Key Points
Question
What is the systemic safety of intravitreal anti-vascular endothelial growth factor (anti-VEGF) drugs?
Findings
In a systematic review with meta-analysis of 74 randomized clinical trials of adult patients with retinal diseases who received intravitreal anti-VEGF drugs, anti-VEGF drugs were not associated with increased arterial or venous thromboembolic events. Small increases in total mortality were noted in patients with diabetes and nonocular hemorrhagic events were apparent in patients with age-related macular degeneration.
Meaning
The findings of this systematic review with meta-analysis suggest that intravitreal anti-VEGF drugs were not associated with an increase in major cardiovascular events.
Abstract
Importance
Systemic safety of intravitreal anti-vascular endothelial growth factor (anti-VEGF) is a matter of debate and regular updates are necessary.
Objective
To evaluate systemic adverse events (SAEs) associated with intravitreal anti-VEGF drugs compared with non–anti-VEGF treatments in patients with ocular diseases.
Data Sources
Electronic searches were conducted in MEDLINE, Embase, and Cochrane Central Register of Controlled Trials databases from inception to July 7, 2020.
Study Selection
Randomized clinical trials conducted in adults with retinal diseases who received intravitreal anti-VEGF drugs.
Data Extraction and Synthesis
Studies and treatment characteristics and outcome data were extracted and analyzed, and study quality was evaluated.
Main Outcomes and Measures
Main outcomes were major cardiovascular events (MACEs) and total mortality. Secondary outcomes included nonocular hemorrhage, components of MACEs, other cardiovascular outcomes, serious SAEs, and all SAEs.
Results
A total of 74 randomized clinical trials were analyzed: 32 trials (43%) included 14 190 patients with age-related macular degeneration (AMD), 24 (32%) included 5424 patients with diabetic retinopathy (diabetic macular edema or proliferative diabetic retinopathy), 17 trials (23%) included 3757 patients with retinal vein occlusion, and 1 trial (1%) included 122 patients with myopic choroidal neovascularization. Anti-VEGF drug administration did not increase MACEs compared with control agents (odds ratio [OR], 1.16; 95% CI, 0.85-1.58) or total mortality (OR, 1.27; 95% CI, 0.82-1.96). There was an interaction (subgroup difference, P = .04) in mortality risk depending on the underlying disease with an increase (OR, 1.80; 95% CI, 1.03-3.16; P = .04) in the risk of death in patients with diabetic retinopathy; however, no increase was observed in patients with AMD or retinal vein occlusion. Administration of anti-VEGF drugs increased the risk of nonocular hemorrhage (OR, 1.46; 95% CI, 1.01-2.10), mainly in patients with AMD.
Conclusions and Relevance
Intravitreal anti-VEGF was not associated with an increase in MACEs in the trials examined herein. Increased mortality in patients with diabetes and nonocular hemorrhages, especially in those with AMD, could represent a safety signal, but the evidence was not strong. However, continued surveillance of SAEs remains warranted.
This systematic review and meta-analysis examines the incidence of systemic adverse events in patients receiving intravitreal administration of anti-vascular endothelial growth factor drugs.
Introduction
Retinal diseases may lead to blindness and functional disability, and represent an important health issue. Age-related macular degeneration (AMD),1 diabetic macular edema or proliferative diabetic retinopathy (DME/PDR), and retinal vein occlusion related edema (RVO)2 are the main causes of blindness in developed countries. The decrease in visual acuity is related to macular edema due to overexpression of vascular endothelial growth factor (VEGF) stimulated by ischemic or hypoxic conditions.3,4 Intravitreal anti-VEGF monoclonal antibodies (bevacizumab and ranibizumab) or fusion proteins (aflibercept) have led to improvement of visual acuity compared with standard of care.5,6
Use of anti-VEGF drugs in oncology practice may increase the risk of serious cardiovascular, venous thromboembolic, hemorrhagic, or infectious systemic adverse events (SAEs).7,8,9,10 When administered intravitreally, even at low doses, a small systemic exposure was observed. The exposure was highest with bevacizumab,11 which was sometimes sufficient to inhibit plasma VEGF12 and could lead to SAEs.
Systematic reviews with meta-analyses have studied the risk of SAEs with intravitreal anti-VEGF drugs, mainly focusing on 1 ocular disease, which limits the power to detect increased risks.13,14,15 Previous findings suggested that anti-VEGF monoclonal antibodies compared with control agents did not increase SAEs, including major adverse cardiovascular events (MACEs), myocardial infarction, stroke, or cardiovascular mortality.16,17 Nevertheless, an increased risk of nonocular hemorrhages in patients with AMD treated with ranibizumab could not be ruled out. Since the publication of the above-mentioned reviews by Thulliez et al,16,17 several clinical trials have been published. Our objective was to update the risk estimation of SAEs, especially mortality and cardiovascular events, associated with intravitreal anti-VEGF drugs compared with non–anti-VEGF treatments in adults.
Methods
We conducted a systematic review and meta-analysis of randomized clinical trials according to Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P)18 and the PRISMA Harms19 recommendations (PRISMA harms). The study protocol is registered and accessible in PROSPERO (CRD42019129864).
Data Sources
We performed electronic searches in MEDLINE, Embase, and Cochrane Central Register of Controlled Trials from inception to March 7, 2019, with an update in MEDLINE and Cochrane Central Register of Controlled Trials to July 7, 2020, without language restrictions. References of eligible trials and meta-analyses were also searched to identify additional studies. The query combined Medical Subject Headings and free terms regarding drugs, administration, and type of study (eTable 1 in the Supplement). References were managed using EndNote (Clarivate Analytics). After removal of duplicate studies, 2 of us (N.N.-N., T.B.-A.) screened articles by title and abstract and assessed the full text of eligible studies for inclusion, with disagreements resolved through discussion.
