Abstract
Background and aim
The incidence of hepatocellular carcinoma (HCC) decreases significantly in chronic hepatitis C (CHC) patients with sustained virologic response (SVR) after pegylated-interferon plus ribavirin (PR) or direct-acting antiviral (DAAs) therapy. We follow-up a single cohort of CHC patients to identify risk factors associated with HCC development post-SVR.
Method
CHC patients with SVR in Beijing/Hong Kong were followed up at 12–24 weekly intervals with surveillance for HCC by ultrasonography and alpha-fetoprotein (AFP). Multivariate Cox proportional hazards regression analysis was used to explore factors associated with HCC occurrence.
Results
Between October 2015 and May 2017, SVR was observed in 519 and 817 CHC patients after DAAs and PR therapy respectively. After a median post -SVR follow-up of 48 months, HCC developed in 54 (4.4%) SVR subjects. By adjusted Cox analysis, older age (≥55 years) [HR 2.4, 95% CI (1.3–4.3)], non-alcoholic fatty liver diseases [HR 2.4, 95%CI (1.3–4.2), higher AFP level (≥20 ng/ml) [HR 3.4, 95%CI (2.0–5.8)], higher liver stiffness measurement (≥14.6 kPa) [HR 4.2, 95%CI (2.3–7.6)], diabetes mellitus [HR 4.2, 95%CI (2.4–7.4)] at pre-treatment were associated with HCC occurrence. HCC patients in the DAAs induced SVR group had a higher prevalence of NAFLD as compared with those in the PR induced SVR group, 62% (18/29) vs 28% (7/25), p = 0.026. A nomogram formulated with the above six independent variables had a Concordance-Index of 0.835 (95% CI 0.783–0.866).
Conclusion
Underlying NAFLD is associated with increased incidence of HCC in chronic HCV patients post-SVR, particularly in those treated with DAA.
Keywords: Chronic hepatitis C, Sustained virologic response, NAFLD, HCC
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; ANGPTL, angiopoietin-like proteins; AST, aspartate aminotransferase; ASV, asunaprevir; BCLC, Barcelona-Clinic Liver Cancer Group; BMI, body mass index; CHC, chronic hepatitis C; CI, confidence intervals (CI); DAAs, direct-acting antiviral agents; DCV, daclatasvir; FGF, fibroblast growth factor; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, Hazard Ratio; IFN, interferon; LDV, ledipasvir; LSM, liver stiffness measurement; Peg-IFN, Pegylated interferon; PLT, platelet count; PR, Peg-IFN-α with RBV; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir; SVR, sustained virologic response; TBIL, total bilirubin; TNF, tumor necrosis factor; ULN, upper limit of normal
Highlights
-
•
Patients with chronic hepatitis C infection are still at risk of HCC after achieving sustained virus clearance (SVR).
-
•
Non-alcoholic liver disease (NAFLD) is emerging as an important risk factor for hepatocellular carcinoma.
-
•
Underlying NAFLD is associated with increased incidence of HCC in patients with chronic HCV infection after sustained virologic response SVR.
1. Introduction
More than seventy million people worldwide suffered from chronic hepatitis C virus (HCV) infection, which if left untreated may culminate in end-stage liver cirrhosis and hepatocellular carcinoma [1]. In China, hepatocellular carcinoma (HCC) is the most diagnosed cancer in individuals under the age of 60 years, and approximately 20% of HCC are attributable to HCV infection. With the significant increase in the number of cases with chronic hepatitis C (CHC) over the past 10 years, the incidence of HCC in China attributable to CHC is projected to increase [2]. Treatment with pegylated interferon (PR)-based therapy or pan-oral direct-acting antiviral agents (DAAs) can achieve sustained virologic response (SVR) in over 65% and 95% of HCV subjects respectively [3,4]. SVR was associated with a more than 70% reduction in the risk of HCC [[5], [6], [7], [8], [9]]. However, patients with CHC and cirrhosis including those with SVR, still had a substantial risk of 1.1 per 1,000 person-years for HCC development [10]. Recent study had shown that the risk of HCC occurrence persisted with a cumulative incidence of 1.1%, 1.9% and 2.8%, at 1, 2, and 3-year post -SVR respectively [11]. Older age, DM, high AFP level and high liver stiffness measurements (LSM) had been identified as risk factors for HCC occurrence in HCV patients with SVR [[12], [13], [14]]. Besides viral hepatitis, alcohol, aflatoxins, aristolochic acid, hemochromatosis are also risk factors for HCC [15]. In the past decade, obesity, insulin resistance and non-alcoholic fatty liver disease (NAFLD) are emerging risk factors for HCC [[16], [17], [18], [19]]. The estimated annual HCC incidence in the progressive form of NAFLD is about 0.3%. With improving living conditions, change in lifestyle and dietary habits, there is a progressive increase in the prevalence of NAFLD in China. NAFLD together with viral hepatitis will be the predominant predisposing factors of hepatocellular carcinoma in China in the upcoming decades. In the current prospective study, we evaluate whether NAFLD is an independent risk factor associated with HCC occurrence in a cohort of treatment-naïve Chinese CHC patients with or without cirrhosis, who had achieved SVR with pan-oral DAAs or PR over a median follow -up of four years.
2. Methods
2.1. Study design
This was an open label prospective observational study with the primary objective to identify clinical factors associated with occurrence of HCC in CHC patients after achievement of SVR. All patients attended The Fifth Medical Center of Chinese PLA General Hospital (302 Hospital)-Hong Kong Humanity and Health Hepatitis C Diagnosis and Treatment Centre, Beijing, China or Humanity and Health Medical Center, Hong Kong SAR, China. The choice of therapy was at each patient’s own discretion after the cost, efficacy and safety had been explained to them. For those who opted for DAAs therapy, DAAs were obtained from Humanity and Health Medical Center, Hong Kong SAR where DAAs were made available by 2015. For those CHC patients treated with PR therapy, treatment was initiated at either The Fifth Medical Center of Chinese PLA General Hospital (302 Hospital)-Hong Kong Humanity and Health Hepatitis C Diagnosis and Treatment Centre, Beijing, China or Humanity and Health Medical Center, Hong Kong SAR, China. This was because DAAs were only approved by The China Food and Drug Administration in mainland China (not including Hong Kong SAR) by mid-2017. Both centers were managed by the medical team under the directorship of the corresponding author (G Lau). HCV RNA were quantitated with COBAS TaqMan 48 analyzer, version 2.0 (Roche Molecular Systems, Branchburg, NJ, USA). HCV genotype and subtype were determined using the Versant HCV Genotype INNOLiPA 2.0 assay (Siemens Healthcare Diagnostics, Tarrytown, NY, USA).
The clinical protocol was registered at ClinicalTrials.gov (NCT02578693) and approval was obtained from the Ethics Committees in Hong Kong (HKCREC) and Beijing (EC of Beijing 302 hospital). Informed consent was obtained from all patients and the study was conducted in compliance with the 1975 Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements.
