7.1071. arXiv:http://care.diabetesjournals.org/content/22/7/1071.full.pdf URL:http://care.diabetesjournals.org/content/22/7/1071. [DOI] [PubMed] [Google Scholar]
- 138.Carnethon M.R., Biggs M.L., Barzilay J.I., Smith N.L., Vaccarino V., Bertoni A.G., Arnold A., Siscovick D. Longitudinal association between depressive symptoms and incident type 2 diabetes mellitus in older adults: the cardiovascular health study. Arch Internal Med. 2007;167(8):802–807. doi: 10.1001/archinte.167.8.802. arXiv:https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/412313/ioi70010_802_807.pdf URL:https://dx.doi.org/10.1001/archinte.167.8.802. [DOI] [PubMed] [Google Scholar]
- 139.Palinkas L., Lee P.P., Barrett-Connor E. A prospective study of type 2 diabetes and depressive symptoms in the elderly: the rancho bernardo study. Diab Med J Br Diabetic Assoc. 2004;21:1185–1191. doi: 10.1111/j.1464-5491.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- 140.Yu M., Zhang X., Lu F., Fang L. Depression and risk for diabetes: a meta-analysis. Can J Diabetes. 2015;39(4):266–272. doi: 10.1016/j.jcjd.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 141.Yeung E.H., Pankow J.S., Astor B.C., Powe N.R., Saudek C.D., Kao W.L. Increased risk of type 2 diabetes from a family history of coronary heart disease and type 2 diabetes. Diabetes Care. 2007;30(1):154–156. doi: 10.2337/dc06-1463. [DOI] [PubMed] [Google Scholar]
- 142.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48(5):937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 143.Mazzone T., Chait A., Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. The Lancet. 2008;371(9626):1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fox C.S. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the framingham heart study. Trends Cardiovasc Med. 2010;20(3):90–95. doi: 10.1016/j.tcm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Olsson A.G., Schwartz G.G., Szarek M., Sasiela W.J., Ezekowitz M.D., Ganz P., Oliver M.F., Waters D., Zeiher A. High-density lipoprotein, but not low-density lipoprotein cholesterol levels influence short-term prognosis after acute coronary syndrome: results from the miracl trial. Eur Heart J. 2005;26(9):890–896. doi: 10.1093/eurheartj/ehi186. [DOI] [PubMed] [Google Scholar]
- 146.Zheng T., Gao Y., Tian H. Relationship between blood lipid profiles and pancreatic islet β) cell function in chinese men and women with normal glucose tolerance: a cross-sectional study. BMC Public Health. 2012;12(1):634. doi: 10.1186/1471-2458-12-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawamoto R., Tabara Y., Kohara K., Miki T., Kusunoki T., Takayama S., Abe M., Katoh T., Ohtsuka N. Relationships between lipid profiles and metabolic syndrome, insulin resistance and serum high molecular adiponectin in japanese community-dwelling adults. Lipids Health Disease. 2011;10(1):79. doi: 10.1186/1476-511X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyer K.A., Kushi L.H., Jacobs D.R., Folsom A.R. Dietary fat and incidence of type 2 diabetes in older iowa women. Diabetes Care. 2001;24(9):1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- 149.Tajima R., Kodama S., Hirata M., Horikawa C., Fujihara K., Yachi Y., Yoshizawa S., Iida K.T., Sone H. High cholesterol intake is associated with elevated risk of type 2 diabetes mellitus–a meta-analysis. Clin Nutr. 2014;33(6):946–950. doi: 10.1016/j.clnu.