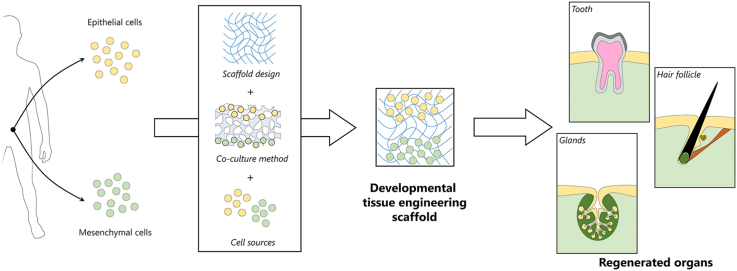
Abstract
Tissue engineering (TE) is a multidisciplinary research field aiming at the regeneration, restoration, or replacement of damaged tissues and organs. Classical TE approaches combine scaffolds, cells and soluble factors to fabricate constructs mimicking the native tissue to be regenerated. However, to date, limited success in clinical translations has been achieved by classical TE approaches, because of the lack of satisfactory biomorphological and biofunctional features of the obtained constructs. Developmental TE has emerged as a novel TE paradigm to obtain tissues and organs with correct biomorphology and biofunctionality by mimicking the morphogenetic processes leading to the tissue/organ generation in the embryo. Ectodermal appendages, for instance, develop in vivo by sequential interactions between epithelium and mesenchyme, in a process known as secondary induction. A fine artificial replication of these complex interactions can potentially lead to the fabrication of the tissues/organs to be regenerated. Successful developmental TE applications have been reported, in vitro and in vivo, for ectodermal appendages such as teeth, hair follicles and glands. Developmental TE strategies require an accurate selection of cell sources, scaffolds and cell culture configurations to allow for the correct replication of the in vivo morphogenetic cues. Herein, we describe and discuss the emergence of this TE paradigm by reviewing the achievements obtained so far in developmental TE 3D scaffolds for teeth, hair follicles, and salivary and lacrimal glands, with particular focus on the selection of biomaterials and cell culture configurations.
Keywords: Developmental, tissue engineering, Cell coculture, Epithelial-mesenchymal interaction, Tooth regeneration, Hair follicle regeneration, Gland regeneration
Graphical abstract
Highlights
-
•
Developmental tissue engineering (TE) mimics morphogenesis to regenerate organs.
-
•
Tooth, hair follicle, and glands successfully regenerated by developmental TE.
-
•
3D cell-seeded/3D cell-laden scaffolds fundamental to regenerate organs by developmental TE.
-
•
Cell source, coculture and scaffold design determine success of developmental TE.
Abbreviations
- 2D:
Bi-dimensional;
- 3D:
Three-dimensional;
- ALP:
Alkaline phosphatase;
- AQP5:
Aquaporin-5;
- BMP:
Bone morphogenic protein;
- DE:
Dental epithelial;
- DM:
Dental mesenchymal;
- DP:
Dermal papilla;
- DS:
Dermal sheath;
- E:
Young's modulus;
- En:
Embryonic day n;
- Eda:
Ectodysplasin;
- ECM:
Extracellular matrix;
- EVAL:
Ethylene vinyl alcohol;
- FGF:
Fibroblast growth factor:
- G′:
Storage modulus;
- G″:
Loss modulus;
- GelMA:
Gelatin methacryloyl (gelatin methacrylate);
- GFP:
Green fluorescent protein;
- H&E:
Haematoxylin and eosin;
- HA:
Hydroxyapatite;
- HF:
Hair follicle;
- HUVEC:
Human umbilical vein endothelial cell;
- iPSC:
Induced pluripotent stem cell;
- PC:
Polycarbonate;
- PDL:
Periodontal ligament;
- PDMS:
Polydimethylsiloxane;
- PEG:
Poly(ethylene glycol);
- PGA:
Polyglycolide acid;
- PLLA:
Poly-l-lactide acid;
- PLGA:
Poly(lactic-co-glycolic acid):
- PMMA:
Polymethyl methacrylate;
- PVA:
Polyvinyl alcohol;
- PVDF:
Polyvinylidene fluoride:
- RGD:
Arg-Gly-Asp;
- Shh:
Sonic hedgehog;
- TCPS:
Tissue culture polystyrene;
- TE:
Tissue engineering;
1. Introduction
Tissue engineering (TE) is ‘an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function’ [1]. TE has originally emerged in response to the clinical need for repair and/or replacement of dysfunctional, damaged or missing tissues and organs, mainly caused by the shortage of available organ donors and problems associated with organ transplantation. Since its first definition in the early 1990s, TE has rapidly evolved and expanded encompassing different research areas, such as biology, chemistry, materials science, design and fabrication engineering, medicine, bioengineering etc. Since then, TE has generated businesses based on start-ups, small- and medium-sized enterprises (SMEs), and commercial companies ($9 billion in sales estimated for companies with TE-related products on market in 2017 in the USA) [2]. The classical TE approach involves the combination of scaffolds, cells and soluble factors to obtain constructs that structurally, mechanically and functionally resemble the mature native tissue to be regenerated [3]. Outstanding progress has been made in the last few decades to shorten the gap between TE research and its clinical applications, with the advent of induced pluripotent cells (iPSCs) [4] and improvements in scaffolds design (e.g. substrate stiffness, advanced fabrication technologies, biomimetic properties, biochemical cues, strategies for vascularization) [5]. However, most advances in TE research have been limited to in vitro studies or in vivo small animal models. Progression towards clinical translation has been relatively limited, and the early predictions of clinical and commercial success of TE is yet to be fully realized [6]. Indeed, regenerating tissues and organs which exhibit adequate morphological and functional characteristics by classical TE approaches still remains highly challenging, and success to date has been mostly observed in simple epithelial structures such as skin. Thus, developmental TE, or developmental engineering [7,8], has recently emerged as a novel TE paradigm to obtain biomorphological and biofunctional constructs by mimicking morphogenesis and by artificially replicating embryonic developmental processes.
2. Developmental tissue engineering
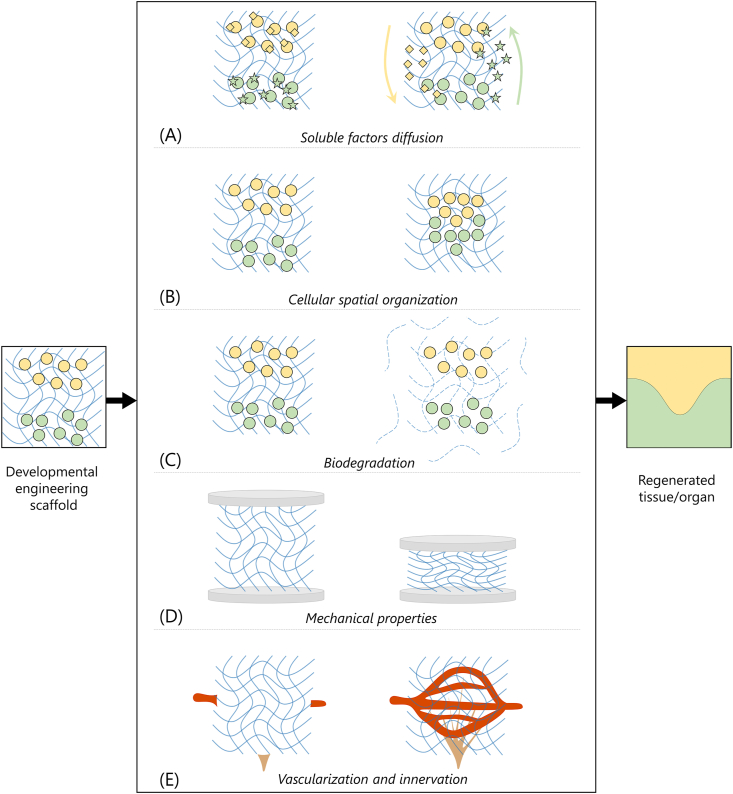
Classical TE approaches aim at fabricating constructs with structural, mechanical and functional properties resembling the mature native tissues to be regenerated [3]. An adequate cell population is selected and combined with an appropriately designed scaffold, soluble factors that are able to direct cell fate can be added; the construct is cultured in vitro to achieve a desired level of maturation and, finally, it is implanted in vivo to ultimately perform its function. In addition to incorporating fundamental features such as biocompatibility and biodegradability, classical TE scaffolds are designed to mimic the microarchitecture and mechanical properties of the mature tissue to be regenerated [9,10]. For instance, different scaffold designs include relatively stiff scaffolds with tuned porous structure for bone [11,12] and gradient porosity for osteochondral regeneration [13,14], scaffolds with preferential orientation for the regeneration of anisotropic tissues [15,16], or scaffolds with relatively soft mechanical properties and big pores for adipose tissue regeneration [17,18]. Classical TE strategies mainly aim at cell growth in a 3D structure and differentiation into the desired phenotype to form the mature tissue, pursued by guiding cell fate by modulating the scaffolds properties (e.g. substrate stiffness) and/or by addition of soluble factors. As an e.g. bone tissue engineering has been generally targeted by direct osteogenic differentiation of mesenchymal stem cells seeded on 3D scaffolds to resemble intramembranous ossification. However, these approaches have hindered clinical applications, caused by lack of vascularization and subsequent core degradation; and developmental TE approaches, on the basis of resolution of similar issues in endochondral ossification, have been alternatively proposed [19]. In general, many classical TE approaches aim at restoring tissues and organs by replacing specialized epithelium through implantation of adult epithelial cells or differentiated stem cells. However, the complex structure of several organs is determined in nature by a series of epithelial and mesenchymal interactions [20]. Thus, tissue and organ development is not only guided by cell growth and differentiation in a 3D environment, as performed by most classical TE approaches [21]; on the contrary, embryonic morphogenesis is guided by a complex spatiotemporal organization and communication between heterogeneous cell populations that eventually results in mature biomorphology and biofunctionality of the tissue/organ [7]. The interplay between tissue mechanics and biochemical signalling orchestrates tissue morphogenesis and patterning in development. Mimicking and replicating these morphogenetic processes represent the core of the developmental TE paradigm [22].
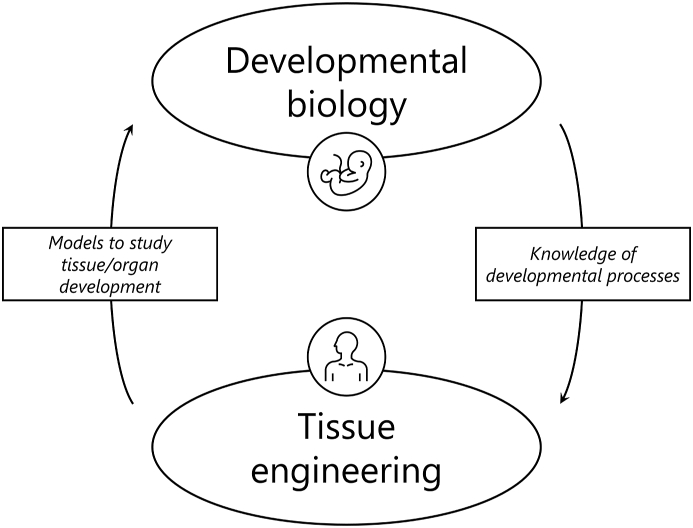
Ectodermal appendages arise from their organ germs by sequential and reciprocal interactions between adjacent layers of epithelial and mesenchymal tissues in the developing embryo [23]. This interaction (i.e. secondary induction) is regulated by conserved families of signalling molecules (e.g. Wnt, bone morphogenic protein, BMP, Hedgehogs, and fibroblast growth factor, FGF), and is accomplished by one group of cells (i.e. inductive cells) that guides the response of the adjacent cell group (i.e. competent cells) [24]. In developmental TE, a cell population retaining inductive capability is cocultured with another cell population competent in receiving the inductive signals to artificially replicate the morphogenesis, eventually leading to the regeneration of the desired tissue or organ [25]. Seminal experiments in the last century demonstrated that the inductive cues are conserved across species, while ectodermal appendage identity is dictated by the signalling processes in the competent epithelium [26]. The success of developmental TE clearly depends on a mutually beneficial interplay between the research fields of TE and developmental biology (Fig. 1). This interplay has the dual aim of improving the outcomes of TE applications and increasing the knowledge of developmental processes of tissues and organs. TE relies on knowledge of morphogenetic processes studied by developmental biology to develop engineered scaffolds and replicate the developmental processes to regenerate tissues/organs. Knowledge of cells involved in developmental processes, their microenvironment, and signalling pathways are fundamental to accurately replicate the morphogenetic processes, to verify that these processes have been adequately replicated, and to eventually regenerate functional organs. In turn, TE can provide reliable, controllable, reproducible, and scalable models to investigate developmental biology processes. Engineered scaffolds can also provide three-dimensional (3D) microenvironments that represent more realistic biochemical and biomechanical microenvironments, compared to traditional bi-dimensional (2D) cultures [27]. The tools offered by developmental TE can be used to study physiology, metabolism, toxicology, tumour development, developmental biology, or for the fabrication of tissue engineered scaffolds for tissue and organ regeneration [28]. The use of this reverse engineering process of morphogenesis can thus be of outstanding importance both for a deeper understanding of biological processes and for the fabrication of biomorphological and biofunctional construct for damaged/missing tissues and organ regeneration [8].
Fig. 1.
Interplay between developmental biology and tissue engineering. Developmental biology provides knowledge on biological developmental processes to be used for human tissue regeneration by tissue engineering, while tissue engineering provides models to investigate developmental processes in developmental biology research (icons © The Noun Project).
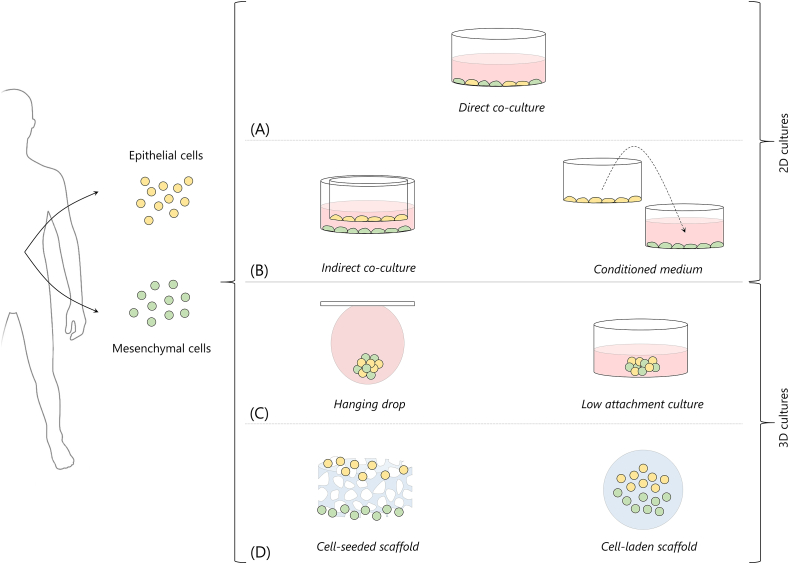
Epithelial-mesenchymal cells interactions can be replicated in vitro to target tissue/organ regeneration by different coculture systems, based on 2D or 3D cell culture configurations (Fig. 2). Combined cell coculture configurations can also be used to obtain more complex culture systems [29,30].
Fig. 2.
Strategies for cell cocultures to replicate developmental processes. 2D cocultures are based on (A) direct and (B) indirect cocultures. Indirect cocultures can be performed by transwell culture systems (left) or by culturing a cell population with a conditioned medium used to previously culture the other cell population (right). 3D cocultures can be based on (C) scaffold-free approaches, such as hanging drop method (left) or ultra-low attachment cultures (right) or (D) scaffold-based approaches, including cell seeding on scaffolds (left) or cells embedding in scaffolds, typically hydrogel-based (right). Culture medium is represented in red; biomaterials/scaffolds are represented in blue.
2D cocultures are performed by direct or indirect coculture. In direct cocultures (Fig. 2A), cells are mixed together or seeded sequentially on tissue culture polystyrene (TCPS) [[31], [32], [33]] and maintained in culture in contact one with the other. In indirect cocultures, the cell populations are not cultured in direct contact, but they generally share the same culture environment. For instance, transwell culture systems (Fig. 2B, left) are used to separately culture the cell populations in two compartments immersed in the same culture medium. These systems have been used, for instance, for the study of the crosstalk between epithelial and mesenchymal cells in tooth [29,30,34] and hair follicle [[35], [36], [37]] development. Alternatively, conditioned medium (Fig. 2B, right), obtained by culturing a cell population and subsequently used to culture the other cell population, has been used [[38], [39], [40]]. Microfluidic platforms have been also developed to optimize the conditioned medium culture technique [41]. Although 2D coculture systems are powerful tools to investigate signalling molecules and study cell populations and signalling involved in the organogenesis process, these systems lack physiological relevance caused by the absence of 3D structure to replicate that of growing tissue. Thus, 3D coculture strategies have emerged to better replicate this complex 3D microenvironment [28].
3D coculture strategies can be scaffold-free or scaffold-based. Scaffold-free approaches (Fig. 2C) rely on culture systems that promote cell-cell interactions to form 3D spheroids, typically without the addition of artificial extracellular matrix (ECM). For instance, in the hanging drop method, cells are seeded and cultured within droplets of culture medium where they develop as coherent 3D cell aggregates (Fig. 2C, left). This versatile and relatively simple culture method [42] has been used to recapitulate the interactions between epithelial and mesenchymal cells during the development of tooth [43], hair follicle [44,45] and salivary gland [46]. Alternatively, low attachment plates (Fig. 2C, right) can be used to prepare 3D spheroids, thanks to the minimized protein and cell attachment on the culture dish that promote the formation of floating cell spheroids and aggregates [47]. As an alternative to scaffold-free strategies, scaffold-based strategies can be used by introducing artificial ECM in the coculture (Fig. 2D). These strategies are based on seeding cells on a porous scaffold (Fig. 2D, left) or on embedding cells in a 3D matrix, typically a hydrogel (Fig. 2D, right). In this review, we discuss recent advances of these latter-mentioned scaffold-based developmental TE approaches for ectodermal organ regeneration, focussing on tooth, hair follicle, and salivary and lacrimal glands.
3. Tooth developmental tissue engineering
Teeth are composed of several hard, differently mineralized tissues (dentin, enamel and cementum), forming a chamber that contains an enclosed soft tissue, known as dental pulp. The dental pulp is predominantly composed of connective tissue that is vascularized and innervated. These different dental tissues form an integrated attachment complex with the alveolar bone, known as periodontium, containing soft tissues, such as the gingiva and the periodontal ligament (PDL) that anchors the cementum of the tooth root to the alveolar bone socket [48].