2.2. Patients
All CHC subjects who had been treated with either DAAs or PR therapy between Oct 2015 to May 2017 were recruited. The inclusion criteria were (1) over 18 years old; (2) diagnosed to have chronic HCV infection based on serum positivity for both anti-HCV and HCV RNA; (3) eligible for both PR and DAAs therapies; Patients with clinically decompensated liver cirrhosis, severe depression, significant neuropsychiatric syndromes, drug addiction, active autoimmune diseases such as lupus erythematosus and pregnant subjects would be excluded. (4) treatment-naïve patients with chronic hepatitis or Child-Pugh A liver cirrhosis; (5) hepatitis B surface antigen negative; (6) human immunodeficiency virus negative; (6) no significant alcohol consumption as defined by Alcohol Use Disorders Identification Test (AUDIT-C) ≥4 (≥3 for women) at the time and six-months prior to anti-HCV therapy initiation [20].
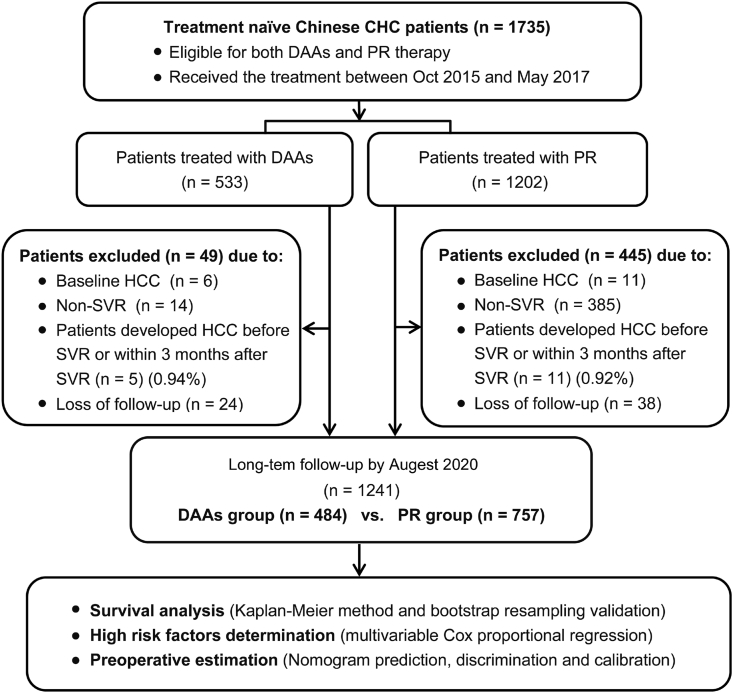
Patients were excluded if they were: (1) diagnosed to have HCC before, at the end-of-treatment (EOT) or within 3 months post-SVR; (2) did not achieve SVR24/12 defined as HCV RNA under the lower limit of quantification (LLOQ, HCV RNA level < 15 IU/ml) at week 12 after completion of the DAAs therapy (SVR12) or week 24 after completion of PR therapy (SVR 24); (3) loss to follow-up longer than 6 months after SVR (Fig. 1).
Fig. 1.
Flow chart of the study population (patients included or excluded in the study). Patients developed HCC before SVR or within 3 months after SVR in DAAs group or PR group was 5 out of 533 patients (0.94%) or 11 out of 1202 patients (0.92%), respectively, and there was no significant difference between these two groups (chi-square test, P = 0.963).
2.3. Treatment
Patients in the DAAs group received various combination of brand-named non-generic US FDA and EU EMEA approved drugs: (1) Daclatasvir (Daklinza, Bristol-Myers Squibb) 60 mg daily and Sofosbuvir (Sovaldi, Gilead Sciences) 400 mg daily for 8–12 weeks; (2) Ledipasvir 90 mg/sofosbuvir 400 mg (Harvoni, Gilead Science) daily for 8–12 weeks; (3) Sofosbuvir (Sovaldi, Gilead Sciences) 400 mg daily, Daclatasvir (Daklinza, Bristol-Myers Squibb) 60 mg daily and asunaprevir (Sunvepra, Bristol-Myers Squibb) 100 mg twice daily for three weeks; (4) Ledipasvir 90 mg/sofosbuvir 400 mg (Harvoni, Gilead Science) and asunaprevir (Sunvepra, Bristol-Myers Squibb) 100 mg twice daily for 3–12 weeks; (5) Sofosbuvir (Sovaldi, Gilead Sciences) 400 mg daily, Daclatasvir (Daklinza, Bristol-Myers Squibb) 60 mg daily and simeprevir 150 mg daily (Olysio, Janssen) for 3 weeks; (6) Ritonavir 50mg/paritaprevir 75 mg/ritonavir 50 mg/, dasabuvir 250 mg (Viekira pak, Abbvie) for 12 weeks. These patients were monitored at a regular 4-weekly intervals during the treatment period and at 12-week after the end of treatment to evaluate whether they achieved SVR (SVR12). Patients in the PR group received Peg-IFN-α (Pegasys, Roche) at a dose of 180 or 135 μg once per week by subcutaneous injection, plus RBV (1,000 to 1,200 mg/day, adjusted by weight) for 48 weeks for none-genotype 2 and 24 weeks for genotype 2 CHC and followed-up at 24 weeks after the end-of-treatment to evaluate whether they achieved SVR (SVR24).
2.4. Assessments
For all studied subjects, standard laboratory tests that included complete blood cell count, alanine aminotransferase [ALT], total bilirubin [TBIL], serum albumin [ALB]), were performed at baseline before initiation of antiviral therapy and each follow-up visit. Ultrasound and liver stiffness measurement (LSM) were performed before initiation of antiviral therapy. Non-alcoholic fatty liver disease (NAFLD) status at time of anti-viral therapy was documented by ultrasound or ultrasound plus hepatic steatosis index. NAFLD was defined as presence of ≥5% hepatic steatosis in the absence of secondary causes of hepatic fat accumulation such as significant alcohol consumption, long-term use of a steatogenic medication, or monogenic hereditary disorders. For cases that the sonographer considered borderline hepatic steatosis, subjects with hepatic steatosis index 36 or above would be considered as having NAFLD. The presence of cirrhosis was established by liver biopsy in patients with informed consent or by LSM (FibroScan®, Echosens, Paris, France) ≥ 14.6 kPa [21.22]. The absence of HCC was confirmed by imaging examinations using ultrasonography (GE, E9, GE Medical System, Milwaukee, WI) and alpha-fetoprotein (AFP), with or without multi-phase dynamic contrast-enhanced magnetic resonance imaging (MRI) scan (GE Signa HD×1.5 T, GE Medical System, Milwaukee, WI), in all patients before initiating antiviral therapy. Surveillance for HCC was performed by ultrasonography and AFP at each 12–24 weekly interval follow-up visit for all subjects who had achieved SVR. HCC was diagnosed by multi-phase dynamic contrast-enhanced MRI according to modified APASL HCC guidelines [23].