Study Selection and Quality
We included parallel-group randomized clinical trials with a follow-up of at least 6 months that randomized adults treated with intravitreal anti-VEGF drugs (all regimens) compared with control agents (sham, no treatment, or non–anti-VEGF standard of care) or with anti-VEGF drugs. If results from one study were reported in several publications, data included in the meta-analysis were extracted from the publication that was considered the most appropriate to avoid using duplicate data. The longest follow-up was considered until 10% or more of the patients (arbitrary threshold) in the control group crossed over to the active group. We excluded studies that evaluated a single intravitreal injection without assessment for retreatment or in the context of ocular surgery, evaluated a combination of anti-VEGF drugs, included children, did not report any SAE, and were crossover studies and studies that randomized eyes, unless the authors reported adverse events data by participants.
Our main comparison was anti-VEGF agent vs control. Secondary comparisons were head-to-head, between-doses (low vs high dose for the same regimen), and between-regimen (as required, or treat-and-extend compared with a regular regimen) comparisons.
The Cochrane Risk of Bias version 2 tool20 was used to assess the quality of each included randomized clinical trial by 2 of us (N.N.-N., T.B.-A), with discrepancies resolved through discussion. The following domains adapted to the assessment of adverse events were considered: randomization process, deviations from the intended intervention, missing outcome, measurement of the outcome, and selective reporting.
Outcomes and Data Extraction
The primary outcomes were total mortality and MACEs using the Antiplatelet Trialists’ collaboration21 criteria, a composite of nonfatal myocardial infarction, nonfatal ischemic or hemorrhagic stroke, or death due to vascular or unknown causes. Secondary outcomes included components of MACEs (cardiovascular death, myocardial infarction, and stroke), nonocular hemorrhagic events, other cardiovascular outcomes (cardiac failure, venous thromboembolism, arterial hypertension, and proteinuria), serious SAEs and all SAEs.
One of us (N.N.-N.) extracted data from the original publication or from the ClinicalTrials.gov database into the RevMan, version 5.3 (Cochrane) file and an Excel 2018 (Microsoft Corp) spreadsheet that was checked for accuracy by another one of us (T.B.-A.). Data extracted included information about study design, population, anti-VEGF drugs, and SAEs. For the main comparison, we pooled data from arms evaluating several doses of the same anti-VEGF agent into a single treatment arm.
Statistical Analysis
We estimated intervention effects for each study by calculating odds ratios (ORs) with 95% CIs. We used a fixed-effects model for pooling ORs using the Peto method. Heterogeneity between studies was assessed using the Cochrane Q statistic (χ2 P values) and the inconsistency I2 statistic. Subgroup analyses by anti-VEGF drug, ocular disease, study quality, and follow-up duration were systematically performed for the main outcomes and whenever needed for secondary outcomes. We assessed publication bias by visually inspecting the funnel plots and using asymmetry tests for the main outcomes. We performed sensitivity analyses by changing the statistical method and model. All analyses were performed with RevMan and RStudio, version 1.2.5033, version (R Foundation for Statistical Computing) software.
Results
Study Characteristics and Quality
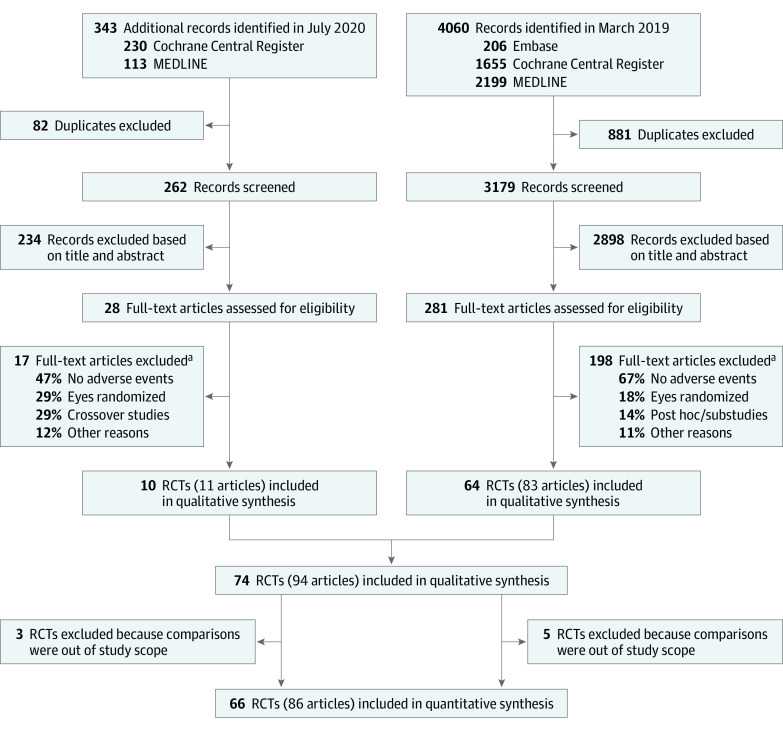
We retrieved 4403 references (Figure 1), assessed 309 full-text articles for eligibility, and selected 94 articles reporting results for 74 trials with 82 comparisons (1 study could participate in more >1 comparison). Among these comparisons, 37 evaluated an anti-VEGF agent vs control, 24 evaluated several doses or regimens of the same anti-VEGF agent, and 21 evaluated several doses or regimens of 2 anti-VEGF agent treatments (Figure 2).
Figure 1. Flow Diagram of Study Selection Process.