Hard dental tissues, organized in a complex 3D structure [49], differ in relation to degree of mineralization and chemical components. Dentin is composed by 65–70 wt.% inorganic matrix [50,51], while the organic component is mainly composed by collagen type I (90%) and proteoglycans [52]. Dentin is produced by odontoblasts, polarized and highly specialized cells, characterized by cytoplasmic processes extended into dentinal tubules, and forms the bulk of the mineralized dental tissue [53]. The enamel is acellular, highly mineralized (96 wt.%) tissue formed by a biomineralization process governed by the ameloblasts (present only during tooth development). The enamel covers dentin in the tooth crown, the part of the tooth visible in the mouth [50,51,54]. Dentin and enamel are characterized by Knoop microhardness values of approximatively 30–70 and 200–350 KHN, respectively [55,56], the latter being the hardest tissue in the human body. The cementum covers dentin in the tooth root region and gives attachment to the PDL and alveolar bone of the socket [57]. The cementum is composed by 45–65 wt.% inorganic components, with organic matrix predominantly composed of collagens [58]. The dental pulp is a highly vascularized and innervated loose connective tissue protected by dentin, cementum and enamel. Fibroblasts, dental pulp stem cells, immune cells are the main cell components of the dental pulp tissue, which is well vascularized and innervated. Odontoblasts are highly specialized cells, lining the periphery of the dental pulp tissue, with their longcytoplasmic processes extending into the dentine tubules. This very specific orientation of the odontoblasts results in dentin and pulp acting together as a complex system, named ‘dentin-pulp complex’. These two dental tissues share anatomical, developmental and functional relationships, resulting in responses to dental injuries. The dental pulp ECM is mainly formed by type I, type III, and type V collagen, phosphorylated and non-phosphorylated proteins, glycosaminoglycans and proteoglycans [59]. Human dental pulp is characterized by a Young's modulus (E) of approximatively 2–5 kPa under compression load, and it exhibits storage modulus (G′) values of 2000–7000 Pa and loss modulus (G″) values of approximatively 1000 Pa when shear stress at different frequencies is applied [60,61].
Tooth loss is a global health problem representing a health cost burden to society and the economy [62,63]. Tooth loss affects an individual's capacity for biting, chewing, smiling, speaking, and impacts on the individual’s psychosocial wellbeing, leading to both oral functional and aesthetic issues [64]. Complete loss of natural teeth is widespread, particularly affecting older people, mainly caused by dental caries, periodontal disease and genetic disorders [65,66]. Current dental treatments that are being used to replace missing tooth structure or missing teeth are based on conservative therapies such as fillings, made of inert dental materials, fixed dental bridges [67] or removable dentures [68] and dental implants [69,70]. Different approaches have emerged, such as concepts of regenerative dentistry, that challenge the modern dentistry to step up the dental research and translate the scientific knowledge of understanding the underlying mechanisms of tooth development and the biological processes of healing and repair. The ultimate aim of these approaches is application of knowledge in harnessing the natural healing potential of the dental tissues or regenerating (engineering) the damaged tissue or organ.
Classical TE approaches for teeth mainly aim to repair/regenerate single tooth components, such as enamel, dentin-pulp complex and different tissues of the periodontium [[71], [72], [73], [74], [75]]. Enamel repair has been principally investigated by targeting the remineralization of the defective enamel, particularly challenging given the acellular structure, inorganic composition, and high mechanical properties. Approaches for enamel repair include physical synthesis, protein matrix-guided crystal growth, and in situ enamel surface mineralization [[76], [77], [78], [79], [80]]. Classical TE approaches have also been used for the regeneration of the dentin-pulp complex, both based on cellular [81] and acellular [82] strategies. In the first case, cells (e.g. dental pulp stem cells, stem cells from apical papilla, stem cells from exfoliated deciduous teeth, stem cells of non-dental origin, and iPSCs [[73], [74], [75],83,84]), morphogenetic factors and scaffolds [[85], [86], [87], [88], [89]] are combined in vitro and then implanted in vivo at targeted site. In the latter case, biodegradable, porous, acellular scaffolds [82], possibly coupled with signalling molecules such as cytokines, are implanted in vivo to promote cell colonization and recruitment from surrounding tissues (‘cell homing’ strategy), as well as proper vascularization and innervation [[90], [91], [92], [93]]. Although these strategies have achieved good research outcomes in restoring specific dental tissues, a whole tooth regeneration approach is highly desirable in treating tooth loss. Aiming at generating a whole biomorphological and biofunctional tooth, surrounded by functional periodontium to guarantee correct functionality and in vivo integration [94], tooth developmental TE strategies have shown unique and promising results.
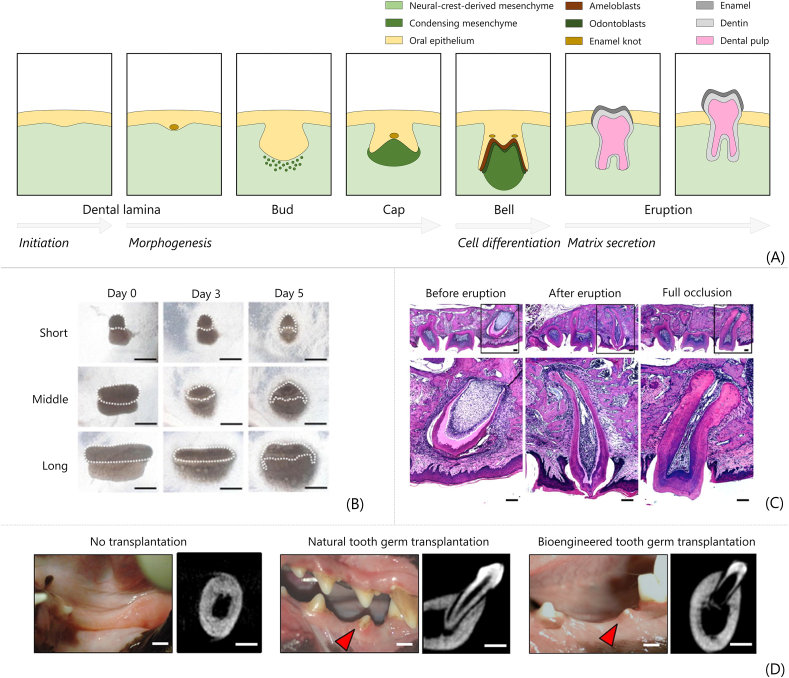
Tooth developmental TE aims at replicating the biological processes that lead to tooth development during embryogenesis [48]. The embryonic ectoderm (epithelium) interacts with the underlying mesenchyme, derived from cranial neural crest, in a sequence of well-orchestrated steps that include initiation, morphogenesis, differentiation and matrix secretion, until the mature tooth erupts in the oral cavity to perform its functions (Fig. 3A) [[95], [96], [97]]. Tooth formation begins when the dental lamina in the oral epithelium thickens (approximatively 7 weeks in humans) and invaginates in the underlying neural-crest-derived mesenchyme. Subsequently (placode stage), the mesenchyme condenses around the invaginated epithelium and gives rise to the tooth bud. The epithelium folds and wraps around the condensing mesenchyme in the following cap and bell stage. The signalling centre known as the ‘enamel knot’, developed at the tip of the late bud, controls this process. During the late bell stage, the mesenchyme is embedded in the epithelium and cytodifferentiation occurs. The epithelial cells close to the mesenchyme differentiate into ameloblasts to eventually produce enamel; adjacent mesenchymal cells differentiate into odontoblasts to produce dentin. Conserved signalling pathways involved in tooth development include Wnt group of signaling pathways, bone morphogenic protein (BMP), fibroblast growth factor (FGF), sonic hedgehog (Shh) and ectodysplasin A (Eda) [98].
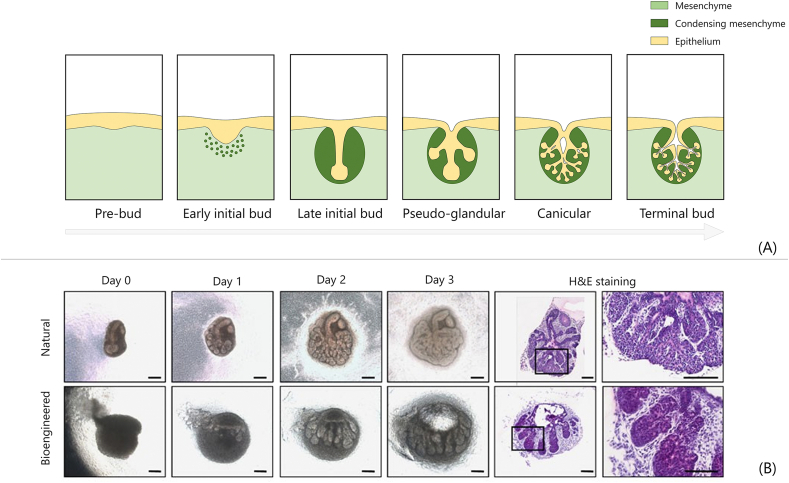
Fig. 3.
Tooth developmental tissue engineering. (A) Fundamental steps of tooth morphogenesis in embryos replicated by developmental TE to regenerate teeth. (B) Phase contrast images of bioengineered tooth germs in collagen drops (the ‘collagen drop organ germ method’). Epithelial (dashed lines) and mesenchymal cells are seeded compartmentalized in collagen drops with different contact areas (short, middle and long); scale bar = 200 μm [99]. (C) Histological sections of bioengineered tooth fabricated by collagen drop method implanted in mice upper first molar, before and immediately after eruption, and at complete occlusion; scale bar = 100 μm [100]. (D) Macroscopic pictures and CT images of canine oral cavity without transplantation, after natural tooth germ transplantation, and after in vitro bioengineering tooth transplantation. Red arrows indicate erupted tooth [101]. All images used with permission.
The regeneration of teeth by developmental TE is based on the coculture of epithelial-mesenchymal cells using an engineered scaffold to sustain the interactions between the cell populations and lead to tooth morphogenesis. The selection of appropriate cell sources and enabling an environment where cell communication and signalling can be established are key aspects. To date, strategies for tooth regeneration by developmental TE [75,[102], [103], [104]] include (i) scaffold-free strategies, (ii) cell-seeded scaffolds and (iii) collagen-drop method/hydrogel-based strategies.
Scaffold-free strategies (i) rely on the use of cell aggregation, for instance by cells pelleting [[105], [106], [107]] or hanging drop method [43]. In cell-seeded scaffold strategies (ii), mixed populations of mesenchymal and epithelial cells are seeded on the scaffold. Alternatively, cell populations are seeded sequentially or compartmentalized in different configurations on fabricated scaffolds [[108], [109], [110], [111], [112], [113]]. In the collagen drop method (iii), also referred to as ‘tooth germ method’, epithelial and mesenchymal cells are loaded in a collagen drop [94,[99], [100], [101],[114], [115], [116], [117], [118]]. Here, we include the ‘collagen drop method’ with all the strategies that are based on loading cells in 3D hydrogel structures [61,[119], [120], [121]], in comparison to the cell-seeded scaffolds where cells are seeded on a pre-fabricated scaffold. In this review, we focus on scaffold-based 3D culture systems for tooth developmental TE, summarized in Table 1 and discussed below.
Table 1.
Scaffold-based developmental tissue engineering strategies for tooth regeneration, based on the coculture of epithelial and mesenchymal cells. A biomaterial is used to prepare a 3D structure and coculture cells. In vitro and in vivo studies are performed to verify the suitability of the engineered construct in regenerating teeth.
| Epithelial cells | Mesenchymal cells | Biomaterial | Culture method | In vivo tests | Main findings | Reference |
|---|---|---|---|---|---|---|
| Cell-seeded scaffold strategies | ||||||
| 3- to 7-day-newborn Lewis rat molar tooth bud (single cells suspension) | PGA fibre mesh with 3% w/w PLLA. PLGA prepared by NaCl crystals leaching. |
Mix of cells seeded on scaffolds | Omentum of 6- to 12-months old Lewis rats | 4-day-newborn cells proliferate in vitro. PGA and PLGA scaffolds support tooth growth in vivo | Dualibi et al., 2004 [110] | |
| Third molars tooth bud from 6-month-old pig jaws (single cells suspension) | PGA fibre mesh with 3% w/w PLLA. PLGA prepared by NaCl crystals leaching. Scaffolds are collagen coated. |
Mix of cells seeded on scaffolds | Omentum of athymic rats | Cells seeded on biodegradable scaffolds regenerate tooth-like structure in vivo | Young et al., 2002 [113] | |
| Third molar from 3.6-month-old Yucatan minipig | PGA/PLLA spheres coated with type I collagen wrapped with gelatin sponge | DM cells seeded on PGA/PLLA scaffolds wrapped and sutured with DE cells seeded gelatin sponge | Full-thickness segmental bone defects in hemimandible of Yucatan minipig | In vivo tooth regeneration with correct structure (1/6 of implants) | Abukawa et al., 2009 [108] | |
| Second molar tooth buds from 6-month-old Yucatan minipig | Electrospun nHA-loaded PLGA | Static seeding of mixed cells (and single cell populations as controls) | Fibres orientation guides DM cells. nHA does not promote cells differentiation and ECM deposition | van Manen, 2014 [112] | ||
| Third molar from 6-month-old pigs | Collagen sponge (75% type I + 25% type III collagen, dry weight) | Different configurations of cells seeded on the collagen sponge | Omentum of 5- to 6-week-old rat | Direct contact between seeded cell populations promotes tooth development | Honda et al., 2007 [109] | |
| Third molar from 6-month-old pig jaws | Third molar from human | Decellularized unerupted tooth bud from 6-month-old pig jaws | DE and DM cells seeded on decellularized enamel and pulp sutured together | Tooth-extracted sockets of 6-month-old Yucatan mini pigs | Regenerated teeth adopt the size of the decellularized scaffold in vivo | Zhang et al., 2017 [111] |
| Cell-laden hydrogel-based strategies | ||||||
| Incisors and molars of E14.5 mice | Type I-A collagen | Dissociated cells in collagen drop (mixed or compartmentalized) | Subrenal capsule of 8-week-old male mice | The organ germ method successfully generates tooth | Nakao et al., 2007 [114] | |
| Human gingival epithelial cells | Molar tooth from E14.5 mouse mesenchyme tissue | Type I-A collagen | Epithelial cells in collagen drop seeded on mesenchyme tissue | Kidney capsules in adult immune-compromised mice | In vivo regeneration of tooth-like structures and root formation | Angelova Volponi et al., 2013 [118] |
| Molar tooth germ from E14.5 mice | Type I-A collagen | Dissociated cells in collagen drop | Bony hole in the upper first molar region of the alveolar bone in an 8-week-old adult murine lost tooth transplantation model | Functional in vivo regeneration of bioengineered tooth | Ikeda et al., 2009 [100] | |
| Premolar tooth germ from 30-day-old beagle dogs | Type I-A collagen | Epithelial tissue and mesenchymal cells in collagen drop | Autologous transplantation into alveolar bone socket of canine mandible | Functional in vivo regeneration of bioengineered tooth (in large animal model) | Ono et al., 2017 [101] | |
| Molar tooth germ from E14.5 mice | Type I-A collagen | Dissociated cells in collagen drop (different contact areas) | Subrenal capsule transplant in mice | Crown width and cusps number depend on contact area between DE and DM cells | Ishida et al., 2011 [99] | |
| Molar tooth germ from E14.5 mice | Type I-A collagen | Dissociated cells in collagen drop | Alveolar bone defect in mouse | Functional in vivo regeneration of size-controlled bioengineered tooth | Oshima et al., 2011 [94] | |
| Molar tooth from E12 mouse | Molar tooth from E14.5 mouse | Type I-A collagen | Cells mixed and recombined with epithelium in collagen drop | Subrenal capsule transplantation in mice | Newborn’s cells > 25% fail to reconstruct tooth. Cells of the offspring after birth not involved in tooth regeneration | Yang et al., 2017 [116] |
| Incisor tooth germ from E14.5 mice | Type I-A collagen | iPCs mixed with DM cells in collagen drop; DE cells seeded adjacent in collagen | Subrenal capsule transplantation in mice | iPSCs’ participation in tooth development | Wen et al., 2012 [117] | |
| Mandibular incisors of 10-day-old Wistar rat offsprings after birth | Mandibular first molar of 10-day-old Wistar rat offsprings after birth | Type I–P collagen | Epithelial cells layered on top of pulp cells laden collagen gel | Cells reciprocally influence their morphology | Notani et al., 2009 [115] | |
| Newborn human oral epithelial cells from gingival tissue | Newborn human dental pulp cells from third molar | Matrigel and collagen gel | Epithelial cells seeded on gels inoculated with dental pulp cells | In vitro reproduction of the epithelium invagination process | Xiao et al., 2012 [121] | |
| Tooth buds from 6-month-old minipigs | Wisdom tooth of 16-year-old Caucasian male | Collagen and Matrigel | Mesenchymal cells in collagen gel + epithelial cells in Matrigel pipetted inside collagen | Subcutaneous into 4-week-old nude rat | Cocultured constructs maintain predetermined shape and size | Zhang et al., 2010 [122] |
| Rat dental epithelial cells (HAT-7) | Human dental pulp stem cells | Type I collagen/chitosan hydrogel blend and matrigel. | Cells separately embedded in two hydrogel blends, divided by Matrigel | Subcutaneous implant in mice | Optimized, stable biomaterial to provide freedom of movement to cells | Ravindran et al., 2010 [119] |
| Unerupted tooth buds from 5-month-old porcine jaws | GelMA | DE-HUVEC loaded hydrogel layered on top of DM-HUVEC hydrogel | Subcutaneously (back of immunocompromised 5-month-old female Rowett Nude rats | GelMA with tunable properties. HUVEC promote capillary-like structure. | Smith et al., 2014 [120], 2017 [61] | |
3.1. Scaffold-based approaches for tooth developmental tissue engineering
The cell-seeded scaffold strategies are based on the fabrication of a scaffold with adequate features, such as cytocompatibility and biocompatibility, possibility of cell-adhesion, degradability, and, generally, a porous structure to allow cell colonization. After the scaffold is fabricated, cells are seeded either as a single cell suspension (i.e. mixed population of epithelial and mesenchymal cells) [110,113] or as separated/compartmentalized cell populations, in different configurations (e.g. epithelial and mesenchymal cells seeded adjacently in contact, or separated) [109,112]. Materials used for the fabrication of these scaffolds include polyglycolide acid (PGA), poly-l-lactide acid (PLLA), and poly(lactic-co-glycolic acid) (PLGA) [108,110,112,113], collagen sponges [109] and decellularized tooth buds [111]. PLGA scaffolds fabricated by sodium chloride particulate leaching (diameter Ø = 75–150 μm) were tested by seeding a single cell suspension derived from tooth bud from rat [110] or pig jaws [113], then subsequently implanted in the omenta of host rats. After a 12-week incubation in vivo, mineralized tissue was observed within rat-derived cell-seeded scaffolds, compared to the absence of regenerated mineralized tissue for acellular scaffold controls. Dentin, enamel and pulp tissues were also present [110]. Comparable outcomes were obtained using either PGA or PLGA scaffolds. The time required for the regeneration using cells from different animals (i.e. 20–30 weeks for pig-derived cells, 12 weeks for rat-derived cells) is in line with the time required for natural tooth development (i.e. tooth eruption in approximatively 80 weeks for pigs and 7 weeks for rats), suggesting the maintenance of different developmental biological programs in cells isolated from different animal sources [113]. PGA/PLGA-based scaffolds with more complex structures were then described to simultaneously target whole tooth and surrounding bone regeneration in mandibular defects [108]. PGA/PLGA sphere scaffolds, coated by type I collagen, were seeded with dental mesenchymal (DM) cells and wrapped with gelatin foam scaffolds seeded with dental epithelial (DE) cells. These scaffolds were coupled with other PLGA porous scaffolds, fabricated by solvent-casting particulate leaching of glucose particles, to regenerate the bone surrounding the tooth. After in vivo implantation in mandibles, regenerated tooth structures including dentin, enamel and pulp were observed, but the regeneration success rate was as low as ∼17% on the total number of implanted scaffolds (i.e. 1 out of 6 implants showed tooth-crown formation). PLGA fibrous scaffolds loaded with nanohydroxyapatite (HA) were also described to guide dental cell alignment, by using the fibrous structure of the electrospun scaffold, and promote cell differentiation and inorganic ECM deposition [112]. Poly(lactic-co-glycolic acid) (PLGA) was electrospun to obtain fibrous scaffolds with random or aligned fibre orientation, loaded with different nano-HA contents (i.e. 0%, 10% and 20%). Aligned fibres successfully guided cell orientation, compared to the random fibres orientation. Dental mesenchymal (DM) and dental epithelial (DE) cells cocultures showed improved ECM deposition and formation of calcified nodules in vitro, compared with single-cell population cultures. However, no evidence of beneficial effects of nano-HA was demonstrated. Naturally derived materials such as collagen [109] and decellularized tooth bud [111] were also used for cell-seeded strategies, as alternatives to the described synthetic polymer-based scaffolds. As an advantage, the choice of naturally derived polymers such as collagen, collagen derivatives and decellularized matrix provides an ECM-biomimetic environment [[123], [124], [125]], thus avoiding the need for coating procedures to promote cell adhesion which is required for synthetic polymer-based scaffolds (e.g. collagen coating) [108,113]. Porous collagen sponges were used to investigate the effects of cell seeding configurations on tooth regeneration [109]. Cells were either seeded on the sponges as single cell populations, sequentially seeded on the same scaffold, seeded on different scaffolds that were then coupled together and divided by a porous membrane, or seeded as single suspension of mixed cells. Both in vitro and in vivo, direct contact between the two cell populations increased the success of tooth regeneration, thus proving the importance of adequate interactions between DE and DM cells to replicate the elements of tooth development. Increased alkaline phosphatase (ALP) activity was measured in vitro when DE and DM cells were cultured in contact on the collagen sponges, compared with spatially separated cells cultured on the sponges. This configuration of cell seeding allowing for cell contact was able to regenerate single tooth-like structures after a 20-week implantation in the omentum of host rats. Moreover, when cells were seeded as single suspension of mixed cells and not as compartmentalized cells in contact one with the other, abnormal teeth structures were formed in vivo, most likely caused by the initiation of several developing teeth in the same scaffold, thus underlining the importance of a controlled contact and compartmentalization of seeded epithelial and mesenchymal cells. A compartmentalized cell seeding configuration of epithelial and mesenchymal cells was also tested using decellularized tooth buds [111]. The decellularization process allows for the removal of immunogenic components, leaving the ECM of the bud to be used as a naturally biomimetic scaffold. Decellularized enamel and pulp scaffolds were separately seeded with porcine DE cells and human DM cells, respectively, and sutured together. Human umbilical vein endothelial cell (HUVEC) were added to promote the in vivo vascularization of the seeded scaffolds. This complex decellularized scaffold showed great in vivo potential in regenerating teeth, by replicating the size and shape of the scaffold with organized mineral structures. However, regenerated teeth were not observed in all the implanted scaffolds.