The SVR date was defined as time zero. Patients were censored at the time of newly diagnosed HCC or death or liver transplantation, whichever occurred earlier. All patients’ information was recorded in a computerized database by a designated research associate at both centers.
2.5. Statistics
2.5.1. Descriptive statistical methods
Continuous variables were expressed as mean ± standard deviation (sd) or median (interquartile range, IQR) and compared using the unpaired, 2-tailed t-test or Mann-Whitney test. Categorical variables were presented as numbers and percentage and compared using the chi-square test. 95% confidence intervals (CI) were calculated for each predictive test and a p-value < 0.05 was considered as significant for all statistical tests. The statistical analyses were performed using R software, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/).The reported cumulative incidence of HCC occurrence was 3% at 3-year post -SVR [11], our sample size of over 1200 post SVR subjects followed for 4 years would have a 0.8 power of detecting a 20% difference of prevalence of various risk factors such as NAFLD, diabetes, LSM≥14.6 kPa between the HCC and non-HCC groups at p<0.05.
2.5.2. HCC incidence
The cumulative rate of HCC occurrence was determined using Kaplan-Meier curves. The significance of treatment baseline variables, such as age, sex, body mass index (BMI), HCV RNA level, HCV RNA genotype, LSM, presence of NAFLD, ALT, TBIL, PLT and AFP, were as risk factors of HCC occurrence, assessed by univariate analysis. All variables associated with HCC at a significant level (p<0.05) were candidates for stepwise multivariate Cox proportional regression analysis. Hazard ratio (HR) of each risk factor was shown in forest plot with its own 95% CI and P value.
2.5.3. Bootstrap resampling
Nonparametric bootstrap test with pooled resampling method was performed to reduce the sample bias, as well as to verify the results of survival analysis. One thousand bootstrap samples were performed followed by Cox proportional regression analysis by using the boot package in R. The hazard ratio of the candidate variable for HCC occurrence is considered significant when the lower end of 95% CI is above zero.
2.6. Prediction with nomogram
A nomogram was formulated based on the results of multivariate Cox regression analysis and by using the rms package of R. The nomogram was based on proportionally converting each regression coefficient in multivariate Cox regression to a 0- to 100-point scale. The effect of the variable with the highest β coefficient was assigned 100 points. The points were added across independent variables to derive total points, which were converted to predicted probabilities. The predictive performance of the nomogram was measured by concordance index (C-index) and calibration with 1000 bootstrap samples to decrease the overfit bias by using the rms package of R [24].
3. Results
3.1. Patient characteristics
Altogether, as shown in Figs. 1 and 1735 treatment naïve Chinese CHC patients who were eligible for both DAAs and PR therapies received the treatment between Oct 2015 and May 2017. Among DAAs subjects, 79 received Daclatasvir/Sofosbuvir, 361 received Ledipasvir/sofosbuvir, 37 received Sofosbuvir/Daclatasvir/Asunaprevir, 46 received Ledipasvir/sofosbuvir/asunaprevir, 6 received Sofosbuvir/Daclatasvir/simeprevir and 4 received Ritonavir/paritaprevir/dasabuvir. During treatment and follow-up period, 49 or 445 patients in DAAs or PR group were excluded from the final analyses due to loss to follow-up and various reasons. Five out of 533 (0.94%) and 11 out of 1202 (0.92%) patients in DAAs group and PR group respectively developed HCC before SVR or within 3 months of achieving SVR, they were also excluded from analysis (Fig. 1).
All remaining 1241 patients (484 in DAAs group and 757 in PR group) with SVR were followed-up for a median duration of 4 years. Similar proportions of subjects in the DAAs and PR groups were lost to follow-up, 4.71% (24/508) and 4.77% (38/7950 respectively. The baseline characteristics of these subjects were summarized in Table 1. 376(30.3%) had LSM≥14.6 kPa at enrolment. 317 (25.5%) patients had NAFLD, among these only 12 were diagnosed with borderline ultrasound findings plus hepatic steatosis index greater than 36. Similar proportions of subjects in the DAAs group and the PR group had NAFLD and LSM>=≥ 14.6 kPa, 36.27.1% (131/484) versus 24.6% (186/757) and 151 31.2%(151/484) and 29.7%(225/757) respectively (p>0.4), Table 2.
Table 1.
Baseline (before antiviral therapy) characteristics of all SVR (HCC + non-HCC) patients.
| Total = 1241 | HCC n = 54 | Non-HCC n = 1187 | P value | |
|---|---|---|---|---|
| Age (yr) | 50.2 ± 12.6 | 58.3 ± 8.0) | 49.8 ± 12.6 | <0.001 |
| Male sex (n, %) | 559 (45.0) | 26 (48.1) | 533 (44.9) | 0.742 |
| BMI (kg/m2) | 23.6 ± 3.2 | 24.0 ± 3.2 | 23.6 ± 3.2 | 0.403 |
| NAFLD (n, %) | 317 (25.5) | 25 (46.3) | 292 (24.6) | 0.001 |
| HCV Genotype (%) | 0.432 | |||
| 1b | 894 (72.0) | 43 (79.6) | 851 (71.7) | |
| 2a | 323 (26.0) | 10 (18.5) | 313 (26.4) | |
| others | 24 (1.9) | 1 (1.9) | 23 (1.9) | |
| HCV RNA (Log10 IU/ml) | 6.0 ± 1.1 | 6.1 ± 1.1 | 6.0 ± 1.1 | 0.484 |
| ALT (U/L) | 74.9 ± 15.7 | 73.2 ± 15.5 | 75.0 ± 15.7 | 0.392 |
| TBIL (μmol/L) | 20.6 ± 9.2 | 23.4 ± 7.4 | 20.5 ± 9.2 | 0.023 |
| CRE (μmol/L) | 74.0 ± 13.8 | 72.4 ± 15.5 | 74.0 ± 13.7 | 0.401 |
| PLT ( × 109/L) | 161.6 ± 63.4± | 145.6 ± 68.0 | 162.4 ± 63.1 | 0.058 |
| Age ≥ 55 yr (n, %) | 483 (38.9) | 37 (68.5) | 446 (37.6) | <0.001 |
| AFP ≥ 20 ng/ml (2 × ULN) | 254 (20.5) | 25 (46.3) | 229 (19.3) | <0.001 |
| Diabetes (n, %) | 116 (9.3) | 18 (33.3) | 98 (8.3) | <0.001 |
| LSM ≥ 14.6 kPa (n, %) | 376 (30.3) | 36 (66.7) | 340 (28.6) | <0.001 |
| Mean LSM (kPa) | 11.1 (7.5–16.0) | 17.4 (13.7–21.3) | 10.8 (7.5–15.3) | <0.001 |
Continuous variables were expressed as mean ± sd and compared using the unpaired, 2-tailed t-test. Categorical variables were presented as numbers and percentages.