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 150.Lotta L.A., Sharp S.J., Burgess S., Perry J.R., Stewart I.D., Willems S.M., Luan J., Ardanaz E., Arriola L., Balkau B. Association between low-density lipoprotein cholesterol–lowering genetic variants and risk of type 2 diabetes: a meta-analysis. Jama. 2016;316(13):1383–1391. doi: 10.1001/jama.2016.14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Besseling J., Kastelein J.J., Defesche J.C., Hutten B.A., Hovingh G.K. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. Jama. 2015;313(10):1029–1036. doi: 10.1001/jama.2015.1206. [DOI] [PubMed] [Google Scholar]
- 152.Zhang N., Hu X., Zhang Q., Bai P., Cai M., Zeng T.S., Zhang J.-Y., Tian S.-H., Min J., Huang H.-T., Zheng J., Peng M.-M., Li M.-J., Chen L.-L. Non-high-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: results from a population-based cohort study. J Diabetes. 2018;10(9):708–714. doi: 10.1111/1753-0407.12650. arXiv:https://onlinelibrary.wiley.com/doi/pdf/10.1111/1753-0407.12650 URL:https://onlinelibrary.wiley.com/doi/abs/10.1111/1753-0407.12650. [DOI] [PubMed] [Google Scholar]
- 153.Song Q., Liu X., Wang A., Wang Y., Zhou Y., Zhou W., Wang X. Associations between non-traditional lipid measures and risk for type 2 diabetes mellitus in a chinese community population: a cross-sectional study. Lipids health Disease. 2016;15(1):70. doi: 10.1186/s12944-016-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haase C.L., Tybjærg-Hansen A., Nordestgaard B.G., Frikke-Schmidt R. Hdl cholesterol and risk of type 2 diabetes: a mendelian randomization study. Diabetes. 2015;64(9):3328–3333. doi: 10.2337/db14-1603. [DOI] [PubMed] [Google Scholar]
- 155.Eaton W.W., Armenian H., Gallo J., Pratt L., Ford D.E. Depression and risk for onset of type ii diabetes: a prospective population-based study. Diabetes Care. 1996;19(10):1097–1102. doi: 10.2337/diacare.19.10.1097. arXiv:http://care.diabetesjournals.org/content/19/10/1097.full.pdf URL:http://care.diabetesjournals.org/content/19/10/1097. [DOI] [PubMed] [Google Scholar]
- 156.Regier D.A., Myers J.K., Kramer M., Robins L.N., Blazer D.G., Hough R.L., Eaton W.W., Locke B.Z. The NIMH epidemiologic catchment area program: historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41(10):934–941. doi: 10.1001/archpsyc.1984.01790210016003. arXiv:https://jamanetwork.com/journals/jamapsychiatry/articlepdf/493431/archpsyc_41_10_003.pdf URL:https://dx.doi.org/10.1001/archpsyc.1984.01790210016003. [DOI] [PubMed] [Google Scholar]
- 157.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: findings from the National Health and Nutrition Examination epidemiologic follow-up study, 1971–1992. Am J Epidemiol 158 (5): 2003; 416–423. arXiv:http://oup.prod.sis.lan/aje/article-pdf/158/5/416/149380/kwg172.pdf, doi:10.1093/aje/kwg172. URL:https://dx.doi.org/10.1093/aje/kwg172. [DOI] [PubMed]
- 158.Golden SH, Williams JE, Ford DE, Yeh H-C, Paton Sanford C, Nieto FJ, Brancati FL. Depressive symptoms and the risk of type 2 diabetes. Diabetes Care 27 (2): 2004; 429–435. arXiv:http://care.diabetesjournals.org/content/27/2/429.full.pdf, doi:10.2337/diacare.27.2.429. URL:http://care.diabetesjournals.org/content/27/2/429. [DOI] [PubMed]
- 159.Fran M, Sowers R, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans DA, Neer R, Matthews KA, Sherman S, Lo A, Weiss G, Kelsey JL. Swan: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition, Sybil L. Crawford.