In cell-laden hydrogel-based strategies, cells are seeded by embedding them in 3D hydrogels during the scaffold fabrication, compared with the previously described methods (i.e. cell-seeded scaffolds) in which cells are seeded on scaffolds fabricated beforehand. These strategies include the ‘collagen-drop’ method, based on the use of collagen as the 3D structure [94,[99], [100], [101],[114], [115], [116], [117], [118]] and other hydrogel-based materials including collagen/chitosan blends [119], Matrigel [121,122] and gelatin methacryloyl (GelMA) [61,120]. The collagen drop method was described in a pioneering study performed by Nakao et al. [114], which also defined this method as the ‘organ germ method’. A collagen drop (approximatively 30 μL of type I collagen) is used as a 3D scaffold in which cells are embedded. In particular, epithelial and mesenchymal cells are manually injected in the collagen drop and compartmentalized in adjacent areas of the drop. Typically, high cell densities are used for the generation of the tooth germ (i.e. ∼108 cell/mL) [114]. Then, the cell-laden collagen drop is transferred at 37 °C in a tissue culture insert and cultured in vitro for the desired time (i.e. 2–7 days) before in vivo implantation or maintenance in culture to validate the in vitro model. When these organ germs are produced by using DE and DM cells from tooth germs of E14.5 mice embryos and transplanted in vivo in subrenal capsules, a 100% rate in tooth regeneration is observed, with properly arranged structures correctly resembling one of the native tooth, including dentin, enamel, dental pulp, alveolar bone and PDL. Dental structures with correct cell types and mineralized tissues were also obtained in vitro [114]. Recombination of adult human gingival epithelial cells with embryonic mouse molar mesenchyme tissue led to the regeneration of fully developed tooth, comprising root, after renal capsule in vivo transplantation in immune deficient mice [118]. This study has shown that human gingival (adult) epithelial cells contributed to the formation of a fully developed tooth, when combined with mouse tooth embryonic mesenchyme. In this particular case, human adult epithelial cells responded to the odontogenic signal from mouse mesenchyme and successfully participated in the development of the tooth, by differentiating into ameloblasts, as well as root sheath cells, known as Malassez cells, found in the tooth root region [118]. Later, a collagen drop loaded with DE and DM cells (i.e. tooth primordia) from mouse embryo was implanted in a bony hole of the alveolar bone surface of mice (i.e. actual site where the scaffolds would be implanted, compared with the subrenal model previously used) and tooth physiological functions were successfully restored [100]. Fully developed, functional tooth with supporting tissues was formed, as shown by histological staining images before eruption, immediately after eruption and at complete occlusion (Fig. 3C). The regenerated tooth was characterized by Knoop hardness values of approximatively 460 and 80 KHN for enamel and dentin, respectively, comparable to those of native mice teeth, thus showing the rehabilitation of masticatory function in the bioengineered tooth. Responsiveness of the bioengineering tooth to noxious stimulation was also proved, demonstrating the presence of innervation in the tooth pulp [101]. However, regenerated teeth showed smaller size compared to native teeth and low control on crown width, cusp position and tooth patterning, fundamental aspects for both functional and aesthetic restoration. A successful regulation of the tooth crown width was later reported by controlling the length of the contact area between DE and DM cells seeded in the collagen drop [99]. By manually injecting cells in the collagen drop, authors achieved different lengths of contact between the cell populations, namely short (up to 450 μm), middle (450–900 μm) and long (900–1500 μm), as shown by phase contrast images of the bioengineering organ germ culture for up to 5 days (Fig. 3B). After subrenal transplantation and proof of tooth regeneration, an increment in crown width and cusp number of regenerated teeth was observed by increasing the contact length between epithelial and mesenchymal cells in the reconstituted organ germ. To further improve the control of regenerated tooth length and shape, bioengineered organ germs in collagen drops were inserted in ring-shaped size-control devices to protect the germ from the surrounding pressure exercised by surrounding tissues after in vivo implantation and allowing for its growth in 3D [94]. The regenerated tooth in mice alveolar bone defects showed correct tissue structure, functionality, and controlled 3D shape. Multiple teeth could also be regenerated by implanting multiple organ germ-containing devices. The collagen drop method was then tested on larger animal models (i.e. canine model) using autologous transplantation of cells isolated from tooth germ of mandibular premolar of offsprings after birth [101]. Teeth were successfully regenerated in the canine model (Fig. 3D), thus shortening the gap between the achieved research progresses and a potential clinical application of the developmental TE method for whole tooth regeneration. However, high rates of teeth regenerated (i.e. 100% tooth regeneration in implants) were achieved only when the organ germs were generated by using either, or both, epithelial and mesenchymal embryonic intact tissues, while low success (i.e. 16.7%) was achieved by reconstructing the organ germ by dissociated DE and DM cells. These results highlight the importance of finding alternative sources to embryonic DE and DM cells to reconstruct the organ germ which, despite some successes [94,99,100,114], represents a practical and ethical barrier to possible clinical application of this tooth developmental TE strategy. Possible alternative and accessible sources for potential clinical applications currently being considered include cells isolated from deciduous or permanent tooth germ of diphyodont mammalian model [101]: dental pulp cells from molars belonging to offsprings after birth, [116], induced pluripotent stem cells (iPSC) [117], human adult epithelial (gingival) cells [117], and bone marrow cells [107]. However, teeth were often regenerated with limitations and low frequencies. In general, the coculture of epithelial and mesenchymal cells allowed for a satisfactory biomimetics of the natural tooth developmental process (Fig. 3A). Formation of primordia is observed after 2–3 days of culture between dissociated epithelial and mesenchymal cells [114]. Bioartificial tooth germs reach the early bell stage of tooth development after 3–7 days of culture, in line with natural tooth development [99,100]. At the end of the mimicked developmental process, accumulation of hard tissue and root extension is observed, proving the regeneration of mature teeth [94].
As an alternative to the adjacent seeding of dental epithelial (DE) and dental mesenchymal (DM) cells in a collagen drop, different configurations of cells seeded in 3D hydrogels, also on the basis of alternatives to collagen, have been described [61,115,[119], [120], [121], [122]]. For instance, DM cells were loaded in a collagen drop, subsequently layered with DE cells and maintained in floating culture [115]. Despite evidence of arrangement and morphology of DE and DM cells driven by reciprocal signalling, optimization of this culture system is required to achieve all tooth components found in vivo. A similar strategy [121] was used to engineer an in vitro model of epithelial invagination in the mesenchyme found in early tooth morphogenesis. Collagen and Matrigel were both tested as biomaterials by first seeding dental pulp stem cells. Then, human oral epithelial cells were inoculated on the cell-seeded gels and the cultures grown at the air-liquid interface. In vitro invagination of epithelial cells in the adjacent mesenchymal cells and expression of some tooth early stage markers (e.g. bone morphogenic proteins BMP4, BMP2, also CD44) was observed. Collagen and Matrigel were also coupled in a more complex configuration [122], where DM cells were suspended in the collagen gel and two drops of DE cells suspended in Matrigel were inoculated inside the DM cell-laden collagen (Fig. 4i and Fig. 4ii). In this study, Matrigel was chosen because of the composition mimicking that of the basal membrane. Despite the constructs maintaining their shape and size during the tests, the observed cell polarization at the Matrigel/collagen interface (Fig. 4iii and Fig. 4iv), and the fact that proper dental tissue markers were expressed in vitro and in vivo, the formed calcified tissues did not resemble the correct native structure found in teeth. In another study [119], a more complex scaffold was fabricated by combining three different layers of biomaterials, using collagen/chitosan blends and Matrigel. The bottom layer consists of dental pulp stem cells embedded in a 1:1 collagen: chitosan blend. The upper layers consist of epithelial cells embedded in a 1:2 collagen: chitosan blend. Between the two cell-laden hydrogels, a Matrigel layer was placed to mimic the basal lamina composition. Chitosan was added to improve the integrity of the hydrogel and to increase and modulate its stiffness. In fact, an increase in E from 1000 to 1600 Pa was detected by changing the collagen: chitosan ratio from 1:2 to 1:3 (values for 1:1 ratio were lower that the cantilever detection capacity). Moreover, the specific ratios of collagen and chitosan in the blend were specifically selected for the mesenchymal and epithelial cells to achieve comparable proliferation rates. The proposed scaffold showed promising results in vitro by demonstrating expression of correct tooth development markers (e.g. ameloblastin from epithelial cells) and calcium deposits, and in vivo, as evidenced by de novo deposition of collagen fibrils and neovascularization in the implanted scaffolds. However, despite Matrigel being a good option for mimicking the ECM composition of the basement membrane, because of the presence of laminin 5, collagen IV, entactin, and heparan sulfate proteoglycans, several barriers exist for the potential clinical translation with use of Matrigel, given its derivation from murine sarcoma (i.e. lot-to-lot variability and in single batch, complex and poorly defined composition, potentially xenogeneic contaminants) [122,126,127]. Finally, gelatin-based scaffolds were used to coculture epithelial and mesenchymal cells to target tooth regeneration. As a collagen derivative, gelatin represents a valid alternative since it maintains important features such as biodegradability and cell adhesive motifs (i.e. Arg-Gly-Asp, RGD, sequences) [128,129], whilst being characterized by lower immunogenicity and antigenicity compared to collagen [130]. Gelatin hydrogel scaffolds were obtained by methacrylation of gelatin and UV-light photo crosslinking. DM and DE cells were embedded in two different formulations of gelatin hydrogels, optimized by varying the gelatin concentration and the amount of photo-initiator [61,120]. In particular, the hydrogel containing DM cells was characterized by E values of 2.45 kPa, comparable to that of dental pulp tissue (2–5 kPa). The two hydrogels were then coupled, by layering a hydrogel on the top of the other one, and subsequently cultured in vitro and implanted in vivo subcutaneously. Despite the implanted bioengineered teeth not showing distinct enamel and dentin layers, robust mineralized tissue was formed, and the implanted construct maintained its shape and size, fundamental for functional and aesthetic tooth reconstruction. Moreover, HUVEC cells embedded in the hydrogel together with DE and DM cells promoted the formation of a capillary-like structure in vitro and in vivo, fundamental for the survival of the implanted scaffold and regenerated tooth [61].
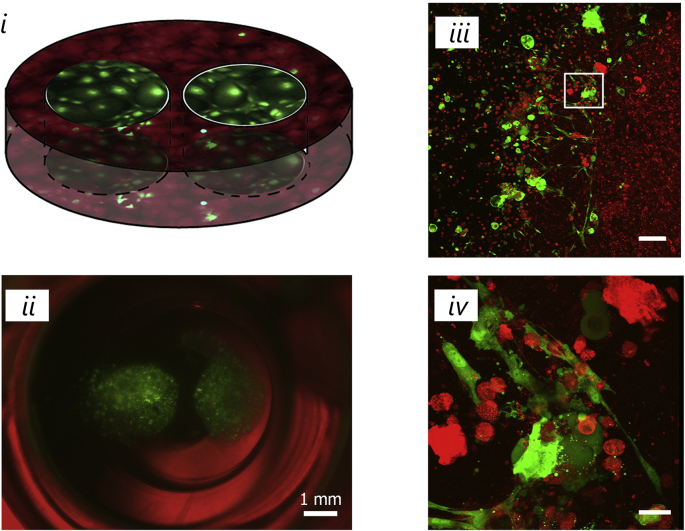
Fig. 4.
Schematic (i) and fluorescent image (ii, scale bar 1 mm) of coculture dental epithelial cells (green) and dental mesenchymal cells. Confocal image of the area at the interface between the hydrogels (iii, scale bar 100 μm) and magnification of the area in the white box showing polarized cells (iv, scale bar 20 μm) [122]. All images used with permission.
4. Hair follicle developmental tissue engineering
Hair follicles (HFs) are epidermal invaginations in the dermis that generate hair shafts, present almost all over mammals’ body surfaces with some exceptions (e.g. sole of the foot, palm of the hand) [131]. HFs are mini-organs that form during embryonic development and undergo continuous dynamic cycles during life consisting of three phases: anagen (i.e. active growth phase), catagen (i.e. transition phase) and telogen (i.e. quiescence phase) [132]. During the cycle, morphological and molecular changes occur in the HFs, so that rapid growth and hair shaft production stages alternate with hair follicle regression and quiescence. Proliferation, differentiation and quiescence during the HF cycle are precisely orchestrated in the HF’s microenvironment, which includes mesenchymal and epithelial cells, as well as adipose tissue, arrector pili muscle, blood vessels and capillaries, nerve fibres, and lymphatics [133].
HFs in the different body areas are characterized by a similar, complex, well architected structure formed by mesenchymal (dermal) and epithelial (epidermal) compartments, separated by a basal lamina [134]. The dermal portion is composed by the dermal papilla (DP), a core cluster of mesenchymal cells at the base of the HFs that coordinates hair growth, and the dermal sheath (DS), which encapsulates the exterior of the follicle and lines with the epithelium [135]. The hair germ, an epithelial population, present in telogen follicles, situated between the dermal papilla and the bulge, is directly involved in the signalling with the mesenchymal population and it is the first to proliferate during the new hair regeneration cycle, followed by the bulge [136]. The upper portions are occupied by the isthmus, an epithelial compartment situated between the bulge and the sebaceous gland, and the infundibulum. Hair shafts are characterized by different features depending on the body area and can be classified depending on diameter, length, cross-sectional shape and colour. The hair shaft is composed by concentric layers that can be divided in the cuticle, cortex and, in some cases, the medulla in the inner region. The hair fibre is made up of dead keratinized cells and 95% keratin, which gives hair good mechanical properties. Hardness values of approximatively 0.2–0.6 GPa and elastic modulus of approximatively 4–8 GPa were measured by nanoindentation, depending on the tested hair cross-section area and on the hair origin [137], data confirmed by tensile testing at the macroscopic scale (i.e. E ∼3 GPa) [138].
Hair play important roles in daily life, including thermoregulation, protection from mechanical insults, cooperation in sensitivity to noxious stimuli and skin homeostasis, and cultural and sociological functions [139]. Hair loss, or alopecia, can be associated to the failure in the regrowth of hair shafts from HFs or to the loss of HFs themselves [140]. Alopecia can be caused by ageing, hormonal disorders, nutrition, genetics, infection, and autoimmune reactions [141] or it can be a consequence of side-effects of medical treatments, such as chemotherapy [142]. Moreover, currently available skin regeneration therapies generally lack appropriate HFs appendages, thus resulting in limited functionality (e.g. delayed wound healing and aesthetically inappropriate regenerated tissues) [143]. Besides, hair loss is associated to decrease in self-esteem and depression, impacting on the life quality of people affected by hair disorders [141]. Nowadays, the global hair loss market is estimated to worth around $2.8 billion [144]. Clinical treatments for alopecia currently involve therapeutic agents (e.g. laser therapy, minoxidil) or surgical transplantation [145]. Limited efficacy and possible side effects of current therapies, and poor availability of transplantable hair led to the development of TE approaches for regeneration of hair [146]. In particular, developmental TE approaches for the regeneration of fully functional HFs have been investigated by combining epithelial and mesenchymal components to mimic HFs embryonic development.
HF develop in embryo (approximatively from week 10–11) by interactions between epithelial and mesenchymal cells (Fig. 5A), broadly classified into induction, organogenesis and cytodifferentiation phases [147,148]. During induction, the ‘first dermal signal’ arises from the mesenchyme (i.e. Wnt/β-catenin signalling), and it stimulates focal epithelial proliferation. Then, placodes form as thickenings of epidermidis [149]. Subsequently, in the organogenesis phase, the mesenchyme condenses underneath the placode, as a result of signals such as FGF20 from the placode, and reciprocal signal from the condensate to the placode causes it to proliferate and downgrow in the underlying dermis (i.e. hair germ). Finally, in the cytodifferentiation phase, the follicular epithelial cells envelop the dermal condensate transitions to become the dermal papilla. After the formation of the primary HF structure, the outer-root sheath, inner-root sheath and the hair shaft, all epithelial in nature, are formed [150]. The HF section is composed of several concentric layers. In the centre is the hair fibre, consisting of the medulla (not always present), the cortex and cuticle, from the inner to the outer layer. Surrounding these are the Huxley's and Henle's layers of the inner root sheath, then the companion layer, outer root sheath and basal layers of the follicle. This basal layer is surrounded by connective tissue sheath, thought to act as a smooth muscle that powers transition through the cycle phases [151]. Eventually, the hair follicle units are composed of the hair follicle, the arrector pili muscle, a smooth muscle involved in hair movements, and the sebaceous gland, involved in sebum secretion for skin protection. Once the HF is fully formed, it undergoes the continuous and dynamic hair cycle.