Table 2.
Baseline (before antiviral therapy) characteristics of all DAAs and PR SVR patients.
| DAAs | PR | P value | |
|---|---|---|---|
| n | 484 | 757 | |
| Age (yr) | 50.6 ± 13.8 | 49.9 ± 11.7 | 0.283 |
| Male sex (n, %) | 225 (46.5) | 334 (44.1) | 0.448 |
| BMI (kg/m2) | 23.7 ± 3.3 | 23.5 ± 3.2 | 0.307 |
| NAFLD (n, %) | 131 (27.1) | 186 (24.6) | 0.359 |
| Genotype (%) | 0.768 | ||
| 1b | 353 (72.9) | 541 (71.5) | |
| 2a | 123 (25.4) | 200 (26.4) | |
| others | 8 (1.7) | 16 (2.1) | |
| HCV RNA (Log10 IU/ml) | 6.1 ± 1.2 | 6.0 ± 1.1 | 0.335 |
| ALT (U/L) | 74.6 ± 15.9 | 75.2 ± 15.5 | 0.540 |
| TBIL (μmol/L) | 20.3 ± 8.9 | 20.9 ± 9.4 | 0.337 |
| CRE (μmol/L) | 74.0 ± 15.0 | 74.0 ± 13.0 | 0.990 |
| PLT ( × 109/L) | 161.0 ± 67.0 | 162.0 ± 61.1 | 0.802 |
| Age ≥ 55 yr (n, %) | 195 (40.3) | 288 (38.0) | 0.465 |
| AFP ≥ 20 ng/ml (2 × ULN) | 104 (21.5) | 150 (19.8) | 0.522 |
| Diabetes (n, %) | 41 (8.5) | 75 (9.9) | 0.455 |
| LSM ≥ 14.6 kPa (n, %) | 151 (31.2) | 225 (29.7) | 0.625 |
| Mean LSM (kPa) | 11.6 (7.4–16.4) | 10.7 (7.6–15.8) | 0.212 |
| Follow-up time (months) | 48.0 (43.5–50.0) | 48.0 (44.5–49.0) | 0.065 |
Continuous variables were expressed as mean ± sd and compared using the unpaired, 2-tailed t-test. Categorical variables were presented as numbers and percentages and compared using the chi-square test.
3.2. HCC incidence rate
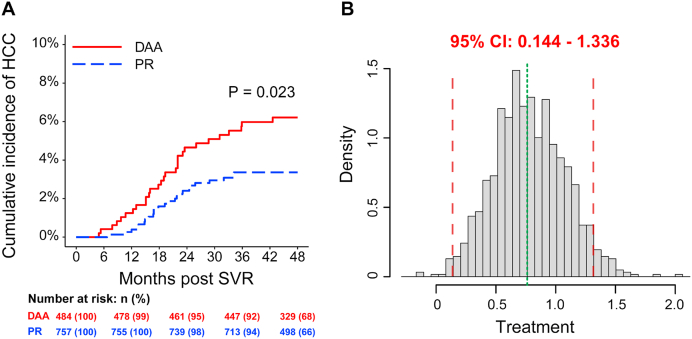
The median follow-up time after SVR was 48.0 months. During the follow-up period, HCC developed in 54 (4.4%) SVR subjects, 29/484 (6.0%) in the DAAs group and 25/757 (3.3%) in the PR group (P = 0.023, Pearson chi-square test) (Fig. 2). Most of the HCC developed within first 2 years after SVR (Table 3). Interestingly, HCC patients in the DAAs induced SVR group had a higher prevalence of NAFLD as compared with HCC subjects in the PR induced SVR group, 62% (18/29) vs 28% (7/25), P = 0.026 (Table 4).
Fig. 2.
Cumulative HCC incidence rates in patients with SVR according to antiviral therapy. The Kaplan-Meier method was used to assess cumulative incidence rate of HCC, and the log-rank test was used to compare them (A). Nonparametric bootstrap test with pooled resampling method was performed to reduce the sample bias, as well as to verify the results of survival analysis. Briefly, the SVR patients were randomly drawn from the study population, and sampling was performed with replacement. From this sample the regression coefficients from COX proportional hazard model analyses were calculated. The resulting sample of effects then was used to calculate the 95% CI. If zero wasn’t included in the 95% CI, it represented the two treatments were different by means of HCC incidence. After 1000 bootstrap samples, HR of DAAs therapy for HCC occurrence was significantly higher than PR therapy for their lower ends of 95% CI were above zero (B).
Table 3.
Characteristics of HCC patients.
| Total | |
|---|---|
| N | 54 |
| Age (yr) | 59.3 ± 8.1 |
| Male sex n (%) | 26 (48.1) |
| BMI (Kg/m2) | 24.0 ± 3.2 |
| aNAFLD (n, %) | 25 (46.3) |
| LSM (>14.6kpa) (n,%) | 36 (66.7) |
| DAA (n, incidence ratea) | 29 (6%) |
| PR (n, incidence ratea) | 25 (3.3%) |
| Incidence n (%) | |
| 12 months | 9 (16.6.) |
| 24 months | 31 (57.4) |
| 36 months | 13 (24) |
| 48 months | 1(1.8) |
| mRECIST n (%) | |
| CR | 30 (55.6) |
| PR | 6 (11.1) |
| SD | 2 (3.7) |
| PD | 16 (29.6) |
| Dead n (%) | 11 (20.4) |
| Follow-up time (months) | 19.1 (14.8–24.3) |
Continuous variables were expressed as mean ± sd or median (IQR) and compared using the unpaired, 2-tailed t-test or Mann-Whitney test. Categorical variables were presented as numbers (percentages) and compared using the chi-square test. CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease.
HCC incidence, 29/484 (6.0%) in the DAAs group and 25/757 (3.3%) in the PR group (P = 0.024, Pearson chi-square test).∗Among the 25 NAFLD patients 16 (64%) had LSM >14.6kpa.
Table 4.