RCTs indicates randomized clinical trials.
aMore than 1 reason for each article.
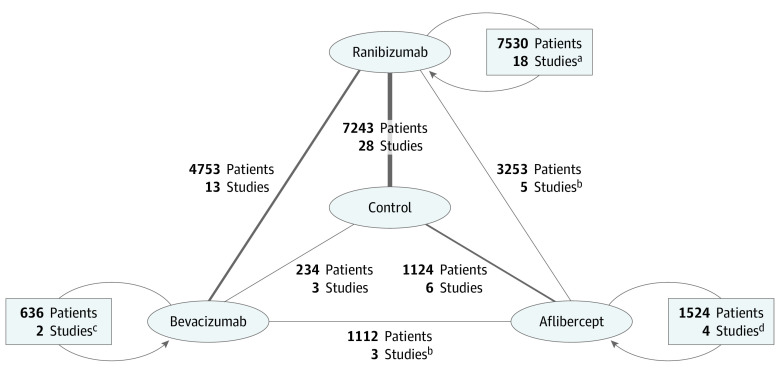
Figure 2. Network of Anti–Vascular Endothelial Growth Factor (anti-VEGF) Studies.
Comparisons (anti-VEGF vs control, anti-VEGF vs another anti-VEGF, and dose or regimen comparison for the same anti-VEGF) for the 66 included studies (some studies participated in more than 1 comparison) with the number of patients randomized for the comparison. The thickness of each line is proportional to the number of studies in each comparison.
aStudies with ranibizumab vs ranibizumab dose or regimen comparisons; 8 studies participated in other comparisons (7 ranibizumab vs control, 1 bevacizumab vs ranibizumab).
bTwo studies participated in another comparison (bevacizumab vs ranibizumab).
cStudies with bevacizumab vs bevacizumab regimen comparisons; 1 study participated in another comparison (bevacizumab vs ranibizumab).
dStudies with aflibercept vs aflibercept dose or regimen comparisons; 3 studies participated in other comparisons (2 aflibercept vs ranibizumab and 1 aflibercept vs control).
In the qualitative analysis, 32 studies (43%) included 14 190 patients (60%) with AMD, 24 studies (32%) included 5424 patients (23%) with DME/PDR, and 17 studies (23%) included 3757 patients (16%) with RVO. Mean age was 77 years (range, 50-101 years), in the AMD studies, 61 years (range, 21-91 years) in the DME/PDR studies, and 64 years (range, 20-91 years) years in the RVO studies. One small study22 included 122 patients with myopic choroidal neovascularization (mean age, 58 years [range, 27-83 years) (eTable 2 in the Supplement). For the quantitative analysis, 32 comparisons were conducted in 25 AMD studies, 28 comparisons in 23 DME/PDR studies, and 21 comparisons in 17 RVO studies (eFigures 1, 2, and 3 in the Supplement).
Among the included studies, safety was the prespecified primary outcome in 3 (4%) and was the secondary outcome in 7 studies (9%); 10 studies (14%) specifically assessed MACEs. All studies prespecified the time frame of SAE monitoring that was the study duration, on average, 16 months for AMD trials, 14 months for DME trials, 9 months for RVO trials, and 6 months for myopic choroidal neovascularization trials. Patients with a history of cardiovascular events were excluded in 33 studies (45%) and information was not reported in 6 studies (8%) (eTable 3 in the Supplement). Two studies23 comparing aflibercept with ranibizumab in AMD were considered together in the qualitative and quantitative analysis because they had similar protocols and reported pooled events.
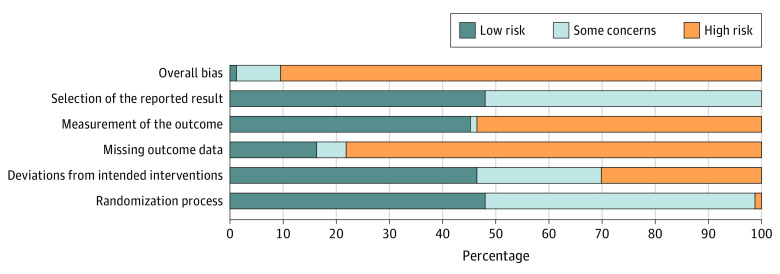
Only 1 study (1%) was considered to be at low overall risk of bias for all criteria. The other studies were considered to be at high (89%) or some concern (8%) overall risk of bias (Figure 3). One study was at high risk for selection bias; 22 (30%) of the studies were at high risk for performance bias, 39 (53%) were at high risk for detection bias, and 58 (78%) were at high risk for attrition bias. No study was at high risk for selective reporting bias (eFigure 4 in the Supplement). Forty-three (58%) of the studies were industry sponsored.
Figure 3. Assessment of Percentage of Each Risk of Bias Across Included Studies.
Main Outcomes
Comparisons of anti-VEGF drugs with controls for major cardiovascular events are shown in Figure 4.22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 Anti-VEGF drugs did not increase the risk of MACEs compared with control agents in 29 trials, with no heterogeneity (OR, 1.16; 95% CI, 0.85-1.58; P = .36; I2 = 0%, P = .99 for heterogeneity) (Figure 4). Follow-up duration, type of drug, disease, and study quality did not influence treatment effect (subgroup differences: duration, P = .91; type of drug, P = .78; disease, P = .82; and study quality, P = .60) (eTable 4 in the Supplement). The result did not change in sensitivity analyses (eTable 5 in the Supplement). No asymmetry was observed in the funnel plot (eFigure 5, eTable 6 in the Supplement).
Figure 4. Comparisons of Anti–Vascular Endothelial Growth Factor (Anti-VEGF) Drugs With Controls for Major Cardiovascular Events.