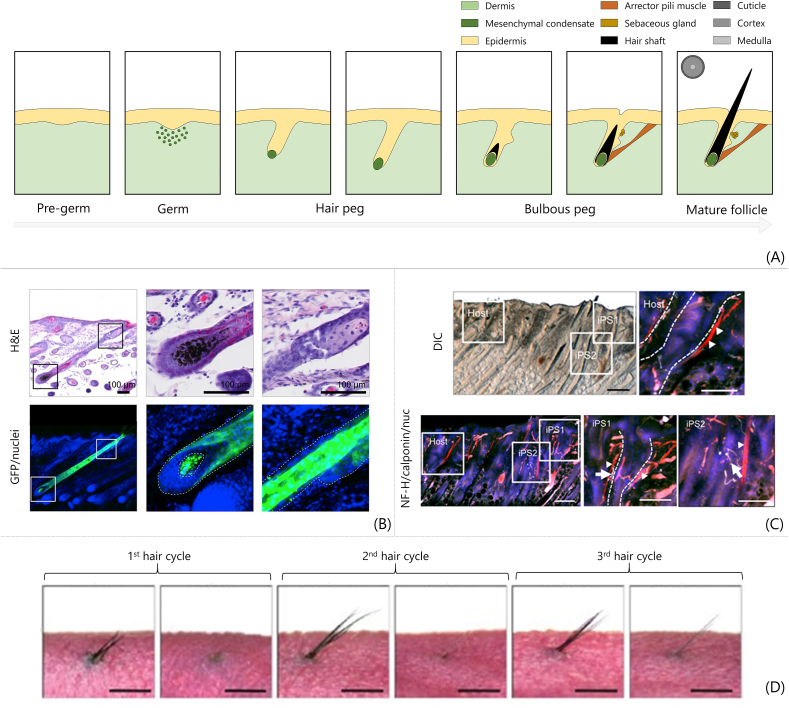
Fig. 5.
Hair follicle developmental tissue engineering. (A) Fundamental steps of hair follicle morphogenesis in embryos replicated by developmental TE. (B) Histological sections of intracutaneously regenerated hair follicle. Haematoxylin and eosin staining (H&E, upper row) and green fluorescent protein (GFP)/nuclei fluorescent images. Panels on the right show higher magnifications of the boxed are in the figures on the left (scale bar 100 μm) [152]. (C) Integration of transplanted HFs with surrounding tissues. Black hair shafts regenerated among native white hair shafts of nude mice. Calponin staining in red for muscle (i.e. arrector pili muscles), neurofilament H staining in white for nerve fibres, and nuclei in blue; scale bar 200 μm for lower magnification and 100 μm for higher magnification (reprinted/adapted from Ref. [153]; © The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. Distributed under a Creative Commons Attribution Non-Commercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/). (D) Morphological images of bioengineered hair follicle cycle in vivo over 80 days period (scale bar 1 mm) [154]. All images used with permission.
HF regeneration by developmental TE has been investigated either by scaffold-free and scaffold-based strategies, including cell-seeded scaffolds and cell-laden scaffolds (Fig. 2). Scaffold-free approaches have been used, for instance, by coculturing epithelial and mesenchymal cells [155], and by hanging drop method [45,156]. Scaffold-based strategies, focus of this review, are summarized in Table 2 and discussed below.
Table 2.
Scaffold-based developmental tissue engineering strategies for hair follicle regeneration, based on the coculture of epithelial and mesenchymal cells. A biomaterial is used to prepare a 3D structure and coculture cells. In vitro and in vivo studies are performed to verify the suitability of the engineered construct in regenerating hair follicles.
| Epithelial cells | Mesenchymal cells | Biomaterial | Culture method | In vivo tests | Main findings | Reference |
|---|---|---|---|---|---|---|
| Cell-seeded scaffold strategies | ||||||
| Adult Wistar rat foot pad keratinocytes | 6-week-old Wistar rat vibrissae dermal papilla cells | Poly(ethylene-co-vinyl alcohol) | Mixed cell suspension seeded on EVAL (and TCPS as control) | Patch assay in female nude mice | EVAL promotes DP cells spheroids formation | Yen et al., 2010 [157] |
| Human foreskin keratinocytes from surgical discards | 2-to 3-month-old adult mice whisker hair dermal papilla cells | Devitalized human abdominal dermis | Mixed cells suspension seeded on devitalized human dermis | Wound in mice back skin | DP condensates observed in vivo in the dermis and shaft-like structures emerging from epidermis | Zhang et al., 2017 [143] |
| Cell-laden hydrogel-based strategies | ||||||
| Whisker follicles of ED14.5 mice | Type I-A collagen | Dissociated cells in collagen drop | Subrenal capsule of 8-week-old male mice | In vivo regeneration of whiskers (shaft, inner and outer root sheath) | Nakao et al., 2007 [114] | |
| Back skin from E18 mouse | Type I-A collagen | Dissociated cells in collagen drop | Intracutaneous implant into 6-week-old mice back skin | Functional regeneration by transplantation of ectopically bioengineered hair follicle | Asakawa et al., 2012 [152] | |
| Back skin from E18 mouse | Type I-A collagen | Dissociated cells in collagen drop | Subrenal capsule of 8-week-old mice. Intracutaneous implant into 6-week-old mice back skin | Functional HFs regeneration (hair cycle, connection with surrounding tissues, piloerection) | Toyoshima et al., 2012 [154] | |
| (Mouse iPS cell line ‘gingiva-derived iPS’) | Type I-A collagen | iPS cell-derived embryoid bodies in collagen drop | Intracutaneous engraftment of iPS cell-derived HFs into back skin of 6-week-old mice | Regenerated HFs and sebaceous glands connected to surrounding tissues | Takagi et al., 2016 [153] | |
| Normal human skin keratinocytes | Human scalp fragments dermal papilla cells; rat vibrissae dermal papilla cells | Rat tail tendon type I collagen | Dermal cells mixed in collagen; keratinocytes seeded on top of the gel | Human DP cells reorganize collagen; keratinocytes organize in tubular structures | Chermnykh et al., 2010 [158] | |
| Outer root sheath keratinocytes from scalp skin | Human dermal fibroblast, dermal papilla fibroblasts | Type I collagen and Matrigel | Fibroblasts in collagen layered with Matrigel (loaded with mixed or sequentially seeded keratinocytes and dermal papilla fibroblasts) | Keratinocytes form spheroid epithelial aggregates; the mixed cell culture configuration improves proliferation and reduces apoptosis | Havlickova et al., 2004 [159] | |
| Outer root sheath keratinocytes | Human dermal papilla fibroblast | Type I collagen mixed with Matrigel (4:1) microspheres | Mixed cells cocultured in microspheres | Development of microsphere-based system to study HFs in vitro | Havlickova et al., 2009 [160] | |
| Epidermal stem cells from neonatal mouse dorsal skin and from epidermidis of adult human foreskin | Skin-derived precursors from neonatal mouse dorsal skin and from epidermidis of adult human foreskin | Matrigel | Dissociated cells mixed in Matrigel | Skin wound on mice back | De novo HFs and functional sebaceous gland formation | Wang et al., 2016 [161] |
| Epidermal stem cells from neonatal mouse dorsal skin | Skin-derived precursors from neonatal mouse dorsal skin | Matrigel | Dissociated cells mixed in Matrigel | Skin wound on mice back | Trichostatin A restores HFs regeneration capability of ex vivo cultured skin-derived precursors | Guo et al., 2019 [162] |
| Face-lift surgery human scalp biopsies keratinocytes | Face-lift surgery human scalp biopsies dermal papilla cells | Different ECM molecules (e.g. collagen, fibronectin) and soluble factors (e.g. BMP6, Wnt3a) | Hanging drop method with different ECM components | Dermal papilla cells spheroids and keratinocytes trabeculae; hyaluronic acid increases cell proliferation | Kalabusheva et al., 2017 [163] | |
| Human HFs keratinocytes | Human HFs dermal papilla cells | Enzymatically crosslinked silk fibroin-gelatin hydrogel | Dissociated cells mixed in the hydrogel | Development of an in vitro model that responds to drug administration | Gupta et al., 2018 [164] | |
| Neonatal mice epidermal cells; epidermal cells from neonatal foreskin | 4- to 5-week-old mice vibrissae dermal papilla cells; human occipital scalp skin | Platelet-rich plasma gel | Dissociated cells in platelet-rich plasma gel | Wound in mice back skin | Platelet-rich plasma increases DPC proliferation and hair induction genes; hair regeneration after 15–16 days in vivo | Xiao et al., 2017 [165] |
| Normal human epidermal keratinocytes | Human follicle dermal papilla cells | Water -soluble chitin from crab shell and sodium alginate | Cell-laden fibrous polyelectrolyte scaffold | Subcutaneous pocket in dorsum of immunodeficient mice | Formation of hair follicle-like structures after in vivo implantation | Lim et al., 2013 [166] |
| Back skin from E18 mouse | Pluronic F-127-coated PDMS chip; type I collagen | Cells cocultured on non-adhesive chips, embedded in collagen with a support mesh | Wound in mice back skin | In vivo regeneration of arrays of HFss | Kageyama et al., 2018 [167] | |
| Back skin from E18 mouse | Back skin from E18 mouse. Human DP cells. |
Type I-A collagen | Mesenchymal cells cultured in collagen drops mixed with epithelial cells | Wound in mice back skin | Increased versican and ALP, increased regenerated shafts in 3D culture | Kageyama et al., 2019 [168] |
| Neonatal foreskin dermal keratinocytes | Dermal papilla cells from discarded human scalp tissues | Type I collagen | Collagen to form a dermal compartment seeded sequentially with dermal papilla cells and keratinocytes | Silicon chambers containing the fabricated scaffolds implanted in the dorsal surface of 8–10-week-old male mice | Controlled spatial organization of regenerated HFs on collagen gels. Spontaneous capillary formation. | Abaci et al., 2018 [169] |
4.1. Scaffold-based approaches for hair follicle developmental tissue engineering
Cell-seeded scaffolds (i.e. materials processed into scaffolds subsequently seeded with cells) were used for developmental hair TE [143,157]. Poly(ethylene-co-vinyl alcohol) (EVAL) was used to coat TCPS and culture a mix of dissociated rat keratinocytes and dermal papilla cells [157]. The presence of hydrophobic and hydrophilic domains on EVAL, compared with uncoated TCPS, promoted the mobility of DP cells, thus decreasing their adhesion to the substrate and subsequently increasing their aggregation and spheroid formation. In the formed spheroids, DP cells preferably occupy the central portion, while keratinocytes distribute on the periphery and successfully differentiate towards follicular phenotype. Devitalized split thickness human dermis has also been tested as cell-seeded scaffold for skin regeneration, seeded with a mixture of human keratinocytes and mouse dermal papilla cells to promote HFs regeneration [143]. After the construct was implanted in vivo in a wound on the mice back, regenerated skin grafts showed follicular structures characterized by dermal papilla cell condensates in the dermis and hair shaft-like structures exposed from the epidermis.
As an alternative to cell seeding on prefabricated scaffolds, cells loading into materials during the scaffold fabrication (i.e. cell-laden scaffolds) has been also widely investigated to regenerate HFs. The ‘organ germ method’, based on the 3D coculture of epithelial and mesenchymal cells in a collagen drop, previously described for tooth developmental TE (see Section 3), has been also tested for HFs regeneration. In a pioneering study, Nakao et al. [114] demonstrated that the whisker follicles can be regenerated in vivo with correct morphology (i.e. shaft, inner and outer root sheath) after implantation of cocultured embryonic mesenchymal and epithelial cells in a collagen drop. By using the collagen drop method, permanent and variable portions of the hair follicles with correct morphology and orientation in skin were histologically identified, at day 22, after in vivo intracutaneous transplantation (Fig. 5B) [152]. Melanin granules were also identified in the hair matrix. Moreover, green fluorescent protein (GFP) staining proved that the successful regeneration of the hair follicle (HF) was attributed to the implanted reconstituted organ germs. The use of a nylon guide to implant the bioengineered germ subsequently allowed prevention of epithelial cyst formation after implant and significantly improved the regenerated HFs frequency (i.e. 94% vs. 38%, with vs. without nylon guide respectively) [154]. Human bioengineered HF germs reconstituted from adult patients and transplanted in the back of mice also showed regeneration of pigmented hair shafts with correct structure (i.e. infundibulum and sebaceous gland, hair matrix and shaft). Piloerection ability comparable to that of native HF was also proved after administration of a neurotransmitter agent. Moreover, the bioengineered HFs showed the ability of alternate growth and regression phases, typical of the hair cycle (Fig. 5D), with cycling times comparable to native HFs. The collagen drop method was subsequently further developed to regenerate integumentary organs by using induced pluripotent stem (iPS) cells [153]. iPS cells were first cultured to generate embryoid bodies in vitro and they differentiated in both epithelial and mesenchymal lineage. Then, the obtained embryoid bodies were transplanted in vivo in mice subrenal capsules. Successfully regenerated HFs were observed together with surrounding tissues including dermis, sebaceous gland, skin, and subcutaneous adipose tissue. The orthotopic transplantation of the bioengineered integumentary organ system in adult mice led to the eruption and growth of hair shafts. Moreover, connections with surrounding tissues were observed (e.g. arrector pili muscles, epidermis, dermis, nerve fibres), as shown in Fig. 5C. The collagen drop method was also used to develop in vitro models by embedding dermal fibroblasts in the collagen gel and seeding keratinocytes on the top of the gel [158]. Morphogenetic events with tubular formations were observed when post-natal dermal fibroblasts were used, while no keratinocytes re-organization was observed when adult dermal papilla cells were used.
Collagen was also used coupled with Matrigel to test different cell coculture configurations [159,160]. Human dermal fibroblasts were first embedded in collagen to form a ‘pseudo-dermis’ [159]. Then, the cell-laded collagen was layered with normal human epidermal keratinocytes and outer root sheath keratinocytes sequentially seeded on collagen or mixed in Matrigel and seeded on collagen (i.e. ‘sandwich’ model) to obtain in vitro models to study HFs epithelial-mesenchymal interactions. Keratinocytes formed spheroidal epithelial cell aggregates in both configurations, but higher cell proliferation and lower apoptosis were detected in the culture systems where cells were mixed. Subsequently, collagen and Matrigel (4:1) were used by the authors to prepare microspheres (Ø = 1–2 mm) laden with a mixture of outer root sheath keratinocytes and dermal papilla fibroblasts [160]. Compared with the previously developed ‘sandwich’ model, the use of microspheres allowed for an easier, faster, highly reproducible and less expensive procedure that also decreased the required number of cells required to develop the in vitro model. Cell migration and matrix reorganization were observed; however, no HFs units spontaneously formed inside the fabricated microspheres. Moreover, cells responded to the administration of different hair growth-modulatory agents, thus proving the potential of the developed microsphere-based coculture system as possible in vitro tool used ahead of screening for the development of new hair loss treatments. Scaffolds prepared using solely Matrigel have also been investigated as possible artificial ECM to recreate an adequate 3D environment. For instance, Matrigel drops were used to prove the possible HFs and sebaceous gland regeneration by coculturing epidermal stem cells and skin-derived precursors derived from adult foreskin and scalp [161]. However, the hair regeneration capability of skin-derived precursors decreased after ex vivo culture. Thus, subsequently, the authors used a similar culture method to prove that trichostatin A supplement in culture restored the hair induction capability of skin-derived precursor, thanks to the increase in BMP expression, thus improving the efficiency of hair regeneration when this cell population is used [162].
Finally, other materials have been used for the development of cell-laden 3D scaffolds to recapitulate the epithelial-mesenchymal interactions of HFs development and target HFs regeneration. Mixed dermal papilla cells and keratinocytes were cultured by hanging drop method and different soluble factors (e.g. BMP6, VD3, VPA, Wnt3a) and ECM molecules (e.g. fibronectin, Matrigel, collagen I) were added to test their influence on the reconstituted hair germ model [163]. When cells were mixed, dermal papilla cells formed aggregates and keratinocytes formed extensions. In presence of proteoglycans and hyaluronic acid, cell proliferation was significantly increased. In another work [164], gelatin and silk fibroin were mixed and enzymatically crosslinked by tyrosinase to fabricate a multicellular coculture hydrogel composed by human HFs dermal papilla cells, keratinocytes and stem cells. Increased proliferation and higher viability were detected for dermal papilla cells cultures and cocultures with keratinocytes in presence of the 3D silk fibroin-gelatin hydrogel. Platelet-rich plasma has been also investigated as potential 3D developmental TE scaffold to regenerate hair [165], because of its emergence for clinical treatments of hair loss [170]. Authors proved that the presence of platelet-rich plasma in culture medium (up to 5%) promoted murine and human DPC proliferation and expression of hair inductive-related genes (e.g. Versican, β-catenin, ALP). Moreover, cell-laden platelet-rich plasma gel showed hair regeneration after in vivo implantation in dorsal wound in mice model. However, hair regeneration was observed only when murine cells were embedded in the gel (i.e. no hair reconstituted in vivo when human cells were embedded in the gel). 3D fibrous scaffolds prepared by polyelectrolyte complexation between water-soluble chitin derived from crab shell and sodium alginate were also used [166], with parallel domains characterized by a resolution of approximatively 50 μm. The structures were designed to first promote homotypic interactions of epithelial cells and mesenchymal cells and, subsequently, heterotypic interactions between the different scaffold domains. After in vivo implantation, the constructs induced HF-like structures. The coculture of epithelial and mesenchymal cells in 3D cell-laden scaffolds allows for the spatiotemporal self-organization of cocultured cells, replicating the HFs developmental processes occurring in vivo. In compartmentalized cocultures, translucent zones at the interface between epithelial and mesenchymal cells are observed, identifying an interaction between the cocultured cell populations [152]. In mixed cell cocultures, mesenchymal cells gradually migrate at the periphery of cell aggregates and the two cell populations separate from each other forming a dumbbell-like configuration [167], observed both in other regenerated HFs [152] and reminiscent of the natural in vivo organogenesis developmental processes [171].
Promising results in HFs regeneration were achieved by engineering cell-laden 3D scaffolds; however, in most cases, the strategies are based on a manual reconstitution of the organ germ, which might represent a limit to the reproducibility of regenerated HFs. Moreover, as multiple HFs are required to cover significant surface of the skin, a scale-up of bioengineered HFs that allows for the fabrication of arrays of HFs is required. Thus, recent works have developed the automation of the production of bioengineered HFs to target the regeneration of multiple HFss [167,168]. Polydimethylsiloxane (PDMS) chips made of 300 round bottom culture wells coated with Pluronic F-127, to make them non-adhesive, were used to culture dissociated epithelial-mesenchymal cells suspensions and promote the spontaneous formation of cell aggregates (Fig. 6A) [167]. After 3 days of culture, a collagen solution (embedded with a surgical mesh to improve its handling) is poured on the chip to obtain a collagen gel sheet loaded with 2D arrays of cultured organ germs. The cocultured cells spontaneously formed cell aggregates when oxygen supply was opportunely provided to the chip during the cell culture (Fig. 6B). Moreover, after 18 days of in vivo transplantation, regenerated HFs aligned on the mice back skin were observed, thus proving the potential of the developed technique in regenerating HFs in desired skin areas (Fig. 6C). Using a similar low-adhesive culture plate [168], the authors demonstrated that by culturing mesenchymal cells in collagen drops, subsequently cocultured with isolated epithelial cells, increased in Versican and ALP expression was detected, and a higher number of hair shafts was regenerated in vivo, compared to self-assembled cells aggregates (i.e. no artificial ECM). Moreover, the developed automatic deposition of collagen drops allowed for a potential industrial scale-up, compared to the manual procedures traditionally performed to reconstitute organ germs (i.e. approximatively 10 min required to deposit 5000 collagen drops). In another work [169], a spatially organized distribution of regenerated HFs was achieved by a fibroblast-laden collagen gel containing HFs-shaped extension fabricated by replica moulding of a 3D printed mould. Dermal papilla cells were seeded in the extensions, allowed to self-aggregate, and keratinocytes were subsequently seeded on the top of them. HFs structures were correctly regenerated; however, cell reprogramming (i.e. overexpression of master regulator gene Lef-1) was necessary to increase the HFs formation rate ex vivo. The addition of HUVEC promoted the formation of a capillary network, necessary for the survival of the fabricated construct.