Comparison of Characteristics of HCC patients in DAAs and PR groups.
| DAAs | PR | P value | |
|---|---|---|---|
| N | 29 | 25 | |
| Age (yr) | 58.0 ± 8.3 | 60.8 ± 7.8 | 0.215 |
| Male sex (n, %) | 14 (48.3) | 12 (48.0) | 1.000 |
| BMI (Kg/m2) | 24.4 ± 3.1 | 23.4 ± 3.3 | 0.273 |
| NAFLD (n, %) | 18 (62.1) | 7 (28.0) | 0.026 |
| Genotype (n, %) | 0.310 | ||
| 1b | 21 (72.4) | 22 (88.0) | |
| 2a | 7 (24.1) | 3 (12.0) | |
| Others | 1 (3.4) | 0 (0.0) | |
| BCLC (n, %) | 0.088 | ||
| 0 | 4 (13.8) | 6 (24.0) | |
| A | 10 (34.5) | 13 (52.0) | |
| B | 10 (34.5) | 6 (24.0) | |
| C | 5 (17.2) | 0 (0.0) | |
| Numbers of nodule (n) | 1.8 ± 1.2 | 1.6 ± 1.2 | 0.723 |
| Cancer treatment (n, %) | 0.021 | ||
| Resection | 8 (27.6) | 2 (8.0) | |
| Ablation | 7 (24.1) | 9 (36.0) | |
| TACE | 8 (27.6) | 6 (24.0) | |
| Liver transplantation | 4 (13.8) | 0 (0.0) | |
| Systematic therapy | 2 (6.9) | 8 (32.0) | |
| mRECIST (n, %) | 0.200 | ||
| CR | 15 (51.7) | 15 (60.0) | |
| PR | 5 (17.2) | 1 (4.0) | |
| SD | 2 (6.9) | 0 (0.0) | |
| PD | 7 (24.1) | 9 (36.0) | |
| Dead (n, %) | 5 (17.2) | 6 (24.0) | 0.782 |
| Follow-up time (months) | 19.0 (13.0–23.5) | 19.2 (14.9–24.5) | 0.815 |
Continuous variables were expressed as mean ± sd or median (IQR) and compared using the unpaired, 2-tailed t-test or Mann-Whitney test. Categorical variables were presented as numbers (percentages) and compared using the chi-square test. CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease.
3.3. Factors associated with HCC development and nomogram
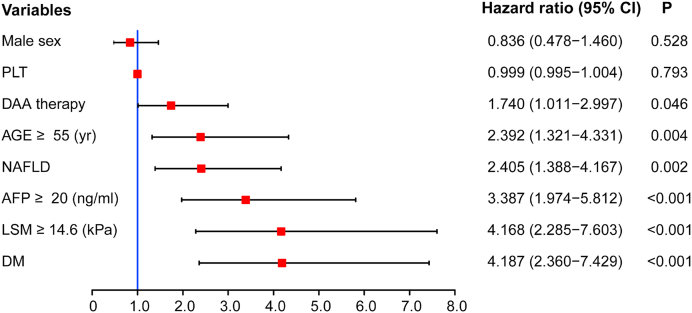
The results of an adjusted multivariate Cox proportional hazards model were presented in Fig. 3, with HR (95% CI) reported. Only the parameters which were significant in the univariate analysis were used in the multivariate model. The occurrence of HCC was not associated with sex, BMI, HCV genotype, PLT, HGB, ALT, TBIL, and CRE (P > 0.05). However, DAAs therapy [HR 1.7, 95%CI(1.0–3.0)], older age (≥55 years) [HR 2.4, 95% 95% CI (1.3–4.3)], NAFLD [HR 2.4, 95%CI (1.3–4.2), higher AFP level (≥20 ng/ml) [HR 3.4, 95%CI (2.0–5.8)], higher liver stiffness measurement (≥14.6 kPa) [HR 4.2, 95%CI (2.3–7.6)], diabetes mellitus [HR 4.2, 95%CI (2.4–7.4)] at initiation of anti-viral therapy were associated with increased risk of HCC (Fig. 3).
Fig. 3.
Risk factors (present at time of initiation of antiviral therapy) associated with development of HCC in patients with HCV who achieved SVR. A stepwise selection procedure and the Breslow method were used in multivariable Cox proportional regression analysis. HRs for HCC in the patients are shown (red solid boxes) with 95% CIs (black line segments). HR: hazard ratio; PLT: platelet; NAFLD: Non-alcoholic fatty liver disease; AFP: Alpha-fetoprotein; LSM: liver stiffness measurement; DM: diabetes mellitus. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
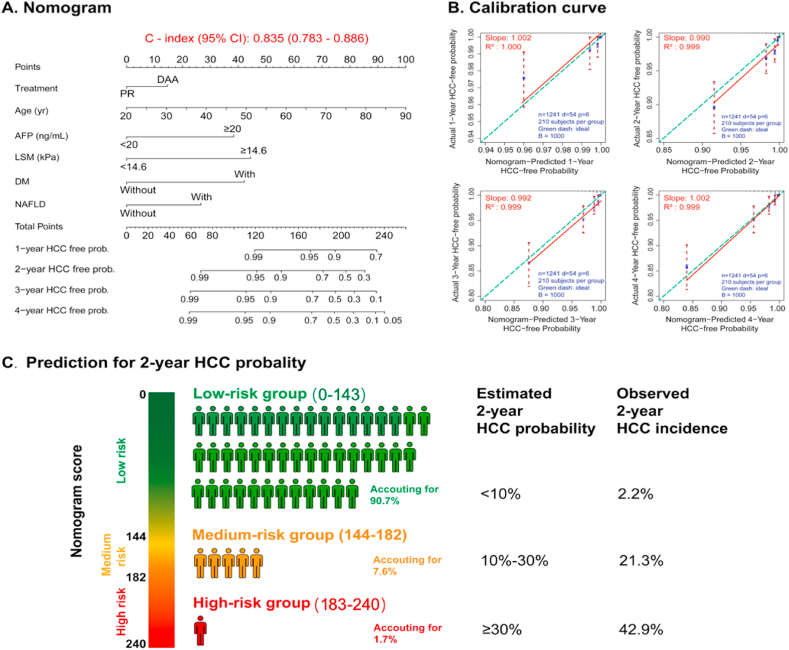
A nomogram was established based on the results of multivariate logistic regression analysis. The predictive performance of the nomogram was measured by concordance index (C-index) and calibration with 1000 bootstrap samples to decrease the overfit bias. The nomogram demonstrated good accuracy in estimating the risk of HCC, with an unadjusted C-index of 0.835 (95%CI:0.783–0.886) (Fig. 4A). Furthermore, calibration plots graphically showed good agreement on the above risk factors. The 1-, 2-, 3-year and 4-year HCC-free probabilities estimation (slopes [R2]) by the nomogram and confirmation of HCC by histopathology or MRI scan were 1.002, 0.990, 0.992,1.002 and 1.000, 0.999, 0.999, 0.999 respectively (Fig. 4B). Two-year HCC incidence after eradication of HCV was showed in Fig. 4C.
Fig. 4.
Nomogram to estimate the risk of HCC incidence in patients with HCV who achieved SVR. A: The nomogram-based prediction scoring model established using Logistic regression. To use the nomogram, find the position of each variable on the corresponding axis, draw a line to the points axis for the number of points, add the points from all of the variables, and draw a line from the total points axis to determine the HCC-free probabilities at the lower line of the nomogram. C-index (0.835) represents the prediction performance of our model is satisfied. Take a 55years man with diabetes, AFP>20 ng/dl, LSM < kPa, without NAFLD and achieved SVR with PR for example, his nomogram score will be 50 + 45+40 + 0+0, total 135 giving a 2-year HCC free probability of 98%. However, if he had NAFLD and DAAs treatment, his total score would be 50 + 45+30 + 15 = 180, giving a 2-year HCC free probability of 94%, an increase of cancer risk by 4%. B: Validity of the predictive performance of the nomogram. C: Two-year HCC incidence after eradication of HCV.