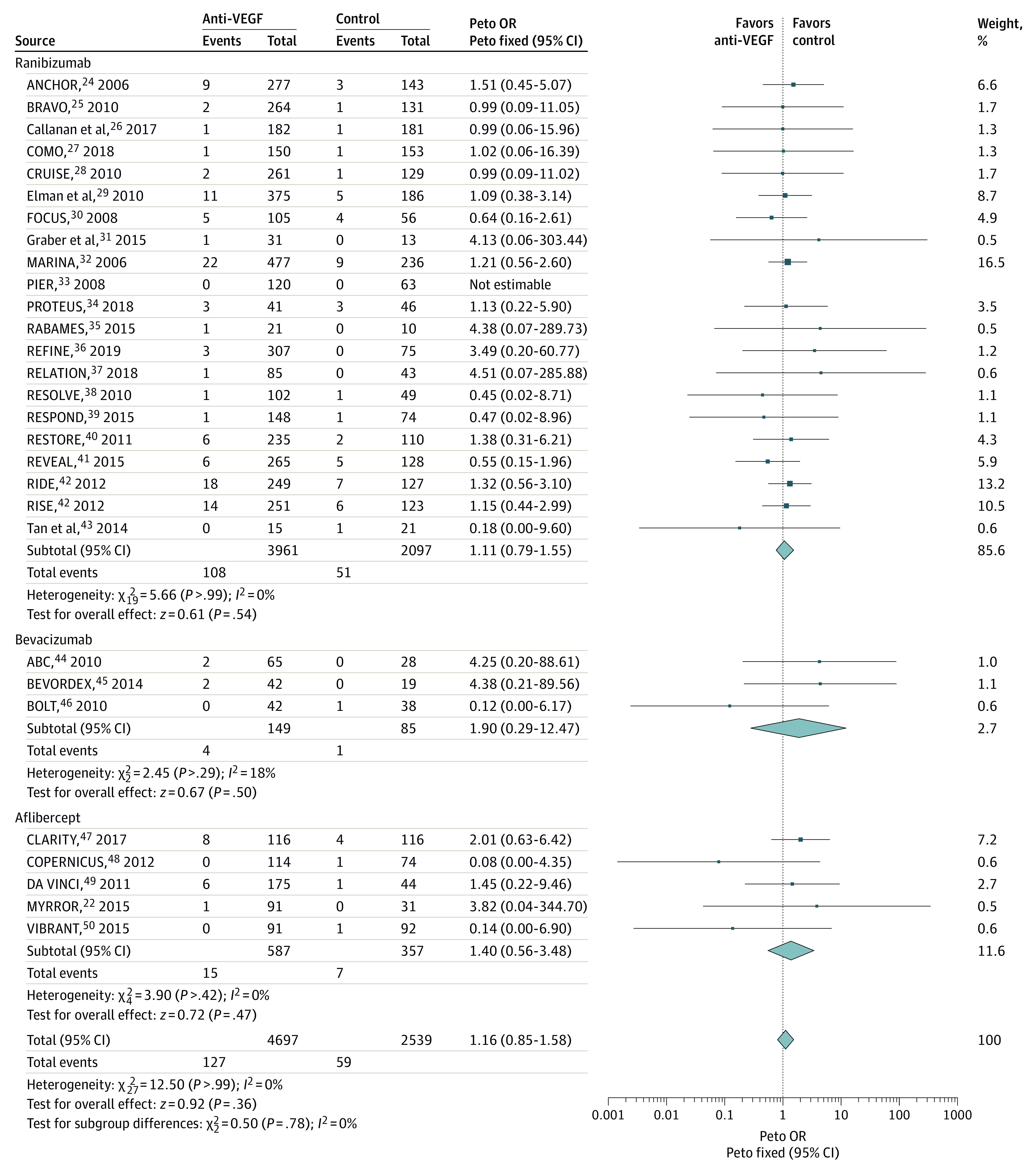
Major cardiovascular outcomes assessed with Antiplatelet Trialists’ collaboration21 criteria, a composite of nonfatal myocardial infarction, nonfatal ischemic or hemorrhagic stroke, or death due to vascular or unknown causes. Squares represent mean values, with the size of the squares indicating weight and horizontal lines representing 95% CIs. Diamonds represent the pooled mean, with the points of the diamonds representing 95% CIs. OR indicates odds ratio.
Comparisons of anti-VEGF drugs with controls for total mortality are shown in Figure 5.24,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 Anti-VEGF drugs did not increase the risk of total mortality compared with control agents in 35 trials, with no heterogeneity (OR, 1.27; 95% CI, 0.82-1.96; P = .29; P = .85 for heterogeneity; I2 = 0%) (Figure 5). The result did not change in sensitivity analyses (eTable 5 in the Supplement). Some asymmetry was apparent in the funnel plot (eFigure 6 in the Supplement), with no asymmetry test in favor of a significant publication bias (eTable 6 in the Supplement). Type of anti-VEGF drug, study quality, and the follow-up duration did not influence treatment effect (subgroup differences: type of anti-VEGF drug, P = .56; study quality, not applicable; and follow-up duration, P = .60) (eTable 4 in the Supplement). There was an interaction (subgroup difference: P = .04) in mortality risk depending on the underlying disease, with an increase (OR, 1.80; 95% CI, 1.03-3.16; P = .04) in the risk of death in patients with DME/PDR, but no increase was observed in patients with AMD or RVO.
Figure 5. Comparisons of Anti–Vascular Endothelial Growth Factor (Anti-VEGF) Drugs With Controls for Total Mortality.