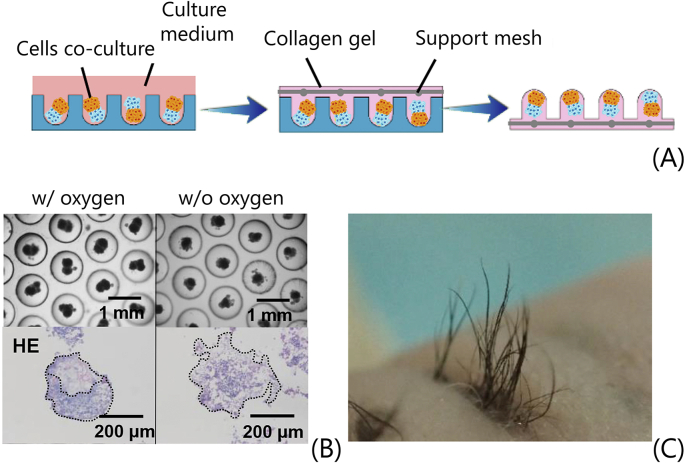
Fig. 6.
Fabrication and transplantation of spatially aligned hair follicle germs. (A) Germs are generated by coculturing cells in non-adhesive wells. Then, a collagen gel with a support mesh is used to embed spatially aligned germs and the obtained collagen sheet is used for transplantation. (B) Phase-contrast (scale bar = 1 mm) and H&E staining images (scale bar = 200 μm) of cell cocultures performed in oxygen-permeable wells PDMS (left) and non-oxygen-permeable polymethyl methacrylate (PMMA) wells (right). (C) Regenerated aligned hair follicles after 18 days of transplantation in back skin of nude mice [167]. All images used with permission.
5. Glands developmental tissue engineering
Salivary and lacrimal glands are exocrine glands that contribute to the maintenance of oral health and eye surface homeostasis, respectively. Both the glands originate from the ectoderm by reciprocal epithelial-mesenchymal interactions during embryogenesis. This developmental process has been mimicked by TE to propose developmental TE strategies for the regeneration of these glands, as summarized in Table 3.
Table 3.
Scaffold-based developmental tissue engineering strategies for salivary and lacrimal glands regeneration, based on the coculture of epithelial and mesenchymal cells. A biomaterial is used to prepare a 3D structure and coculture cells. In vitro and in vivo studies are performed to verify the suitability of the engineered construct in regenerating the glands.
| Epithelial cells | Mesenchymal cells | Biomaterial | Culture method | In vivo tests | Main findings | Reference |
|---|---|---|---|---|---|---|
| Salivary gland | ||||||
| Submandibular gland from E13 mice | PVA, EVAL, PVDF, PC | Tissue recombination cultured on biomaterials | PVDF supports morphogenesis in serum-free in vitro culture | Yang et al., 2010 [172] | ||
| Submandibular and sublingual gland from E14.5 mice | Type I-A collagen | Dissociated cells in collagen drop | Mice masseter muscle (with PGA monofilament guide) | In vitro branching morphogenesis. In vivo functional restoration (saliva production) | Ogawa et al., 2013 [173] | |
| Lacrimal gland | ||||||
| Lacrimal and harderian gland from E16.5 mice | Type I-A collagen | Dissociated cells in collagen drop | Mice lacrimal gland defect (with PGA monofilament guide) | Lacrimal gland develops in vivo with correct functionality (tear secretion) | Hirayama et al., 2013 [174] | |
| Lacrimal glands from 8-week-old pigs | Matrigel | Mixture of dissociated cells seeded on Matrigel | Cells organize in spheroid on Matrigel (not on culture plastic) | Massie et al., 2017 [175] | ||
5.1. Salivary gland
Salivary glands are exocrine glands categorized in three pairs of major salivary glands (i.e. parotid, submandibular, and sublingual glands, producing approximatively 90% of secreted saliva) and other 600 to 1000 minor salivary glands [176]. All glands share a similar architecture and are composed of parenchymal and stromal components. Salivary gland end pieces (i.e. serous or mucous acini) are composed of serous or mucous cells, arranged around a lumen, that produce primary saliva. The acini are connected to a ductal structure opening in mouth, with different length and diameter depending on the gland type. Myoepithelial cells, connected to the nervous system, promote saliva secretion. Other cell types include myofibroblasts, immune cells, endothelial cells, stromal cells, and nerve fibres [177].
The main role of salivary glands is the production and secretion of saliva in the oral cavity (approximatively 0.6–1.5 L daily production), composed of 99% water and 1% proteins and salts. Saliva protects teeth and oral mucosa, it contributes to speech articulation, lubrication, and pH buffering, it is fundamental for mastication, digestion and swallowing, and it is involved in electrolyte, mucus, enzymes and antibacterial compound supply [178]. Salivary glands can be affected by a variety of diseases, including obstructions, infections, inflammatory disorders, malignant or benign tumours, leading to loss of salivary gland function and xerostomia (dry mouth). Radiation therapy, ageing or Sjögren's syndrome can also affect salivary glands function [179]. These conditions can lead to bacterial infection, dental decay, mastication and swallowing hypofunction or dysfunction, eventually leading to a loss of life quality (e.g. oral dryness, disturbed sleep at night), increased tooth decay, oral infections, and disturbed taste sensation [180]. Current clinical treatments are based on salivary substitutes or salivary stimulants [181]; however, these treatments do not target the regeneration of functional salivary glands that would naturally provide saliva. TE may allow for the restoration of functional salivary glands. Classical TE approaches for salivary gland regeneration rely on the transplantation of cells in a 3D matrix, typically hydrogel-based, to restore the salivary gland functions [182]. For instance, stem/progenitor cells were 3D cultured in decellularized salivary gland ECM-derived hydrogels [183], and primary submandibular gland cells were encapsulated in poly(ethylene glycol) (PEG) hydrogels [184] or laminin I peptide-decorated fibrin hydrogels [185]. However, reproducing the branching morphogenesis characterizing the salivary gland tissue to achieve a correct morphology and biofunctionality is still challenging [182,185]. Thus, developmental TE approaches, mimicking the branching morphogenesis process in the embryo, have been investigated.
In humans, salivary gland morphogenesis initiates 6–8 weeks after gestation. Salivary gland development consists of a branching morphogenesis of the epithelium, guided by growth factors provided by the neural crest-derived mesenchyme, which develops by subsequent changes in ECM proteins synthesis and arrangement, and in cell-cell and cell-ECM interactions. As for tooth and hair follicle, mouse embryos are often used as models for the study of salivary gland developmental biology (Fig. 7A) [180]. After the oral epithelium thickens (E11), the mesenchyme condenses, and a spherical epithelial structure connected to a stalk forms (E12). Branching initiates (E13) and the epithelium divides into different buds. Finally, a highly branched structure comprising acini, contributing to saliva production, and ducts, allowing for saliva secretion, is formed. Salivary gland regeneration by developmental TE (Table 3), mimicking in vivo gland morphogenesis, was initially targeted by recombination of rudiments of epithelium and mesenchyme derived from E13 mice submandibular glands [172]. The recombinants were cultured on different biomaterials, namely polyvinyl alcohol (PVA), ethylene vinyl alcohol (EVAL), polycarbonate (PC), and polyvinylidene fluoride (PVDF), to test their potential in promoting correct salivary gland morphogenesis in serum-free in vitro cultures. When recombinants were cultured on PVDF, tissue morphogenesis and correct marker expression was detected and related to an increased adsorption on the culture substrate of FGF7, key factor in mediating epithelial morphogenesis. In more recent studies, the collagen drop method was used to recombine dissociated epithelial and mesenchymal cells from embryonic mice salivary glands [173]. After 1 day of in vitro culture, epithelial-mesenchymal cells interact and develop to an initial bud stage, also observed in natural gland morphogenesis. Recombined organ germs showed successful branching morphogenesis after 3 days of in vitro culture, followed by stalk elongation and cleft formation, as observed in vivo (Fig. 7B). These observations suggest that, morphologically, the bioartificial germ develops replicating the morphogenetic steps occurring in native gland development. The germs were then implanted in vivo in mice masseter muscle, with the help of a biodegradable PGA monofilament thread guide inserted in the epithelial/mesenchymal portion of the germ. The implanted bioengineered salivary glands showed a morphology replicating one of native glands, including presence of membrane water channel protein aquaporin-5 (AQP5), E-cadherin, calponin, and innervation. The presence of functional innervation was proved by saliva secretion after stimulation by pilocarpine and gustatory stimulation by citrate. Reduced bacterial colonies and improved swallowing ability and survival rate were detected for mice that received the bioengineered gland, compared to salivary gland-defected mice. However, the secreted saliva contained a lower amount of amylase compared to saliva produced by native glands. The authors later demonstrated that a fully functional salivary gland was successfully regenerated in vivo by transplanting an induced salivary gland primordium combined with mesenchyme tissue [186]. The engineered salivary gland was also used for the in vitro study of transcription factors (e.g. Sox9, Foxc1) involved in salivary gland development.
Fig. 7.
Salivary gland developmental tissue engineering. (A) Fundamental steps of salivary gland in vivo morphogenesis. (B) Phase contrast images and H&E staining of natural and bioengineered salivary glands, obtained by developmental TE, cultured in vitro up to 3 days; scale bar = 200 μm [173]. All images used with permission.
5.2. Lacrimal gland
Lacrimal glands are almond-shaped, bilobed, exocrine glands that secrete the aqueous part of the tear film. Lacrimal glands are composed by lobules separated by loose connective tissue. Each lobule is composed by multiple acini lined by columnar secretory cells separated by intralobular ducts that drain into tubules or excretory ducts that direct the lacrimal fluid towards the ocular surface. The lacrimal branch of the ophthalmic artery supplies blood to the lacrimal gland. Fibres from the trigeminal nerve innervate the gland. Main cell components of the lacrimal gland are acinar cells that secrete lacrimal gland primary fluid, ductal cells that modify the fluid composition, and myoepithelial cells that help in fluid secretion [187].
Lacrimal glands are the primary contributors to the aqueous layer of the tear film. Lacrimal glands are involved in ocular surface homeostasis, integrity and health by tear secretion stimulated by sensory nerves, as secreted tears protect the ocular surface from pathogens, because of the presence of bacteria and fungicidal agents and promote the removal of debris from the ocular surface. Lacrimal gland dysfunction can be caused by ageing, infection, inflammation given by pathological conditions such as Sjögren's syndrome, radiation, ocular cicatricial pemphigoid, long-term work with displays, dry-air environments, use of contact lenses, and refractive surgery [188]. Perturbations of the physiological functions of the lacrimal gland can lead to dry eye syndrome, resulting in changing of the tear film composition, ocular damage and potential decline of vision [189]. Current available treatments include lubrication of the eye surface or pharmacological stimulation to promote tear secretion, mainly aiming at temporary symptomatic relief and treatment of ocular surface inflammation. However, these solutions have limited outcomes to restore the ocular surface homeostasis in the long term [190]. The regeneration of a functional lacrimal gland, allowing for native and natural tear secretion without the need of artificial external stimuli, may be achieved by TE. In fact, regenerated functional lacrimal glands would allow for the restoration of the ocular integrity and healthy long-term homeostasis. Classical TE approaches described for the regeneration of lacrimal glands rely on the culture of epithelial cells on scaffolds, such as decellularized lacrimal glands [191,192]. Much progress has been made to develop culture systems for lacrimal epithelial cells or precursor cells that can promote cell differentiation and correct functionality or that can allow for cell proliferation during in vitro culture, respectively [193,194]. Developmental TE approaches have been also described for lacrimal gland regeneration by mimicking the epithelial-mesenchymal interactions occurring during embryogenesis (Table 3) [195].
Lacrimal glands start developing in mice at E13.5, with the invagination of the conjunctival epithelium and subsequent formation of a tubular structure thickened at the tip (E15.5). The branching morphogenesis then begins (E16.5) and a complete lobular structure forms (E19) [196]. The collagen drop method was used for lacrimal gland regeneration by coculturing E16.5 mice lacrimal and harderian gland dissociated epithelial and mesenchymal cells [174]. During in vitro culture, epithelial-mesenchymal interactions were observed after 1 day, while branching morphogenesis successfully initiated after 3 days of culture (frequency > 90%), followed by stalk elongation and cleft formation. As observed for salivary glands, the bioengineered lacrimal glands recapitulate in vitro morphogenetic steps naturally occurring in vivo during embryonic morphogenesis. After in vivo engraftment in lacrimal gland-defected mice, functional lacrimal glands secreting tears were successfully regenerated (frequency > 70%, Fig. 8A). Tear secretion was improved, after nervous stimulation, in mice that received the bioengineered gland, compared to mice with lacrimal gland defects. The bioengineered gland correctly connected in vivo to the recipient's excretory duct. The regenerated gland was characterized by the correct morphological structure, mimicking that of native lacrimal glands, with acinar and duct cells expressing E-cadherin and AQP5 water channels (green and red in Fig. 8B, respectively). Moreover, lactoferrin, a protein secreted by lacrimal glands, was detected in bioengineered lacrimal glands (Fig. 8C). To avoid the use of the embryonic cells with the collagen drop method, epithelial and mesenchymal cells belonging to the offspring after birth from lacrimal glands were dissociated and mixed at different ratios with endothelial cells [175]. The mixed cell suspension was seeded on Matrigel and, compared to cells cultured on TCPS, spheroids with organized cell distribution formed in vitro. Despite formation of an organized cellular spheroid correctly responding to parasympathetic stimulation, the use of Matrigel is affected by limited potential clinical applications and in vivo proof of functionality (e.g. tear secretion, response to nervous stimulation) of these bioengineered lacrimal glands is required.
Fig. 8.
Lacrimal gland developmental tissue engineering. (A) Tear secretion from mice with natural lacrimal gland (left) and bioengineered lacrimal gland (right). (B) Immunohistochemical images of acinar and duct cells in natural and bioengineered lacrimal glands stained for aquaporin-5 (AQP5, red) and E-cadherin (green); scale bar = 50 μm. (C) Lactoferrin in the acini of natural and bioengineered implanted lacrimal gland; scale bar = 50 μm [174]. All images used with permission.
6. Discussion and conclusions
Developmental TE is emerging as a specific paradigm of TE aiming at tissue and organ regeneration by mimicking the developmental biological processes that naturally occur in vivo, in embryos, during tissue/organ formation. As several organs (e.g. teeth, HFs, lacrimal and salivary gland) originate from finely orchestrated reciprocal interactions between epithelial and mesenchymal compartments [23], developmental TE approaches aim at replicate these interactions to regenerate the tissue/organ. Adequate cell populations must be selected, appropriate cell coculture strategies must be applied, and a biomimetic microenvironment should be engineered to allow for the replication of the developmental process.
The selection of appropriate cell sources is an ongoing challenge [197]. Embryonic cells and murine cells have been the preferred cell populations used in the described works, potentially hindering future clinical translation [75,198]. For instance, considering tooth regeneration, classical tissue recombination experiments demonstrated sequential signalling between the dental epithelium and the mesenchyme of different stages and origins, where the epithelium acted as an inductive tissue [199,200]. The odontogenic capacity of the epithelium is present only in the early stages of tooth development and then switches to mesenchyme, as it naturally happens during embryonic tooth development. Thus, the mesenchyme becomes the odontogenic inductive source in the following stages [201]. Embryonic tooth germ cells were shown to re-aggregate and form teeth, following dissociation [105]. In other studies, epithelium and mesenchyme tissues from mouse embryonic tooth germs (E14.5 to E12.5 stage) were separated and the cells dissociated and recombined to form teeth [100,114]. These engineered teeth were shown to be vascularized [202] and further studies indicated that immunomodulation might play a role in their innervation [203]. While these studies focused on usage of embryonic tissues and cells derived from embryonic tooth germ at different stages, the reciprocal tissue induction has been utilized to suggest a basis for whole-tooth bioengineering that could employ adult cells [107,204]. Although the adult stem cells were shown to respond to an inductive odontogenic signal and participate in tooth formation, the cells that induced tooth formation in vitro were derived from inductive embryonic tooth-germ tissue (epithelium or mesenchyme) [73,205]. Different strategies were proposed to preserve the odontogenic signal of cells that have been expanded in vitro, such as employment of a three-dimensional micro-culture system [43] and utilizing the community effect of cells [116], aiming at three-dimensional microenvironmental reprogramming and preserving of inductive odontogenic capacity of the cells. Another important aspect, in prospect to translating the engineered teeth in future clinical treatments, is the fact that the majority of the studies utilized mouse embryonic teeth. Mouse dentition differs from human diphyodont dentition, with continuously growing incisors and only one set of molars (monophyodont). Mouse incisor has been extensively used as a model to study and characterize dental pulp stem cells [206,207], while whole tooth engineering experiments utilized cells and tissues derived from mouse molar, tooth germ [73,205]. Nevertheless, the biological time for tooth development in mice and human differs, with human tooth development being eight times slower than in mice, prompting another obstacle in securing long-term in vitro conditions in engineering tooth germ. In general, the selected cell populations (i) must guarantee adequate cross-talk, signalling, differentiation and desired ECM production, eventually leading to the actual organ regeneration, (ii) should be expanded in vitro to achieve the required number of cells to obtain the biological construct (i.e. ‘high cell densities’ generally required [114]) without losing their potential, and (iii) their origin and isolation should be compatible with possible clinical applications, without arising ethical concerns or restriction (e.g. difficulties/impossibilities of directly obtaining suitable human embryonic cells [122]).
Once cell populations are isolated and expanded, an adequate culture system (Fig. 2) must be selected to allow for cell crosstalk and tissue/organ regeneration. 2D culture systems have been tested to replicate epithelial-mesenchymal interactions for the development of different tissue/organs [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. These culture systems are relatively simple but lack the complexity of the 3D native tissue microenvironment and, thus, might fail in guaranteeing an adequate cell spatial-temporal organization and, eventually, tissue/organ development [28]. 3D cultures have alternatively been adopted to reproduce a more physiological-like microenvironment, either by scaffold-free or scaffold-based strategies, based on the culture of cells on a pre-formed scaffold or on the embedding of cells inside the scaffold during its fabrication. Scaffold-free 3D culture systems [[43], [44], [45], [46], [47]] represent a step forward compared to 2D traditional cultures, since they allow for cells re-organization and for the formation of spheroids and tissue/organ-like structure. However, these systems are mainly based on the use of culture medium and no ECM is used to simulate the actual microenvironment that cells natively experiment during tissue/organ development. Thus, scaffold-based approaches, discussed in this review, have been developed with promising results and represent an optimal and versatile strategy to artificially recreate an environment to opportunely support cells interaction and tissue/organ development. Regardless the two different 3D scaffold-based methods, developmental TE scaffolds should be designed and fabricated to target fundamental requirements, such as the possible diffusion of signalling molecules, a structure allowing for cells spatial reorganization, biodegradation, adequate mechanical properties, and promotion of vascularization and innervation (Fig. 9).
Fig. 9.
Developmental tissue engineering scaffold requirements. (A) Diffusion of nutrients and oxygen for cell survival as well as soluble factors fundamental for epithelial-mesenchymal interactions. (B) Possibility for cells to remodel the artificial ECM and correctly organize in space to develop the regenerated tissue/organ. (C) Biodegradability of the scaffold that should be, in time, substituted with the newly formed ECM of the mature tissue/organ. (D) Adequate mechanical properties. (E) Promotion of vascularization and innervation to allow for the mature tissue/organ survival and functionality. Epithelial and mesenchymal cells are depicted in yellow and green; light blue lines represent a generic 3D developmental tissue engineering scaffold.