PLT: platelet; ALT: alanine aminotransferase; TBIL: total bilirubin; CRE: creatinine; AFP: Alpha-fetoprotein; BMI: body mass index; LSM: liver stiffness measurement; DM: diabetes mellitus.
For example, a 55 years-old man with diabetes, AFP>20 ng/dl, LSM <14.6 kPa, without NAFLD and achieved SVR with PR, his nomogram score will be 50 + 45+40 + 0+0 + 0, total 135 giving a 2-year HCC free probability of 98%. However, if he had NAFLD and DAAs treatment, his total score would be 50 + 45+40 + 0 +30 + 15 = 180, giving a 2-year HCC free probability of 94%, an increase of cancer risk by 4%. If the same person LSM was ≥ 14.6 kPa, the corresponding nomogram scores with and without NAFLD plus DAAs treatment would be 225 and 180 respectively, with 2-year HCC free probability of 94% and 75% respectively, a difference of 19%. Overall, the presence of NAFLD and use of DAAs therapy would further increase the likelihood of HCC occurrence by three to 30%.
4. Discussion
Consistent with other reports, we have identified certain baseline characteristics, such as older age (≥55 years), higher AFP level (≥20 ng/ml), DM, and higher LSM (≥14.6 kPa) as risk factors for HCC development in such patients [[12], [13], [14]]. We also found that the occurrence of HCC by 4 years of SVR was also associated with DAAs therapy and NAFLD. It remains controversial whether there is any difference in HCC incidence after SVR using direct-acting antiviral agents (DAAs) versus PR therapy. Two large-scale retrospective studies performed in the USA [25,26] reported that the reduction of HCC risk in DAAs or PR induced SVR were similar. However, in these studies, based on Veterans Affairs (VA) healthcare electronic system, a substantial number of patients were treated with variable and incomplete courses of therapy or were lost to follow-up from the original cohort due to missing data following SVR. Moreover, the occurrence of HCC was based on different versions of ICD-coding obtained from chart extraction, thus affecting the accuracies. On the other hand, in the French ANRS CirVir prospective study, the crude 3-year cumulative incidences of HCC after SVR were significantly higher in the DAAs group as compared with IFN group, 5.9% and 3.1% respectively [27]. However, this apparent increase in HCC incidence might be due to different patient characteristics such as age, diabetes, and reduced liver function. Also, the HCC surveillance intensity was different in the DAAs and IFN groups and this might have affected the rate of HCC detection [27]. In a recent meta-analysis that included 26 studies (9 with DAAs and 17 with IFN-based therapy, total 91,249 DAAs-treated and 71,443 IFN-treated CHC patients), no difference in HCC occurrence was observed between patients treated with DAA or IFN therapy [14]. However, the follow-up duration was much shorter for those treated with DAAs as compared to those treated with IFN (1.3 years versus 5 years). Unlike other studies which were mainly retrospective and historical IFN-treated CHC was used for comparison, our study was prospectively conducted with DAAs and PR therapy being used in parallel. Therefore, additional confounding risk factors for HCC occurrence, such as diabetes mellitus, obesity, alcoholic and non-alcoholic fatty liver disease that are increasing in prevalence in Asia due to changing lifestyles, could be accounted for as well.
NAFLD has recently been recognized as an important etiology risk factor contributing to the increased incidence of hepatocellular carcinoma (HCC). Our data also suggested that NAFLD is a high-risk factor of HCC in CHC patients after HCV clearance. The development of HCC in NAFLD is most likely multifactorial and involves obesity-mediated mechanisms including low-grade chronic inflammatory response, increased lipid storage and lipo-toxicity, alteration of gut microbiota with increased levels of lipopolysaccharide, insulin resistance with hyperinsulinemia and increased IGF levels [[28], [29], [30], [31]]. Human gut microbiota plays a pivotal role in the development of NAFLD and HCC. Gut microbiota generates a variety of bioactive substances such as lipopolysaccharides, peptidoglycan, DNA, and extracellular vesicles, short-chain fatty acids, secondary bile acids, indole and its derivatives, trimethylamine, carotenoids, and phenolic compounds [[31], [32], [33]]. Gut microbiota alteration might lead to increased gut permeability, translocation of microbiota products from the gut to liver via the portal vein. The various Toll-like receptors (TLRs) present on the Kupffer cells, hepatic stellate cells and hepatocytes respond to these microbiota products, triggering downstream inflammatory responses, cytokine production and modulating hepatic redox hemostasis. These in turn can cause fibrosis and contribute to the pathogenesis of HCC [31]. Hepatokines and adipokines have been shown to be associated with disease progression in NAFLD or may even be early markers of HCC development [34]. Increases in serum angiopoietin-like proteins (ANGPTL) 1, 2, and 8, fibroblast growth factor (FGF) 2,19, and 21, apelin, chemerin, leptin, and visfatin have been associated either with HCC development or a poor prognosis in NAFLD-related HCC [35,36]. The increased levels of tumor necrosis factor (TNF) superfamily members, transforming growth factor β (TGFβ) and IL-18, in the setting of hepatocyte cell death and compensatory proliferation, contribute to increased hepatocellular carcinoma (HCC) risk [35]. Analysis of the hepatokines and adipokines profile in HCV post SVR subjects with NAFLD and HCC, HCV post SVR subjects with NAFLD but no HCC, HCV post SVR subjects without NAFLD and HCC and NAFLD/HCC subjects without HCV will shed light on the interplay between HCV, NAFLD and HCC development. Gut microbiota of HCC patients with NAFLD cirrhosis had increased Bacteroides vulgatus, Ruminococcus Blautia and decreased Bifidobacterium when compared with that of NAFLD cirrhosis patients without HCC [36]. CHC is also associated with an altered gut microbiota profile with increased Genus Streptococcus, Lactobacillus, Bacteroidetes, and decreased Bifidobacterium, a profile similar to that observed in HCC patients with NAFLD cirrhosis [37,38]. This gut dysbiosis persisted in patients who achieved a sustained virologic response after treatment with pegylated interferon and ribavirin [39]. On the other hand, DAA’s induced SVR was associated with improvement of gut microbiota alpha diversity, but not intestinal barrier functions [40]. DAA therapy seems to have little impact on glycol or lipid metabolism, but previous studies had shown that Peg-IFN-alpha could improve these two metabolisms [41,42], and these differences may explain why HCC patients in the DAA induced SVR group had higher prevalence of NAFLD than those in PR group. Further studies of