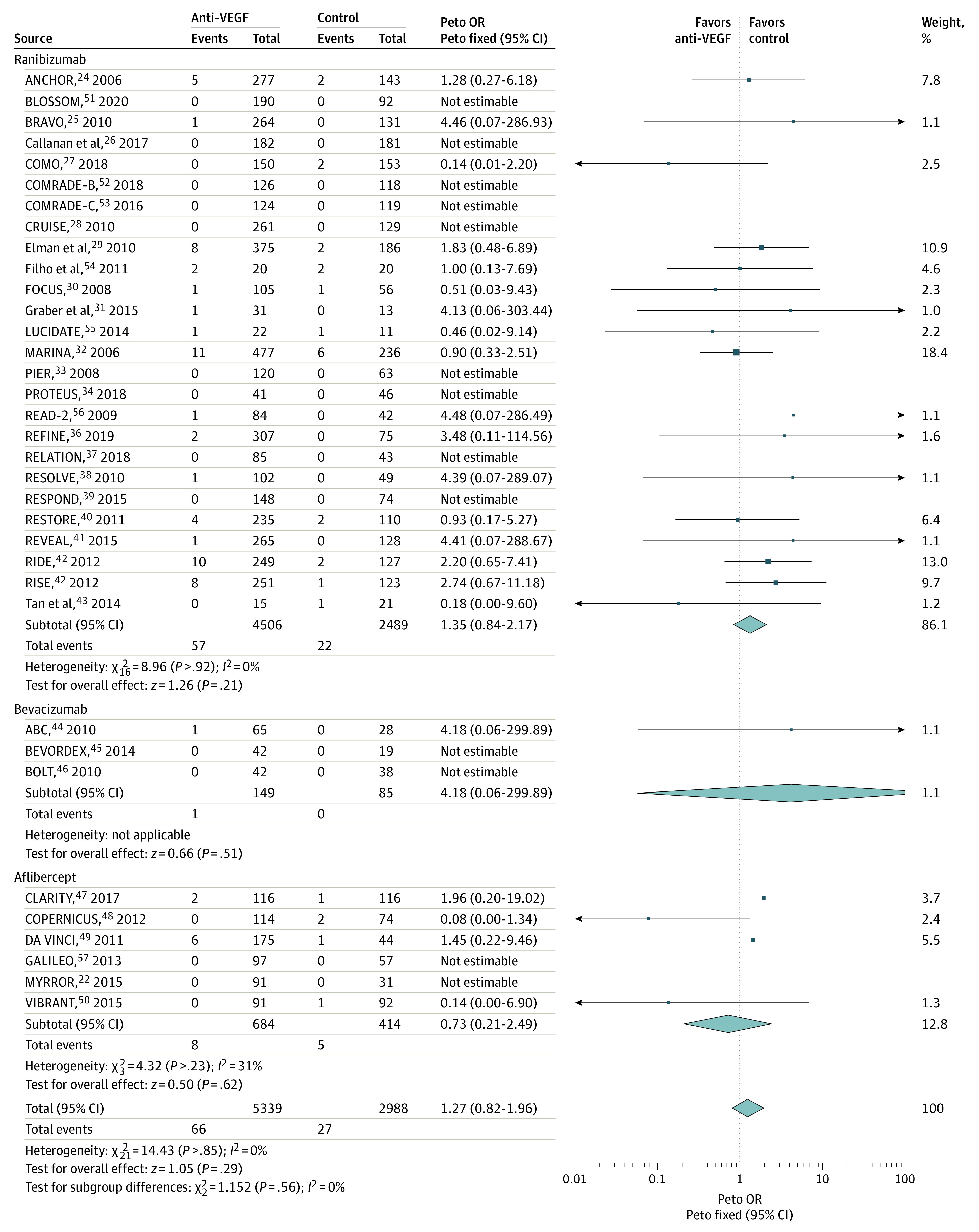
Squares represent mean values, with the size of the squares indicating weight and horizontal lines representing 95% CIs. Diamonds represent the pooled mean, with the points of the diamonds representing 95% CIs. OR indicates odds ratio.
Secondary Outcomes
Anti-VEGF drugs compared with control agents did not increase the risks of components of MACEs: cardiovascular mortality (OR, 1.21; 95% CI, 0.69-2.10; 33 trials), myocardial infarction (OR, 0.86; 95% CI, 0.55-1.33; 26 trials), or stroke (OR, 1.50; 95% CI, 0.91-2.48; 30 trials). Anti-VEGF drugs also did not increase other cardiovascular outcomes: cardiac failure (OR, 0.93; 95% CI, 0.54-1.58; 17 trials), venous thromboembolism (OR, 1.23; 95% CI, 0.40-3.79; 9 trials), arterial hypertension (OR, 0.94; 95% CI, 0.76-1.17; 28 trials), proteinuria (OR, 2.30; 95% CI, 0.47-11.23; 9 trials with ranibizumab only), all serious SAEs (OR, 0.99; 95% CI, 0.83-1.18; 19 trials), and all SAEs (OR, 0.93; 95% CI, 0.78-1.10; 11 trials) (eTable 4 in the Supplement).
Anti-VEGF drugs, mainly ranibizumab, increased the risks of nonocular hemorrhage compared with control agents (OR, 1.46; 95% CI, 1.01-2.10; P = .04; P = .81 for heterogeneity; I2 = 0%). This association was stronger in patients with AMD (OR, 1.57; 95% CI, 1.01-2.44), but the underlying disease did not seem to influence the risk (subgroup difference: P = .88). Type of anti-VEGF drug, study quality, and follow-up duration did not influence the risk of nonocular hemorrhage (subgroup differences: type of anti-VEGF drug, P = .78; study quality, P = .47 and follow-up duration, P = .57) (eTable 4 in the Supplement). The overall quality of evidence for the main outcomes and for nonocular hemorrhage risk was considered low, mainly owing to the risk of bias and indirectness (eTable 7 in the Supplement).