Diffusion of nutrients and oxygen is essential for scaffolds designed either for classical or developmental TE approaches (Fig. 9A), as only adequate nutrient/oxygen diffusion can guarantee the survival of cells colonizing the scaffold or embedding into it. Moreover, for developmental TE approaches, it is fundamental that the scaffold allows for signalling molecules diffusion, since the interaction between epithelial and mesenchymal cells leading to tissue/organ development is based on the exchange of conserved families of signalling molecules that guide the different morphogenetic stages [24]. Thus, the material used to fabricate the scaffold and/or the structure of the fabricated scaffold not only must guarantee oxygen and nutrient diffusion, but they should also allow for diffusion of signalling molecules to allow for the replication of the signalling pathways guiding morphogenesis in vivo. The regeneration of the tissue/organ with correct biomorphological features is highly dependent on the possibility for cells to migrate and self-organize in the scaffold (Fig. 9B). Cell growth and colonization of the scaffold 3D structure is a core concept for all TE approaches and it depends on a variety of scaffold design parameters, including porosity, pore size, topography, biochemical cues, and mechanical properties [208]. Cell mobility, migration and reorganization is particularly crucial in developmental TE. In fact, cocultured cell populations should be able to migrate and self-reorganize to replicate the different morphogenetic steps observed in vivo (e.g., mesenchyme condensation around invaginated epithelium in teeth, follicular epithelial cells enveloping mesenchymal condensates in HFs morphogenesis, cells reorganization during branching morphogenesis of regenerated glands). Cell mobility is also strictly dependent on the possible remodelling of the artificial ECM used to fabricate the scaffold. Thus, the scaffold should be biodegradable, to allow for cell remodelling and, eventually, to its substitution by newly formed ECM (Fig. 9C). The mechanical properties of the scaffold should also be adequately tuned to promote organ regeneration by developmental TE (Fig. 9D), as for all TE approaches the mechanical properties of the cellular microenvironment are known to contribute in determining cell fate and guiding cell response [209,210]. To date, there are limited reports which investigate the mechanical properties of the scaffolds used for developmental TE [61,119], reporting elastic modulus values in the range of few kPa (i.e. approximatively E = 1–4 kPa). In most works, the mechanical properties are only tested after tissue/organ regeneration and compared to those of native mature tissues to demonstrate functionality has been achieved [100]. In classical TE approaches, scaffolds are mainly designed to target the properties of the mature tissue to be regenerated [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18]]. For developmental TE approaches, it is more likely that the properties of the scaffold should mimic those of the metastable environment where the native developmental process occurs and that the mechanical properties of the construct dynamically change following the developmental regeneration process [20]. More studies investigating the mechanical properties of developmental TE scaffolds and their effects in guiding epithelial-mesenchymal cell interactions are required. We believe the interplay between TE and developmental biology is fundamental to understand the mechanical properties of the cell microenvironment during organogenesis which can be used to design developmental TE approaches. Finally, the scaffolds should be able to sustain an adequate vascularization and innervation (Fig. 9E). Indeed, vascularization is required for long-term survival of the regenerated tissue/organ [61,169,211,212]. Innervation is required for a correct integration of the regenerated tissue/organ with the surrounded tissues and for adequate response to stimuli that contribute to its physiological functions [[213], [214], [215]]. For instance, a correct innervation and integration with surrounded tissues was proved to be fundamental to regenerate functional HFs [153], salivary [173] and lacrimal gland [174] by developmental TE approaches.
Materials selection and scaffold design are fundamental to achieve the above-mentioned criteria. Polymers from both synthetic and natural origin have been proposed to fabricate developmental TE scaffolds. Among these, collagen has been extensively investigated with promising outcomes for the regeneration of functional teeth [94,[99], [100], [101],[114], [115], [116], [117], [118]], HFs [114,[152], [153], [154],158,167,168], and glands [173,174], being the major component of native ECM [216]. The bioartificial ECM, in fact, should allow cell adhesion and functionality; thus, natural polymers possessing ECM-biomimetic sequences or synthetic polymers artificially biofunctionalized to replicate them might represent the preferred choices. Particularly promising results were achieved by using cell-laden hydrogel-based strategies, as cells are directly embedded in a 3D microenvironment that allows for their mobility and self-organization to replicate the developmental processes that lead to the organs development. In cell-seeded scaffold approaches, on the contrary, cells are seeded on (macro)porous scaffolds that, despite potentially promoting tissue ingrowth after in vivo implantation, require particular efforts in the design of pores, porosity and interconnectivity to allow for full scaffold colonization, which remains challenging [217], and subsequent cell self-organization. Finally, most of the described approaches require manually prepared cell-laden scaffolds. Manual procedures may represent a limitation for reproducibility, scale up and clinical translation. Thus, automated procedures for scaffolds production and sterilization are required, in future, to standardize scaffold production. For instance, additive manufacturing technologies have been poorly investigated in developmental TE approaches and they may represent a step forward in the field, allowing for a compartmentalized cell coculture by a reproducible and scalable approach. Altogether, the developmental TE paradigm represents a promising approach that might allow for the regeneration of tissues and organs with correct biomorphological and biofunctional features by the combination of adequate cells sources, seeded in an appropriate configuration on a scaffold designed with the previously stated features.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgements
Confidence in Collaboration in Advanced Therapies Award - Project CiC007 (AC), UKRI Future Leaders Fellowship MR/S034757/1 (AC).
References
- 1.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. (80-.) [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.S., Smoak M.M., Melchiorri A.J., Mikos A.G. An overview of the tissue engineering market in the United States from 2011 to 2018. Tissue Eng. 2019;25:1–8. doi: 10.1089/ten.tea.2018.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caddeo S., Boffito M., Sartori S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017;5:1–22. doi: 10.3389/fbioe.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Khademhosseini A., Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11:1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 6.Williams D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019;7:1–10. doi: 10.3389/fbioe.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenas P., Moos M., Luyten F.P. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in Vivo development. Tissue Eng. B Rev. 2009;15:381–394. doi: 10.1089/ten.teb.2008.0575. [DOI] [PubMed] [Google Scholar]
- 8.Marcucio R.S., Qin L., Alsberg E., Boerckel J.D. Reverse engineering development: crosstalk opportunities between developmental biology and tissue engineering. J. Orthop. Res. 2017;35:2356–2368. doi: 10.1002/jor.23636. [DOI] [PubMed] [Google Scholar]
- 9.McDermott A.M., Herberg S., Mason D.E., Collins J.M., Pearson H.B., Dawahare J.H., Tang R., Patwa A.N., Grinstaff M.W., Kelly D.J., Alsberg E., Boerckel J.D. Recapitulating bone development through engineered mesenchymal condensations and mechanical cues for tissue regeneration. Sci. Transl. Med. 2019;11:1–16. doi: 10.1126/scitranslmed.aav7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negrini N.C., Toffoletto N., Farè S., Altomare L. Plant tissues as 3D natural scaffolds for adipose , bone and tendon tissue regeneration. Front. Bioeng. Biotechnol. 2020;8:1–15. doi: 10.3389/fbioe.2020.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi K., Kishida R., Tsuchiya A., Ishikawa K. Honeycomb blocks composed of carbonate apatite, β-tricalcium phosphate, and hydroxyapatite for bone regeneration: effects of composition on biological responses. Mater. Today Bio. 2019;4:100031. doi: 10.1016/j.mtbio.2019.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Zhang X., Dai C., Yin Y., Gong L., Pan W., Huang R., Bu Y., Liao X., Guo K., Gao F. Bioactive three-dimensional graphene oxide foam/polydimethylsiloxane/zinc silicate scaffolds with enhanced osteoinductivity for bone regeneration. ACS Biomater. Sci. Eng. 2020 doi: 10.1021/acsbiomaterials.9b01931. [DOI] [PubMed] [Google Scholar]
- 13.Bittner S.M., Smith B.T., Diaz-Gomez L., Hudgins C.D., Melchiorri A.J., Scott D.W., Fisher J.P., Mikos A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019;90:37–48. doi: 10.1016/j.actbio.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuckensen K., Schwab A., Knauer M., Muiños-López E., Ehlicke F., Reboredo J., Granero-Moltó F., Gbureck U., Prósper F., Walles H., Groll J. Tissue mimicry in morphology and composition promotes hierarchical matrix remodeling of invading stem cells in osteochondral and meniscus scaffolds. Adv. Mater. 2018;30:1–7. doi: 10.1002/adma.201706754. [DOI] [PubMed] [Google Scholar]
- 15.Rinoldi C., Fallahi A., Yazdi I.K., Campos Paras J., Kijeńska-Gawrońska E., Trujillo-De Santiago G., Tuoheti A., Demarchi D., Annabi N., Khademhosseini A., Swieszkowski W., Tamayol A. Mechanical and biochemical stimulation of 3D multilayered scaffolds for tendon tissue engineering. ACS Biomater. Sci. Eng. 2019;5:2953–2964. doi: 10.1021/acsbiomaterials.8b01647. [DOI] [PubMed] [Google Scholar]
- 16.Miao S., Nowicki M., Cui H., Lee S.J., Zhou X., Mills D.K., Zhang L.G. 4D anisotropic skeletal muscle tissue constructs fabricated by staircase effect strategy. Biofabrication. 2019;11 doi: 10.1088/1758-5090/ab1d07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contessi Negrini N., Celikkin N., Tarsini P., Farè S., Święszkowski W. Three-dimensional printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering. Biofabrication. 2020;12 doi: 10.1088/1758-5090/ab56f9. [DOI] [PubMed] [Google Scholar]
- 18.Tytgat L., Van Damme L., Van Hoorick J., Declercq H., Thienpont H., Ottevaere H., Blondeel P., Dubruel P., Van Vlierberghe S. Additive manufacturing of photo-crosslinked gelatin scaffolds for adipose tissue engineering. Acta Biomater. 2019;94:340–350. doi: 10.1016/j.actbio.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 19.Sheehy E.J., Kelly D.J., O'Brien F.J. Biomaterial-based endochondral bone regeneration: a shift from traditional tissue engineering paradigms to developmentally inspired strategies. Mater. Today Bio. 2019;3 doi: 10.1016/j.mtbio.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingber D.E., Mow V.C., Butler D., Niklason L., Huard J., Mao J., Yannas I., Kaplan D., Vunjak-Novakovic G. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265–3283. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 21.Marga F., Neagu A., Kosztin I., Forgacs G. Developmental biology and tissue engineering. Birth Defects Res. Part C Embryo Today - Rev. 2007;81:320–328. doi: 10.1002/bdrc.20109. [DOI] [PubMed] [Google Scholar]
- 22.Lenas P. Developmental biology in bioartificial tissue design: manufacturing and regulatory considerations. Regen. Med. 2018;13:7–11. doi: 10.2217/rme-2017-0126. [DOI] [PubMed] [Google Scholar]
- 23.Wan A.C.A. Recapitulating cell–cell interactions for organoid construction – are biomaterials dispensable? Trends Biotechnol. 2016;34:711–721. doi: 10.1016/j.tibtech.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Pispa J., Thesleff I. Mechanisms of ectodermal organogenesis. Dev. Biol. 2003;262:195–205. doi: 10.1016/S0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 25.Takeo M., Tsuji T. Organ regeneration based on developmental biology: past and future. Curr. Opin. Genet. Dev. 2018;52:42–47. doi: 10.1016/j.gde.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Dhouailly D. Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds and mammals. Wilhelm Roux’s Arch. Dev. Biol. 1975;177:323–340. doi: 10.1007/BF00848183. [DOI] [PubMed] [Google Scholar]
- 27.Duval K., Grover H., Han L.H., Mou Y., Pegoraro A.F., Fredberg J., Chen Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32:266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 28.Belair D.G., Abbott B.D. Engineering epithelial-stromal interactions in vitro for toxicology assessment. Toxicology. 2017;382:93–107. doi: 10.1016/j.tox.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He P., Zhang Y., Kim S.O., Radlanski R.J., Butcher K., Schneider R.A., DenBesten P.K. Ameloblast differentiation in the human developing tooth: effects of extracellular matrices. Matrix Biol. 2010;29:411–419. doi: 10.1016/j.matbio.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto A., Harada H., Saito M., Taniguchi A. Induction of enamel matrix protein expression in an ameloblast cell line co-cultured with a mesenchymal cell line in vitro. In Vitro Cell. Dev. Biol. Anim. 2011;47:39–44. doi: 10.1007/s11626-010-9362-7. [DOI] [PubMed] [Google Scholar]
- 31.Yu J., Jin F., Deng Z., Li Y., Tang L., Shi J., Jin Y. Epithelial-mesenchymal cell ratios can determine the crown morphogenesis of dental pulp stem cells. Stem Cell. Dev. 2008;17:475–482. doi: 10.1089/scd.2007.0120. [DOI] [PubMed] [Google Scholar]
- 32.Morotomi T., Kawano S., Toyono T., Kitamura C., Terashita M., Uchida T., Toyoshima K., Harada H. In vitro differentiation of dental epithelial progenitor cells through epithelial-mesenchymal interactions. Arch. Oral Biol. 2005;50:695–705. doi: 10.1016/j.archoralbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Shiba H., Mouri Y., Komatsuzawa H., Mizuno N., Xu W., Noguchi T., Nakamura S., Sugai M., Kato Y., Kurihara H. Enhancement of alkaline phosphatase synthesis in pulp cells co-cultured with epithelial cells derived from lower rabbit incisors. Cell Biol. Int. 2003;27:815–823. doi: 10.1016/S1065-6995(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 34.Lee H.K., Park J.W., Seo Y.M., Kim H.H., Lee G., Bae H.S., Park J.C. Odontoblastic inductive potential of epithelial cells derived from human deciduous dental pulp. J. Mol. Histol. 2016;47:345–351. doi: 10.1007/s10735-016-9676-1. [DOI] [PubMed] [Google Scholar]
- 35.Veraitch O., Kobayashi T., Imaizumi Y., Akamatsu W., Sasaki T., Yamanaka S., Amagai M., Okano H., Ohyama M. Human induced pluripotent stem cell-derived ectodermal precursor cells contribute to hair follicle morphogenesis in vivo. J. Invest. Dermatol. 2013;133:1479–1488. doi: 10.1038/jid.2013.7. [DOI] [PubMed] [Google Scholar]
- 36.Veraitch O., Mabuchi Y., Matsuzaki Y., Sasaki T., Okuno H., Tsukashima A., Amagai M., Okano H., Ohyama M. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep42777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohyama M., Kobayashi T., Sasaki T., Shimizu A., Amagai M. Restoration of the intrinsic properties of human dermal papilla in vitro. J. Cell Sci. 2012;125:4114–4125. doi: 10.1242/jcs.105700. [DOI] [PubMed] [Google Scholar]
- 38.Yu J., Deng Z., Shi J., Zhai H., Nie X., Zhuang H., Li Y., Jin Y. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 2006;12:3097–3105. doi: 10.1089/ten.2006.12.3097. [DOI] [PubMed] [Google Scholar]
- 39.Ning F., Guo Y., Tang J., Zhou J., Zhang H., Lu W., Gao Y., Wang L., Pei D., Duan Y., Jin Y. Differentiation of mouse embryonic stem cells into dental epithelial-like cells induced by ameloblasts serum-free conditioned medium. Biochem. Biophys. Res. Commun. 2010;394:342–347. doi: 10.1016/j.bbrc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Shim J.H. Hair growth-promoting effect of human dermal stem/progenitor cell-derived conditioned medium. Tissue Eng. Regen. Med. 2015;12:268–275. doi: 10.1007/s13770-015-0012-8. [DOI] [Google Scholar]
- 41.Kang K.J., Ju S.M., Jang Y.J., Kim J. Indirect co-culture of stem cells from human exfoliated deciduous teeth and oral cells in a microfluidic platform. Tissue Eng. Regen. Med. 2016;13:428–436. doi: 10.1007/s13770-016-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breslin S., O'Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov. Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Kuchler-Bopp S., Bécavin T., Kökten T., Weickert J.L., Keller L., Lesot H., Deveaux E., Benkirane-Jessel N. Three-dimensional micro-culture system for tooth tissue engineering. J. Dent. Res. 2016;95:657–664. doi: 10.1177/0022034516634334. [DOI] [PubMed] [Google Scholar]
- 44.Kalabusheva E.P., Vorotelyak E.A. Hair follicle regeneration in vitro. Paleontol. J. 2016;50:1656–1664. doi: 10.1134/S0031030116140045. [DOI] [Google Scholar]
- 45.Lin B., Miao Y., Wang J., Fan Z., Du L., Su Y., Liu B., Hu Z., Xing M. Surface tension guided hanging-drop: producing controllable 3D spheroid of high-passaged human dermal papilla cells and forming inductive microtissues for hair-follicle regeneration. ACS Appl. Mater. Interfaces. 2016;8:5906–5916. doi: 10.1021/acsami.6b00202. [DOI] [PubMed] [Google Scholar]
- 46.Bécavin T., Kuchler-Bopp S., Kökten T., Huck O., Messaddeq N., Lesot H., Deveaux E., Benkirane-Jessel N., Laetitia K. Well-organized spheroids as a new platform to examine cell interaction and behaviour during organ development. Cell Tissue Res. 2016;366:601–615. doi: 10.1007/s00441-016-2487-6. [DOI] [PubMed] [Google Scholar]
- 47.Rosowski J., Bräunig J., Amler A.K., Strietzel F.P., Lauster R., Rosowski M. Emulating the early phases of human tooth development in vitro. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-43468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nanci A. 2018. Ten Cate's Oral Histology : Development, Structure, and Function. [Google Scholar]
- 49.Sharpe P.T. Dental mesenchymal stem cells. Dev. 2016;143:2273–2280. doi: 10.1242/dev.134189. [DOI] [PubMed] [Google Scholar]
- 50.Guentsch A., Fahmy M.D., Wehrle C., Nietzsche S., Popp J., Watts D.C., Kranz S., Krafft C., Sigusch B.W. Effect of biomimetic mineralization on enamel and dentin: a Raman and EDX analysis. Dent. Mater. 2019;35:1300–1307. doi: 10.1016/j.dental.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y.R., Du W., Zhou X.D., Yu H.Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014;6:61–69. doi: 10.1038/ijos.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertassoni L.E., Orgel J.P.R., Antipova O., Swain M.V. The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012;8:2419–2433. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawashima N., Okiji T. Odontoblasts: specialized hard-tissue-forming cells in the dentin-pulp complex. Congenital. Anom. 2016;56:144–153. doi: 10.1111/cga.12169. [DOI] [PubMed] [Google Scholar]