gut microbiota dysbiosis, gut barrier permeability, in CHC NAFLD patients pre and post SVR will be of interest. Peleg et al. in a study of 515 CHC patients who achieved SVR following treatment with DAA, observed that subjects with liver steatosis had a much higher incidence of HCC when compared with SVR subjects without liver steatosis, 10.4% (22/211) versus 1.66% (5/304), p<0.001, after a median of follow-up of 24 months [43]. Both Peleg and our study showed that NAFLD or hepatic steatosis was independent risk fosters of HCC in subjects who had achieved SVR irrespective of degree of liver stiffness or fibrosis. One third of our HCC subjects with NAFLD had LSM<14.6 kPa. Perhaps, both NAFLD/hepatic steatosis and LSM/fibrosis should be included in risk factors stratification for HCC occurrence after SVR in CHC patients. Our nomogram score could stratify SVR subjects into different risk groups, with LSM and presence of NAFLD contributed independently to calculation of HCC risks. Desgasperi et al., in a series of 509 DAA-treated patients with HCV cirrhosis noted that a genetic risk score comprising of variants in PNPLA3, MBOAT7, TM6SF2, GCKR genes was a risk factor for HCC occurrence independent of other classical risk factors such as diabetes, male sex, liver stiffness measurements [44]. The combination of the above genetic risk score of hepatic fat accumulation and classical risk factors may allow better stratification of HCC risks. However, genetic risk scoring may not be feasible in many hospitals. Alonso-Lopez et al. suggested that DAA pre-treatment albumin levels together with dynamic changes in LSM between base line and at 1-year follow-up may identify a group of HCV-post SVR patients with extremely low risk of HCC and such patients require much less intensive surveillance after the first year [45]. The American Association for the Study of Liver Diseases (AASLD), the Asian Pacific Association for the Study of the Liver (APASL), and the European Association for the Study of the Liver (EASL) have all published guidelines for HCC surveillance in high groups at 6 months intervals with ultrasound [23, [46], [47], [48]. All three associations recommend surveillance for patients with cirrhosis. The APASL and EASL guidelines state that surveillance should be offered to chronic HCV who have advanced fibrosis or cirrhosis even after achieving SVR. Surveillance can also be considered in chronic HCV post-SVR patients with bridging fibrosis and NAFLD patients without underlying cirrhosis, however, further data are needed before making this recommendation [23,48]. The AASLD guidelines do not address the issue of HCC surveillance in non-cirrhotic patients with NAFLD or CHC. Our data as well as others [43,44] suggest that CHC patients with DAAs induced SVR and NAFLD may also benefit from more intensive surveillance and the current one-size fit all surveillance guidelines may need further tuning. Our data showed that the presence of NAFLD and use of DAAs therapy would further increase the likelihood of HCC occurrence by three to thirty percent In the future, a risk stratification score including NAFLD/hepatic steatosis or genetic hepatic fat accumulation score, dynamic LSM and perhaps gut microbiota changes may allow better and more individualized HCC surveillance strategy.
The major strength of our study is that it was a large cohort of HCC post SVR patients followed-up for 4 years in two major centers under one principal investigator, thus avoiding the center-to-center variations in protocol adherence that occur in many studies involving multiple centers. Also, all patients were enrolled within 18 months (Oct 2015 to May 2017), with a longer enrollment period, the prevalence of some baseline confounding variables such as obesity, BMI, NAFLD, might change substantially during the recruitment, thus skewing the comparisons. One of the limitations of our study is diagnosis of NAFLD and fibrosis were based on ultrasound and LSM, instead of liver biopsies, therefore the issue of non-alcoholic steatosis hepatitis could not be addressed.
5. Conclusions
Underlying NAFLD is associated with increased incidence of HCC in chronic HCV patients post-SVR, particularly in those treated with DAA. In future, a risk stratification scoring system including NAFLD/hepatic steatosis may allow better and more individualized HCC surveillance strategy.
Funding
Capital characteristic clinic project of the Beijing municipal science and technology commission (Grant No. Z181100001718034), Key Project of Jumei Special Fund for Hepatobiliary Disease Prevention and Treatment (2018JM12603003), Chinese Medical Association (Grant No.13071110496) and AIDS Research NIH (Grant No. 2P30-AI-050409).
CRediT author contribution statement
Dong Ji: Conceptualization, Formal analysis, Data curation, Methodology, Writing – original draft, Writing – review & editing, Conception and design, Development of methodology, Acquisition, analysis and interpretation of data, Writing, review, and/or revision of the manuscript, Administrative, technical, or material support. Xiao-xia Niu: Formal analysis, Data curation, Acquisition, analysis and interpretation of data. Mingjie Zhang: Formal analysis, Data curation, Acquisition, analysis and interpretation of data. Cheng Wang: Administrative, technical, or material support. Qing Shao: Methodology, Development of methodology. Vanessa Wu: Administrative, technical, or material support. Yudong Wang: Methodology, Development of methodology. Gregory Cheng: Conceptualization, Writing – original draft, Writing – review & editing, Conception and design, Writing, review, and/or revision of the manuscript, Formal analysis, Data curation, Acquisition, analysis and interpretation of data. Selwyn J. Hurwitz: Writing – original draft, Writing – review & editing, Writing, review, and/or revision of the manuscript. Raymond F. Schinazi: Writing – original draft, Writing – review & editing, Writing, review, and/or revision of the manuscript. George Lau: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Conception and design, Development of methodology, Writing, review, and/or revision of the manuscript, Study supervision.
Declaration of competing interest
We declare no conflict of interest.
Acknowledgements
The authors want to acknowledge Chief Nurse Ms. April Wong in Division of Gastroenterology and Hepatology, Humanity and Health Medical Center, Hong Kong SAR for great support in recruiting patients and assessing patients during the study; Prof Sheung-tat Fan and Prof See-ching Chan, Department of Hepatobiliary Surgery, Hong Kong Sanatorium and Hospital, Hong Kong SAR and Prof Zheng-Wen Liu, Department of liver transplant and Research Center, The Fifth Medical Center of Chinese PLA General Hospital (302 Hospital), Beijing, China for surgical management of HCC.