Secondary Comparisons and Post Hoc Analyses
Few studies compared one anti-VEGF with another, with the exception of bevacizumab vs ranibizumab (eTable 8 in the Supplement). No drug was associated with an increased or decreased risk of mortality, MACEs, or the components of MACEs when compared. Arterial hypertension seemed to be lower (OR, 0.69; 95% CI, 0.50-0.95; P = .02; I2 = 0%) in patients who received bevacizumab compared with those who received ranibizumab. There was a small increase in serious SAEs in bevacizumab vs ranibizumab studies (OR, 1.19; 95% CI, 1.03-1.39; P = .02, I2 = 0%).
Comparison between low and high doses for the monthly regimen could be analyzed in ranibizumab and aflibercept studies (eTable 9 in the Supplement). There were no statistically significant differences in mortality, MACEs or the components of MACEs, and other cardiovascular outcomes in low-dose (0.3 and 0.5 mg) compared with high-dose (0.5 and 2 mg) studies except for the risk of stroke and all serious SAEs. These risks were lower with lower dose ranibizumab (OR, 0.55; 95% CI, 0.33-0.93 for stroke, and OR, 0.70; 95% CI, 0.52-0.96 for all serious SAEs). With aflibercept, we observed an increased risk of myocardial infarction with the lower dose (0.5 mg) compared with the higher dose (2 mg) (OR 2.43; 95% CI, 1.03-5.75), but the number of events was low (21); therefore, this result should be interpreted with caution. The as-needed or treat-and-extend regimen was not associated with a lower risk of MACEs, mortality, or other outcomes compared with the monthly regimen (eTable 10 in the Supplement).
A post hoc analysis of subgroups in trials that excluded or included patients with cardiovascular diseases showed no potential enrollment bias for the MACEs, total mortality, and nonocular hemorrhage (subgroup differences: MACEs, P = .58; total mortality, P = .37; and nonocular hemorrhage, P = .48) (eTable 4 in the Supplement).
Discussion
This systematic review and meta-analysis investigated several cardiovascular SAEs associated with intravitreal anti-VEGF drugs in a large population of patients with ocular diseases included in clinical trials. Our findings suggest that intravitreal anti-VEGF agents were not associated with an increased risk of MACEs or its components compared with non–anti-VEGF controls, with no influence of type of drug, ocular disease, study quality, or duration of follow-up. These results are consistent with other systematic reviews.16 The incidence of overall mortality was low, 0.9% in the control group, which is in accordance with previous data.58,59 An increase in overall mortality was only observed in patients with diabetes, a high-risk population, but these outcomes were not adjusted for in multiple analyses. One recent retrospective cohort study suggested that, in patients with a history of myocardial infarction, use of intravitreal bevacizumab was associated with an increase in overall mortality compared with age- and sex-matched controls who experienced a myocardial infarction but did not receive bevacizumab.60 Our meta-analysis was based on aggregated data, and no adjustment for the history of cardiovascular disease was possible. We included studies with a minimum of 6 months follow-up and cannot ascertain whether an increased risk was apparent in short-term studies. A recent meta-analysis61 evaluating the risk of death associated with intravitreal anti-VEGF drugs described similar results with fewer studies, and studies with more than 30% crossover between groups, which might have influenced the risk estimate in the control group.
Our results support previous finding of an increased risk of nonocular hemorrhagic events,16,62 mainly with ranibizumab in patients with AMD. The prevalence of nonocular hemorrhagic events was 2.4% and most of these events were considered serious. However, we could not adjust our findings on treatments that may increase hemorrhagic risk, such as antithrombotic drugs, that are commonly prescribed in patients with diabetes and people older than 60 years at high cardiovascular risk.
Direct comparisons between bevacizumab and ranibizumab showed an increased risk of SAEs and a decreased risk of hypertension with bevacizumab. No significant differences were observed between aflibercept and bevacizumab or ranibizumab, but the number of studies was low. Pharmacokinetics and pharmacodynamics data after intravitreal administration in humans and monkeys reported that serum levels were higher for bevacizumab than for ranibizumab, with a corresponding persistent and more important decrease in VEGF plasma levels for bevacizumab than for ranibizumab.11,12,63 The increased risk of SAEs with bevacizumab was, however, not observed in a meta-analysis based on individualized data in patients with AMD.64 In this meta-analysis, the risk of SAEs was lower than that reported in original publications, possibly because of the process of data check before pooling. Furthermore, in our main comparison, the type of drug did not appear to influence the risks of MACEs, total mortality, and nonocular hemorrhage of anti-VEGF agents compared with controls. This finding is in accordance with real-world data58,59,65 and a recent head-to-head comparison meta-analysis.66 In addition, our between-doses comparisons found no significant differences in risks except for a lower stroke and serious SAEs risk with low dose (0.3 mg) ranibizumab compared with standard dose (0.5 mg). Stroke risks were low (1.2% for 0.3 mg and 1.6% for 0.5 mg) with few events, mainly owing to the Safety Assessment of Intravitreous Lucentis for AMD (SAILOR)67 and RISE-RIDE68 studies. These results were in agreement with individual patient data pooled analyses,69,70 even if inclusion criteria and the method used to estimate these risks could vary and explain some apparent differences. An unexpected increase in the risk of myocardial infarction was observed with low-dose aflibercept (0.5 mg) compared with the standard (2 mg) dose, but this finding should be considered with caution given the low number of studies (3) and events (21). Furthermore, the between-doses direct comparisons may be of limited validity, especially in the absence of knowledge regarding the plasma concentration, because the dose is sometimes poorly associated with plasma concentrations, obscuring an existing concentration-response relationship. No differences in risks were observed for an as-needed regimen compared with a monthly regimen.