- 54.McGuire J.D., Walker M.P., Dusevich V., Wang Y., Gorski J.P. Enamel organic matrix: potential structural role in enamel and relationship to residual basement membrane constituents at the dentin enamel junction. Connect. Tissue Res. 2014;55:33–37. doi: 10.3109/03008207.2014.923883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonçalves L.M.N., Palma-Dibb R.G., Paula-Silva F.W.G., De Oliveira H.F., Nelson-Filho P., Da Silva L.A.B., De Queiroz A.M. Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J. Dent. 2014;42:986–992. doi: 10.1016/j.jdent.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Gutiérrez-Salazar M. del P., Reyes-Gasga J. Microhardness and chemical composition of human tooth. Mater. Res. 2003;6:367–373. doi: 10.1590/s1516-14392003000300011. [DOI] [Google Scholar]
- 57.Foster B.L. On the discovery of cementum. J. Periodontal. Res. 2017;52:666–685. doi: 10.1111/jre.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto T., Hasegawa T., Yamamoto T., Hongo H., Amizuka N. Histology of human cementum: its structure, function, and development. Jpn. Dent. Sci. Rev. 2016;52:63–74. doi: 10.1016/j.jdsr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veis A., Goldberg M. Pulp extracellular matrix. In: Goldberg M., editor. Dent. Pulp Biol. Pathol. Regen. Ther. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. pp. 35–46. [DOI] [Google Scholar]
- 60.Ozcan B., Bayrak E., Erisken C. Characterization of human dental pulp tissue under oscillatory shear and compression. J. Biomech. Eng. 2016;138:1–5. doi: 10.1115/1.4033437. [DOI] [PubMed] [Google Scholar]
- 61.Smith E.E., Zhang W., Schiele N.R., Khademhosseini A., Kuo C.K., Yelick P.C. Developing a biomimetic tooth bud model. J. Tissue Eng. Regen. Med. 2017;11:3326–3336. doi: 10.1002/term.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Righolt A.J., Jevdjevic M., Marcenes W., Listl S. Global-, regional-, and country-level economic impacts of dental diseases in 2015. J. Dent. Res. 2018;97:501–507. doi: 10.1177/0022034517750572. [DOI] [PubMed] [Google Scholar]
- 63.Hosseinpoor A.R., Itani L., Petersen P.E. Socio-economic inequality in oral healthcare coverage: results from the world health survey. J. Dent. Res. 2012;91:275–281. doi: 10.1177/0022034511432341. [DOI] [PubMed] [Google Scholar]
- 64.Hong D.G.K., Oh J. Recent advances in dental implants. Maxillofac. Plast. Reconstr. Surg. 2017;39 doi: 10.1186/s40902-017-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global burden of severe tooth loss: a systematic review and meta-analysis. J. Dent. Res. 2014;93:20S–28S. doi: 10.1177/0022034514537828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kassebaum N.J., Smith A.G.C., Bernabé E., Fleming T.D., Reynolds A.E., Vos T., Murray C.J.L., Marcenes W. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990-2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017;96:380–387. doi: 10.1177/0022034517693566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miettinen M., Millar B.J. A review of the success and failure characteristics of resin-bonded bridges. Br. Dent. J. 2013;215 doi: 10.1038/sj.bdj.2013.686. [DOI] [PubMed] [Google Scholar]
- 68.AlHelal A., AlRumaih H.S., Kattadiyil M.T., Baba N.Z., Goodacre C.J. Comparison of retention between maxillary milled and conventional denture bases: a clinical study. J. Prosthet. Dent. 2017;117:233–238. doi: 10.1016/j.prosdent.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 69.Rasouli R., Barhoum A., Uludag H. A review of nanostructured surfaces and materials for dental implants: surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018;6:1312–1338. doi: 10.1039/c8bm00021b. [DOI] [PubMed] [Google Scholar]
- 70.Jiang X., Yao Y., Tang W., Han D., Zhang L., Zhao K., Wang S., Meng Y. Design of dental implants at materials level: an overview. J. Biomed. Mater. Res. 2020:1–28. doi: 10.1002/jbm.a.36931. [DOI] [PubMed] [Google Scholar]
- 71.Proksch S., Galler K.M. Scaffold materials and dental stem cells in dental tissue regeneration. Curr. Oral Heal. Reports. 2018;5:304–316. doi: 10.1007/s40496-018-0197-8. [DOI] [Google Scholar]
- 72.Ahmed G.M., Abouauf E.A., Abubakr N., Dörfer C.E., El-Sayed K.F. Tissue engineering approaches for enamel, dentin, and pulp regeneration: an update. Stem Cell. Int. 2020;2020 doi: 10.1155/2020/5734539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volponi A.A., Pang Y., Sharpe P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20:715–722. doi: 10.1016/j.tcb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Angelova Volponi A., Zaugg L.K., Neves V., Liu Y., Sharpe P.T. Tooth repair and regeneration. Curr. Oral Heal. Reports. 2018;5:295–303. doi: 10.1007/s40496-018-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yelick P.C., Sharpe P.T. Tooth bioengineering and regenerative dentistry. J. Dent. Res. 2019;98:1173–1182. doi: 10.1177/0022034519861903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pandya M., Diekwisch T.G.H. Enamel biomimetics—fiction or future of dentistry. Int. J. Oral Sci. 2019;11:1–9. doi: 10.1038/s41368-018-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H., Xiao Z., Yang J., Lu D., Kishen A., Li Y., Chen Z., Que K., Zhang Q., Deng X., Yang X., Cai Q., Chen N., Cong C., Guan B., Li T., Zhang X. Oriented and ordered biomimetic remineralization of the surface of demineralized dental enamel using HAP@ACP nanoparticles guided by Glycine. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep40701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prajapati S., Ruan Q., Mukherjee K., Nutt S., Moradian-Oldak J. The presence of MMP-20 reinforces biomimetic enamel regrowth. J. Dent. Res. 2018;97:84–90. doi: 10.1177/0022034517728504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elsharkawy S., Al-Jawad M., Pantano M.F., Tejeda-Montes E., Mehta K., Jamal H., Agarwal S., Shuturminska K., Rice A., Tarakina N.V., Wilson R.M., Bushby A.J., Alonso M., Rodriguez-Cabello J.C., Barbieri E., Del Río Hernández A., Stevens M.M., Pugno N.M., Anderson P., Mata A. Protein disorder-order interplay to guide the growth of hierarchical mineralized structures. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-04319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kind L., Stevanovic S., Wuttig S., Wimberger S., Hofer J., Müller B., Pieles U. Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 2017;96:790–797. doi: 10.1177/0022034517698419. [DOI] [PubMed] [Google Scholar]
- 81.Hashemi-Beni B., Khoroushi M., Foroughi M.R., Karbasi S., Khademi A.A. Tissue engineering: dentin – pulp complex regeneration approaches (A review) Tissue Cell. 2017;49:552–564. doi: 10.1016/j.tice.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Medina-Fernandez I., Celiz A.D. Acellular biomaterial strategies for endodontic regeneration. Biomater. Sci. 2019;7:506–509. doi: 10.1039/c8bm01296b. [DOI] [PubMed] [Google Scholar]
- 83.Gong T., Heng B.C., Lo E.C.M., Zhang C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/9204574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu T., Volponi A.A., Babb R., An Z., Sharpe P.T. first ed. Elsevier Inc.; 2015. Stem Cells in Tooth Development, Growth, Repair, and Regeneration. [DOI] [PubMed] [Google Scholar]
- 85.Jazayeri H.E., Lee S.M., Kuhn L., Fahimipour F., Tahriri M., Tayebi L. Polymeric scaffolds for dental pulp tissue engineering: a review. Dent. Mater. 2020;36:e47–e58. doi: 10.1016/j.dental.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 86.Han J., Kim D.S., Jang H., Kim H.R., Kang H.W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019;10 doi: 10.1177/2041731419845849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song J.S., Takimoto K., Jeon M., Vadakekalam J., Ruparel N.B., Diogenes A. Decellularized human dental pulp as a scaffold for regenerative endodontics. J. Dent. Res. 2017;96:640–646. doi: 10.1177/0022034517693606. [DOI] [PubMed] [Google Scholar]
- 88.Gangolli R.A., Devlin S.M., Gerstenhaber J.A., Lelkes P.I., Yang M. A bilayered poly (lactic-Co-glycolic acid) scaffold provides differential cues for the differentiation of dental pulp stem cells. Tissue Eng. 2019;25:224–233. doi: 10.1089/ten.tea.2018.0041. [DOI] [PubMed] [Google Scholar]
- 89.Zou H., Wang G., Song F., Shi X. Investigation of human dental pulp cells on a potential injectable poly(lactic-co-glycolic acid) microsphere scaffold. J. Endod. 2017;43:745–750. doi: 10.1016/j.joen.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 90.Eramo S., Natali A., Pinna R., Milia E. Dental pulp regeneration via cell homing. Int. Endod. J. 2018;51:405–419. doi: 10.1111/iej.12868. [DOI] [PubMed] [Google Scholar]
- 91.Ruangsawasdi N., Zehnder M., Patcas R., Ghayor C., Siegenthaler B., Gjoksi B., Weber F.E. Effects of stem cell factor on cell homing during functional pulp regeneration in human immature teeth. Tissue Eng. 2017;23:115–123. doi: 10.1089/ten.tea.2016.0227. [DOI] [PubMed] [Google Scholar]
- 92.Pan S., Dangaria S., Gopinathan G., Yan X., Lu X., Kolokythas A., Niu Y., Luan X. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling - potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev. Reports. 2013;9:655–667. doi: 10.1007/s12015-013-9442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neves V.C.M., Babb R., Chandrasekaran D., Sharpe P.T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci. Rep. 2017;7:1–7. doi: 10.1038/srep39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oshima M., Mizuno M., Imamura A., Ogawa M., Yasukawa M., Yamazaki H., Morita R., Ikeda E., Nakao K., Takano-Yamamoto T., Kasugai S., Saito M., Tsuji T. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PloS One. 2011;6 doi: 10.1371/journal.pone.0021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 96.Tucker A., Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat. Rev. Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 97.Svandova E., Peterkova R., Matalova E., Lesot H. Formation and developmental specification of the odontogenic and osteogenic mesenchymes. Front. Cell Dev. Biol. 2020;8:1–16. doi: 10.3389/fcell.2020.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balic A., Thesleff I. Tissue interactions regulating tooth development and renewal. Curr. Top. Dev. Biol. 2015;115:157–186. doi: 10.1016/bs.ctdb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Ishida K., Murofushi M., Nakao K., Morita R., Ogawa M., Tsuji T. The regulation of tooth morphogenesis is associated with epithelial cell proliferation and the expression of Sonic hedgehog through epithelial-mesenchymal interactions. Biochem. Biophys. Res. Commun. 2011;405:455–461. doi: 10.1016/j.bbrc.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 100.Ikeda E., Morita R., Nakao K., Ishida K., Nakamura T., Takano-Yamamoto T., Ogawa M., Mizuno M., Kasugai S., Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ono M., Oshima M., Ogawa M., Sonoyama W., Satoshi Hara E., Oida Y., Shinkawa S., Nakajima R., Mine A., Hayano S., Fukumoto S., Kasugai S., Yamaguchi A., Tsuji T., Kuboki T. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017;7:1–11. doi: 10.1038/srep44522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakajima K., Oshima M., Tsuji T. Whole tooth regenerative therapy using a bioengineered tooth germ. Curr. Oral Heal. Reports. 2014;1:43–49. doi: 10.1007/s40496-013-0004-5. [DOI] [Google Scholar]
- 103.Oshima M., Tsuji T. Functional tooth regenerative therapy: tooth tissue regeneration and whole-tooth replacement. Odontology. 2014;102:123–136. doi: 10.1007/s10266-014-0168-z. [DOI] [PubMed] [Google Scholar]
- 104.Smith E.E., Yelick P.C. Progress in bioengineered whole tooth research: from bench to dental patient chair. Curr. Oral Heal. Reports. 2016;3:302–308. doi: 10.1007/s40496-016-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto H., Kim E.J., Cho S.W., Jung H.S. Analysis of tooth formation by reaggregated dental mesenchyme from mouse embryo. J. Electron Microsc. (Tokyo) 2003;52:559–566. doi: 10.1093/jmicro/52.6.559. [DOI] [PubMed] [Google Scholar]
- 106.Song Y., Zhang Z., Yu X., Yan M., Zhang X., Gu S., Stuart T., Liu C., Reiser J., Zhang Y., Chen Y. Application of lentivirus-mediated RNAi in studying gene function in mammalian tooth development. Dev. Dynam. 2006;235:1347–1357. doi: 10.1002/dvdy.20706. [DOI] [PubMed] [Google Scholar]
- 107.Ohazama A., Modino S.A., Miletich I., Sharpe P.T. Stem-cell-based tissue engineering of murine teeth. J. Dent. Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 108.Abukawa H., Zhang W., Young C.S., Asrican R., Vacanti J.P., Kaban L.B., Troulis M.J., Yelick P.C. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J. Oral Maxillofac. Surg. 2009;67:335–347. doi: 10.1016/j.joms.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Honda M.J., Tsuchiya S., Sumita Y., Sagara H., Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007;28:680–689. doi: 10.1016/j.biomaterials.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 110.Dualibi M.T., Dualibi S.E., Young C.S., Bartlett J.D., Vacanti J.P., Yelick P.C. Bioengineered teeth from and seeding of rat tooth. J. Dent. Res. 2004;83:3–8. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 111.Zhang W., Vazquez B., Oreadi D., Yelick P.C. Decellularized tooth bud scaffolds for tooth regeneration. J. Dent. Res. 2017;96:516–523. doi: 10.1177/0022034516689082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Manen E.H.C., Zhang W., Walboomers X.F., Vazquez B., Yang F., Ji W., Yu N., Spear D.J., Jansen J.A., Yelick P.C. The influence of electrospun fibre scaffold orientation and nano-hydroxyapatite content on the development of tooth bud stem cells in vitro. Odontology. 2014;102:14–21. doi: 10.1007/s10266-012-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Young C.S., Terada S., Vacanti J.P., Honda M., Bartlett J.D., Yelick P.C. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J. Dent. Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 114.Nakao K., Morita R., Saji Y., Ishida K., Tomita Y., Ogawa M., Saitoh M., Tomooka Y., Tsuji T. The development of a bioengineered organ germ method. Nat. Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 115.Notani T., Tabata M.J., Iseki H., Baba O., Takano Y. Introduction of a three-dimensional and layered (TDL) culture, a novel primary co-culture method for ameloblasts and pulp-derived cells. Arch. Histol. Cytol. 2009;72:187–198. doi: 10.1679/aohc.72.187. [DOI] [PubMed] [Google Scholar]
- 116.Yang L., Angelova Volponi A., Pang Y., Sharpe P.T. Mesenchymal cell community effect in whole tooth bioengineering. J. Dent. Res. 2017;96:186–191. doi: 10.1177/0022034516682001. [DOI] [PubMed] [Google Scholar]
- 117.Wen Y., Wang F., Zhang W., Li Y., Yu M., Nan X., Chen L., Yue W., Xu X., Pei X. Application of induced pluripotent stem cells in generation of a tissue-engineered tooth-like structure. Tissue Eng. 2012;18:1677–1685. doi: 10.1089/ten.tea.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Angelova Volponi A., Kawasaki M., Sharpe P.T. Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J. Dent. Res. 2013;92:329–334. doi: 10.1177/0022034513481041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ravindran S., Song Y., George A. Development of three-dimensional biomimetic scaffold to study epithelial-mesenchymal interactions. Tissue Eng. 2010;16:327–342. doi: 10.1089/ten.tea.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith E.E., Yelick P.C., Khademhosseini A. Optimization of a biomimetic model for tooth regeneration. Proc. IEEE Annu. Northeast Bioeng. Conf. NEBEC. 2014:3–4. doi: 10.1109/NEBEC.2014.6972943. [DOI] [Google Scholar]
- 121.Xiao L., Tsutsui T. Three-dimensional epithelial and mesenchymal cell co-cultures form early tooth epithelium invagination-like structures: expression patterns of relevant molecules. J. Cell. Biochem. 2012;113:1875–1885. doi: 10.1002/jcb.24056. [DOI] [PubMed] [Google Scholar]
- 122.Zhang W., Ahluwalia I.P., Yelick P.C. Three dimensional dental epithelial-mesenchymal constructs of predetermined size and shape for tooth regeneration. Biomaterials. 2010;31:7995–8003. doi: 10.1016/j.biomaterials.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Capella-Monsonís H., Tilbury M.A., Wall J.G., Zeugolis D.I. Porcine mesothelium matrix as a biomaterial for wound healing applications. Mater. Today Bio. 2020;7:100057. doi: 10.1016/j.mtbio.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Contessi Negrini N., Lipreri M.V., Tanzi M.C., Farè S. In vitro cell delivery by gelatin microspheres prepared in water-in-oil emulsion. J. Mater. Sci. Mater. Med. 2020;31 doi: 10.1007/s10856-020-6363-2. [DOI] [PubMed] [Google Scholar]
- 125.Naka Y., Kitano S., Irie S., Matsusaki M. Wholly vascularized millimeter-sized engineered tissues by cell-sized microscaffolds. Mater. Today Bio. 2020;6:100054. doi: 10.1016/j.mtbio.2020.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ogasawara T., Okano S., Ichimura H., Kadota S., Tanaka Y., Minami I., Uesugi M., Wada Y., Saito N., Okada K., Kuwahara K., Shiba Y. Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-09217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aisenbrey E.A., Murphy W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020 doi: 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tytgat L., Kollert M.R., Van Damme L., Thienpont H., Ottevaere H., Duda G.N., Geissler S., Dubruel P., Van Vlierberghe S., Qazi T.H. Evaluation of 3D printed gelatin-based scaffolds with varying pore size for MSC-based adipose tissue engineering. Macromol. Biosci. 2020;20:1–6. doi: 10.1002/mabi.201900364. [DOI] [PubMed] [Google Scholar]
- 129.Contessi Negrini N., Tarsini P., Tanzi M.C., Farè S. Chemically crosslinked gelatin hydrogels as scaffolding materials for adipose tissue engineering. J. Appl. Polym. Sci. 2019;47104:1–12. doi: 10.1002/app.47104. [DOI] [Google Scholar]
- 130.Gorgieva S., Kokol V. Biomater. Appl. Nanomedicine. 2011. Collagen- vs. gelatine-based biomaterials and their biocompatibility: review and perspectives; pp. 17–52. [DOI] [Google Scholar]
- 131.Buffoli B., Rinaldi F., Labanca M., Sorbellini E., Trink A., Guanziroli E., Rezzani R., Rodella L.F. The human hair: from anatomy to physiology. Int. J. Dermatol. 2014;53:331–341. doi: 10.1111/ijd.12362. [DOI] [PubMed] [Google Scholar]
- 132.Schneider M.R., Schmidt-Ullrich R., Paus R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 133.Wang E.C.E., Higgins C.A. Immune cell regulation of the hair cycle. Exp. Dermatol. 2020;29:322–333. doi: 10.1111/exd.14070. [DOI] [PubMed] [Google Scholar]
- 134.Sennett R., Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 2012;23:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang C.C., Cotsarelis G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rompolas P., Greco V. Stem cell dynamics in the hair follicle niche. Semin. Cell Dev. Biol. 2014;25–26:34–42. doi: 10.1016/j.semcdb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wei G., Bhushan B., Torgerson P.M. Nanomechanical characterization of human hair using nanoindentation and SEM. Ultramicroscopy. 2005;105:248–266. doi: 10.1016/j.ultramic.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 138.Sayahi E., Harizi T., Msahli S., Sakli F. Physical and mechanical properties of Tunisian women hair. Int. J. Cosmet. Sci. 2016;38:470–475. doi: 10.1111/ics.12313. [DOI] [PubMed] [Google Scholar]