- 160.Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. J Psychosomat Res. 2007;62(1):31–38. doi: 10.1016/j.jpsychores.2006.07.009. URL:http://www.sciencedirect.com/science/article/pii/S002239990600331X. [DOI] [PubMed] [Google Scholar]
- 161.Fried L., Borhani N., Enright P., Furberg C., Gardin J., Kronmal R., Kuller L., Manolio T., Mittelmark M., Newman A., O’Leary D., Psaty B., Rautaharju P., Tracy R., Weiler P. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 162.Folsom A.R., Prineas R.J., Kaye S.A., Soler J.T. Body fat distribution and self-reported prevalence of hypertension, heart attack, and other heart disease in older women. Int J Epidemiol. 1989;18(2):361–367. doi: 10.1093/ije/18.2.361. [DOI] [PubMed] [Google Scholar]
- 163.Aguib Y, Al Suwaidi J. The copenhagen city heart study (Østerbroundersøgelsen). Global Cardiol Sci Pract 2015 (3): 2015; 33. doi:10.5339/gcsp.2015.33. URL:http://europepmc.org/articles/PMC4625209. [DOI] [PMC free article] [PubMed]
- 164.Bergholdt H., Bathum L., Kvetny J., Rasmussen D.B., Moldow B., Hoeg T., Jemec G., Berner-Nielsen H., Nordestgaard B.G., Ellervik C. Study design, participation and characteristics of the danish general suburban population study. Dan Med J. 2013;60(9):A4693. [PubMed] [Google Scholar]
- 165.Ning G, RS Group. Risk evaluation of cancers in chinese diabetic individuals: a longitudinal (reaction) study. J Diabetes 4 (2): 2012; 172–173 [DOI] [PubMed]
- 166.Hayashi T., Tsumura K., Suematsu C., Endo G., Fujii S., Okada K. High normal blood pressure, hypertension, and the risk of type 2 diabetes in japanese men. The osaka health survey. Diabetes Care. 1999;22(10):1683–1687. doi: 10.2337/diacare.22.10.1683. [DOI] [PubMed] [Google Scholar]
- 167.Gress T.W., Nieto F.J., Shahar E., Wofford M.R., Brancati F.L. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. New England J Med. 2000;342(13):905–912. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 168.Conen D., Ridker P.M., Mora S., Buring J.E., Glynn R.J. Blood pressure and risk of developing type 2 diabetes mellitus: the women’s health study. Eur Heart J. 2007;28(23):2937–2943. doi: 10.1093/eurheartj/ehm400. [DOI] [PubMed] [Google Scholar]
- 169.Kim M.-J., Lim N.-K., Choi S.-J., Park H.-Y. Hypertension is an independent risk factor for type 2 diabetes: the korean genome and epidemiology study. Hypertension Res. 2015;38(11):783. doi: 10.1038/hr.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wei G.S., Coady S.A., Goff D.C., Brancati F.L., Levy D., Selvin E., Vasan R.S., Fox C.S. Blood pressure and the risk of developing diabetes in african americans and whites: Aric, cardia, and the framingham heart study. Diabetes Care. 2011;34(4):873–879. doi: 10.2337/dc10-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ageing and health. URL:https://www.who.int/news-room/fact-sheets/detail/ageing-and-health [Accessed on 09/30/2020].