References
- 1.Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Wang F.S., Fan J.G., Zhang Z., Gao B., Wang H.Y. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldt B.J., Heathcote E.J., Wedemeyer H. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 4.Falade-Nwulia O., Suarez-Cuervo C., Nelson D.R., Fried M.W., Segal J.B., Sulkowski M.S. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2007;166:637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanwal F., Kramer J., Asch S.M., Chayanupatkul M., Cao Y., El-Serag H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Carrat F., Fontaine H., Dorival C., Simony M., Diallo A., Hezode C. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453–1464. doi: 10.1016/S0140-6736(18)32111-1. [DOI] [PubMed] [Google Scholar]
- 7.Calvaruso V., Cabibbo G., Cacciola I., Petta S., Madonia S., Bellia A. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155:411–421. doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Akuta N., Kobayashi M., Suzuki F., Sezaki H., Fujiyama S., Kawamura Y. Liver fibrosis and body mass index predict hepatocarcinogenesis following eradication of hepatitis C virus RNA by direct-acting antivirals. Oncology. 2016;91:341–347. doi: 10.1159/000450551. [DOI] [PubMed] [Google Scholar]
- 9.Calleja J.L., Crespo J., Rincón D., Ruiz-Antorán B., Fernandez I., Perelló C. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real-world cohort. J Hepatol. 2017;66:1138–1148. doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Kanwal F., Kramer J.R., Asch S.M., Cao Y., Liang L. El-serag HB long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology. 2020;71:44–55. doi: 10.1002/hep.30823. [DOI] [PubMed] [Google Scholar]
- 11.Terrault N.A., Hassanein T.I. Management of the patient with SVR. J Hepatol. 2016;65:120–129. doi: 10.1016/j.jhep.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag H.B., Kanwal F., Richardson P., Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanwal F., Kramer J., Asch S.M., Chayanupatkul M., Cao Y., El-Serag H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Waziry R., Hajarizadeh B., Grebely J., Amin J., Law M., Danta M., George J., Dores G.J. Hepatocellular carcinoma risk following direct acting antiviral HCV therapy: a systematic review, meta-analyses and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka K., Tsuji I., Tamakoshi A., Matsuo K., Ito H., Wakai K. Obesity and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:212–221. doi: 10.1093/jjco/hyr198. [DOI] [PubMed] [Google Scholar]
- 17.Wang P., Kang D., Cao W., Wang Y., Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 18.Scalera A., Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217–9228. doi: 10.3748/wjg.v20.i28.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 20.Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 21.Degos F., Perez P., Roche B., Mahmoudi A., Asselineau J., Voitot H. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;158:1013–1021. doi: 10.1016/j.jhep.2010.05.035. 53. [DOI] [PubMed] [Google Scholar]
- 22.Chinese Foundation for Hepatitis Prevention and Control Chinese society of infectious disease and Chinese society of Hepatology, Chinese medical association; liver disease committee of Chinese research hospital association. Consensus on clinical application of transient elastography detecting liver fibrosis: a 2018 update. Gan Zang Bing Za Zhi. 2019;27:182–191. doi: 10.3760/cma.j.issn.1007-3418.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Omata M., Cheng A.L., Kokudo N., Kudo M., Lee J.M., Jia J. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steyerberg E.W., Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–1931. doi: 10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannou G.N., Green P.K., Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2018;68:25–32. doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D.K., Ren Y., Fierer D.S., Rutledge S., Shaikh O.S., Lo Re V., 3rd The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: an ERCHIVES study. Hepatology. 2018;67:2244–2453. doi: 10.1002/hep.29707. [DOI] [PubMed] [Google Scholar]
- 27.Nahon P., Layese R., Bourcier V., Cagnot C., Marcellin P., Guyader D., ANRS CO12 CirVir Group Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155:1436–1450. doi: 10.1053/j.gastro.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Margini C., Dufour J.F. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317–324. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 29.Massoud O., Charlton M. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin Liver Dis. 2018;22:201–211. doi: 10.1016/j.cld.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Zoller H., Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism. 2016;65:1151–1160. doi: 10.1016/j.metabol.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Kolodziejczyk A.A., Zheng D., Shibolet O., Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11 doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji Y., Yin Y., Li Z., Zhang W. Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD) Nutrients. 2019;11:1712. doi: 10.3390/nu11081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong E.K., Puri P. Gut microbiome changes in Nonalcoholic fatty liver disease & alcoholic liver disease. Transl Gastroenterol Hepatol. 2021 doi: 10.21037/tgh.2020.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kucukoglu O., J Sowa J.P., Mazzolini G.D., Syn W.K., Canbay Hepatokines and adipokines in NASH-related hepatocellular carcinoma. J Hepatol. 2021;74:442–457. doi: 10.1016/j.jhep.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Anstee Q.M., Reeves H.L., Kotsiliti E., Govaere O., Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 36.Ponziani F.R., Bhoori S., Castelli C. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 37.Aly A.M., Adel A., El-Gendy A.O., Essam T.M., Aziz R.K. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog. 2016;8:42. doi: 10.1186/s13099-016-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue T., Nakayama J., Moriya K., Kawaratani H., Momoda R., Ito K. Gut dysbiosis associated with hepatitis C virus infection. Clin Infect Dis. 2018;67:869–877. doi: 10.1093/cid/ciy205. [DOI] [PubMed] [Google Scholar]
- 39.Bajaj J.S., Sterling R.K., Betrapally N.S., Nixon D.E., Fuchs M., Daita K. HCV eradication does not impact gut dysbiosis or systemic inflammation in cirrhotic patients. Aliment Pharmacol Ther. 2016;44:638–643. doi: 10.1111/apt.13732. [DOI] [PubMed] [Google Scholar]
- 40.Ponziani F.R., Putignani L., Paroni Sterbini F., Petito V., Picca A., Del Chierico F. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther. 2018;48:1301–1311. doi: 10.1111/apt.15004. [DOI] [PubMed] [Google Scholar]
- 41.Qing S., Ji D., Li B., Li F., Wang Y., Niu X. Improvement of glucose and lipid metabolism with pegylated interferon-a plus ribavirin therapy in Chinese patients chronically infected with genotype 1b hepatitis C virus. Ann Saudi Med. 2015;35:293–297. doi: 10.5144/0256-4947.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butt A.A., Umbleja T., Andersen J.W., Sherman K.E., Chung R.T. ACTG A5178 Study Team. Impact of peginterferon alpha and ribavirin treatment on lipid profiles and insulin resistance in Hepatitis C virus/HIV-coinfected persons: the AIDS Clinical Trials Group A5178 Study. Clin Infect Dis. 2012;55:631–638. doi: 10.1093/cid/cis463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peleg N., Issachar A., Sneh Arbib O., Cohen-Naftaly M., Harif Y., Oxtrud E., Braun M., Leshno M., Barsheshet A., Shlomai A. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepat. 2019;26:1257–1265. doi: 10.1111/jvh.13167. [DOI] [PubMed] [Google Scholar]
- 44.Degasperi E., Galmozzi E., Pelusi S., D’Ambrosio R., Soffredini R., Borghi M. Hepatic fat—genetic risk score predicts hepatocellular carcinoma in patients with cirrhotic HCV treated with DAAs. Hepatology. 2020;72:1912–1923. doi: 10.1002/hep.31500. [DOI] [PubMed] [Google Scholar]
- 45.Alonso López S., Manzano M.L., Gea F., Gutiérrez M.L., Ahumada A.M., Devesa M.J. A model based on noninvasive markers predicts very low hepatocellular carcinoma risk after viral response in hepatitis C virus–advanced fibrosis. Hepatology. 2020;72:1924–1934. doi: 10.1002/hep.31588. [DOI] [PubMed] [Google Scholar]
- 46.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 47.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M. Diagnosis, staging, and management of hepatocellular carcinoma: practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 48.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]