Strengths and Limitations
One of the strengths of our systematic review with meta-analyses is that, contrary to previously published meta-analysis, we did not restrict our analyses to a single ocular disease.16 Instead, we considered all adult patients included in trials that evaluated anti-VEGF drugs to increase the power to detect safety signals. Some of these diseases are also associated with a high cardiovascular risk,71 a population in whom SAEs of anti-VEGF drugs are more likely to be detected. Although cardiovascular risks may differ between populations, randomization allows group comparability and OR estimation.
There are several limitations of our meta-analysis. The incidence of SAEs was considered as primary outcome in only 3 studies, leaving the other studies open to potential detection bias, which was difficult to evaluate because of limited information on SAEs monitoring and on the safety outcome assessor’s blinding was available. Methods for SAE reporting were heterogeneous and data reported by using the MedDRA system could not be always included in our meta-analysis, since MedDRA focus on event reporting without analyzing this event in the context of a patient’s diagnosis. A number of studies reported no events for different outcomes, which could also increase the difficulty of analysis, but our results did not vary when different methods to pool the data were tested. We only used published data, with no unpublished trials retrieved; therefore, a publication bias cannot be excluded. The average follow-up of the included studies was 16 months, which could be too short to detect an increase in rare SAEs. Exclusion of patients with a history of cardiovascular disease from several clinical trials, the absence of data regarding patients’ comorbidities, and the impossibility to adjust for such comorbidities, limit the validity of the risk assessment for patients in clinical practice. Because of the low incidence, the lack of adequate power to show an increased risk, the absence of adjudication of SAEs, exclusion of patients with cardiovascular disease history, and potential attrition bias in included studies (loss to follow-up), our results should be interpreted with caution and considered as safety signals.
Conclusions
The findings of our systematic review with meta-analysis suggest that intravitreal anti-VEGF drugs were not associated with an increase in MACEs. Increased mortality in patients with diabetes and nonocular hemorrhages, especially in patients with AMD, could represent a safety signal, but the evidence is not strong and the studies were not sufficiently powered to correctly assess a small increase in the incidence of SAEs. Cardiologists and ophthalmologists should be aware of these safety signals, especially in patients at high risk. Continued surveillance for SAEs in patients treated with intravitreal anti-VEGF drugs by means of population-based studies remains warranted.
eFigure 1. Network of Anti-VEGF Comparisons (Anti-VEGF vs Control, Anti-VEGF vs Another Anti-VEGF and Dose or Regimen Comparison for the Same Anti-VEGF) for the 25 Included AMD Studies
eFigure 2. Network of Anti-VEGF Comparisons (Anti-VEGF vs Control, Anti-VEGF vs Another Anti-VEGF and Dose Regimen Comparison for the Same Anti-VEGF) for the 23 Included DME/PDR Studies
eFigure 3. Network of Anti-VEGF Comparisons (Anti-VEGF vs Control, Anti-VEGF vs Another Anti-VEGF and Dose Regimen Comparison for the Same Anti-VEGF) for the 17 Included RVO Studies
eFigure 4. Risk of Bias Summary: Review Authors' Judgements About Each Risk of Bias Item for Each Included Study
eFigure 5. Funnel Plot for the Major Cardiovascular Events (APTC Criteria) Outcome, Comparison Anti-VEGF vs Control
eFigure 6. Funnel Plot for Total Mortality Outcome, Comparison Anti-VEGF vs Control
eTable 1. Search strategy for Medline and Embase
eTable 2. Characteristics of Included Studies, Population and Intervention
eTable 3. Methodology and Systemic Safety of Included Studies
eTable 4. Summary Statistics of Anti-VEGF Treatments (Aflibercept, Bevacizumab, Ranibizumab) Versus Control Comparisons for Primary and Secondary Outcomes, and Sub-group Analyses
eReferences
eTable 5. Sensitivity Analysis for Primary Outcomes by Changing Methods and Models
eTable 6. Funnel Plot Asymmetry Tests (With Continuity Correction if Necessary) for Primary Outcomes
eTable 7. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence Table for Primary Outcomes and Non-Ocular Hemorrhages
eTable 8. Summary Statistics of Aflibercept vs Ranibizumab, Aflibercept vs Bevacizumab, Bevacizumab vs Ranibizumab Comparison for Primary and Secondary Outcomes
eTable 9. Summary Statistics Of Between Doses (Ranibizumab 0.5 mg vs 2 mg; 0.3 mg vs 0.5 mg and Aflibercept 0.5 mg vs 2.0 mg) Comparisons for Primary and Secondary Outcomes
eTable 10. Summary Statistics of Anti-VEGF Drugs (Aflibercept, Bevacizumab, Ranibizumab) As Needed (PRN) or Treat and Extend (TE) Regimens vs Monthly Regimens Comparisons for Primary And Secondary Outcomes
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614-618. doi: 10.1136/bjophthalmol-2011-300539 [DOI] [PubMed] [Google Scholar]
- 2.Bourne RRA, Stevens GA, White RA, et al. ; Vision Loss Expert Group . Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339-e349. doi: 10.1016/S2214-109X(13)70113-X [DOI] [PubMed] [Google Scholar]
- 3.Pe’er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72(6):638-645. [PubMed] [Google Scholar]
- 4.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142(6):961-969. doi: 10.1016/j.ajo.2006.06.068 [DOI] [PubMed] [Google Scholar]
- 5.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359. doi: 10.1016/j.ophtha.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ip MS, Scott IU, Brown GC, et al. ; American Academy of Ophthalmology . Anti-vascular endothelial growth factor pharmacotherapy for age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(10):1837-1846. doi: 10.1016/j.ophtha.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 7.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300(19):2277-2285. doi: 10.1001/jama.2008.656 [DOI] [PubMed] [Google Scholar]