- 172.Sarkar D., Lebedeva I.V., Emdad L., Kang D.-C., Baldwin A.S., Fisher P.B. Human polynucleotide phosphorylase (hpnpaseold-35): a potential link between aging and inflammation. Cancer Res. 2004;64(20):7473–7478. doi: 10.1158/0008-5472.CAN-04-1772. [DOI] [PubMed] [Google Scholar]
- 173.Suastika K, Dwipayana P, Semadi MS, Kuswardhani RT. Age is an important risk factor for type 2 diabetes mellitus and cardiovascular diseases. Glucose Tolerance: Intech Open 2012; 67–76
- 174.Choi B., Shi F. Risk factors for diabetes mellitus by age and sex: results of the national population health survey. Diabetologia. 2001;44(10):1221–1231. doi: 10.1007/s001250100648. [DOI] [PubMed] [Google Scholar]
- 175.McNeely M.J., Boyko E.J. Type 2 diabetes prevalence in asian americans: results of a national health survey. Diabetes Care. 2004;27(1):66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 176.Shai I., Jiang R., Manson J.E., Stampfer M.J., Willett W.C., Colditz G.A., Hu F.B. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29(7):1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 177.Zimmet P., Taylor R., Ram P., King H., Sloman G., Raper L.R., Hunt D. Prevalence of diabetes and impaired glucose tolerance in the biracial (melanesian and indian) population of fiji: a rural-urban comparison. Am J Epidemiol. 1983;118(5):673–688. doi: 10.1093/oxfordjournals.aje.a113678. [DOI] [PubMed] [Google Scholar]
- 178.Zimmet P., Faaiuso S., Ainuu J., Whitehouse S., Milne B., DeBoer W. The prevalence of diabetes in the rural and urban polynesian population of western samoa. Diabetes. 1981;30(1):45–51. doi: 10.2337/diab.30.1.45. [DOI] [PubMed] [Google Scholar]
- 179.Mather H.M., Keen H. The southall diabetes survey: prevalence of known diabetes in asians and Europeans. Br Med J (Clin Res Ed) 1985;291(6502):1081–1084. doi: 10.1136/bmj.291.6502.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McKeigue P., Marmot M., Cottier D., Rahman S., Riemersma R. Diabetes, hyperinsulinaemia, and coronary risk factors in bangladeshis in east London. Heart. 1988;60(5):390–396. doi: 10.1136/hrt.60.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Simmons D., Williams D., Powell M. Prevalence of diabetes in a predominantly asian community: preliminary findings of the coventry diabetes study. BMJ. 1989;298(6665):18–21. doi: 10.1136/bmj.298.6665.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Deurenberg P., Deurenberg-Yap M., Guricci S. Asians are different from caucasians and from each other in their body mass index/body fat per cent relationship. Obesity Rev. 2002;3(3):141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 183.TORRens JI, Skurnick J, Davidow AL, Korenman SG, Santoro N, Soto-Greene M, Lasser N, Weiss G. Ethnic differences in insulin sensitivity and -cell function in premenopausal or early perimenopausal women without diabetes: the study of women’s health across the nation (swan). Diabetes Care 27 (2): 2004; 354–361. [DOI] [PubMed]
- 184.Haffner S.M., Saad M.F., Rewers M., Mykkänen L., Selby J., Howard G., Savage P.J., Hamman R.F., Wegenknecht L.E., Bergman R.N. Increased insulin resistance and insulin secretion in nondiabetic african-americans and hispanics compared with non-hispanic whites: the insulin resistance atherosclerosis study. Diabetes. 1996;45(6):742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 185.Rexrode K.M., Lee I.-M., Cook N.R., Hennekens C.H., Buring J.E. Baseline characteristics of participants in the women’s health study. J Women’s Health Gender-based Med. 2000;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 186.Home. URL: https://www.cardia.dopm.uab.edu/ [Accessed on 03/03/2019].
- 187.Mahmood S.S., Levy D., Vasan R.S., Wang T.J. The framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. The Lancet. 2014;383(9921):999–1008. doi: 10.1016/S0140-6736(13)61752-3. URL:http://www.sciencedirect.com/science/article/pii/S0140673613617523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stahl C.H., Novak M., Lappas G., Wilhelmsen L., Björck L., Hansson P.-O., Rosengren A. High-normal blood pressure and long-term risk of type 2 diabetes: 35-year prospective population based cohort study of men. BMC Cardiovasc Disorders. 2012;12(1):89. doi: 10.1186/1471-2261-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wilhelmsen L., Berglund G., Elmfeldt D., Tibblin G., Wedel H., Pennert K., Vedin A., Wilhelmsson C., Werkö L. The multifactor primary prevention trial in göteborg, Sweden. Eur Heart J. 1986;7(4):279–288. doi: 10.1093/oxfordjournals.eurheartj.a062065. [DOI] [PubMed] [Google Scholar]
- 190.Kim B., Park J., Ahn Y., Kimm K., Shin C. Geographical difference in the prevalence of isolated systolic hypertension in middle-aged men and women in korea: the korean health and genome study. J Human Hypertension. 2005;19(11):877. doi: 10.1038/sj.jhh.1001904. [DOI] [PubMed] [Google Scholar]
- 191.C. for Disease Control, Prevention, et al. Behavioral risk factor surveillance system survey data. http://apps.nccd.cdc.gov/brfss/list.asp?cat=OH&yr-2008&qkey=6610&state=All.