- 139.Kiani M.T., Higgins C.A., Almquist B.D. The hair follicle: an underutilized source of cells and materials for regenerative medicine. ACS Biomater. Sci. Eng. 2018;4:1193–1207. doi: 10.1021/acsbiomaterials.7b00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chueh S.C., Lin S.J., Chen C.C., Lei M., Wang L.M., Widelitz R., Hughes M.W., Jiang T.X., Chuong C.M. Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expet Opin. Biol. Ther. 2013;13:377–391. doi: 10.1517/14712598.2013.739601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin R.L., Garibyan L., Kimball A.B., Drake L.A. Systemic causes of hair loss. Ann. Med. 2016;48:393–402. doi: 10.1080/07853890.2016.1180426. [DOI] [PubMed] [Google Scholar]
- 142.Watanabe T., Yagata H., Saito M., Okada H., Yajima T., Tamai N., Yoshida Y., Takayama T., Imai H., Nozawa K., Sangai T., Yoshimura A., Hasegawa Y., Yamaguchi T., Shimozuma K., Ohashi Y. A multicenter survey of temporal changes in chemotherapy-induced hair loss in breast cancer patients. PloS One. 2019;14:1–12. doi: 10.1371/journal.pone.0208118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang L., Wang W.H., Jin J.Y., Degan S., Zhang G.Q., Erdmann D., Hall R.P., Zhang J.Y. Induction of hair follicle neogenesis with cultured mouse dermal papilla cells in de novo regenerated skin tissues. J. Tissue Eng. Regen. Med. 2019;13:1641–1650. doi: 10.1002/term.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pantelireis N., Higgins C.A. A bald statement — current approaches to manipulate miniaturisation focus only on promoting hair growth. Exp. Dermatol. 2018;27:959–965. doi: 10.1111/exd.13690. [DOI] [PubMed] [Google Scholar]
- 145.Vañó-Galván S., Camacho F. New treatments for hair loss. Actas Dermosifiliogr. 2017;108:221–228. doi: 10.1016/j.adengl.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 146.Castro A.R., Logarinho E. Tissue engineering strategies for human hair follicle regeneration: how far from a hairy goal? Stem Cells Transl. Med. 2020;9:342–350. doi: 10.1002/sctm.19-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rishikaysh P., Dev K., Diaz D., Shaikh Qureshi W.M., Filip S., Mokry J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014;15:1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stenn K., Parimoo S., Zheng Y., Barrows T., Boucher M., Washenik K. Bioengineering the hair follicle. Organogenesis. 2007;3:6–13. doi: 10.4161/org.3.1.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang X., Tredget E.E., Wu Y. Dynamic signals for hair follicle development and regeneration. Stem Cell. Dev. 2012;21:7–18. doi: 10.1089/scd.2011.0230. [DOI] [PubMed] [Google Scholar]
- 150.Millar S.E. Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 151.Heitman N., Sennett R., Mok K.W., Saxena N., Srivastava D., Martino P., Grisanti L., Wang Z., Ma’ayan A., Rompolas P., Rendl M. Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science. 2020;367:161–166. doi: 10.1126/science.aax9131. (80-.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Asakawa K., Toyoshima K.E., Ishibashi N., Tobe H., Iwadate A., Kanayama T., Hasegawa T., Nakao K., Toki H., Noguchi S., Ogawa M., Sato A., Tsuji T. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci. Rep. 2012;2 doi: 10.1038/srep00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takagi R., Ishimaru J., Sugawara A., Toyoshima K.E., Ishida K., Ogawa M., Sakakibara K., Asakawa K., Kashiwakura A., Oshima M., Minamide R., Sato A., Yoshitake T., Takeda A., Egusa H., Tsuji T. Bioengineering a 3D integumentary organ system from iPS cells using an in vivo transplantation model. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1500887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Toyoshima K.E., Asakawa K., Ishibashi N., Toki H., Ogawa M., Hasegawa T., Irié T., Tachikawa T., Sato A., Takeda A., Tsuji T. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat. Commun. 2012;3 doi: 10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nilforoushzadeh M., Jameh E.R., Jaffary F., Abolhasani E., Keshtmand G., Zarkob H., Mohammadi P., Aghdami N. Hair follicle generation by injections of adult human follicular epithelial and dermal papilla cells into nude mice. Cell J. 2017;19:259–268. doi: 10.22074/cellj.2016.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Higgins C.A., Chen J.C., Cerise J.E., Jahoda C.A.B., Christiano A.M. Microenvironmental reprogramming by threedimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. U.S.A. 2013;110:19679–19688. doi: 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yen C.M., Chan C.C., Lin Sung Jan S.J. High-throughput reconstitution of epithelial-mesenchymal interaction in folliculoid microtissues by biomaterial-facilitated self-assembly of dissociated heterotypic adult cells. Biomaterials. 2010;31:4341–4352. doi: 10.1016/j.biomaterials.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 158.Chermnykh E.S., Vorotelyak E.A., Gnedeva K.Y., Moldaver M.V., Yegorov Y.E., Vasiliev A.V., Terskikh V.V. Dermal papilla cells induce keratinocyte tubulogenesis in culture. Histochem. Cell Biol. 2010;133:567–576. doi: 10.1007/s00418-010-0691-0. [DOI] [PubMed] [Google Scholar]
- 159.Havlickova B., Bíró T., Mescalchin A., Arenberger P., Paus R. Towards optimization of an organotypic assay system that imitates human hair follicle-like epithelial-mesenchymal interactions. Br. J. Dermatol. 2004;151:753–765. doi: 10.1111/j.1365-2133.2004.06184.x. [DOI] [PubMed] [Google Scholar]
- 160.Havlickova B., Bíró T., Mescalchin A., Tschirschmann M., Mollenkopf H., Bettermann A., Pertile P., Lauster R., Bodó E., Paus R. A human folliculoid microsphere assay for exploring epithelial- mesenchymal interactions in the human hair follicle. J. Invest. Dermatol. 2009;129:972–983. doi: 10.1038/jid.2008.315. [DOI] [PubMed] [Google Scholar]
- 161.Wang X., Wang X., Liu J., Cai T., Guo L., Wang S., Wang J., Cao Y., Ge J., Jiang Y., Tredget E.E., Cao M., Wu Y. Hair follicle and sebaceous gland de novo regeneration with cultured epidermal stem cells and skin-derived precursors. Tissue Eng. Regen. Med. 2016;5:1695–1706. doi: 10.5966/sctm.2015-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo L., Wang X., Yuan J., Zhu M., Fu X., Xu R.H., Wu C., Wu Y. TSA restores hair follicle-inductive capacity of skin-derived precursors. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-39394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kalabusheva E., Terskikh V., Vorotelyak E. Hair germ model in vitro via human postnatal keratinocyte-dermal papilla interactions: impact of hyaluronic acid. Stem Cell. Int. 2017;2017 doi: 10.1155/2017/9271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gupta A.C., Chawla S., Hegde A., Singh D., Bandyopadhyay B., Lakshmanan C.C., Kalsi G., Ghosh S. Establishment of an in vitro organoid model of dermal papilla of human hair follicle. J. Cell. Physiol. 2018;233:9015–9030. doi: 10.1002/jcp.26853. [DOI] [PubMed] [Google Scholar]
- 165.Xiao S.E., Miao Y., Wang J., Jiang W., Fan Z.X., Liu X.M., Hu Z.Q. As a carrier-Transporter for hair follicle reconstitution, platelet-rich plasma promotes proliferation and induction of mouse dermal papilla cells. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-01105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lim T.C., Leong M.F., Lu H., Du C., Gao S., Wan A.C.A., Ying J.Y. Follicular dermal papilla structures by organization of epithelial and mesenchymal cells in interfacial polyelectrolyte complex fibers. Biomaterials. 2013;34:7064–7072. doi: 10.1016/j.biomaterials.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 167.Kageyama T., Yoshimura C., Myasnikova D., Kataoka K., Nittami T., Maruo S., Fukuda J. Spontaneous hair follicle germ (HFG) formation in vitro, enabling the large-scale production of HFGs for regenerative medicine. Biomaterials. 2018;154:291–300. doi: 10.1016/j.biomaterials.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 168.Kageyama T., Yan L., Shimizu A., Maruo S., Fukuda J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials. 2019;212:55–63. doi: 10.1016/j.biomaterials.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 169.Abaci H.E., Coffman A., Doucet Y., Chen J., Jacków J., Wang E., Guo Z., Shin J.U., Jahoda C.A., Christiano A.M. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-07579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gentile P., Garcovich S., Bielli A., Scioli M.G., Orlandi A., Cervelli V. The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial, stem cells transl. Med. 2015;4:1317–1323. doi: 10.5966/sctm.2015-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ohyama M., Zheng Y., Paus R., Stenn K.S. The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp. Dermatol. 2010;19:89–99. doi: 10.1111/j.1600-0625.2009.00935.x. [DOI] [PubMed] [Google Scholar]
- 172.Yang T.L., Hsiao Y.C., Lin S.J., Lee H.W., Lou P.J., Ko J.Y., Young T.H. Biomaterial mediated epithelial-mesenchymal interaction of salivary tissue under serum free condition. Biomaterials. 2010;31:288–295. doi: 10.1016/j.biomaterials.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 173.Ogawa M., Oshima M., Imamura A., Sekine Y., Ishida K., Yamashita K., Nakajima K., Hirayama M., Tachikawa T., Tsuji T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013;4:1–10. doi: 10.1038/ncomms3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hirayama M., Ogawa M., Oshima M., Sekine Y., Ishida K., Yamashita K., Ikeda K., Shimmura S., Kawakita T., Tsubota K., Tsuji T. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013;4 doi: 10.1038/ncomms3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Massie I., Spaniol K., Barbian A., Geerling G., Metzger M., Schrader S. Development of lacrimal gland spheroids for lacrimal gland tissue regeneration. J. Tissue Eng. Regen. Med. 2018;12:e2001–e2009. doi: 10.1002/term.2631. [DOI] [PubMed] [Google Scholar]
- 176.Holmberg K.V., Hoffman M.P. Anatomy, biogenesis and regeneration of salivary glands. Monogr. Oral Sci. 2014;24:1–13. doi: 10.1159/000358776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Paula F., Teshima T.H.N., Hsieh R., Souza M.M., Nico M.M.S., Lourenco S.V. Overview of human salivary glands: highlights of morphology and developing processes. Anat. Rec. 2017;300:1180–1188. doi: 10.1002/ar.23569. [DOI] [PubMed] [Google Scholar]
- 178.Pedersen A.M.L., Sørensen C.E., Proctor G.B., Carpenter G.H., Ekström J. Salivary secretion in health and disease. J. Oral Rehabil. 2018;45:730–746. doi: 10.1111/joor.12664. [DOI] [PubMed] [Google Scholar]
- 179.Ogle O.E. Salivary gland diseases. Dent. Clin. 2020;64:87–104. doi: 10.1016/j.cden.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 180.feng Hsu J.C., Yamada K.M. Salivary gland branching morphogenesis–recent progress and future opportunities. Int. J. Oral Sci. 2010;2:117–126. doi: 10.4248/IJOS10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cassolato S.F., Turnbull R.S. Xerostomia: clinical aspects and treatment. Gerodontology. 2003;20:64–77. doi: 10.1111/j.1741-2358.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 182.Ozdemir T., Fowler E.W., Hao Y., Ravikrishnan A., Harrington D.A., Witt R.L., Farach-Carson M.C., Pradhan-Bhatt S., Jia X. Biomaterials-based strategies for salivary gland tissue regeneration. Biomater. Sci. 2016;4:592–604. doi: 10.1039/c5bm00358j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shin K., Koo K.H., Jeong J., Park S.J., Choi D.J., Ko Y.G., Kwon H. Three-dimensional culture of salivary gland stem cell in orthotropic decellularized extracellular matrix hydrogels. Tissue Eng. 2019;25:1396–1403. doi: 10.1089/ten.tea.2018.0308. [DOI] [PubMed] [Google Scholar]
- 184.Shubin A.D., Felong T.J., Graunke D., Ovitt C.E., Benoit D.S.W. Development of poly(ethylene glycol) hydrogels for salivary gland tissue engineering applications. Tissue Eng. 2015;21:1733–1751. doi: 10.1089/ten.tea.2014.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nam K., Wang C.S., Maruyama C.L.M., Lei P., Andreadis S.T., Baker O.J. L1 peptide-conjugated fibrin hydrogels promote salivary gland regeneration. J. Dent. Res. 2017;96:798–806. doi: 10.1177/0022034517695496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tanaka J., Ogawa M., Hojo H., Kawashima Y., Mabuchi Y., Hata K., Nakamura S., Yasuhara R., Takamatsu K., Irié T., Fukada T., Sakai T., Inoue T., Nishimura R., Ohara O., Saito I., Ohba S., Tsuji T., Mishima K. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-06469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Garg A., Zhang X. Lacrimal gland development: from signaling interactions to regenerative medicine. Dev. Dynam. 2017;246:970–980. doi: 10.1002/dvdy.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Conrady C.D., Joos Z.P., Patel B.C.K. Review: the lacrimal gland and its role in dry eye. J. Ophthalmol. 2016;2016 doi: 10.1155/2016/7542929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ding J., Sullivan D.A. Aging and dry eye disease. Exp. Gerontol. 2012;47:483–490. doi: 10.1016/j.exger.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dietrich J., Schrader S. Towards lacrimal gland regeneration: current concepts and experimental approaches. Curr. Eye Res. 2020;45:230–240. doi: 10.1080/02713683.2019.1637438. [DOI] [PubMed] [Google Scholar]
- 191.Lin H., Sun G., He H., Botsford B., Li M., Elisseeff J.H., Yiu S.C. Three-dimensional culture of functional adult rabbit lacrimal gland epithelial cells on decellularized scaffold. Tissue Eng. 2016;22:65–74. doi: 10.1089/ten.tea.2015.0286. [DOI] [PubMed] [Google Scholar]
- 192.Spaniol K., Metzger M., Roth M., Greve B., Mertsch S., Geerling G., Schrader S. Engineering of a secretory active three-dimensional lacrimal gland construct on the basis of decellularized lacrimal gland tissue. Tissue Eng. 2015;21:2605–2617. doi: 10.1089/ten.tea.2014.0694. [DOI] [PubMed] [Google Scholar]
- 193.Tiwari S., Ali M.J., Vemuganti G.K. Human lacrimal gland regeneration Perspectives and review of literature. Saudi J. Ophthalmol. 2014;28:12–18. doi: 10.1016/j.sjopt.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu C.Y., Hirayama M., Ali M., Shah D., Aakalu V.K. Strategies for regenerating the lacrimal gland. Curr. Ophthalmol. Rep. 2017;5:193–198. doi: 10.1007/s40135-017-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hirayama M., Tsubota K., Tsuji T. Bioengineered lacrimal gland organ regeneration in vivo. J. Funct. Biomater. 2015;6:634–649. doi: 10.3390/jfb6030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Makarenkova H.P., Dartt D.A. Myoepithelial cells: their origin and function in lacrimal gland morphogenesis, homeostasis, and repair. Curr. Mol. Biol. Reports. 2015;1:115–123. doi: 10.1007/s40610-015-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Keller L., Kuchler-Bopp S., Mendoza S.A., Poliard A., Lesot H. Tooth engineering: searching for dental mesenchymal cells sources. Front. Physiol. MAR. 2011:1–10. doi: 10.3389/fphys.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reisman M., Adams K.T. Stem cell therapy: a look at current research, regulations, and remaining hurdles. P T. 2014;39:846–857. [PMC free article] [PubMed] [Google Scholar]
- 199.Lumsden A.G.S. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103:155–169. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- 200.Mina M., Kollar E.J. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 1987;32:123–127. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- 201.Tummers M., Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J. Exp. Zool. B Mol. Dev. Evol. 2009;312:309–319. doi: 10.1002/jez.b.21280. [DOI] [PubMed] [Google Scholar]
- 202.Nait Lechguer A., Kuchler-Bopp S., Hu B., Haïkel Y., Lesot H. Vascularization of engineered teeth. J. Dent. Res. 2008;87:1138–1143. doi: 10.1177/154405910808701216. [DOI] [PubMed] [Google Scholar]
- 203.Kökten T., Bécavin T., Keller L., Weickert J.L., Kuchler-Bopp S., Lesot H. Immunomodulation stimulates the innervation of engineered tooth organ. PloS One. 2014;9:1–12. doi: 10.1371/journal.pone.0086011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang Y.D., Chen Z., Song Y.Q., Liu C., Chen Y.P. Making a tooth: growth factors, transcription factors, and stem cells. Cell Res. 2005;15:301–316. doi: 10.1038/sj.cr.7290299. [DOI] [PubMed] [Google Scholar]
- 205.Oshima M., Tsuji T. Whole tooth regeneration as a future dental treatment. In: Bertassoni L.E., Coelho P.G., editors. Eng. Miner. Load Bear. Tissues. Springer International Publishing; Cham: 2015. pp. 255–269. [DOI] [Google Scholar]
- 206.Kaukua N., Shahidi M.K., Konstantinidou C., Dyachuk V., Kaucka M., Furlan A., An Z., Wang L., Hultman I., Ährlund-Richter L., Blom H., Brismar H., Lopes N.A., Pachnis V., Suter U., Clevers H., Thesleff I., Sharpe P., Ernfors P., Fried K., Adameyko I. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–554. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- 207.Feng J., Mantesso A., De Bari C., Nishiyama A., Sharp P.T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lawrence B.J., Madihally S.V. Cell colonization in degradable 3D porous matrices. Cell Adhes. Migrat. 2008;2:9–16. doi: 10.4161/cam.2.1.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu J., Li P., Dong C., Jiang H., Xue B., Gao X., Qin M., Wang W., Chen B., Cao Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-02917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Patel A.K., Tibbitt M.W., Celiz A.D., Davies M.C., Langer R., Denning C., Alexander M.R., Anderson D.G. High throughput screening for discovery of materials that control stem cell fate. Curr. Opin. Solid State Mater. Sci. 2016;20:202–211. doi: 10.1016/j.cossms.2016.02.002. [DOI] [Google Scholar]
- 211.Contessi Negrini N., Bonnetier M., Giatsidis G., Orgill D.P., Farè S., Marelli B. Tissue-mimicking gelatin scaffolds by alginate sacrificial templates for adipose tissue engineering. Acta Biomater. 2019;87:61–75. doi: 10.1016/j.actbio.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 212.Fleischer S., Tavakol D.N., Vunjak-Novakovic G. From arteries to capillaries: approaches to engineering human vasculature. Adv. Funct. Mater. 2020;1910811:1–23. doi: 10.1002/adfm.201910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kuchler-Bopp S., Larrea A., Petry L., Idoux-Gillet Y., Sebastian V., Ferrandon A., Schwinté P., Arruebo M., Benkirane-Jessel N. Promoting bioengineered tooth innervation using nanostructured and hybrid scaffolds. Acta Biomater. 2017;50:493–501. doi: 10.1016/j.actbio.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 214.Das S., Gordián-Vélez W.J., Ledebur H.C., Mourkioti F., Rompolas P., Chen H.I., Serruya M.D., Cullen D.K. Innervation: the missing link for biofabricated tissues and organs. Npj Regen. Med. 2020;5 doi: 10.1038/s41536-020-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Strub M., Keller L., Idoux-Gillet Y., Lesot H., Clauss F., Benkirane-Jessel N., Kuchler-Bopp S. Bone marrow stromal cells promote innervation of bioengineered teeth. J. Dent. Res. 2018;97:1152–1159. doi: 10.1177/0022034518779077. [DOI] [PubMed] [Google Scholar]
- 216.Milazzo M., Contessi Negrini N., Scialla S., Marelli B., Farè S., Danti S., Buehler M.J. Additive manufacturing approaches for hydroxyapatite-reinforced composites. Adv. Funct. Mater. 2019:1–26. doi: 10.1002/adfm.201903055. [DOI] [Google Scholar]
- 217.Bakry A. Synergistic effects of surface aminolysis and hydrolysis on improving fibroblast cell colonization within poly(L-lactide) scaffolds. J. Appl. Polym. Sci. 2020;138 doi: 10.1002/app.49643. [DOI] [Google Scholar]