- 192.Yoon P.W., Scheuner M.T., Peterson-Oehlke K.L., Gwinn M., Faucett A., Khoury M.J. Can family history be used as a tool for public health and preventive medicine? Gen Med. 2002;4(4):304–310. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 193.Tsenkova V.K., Karlamangla A.S., Ryff C.D. Parental history of diabetes, positive affect, and diabetes risk in adults: findings from midus. Ann Behav Med. 2016;50(6):836–843. doi: 10.1007/s12160-016-9810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Burchfiel C.M., Curb J.D., Rodriguez B.L., Yano K., Hwang L.-J., Fong K.-O., Marcus E.B. Incidence and predictors of diabetes in japanese-american men the honolulu heart program. Ann Epidemiol. 1995;5(1):33–43. doi: 10.1016/1047-2797(94)00038-u. [DOI] [PubMed] [Google Scholar]
- 195.Mitchell B.D., Valdez R., Hazuda H.P., Haffner S.M., Monterrosa A., Stern M.P. Differences in the prevalence of diabetes and impaired glucose tolerance according to maternal or paternal history of diabetes. Diabetes Care. 1993;16(9):1262–1267. doi: 10.2337/diacare.16.9.1262. [DOI] [PubMed] [Google Scholar]
- 196.Rodríguez-Moran M., Guerrero-Romero F., Aradillas-García C., Violante R., Simental-Mendia L.E., Monreal-Escalante E., De La Cruz Mendoza E. Obesity and family history of diabetes as risk factors of impaired fasting glucose: implications for the early detection of prediabetes. Pediatr Diabetes. 2010;11(5):331–336. doi: 10.1111/j.1399-5448.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- 197.Valdez R., Yoon P.W., Liu T., Khoury M.J. Family history and prevalence of diabetes in the us population: the 6-year results from the national health and nutrition examination survey (1999–2004) Diabetes Care. 2007;30(10):2517–2522. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- 198.Sargeant L., Wareham N., Khaw K. Family history of diabetes identifies a group at increased risk for the metabolic consequences of obesity and physical inactivity in epic-norfolk: a population-based study. Int J Obesity. 2000;24(10):1333. doi: 10.1038/sj.ijo.0801383. [DOI] [PubMed] [Google Scholar]
- 199.Suchindran S., Vana A.M., Shaffer R.A., Alcaraz J.E., McCarthy J.J. Racial differences in the interaction between family history and risk factors associated with diabetes in the national health and nutritional examination survey, 1999–2004. Genet Med. 2009;11(7):542. doi: 10.1097/GIM.0b013e3181a70917. [DOI] [PubMed] [Google Scholar]
- 200.Shaten B.J., Smith G.D., Kuller L.H., Neaton J.D. Risk factors for the development of type ii diabetes among men enrolled in the usual care group of the multiple risk factor intervention trial. Diabetes Care. 1993;16(10):1331–1339. doi: 10.2337/diacare.16.10.1331. [DOI] [PubMed] [Google Scholar]
- 201.Bjørnholt J.V., Erikssen G., Liestøl K., Jervell J., Thaulow E., Erikssen J. Type 2 diabetes and maternal family history: an impact beyond slow glucose removal rate and fasting hyperglycemia in low-risk individuals? results from 22.5 years of follow-up of healthy nondiabetic men. Diabetes Care. 2000;23(9):1255–1259. doi: 10.2337/diacare.23.9.1255. [DOI] [PubMed] [Google Scholar]
- 202.Meigs J.B., Cupples L.A., Wilson P.W. Parental transmission of type 2 diabetes: the framingham offspring study. Diabetes. 2000;49(12):2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 203.Thorand B., Liese A.D., Metzger M.-H., Reitmeir P., Schneider A., Löwel H. Can inaccuracy of reported parental history of diabetes explain the maternal transmission hypothesis for diabetes? Int J Epidemiol. 2001;30(5):1084–1089. doi: 10.1093/ije/30.5.1084. [DOI] [PubMed] [Google Scholar]
- 204.Knowler W.C., Pettitt D.J., Savage P.J., Bennett P.H. Diabetes incidence in pima Indians: contributions of obesity and parental diabetes. Am J Epidemiol. 1981;113(2):144–156. doi: 10.1093/oxfordjournals.aje.a113079. [DOI] [PubMed] [Google Scholar]
- 205.Lin R.S., Lee W.C., Lee Y.-T., Chou P., FU C.-C. Maternal role in type 2 diabetes mellitus: indirect evidence for a mitochondrial inheritance. Int J Epidemiol. 1994;23(5):886–890. doi: 10.1093/ije/23.5.886. [DOI] [PubMed] [Google Scholar]
- 206.Ishikawa-Takata K., Ohta T., Moritaki K., Gotou T., Inoue S. Obesity, weight change and risks for hypertension, diabetes and hypercholesterolemia in japanese men. Eur J Clin Nutr. 2002;56(7):601–607. doi: 10.1038/sj.ejcn.1601364. [DOI] [PubMed] [Google Scholar]
- 207.Collaboration A.P.C.S. Body mass index and risk of diabetes mellitus in the asia-pacific region. Asia Pacific. 2006;15(2):127–133. [PubMed] [Google Scholar]
- 208.Ohnishi H., Saitoh S., Takagi S., Katoh N., Chiba Y., Akasaka H., Nakamura Y., Shimamoto K. Incidence of type 2 diabetes in individuals with central obesity in a rural japanese population: the tanno and sobetsu study. Diabetes Care. 2006;29(5):1128–1129. doi: 10.2337/diacare.2951128. [DOI] [PubMed] [Google Scholar]
- 209.Hadaegh F., Zabetian A., Harati H., Azizi F. The prospective association of general and central obesity variables with incident type 2 diabetes in adults, tehran lipid and glucose study. Diabetes Res Clin Pract. 2007;76(3):449–454. doi: 10.1016/j.diabres.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 210.He Y.-H., Jiang G.-X., Yang Y., Huang H.-E., Li R., Li X.-Y., Ning G., Cheng Q. Obesity and its associations with hypertension and type 2 diabetes among chinese adults age 40 years and over. Nutrition. 2009;25(11–12):1143–1149. doi: 10.1016/j.nut.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 211.Bermudez O.I., Tucker K.L. Total and central obesity among elderly hispanics and the association with type 2 diabetes. Obesity Res. 2001;9(8):443–451. doi: 10.1038/oby.2001.58. [DOI] [PubMed] [Google Scholar]
- 212.Midus – midlife in the united states, a national longitudinal study of health and well-being. URL: http://midus.wisc.edu/ [Accessed on 03/04/2019].
- 213.Krishnan S., Rosenberg L., Djoussé L., Cupples L.A., Palmer J.R. Overall and central obesity and risk of type 2 diabetes in us black women. Obesity. 2007;15(7):1860–1866. doi: 10.1038/oby.2007.220. [DOI] [PubMed] [Google Scholar]
- 214.Flegal K.M., Carroll M.D., Ogden C.L., Johnson C.L. Prevalence and trends in obesity among us adults, 1999–2000. Jama. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 215.Goryakin Y, Rocco L, Suhrcke M. The contribution of urbanization to non-communicable diseases: Evidence from 173 countries from 1980 to 2008. Econom Human Biol 26: 2017; 151–163. [DOI] [PubMed]
- 216.Hu F.B. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Association A.D. Standards of medical care in diabetes: 2012. Diabetes Care. 2012;35(1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]