- 8.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305(5):487-494. doi: 10.1001/jama.2011.51 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Ran Y, Shao Y, Wang K, Zhu Y. Incidence and risk of severe infections associated with aflibercept in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81(1):33-40. doi: 10.1111/bcp.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Totzeck M, Mincu RI, Rassaf T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20 000 patients. J Am Heart Assoc. 2017;6(8):e006278. doi: 10.1161/JAHA.117.006278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics and pharmacodynamics of intravitrealaflibercept, bevacizumab, and ranibizumab. Retina. 2017;37(10):1847-1858. doi: 10.1097/IAE.0000000000001493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano T, Toriyama Y, Iesato Y, Imai A, Murata T. Changes in plasma vascular endothelial growth factor level after intravitreal injection of bevacizumab, aflibercept, or ranibizumab for diabetic macular edema. Retina. 2018;38(9):1801-1808. doi: 10.1097/IAE.0000000000002004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korobelnik J-F, Kleijnen J, Lang SH, et al. Systematic review and mixed treatment comparison of intravitreal aflibercept with other therapies for diabetic macular edema (DME). BMC Ophthalmol. 2015;15:52. doi: 10.1186/s12886-015-0035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarwar S, Clearfield E, Soliman MK, et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2:CD011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song WT, Xia XB. Ranibizumab for macular edema secondary to retinal vein occlusion: a meta-analysis of dose effects and comparison with no anti-VEGF treatment. BMC Ophthalmol. 2015;15:31. doi: 10.1186/s12886-015-0017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thulliez M, Angoulvant D, Pisella P-J, Bejan-Angoulvant T. Overview of systematic reviews and meta-analyses on systemic adverse events associated with intravitreal anti-vascular endothelial growth factor medication use. JAMA Ophthalmol. 2018;136(5):557-566. doi: 10.1001/jamaophthalmol.2018.0002 [DOI] [PubMed] [Google Scholar]
- 17.Thulliez M, Angoulvant D, Le Lez ML, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132(11):1317-1326. doi: 10.1001/jamaophthalmol.2014.2333 [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zorzela L, Loke YK, Ioannidis JP, et al. ; PRISMAHarms Group . PRISMA Harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;352:i157. doi: 10.1136/bmj.i157 [DOI] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21.Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6921):81-106. doi: 10.1136/bmj.308.6921.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikuno Y, Ohno-Matsui K, Wong TY, et al. ; MYRROR Investigators . Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR Study. Ophthalmology. 2015;122(6):1220-1227. doi: 10.1016/j.ophtha.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193-201. doi: 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 24.Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 25.Campochiaro PA, Heier JS, Feiner L, et al. ; BRAVO Investigators . Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112.e1. doi: 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 26.Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):463-473. doi: 10.1007/s00417-016-3472-1 [DOI] [PubMed] [Google Scholar]
- 27.Bandello F, Augustin A, Tufail A, Leaback R. A 12-month, multicenter, parallel group comparison of dexamethasone intravitreal implant versus ranibizumab in branch retinal vein occlusion. Eur J Ophthalmol. 2018;28(6):697-705. doi: 10.1177/1120672117750058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DM, Campochiaro PA, Singh RP, et al. ; CRUISE Investigators . Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133.e1. doi: 10.1016/j.ophtha.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 29.Elman MJ, Aiello LP, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network . Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. doi: 10.1016/j.ophtha.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antoszyk AN, Tuomi L, Chung CY, Singh A; FOCUS Study Group . Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008;145(5):862-874. doi: 10.1016/j.ajo.2007.12.029 [DOI] [PubMed] [Google Scholar]
- 31.Graber M, Glacet-Bernard A, Fardeau C, et al. [Comparison of early management of central retinal vein occlusion with ranibizumab versus hemodilution]. J Fr Ophtalmol. 2015;38(9):815-821. doi: 10.1016/j.jfo.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 33.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239-248. doi: 10.1016/j.ajo.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 34.Figueira J, Fletcher E, Massin P, et al. ; EVICR.net Study Group . ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS Study). Ophthalmology. 2018;125(5):691-700. doi: 10.1016/j.ophtha.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Pielen A, Mirshahi A, Feltgen N, et al. ; RABAMES Study Group . Ranibizumab for Branch Retinal Vein Occlusion Associated Macular Edema Study (RABAMES): six-month results of a prospective randomized clinical trial. Acta Ophthalmol. 2015;93(1):e29-e37. doi: 10.1111/aos.12488 [DOI] [PubMed] [Google Scholar]
- 36.Li X, Dai H, Li X, et al. ; REFINE study group . Efficacy and safety of ranibizumab 0.5 mg in Chinese patients with visual impairment due to diabetic macular edema: results from the 12-month REFINE study. Graefes Arch Clin Exp Ophthalmol. 2019;257(3):529-541. doi: 10.1007/s00417-018-04213-x [DOI] [PubMed] [Google Scholar]
- 37.Lang GE, Liakopoulos S, Vögeler J, et al. The RELATION study: efficacy and safety of ranibizumab combined with laser photocoagulation treatment versus laser monotherapy in NPDR and PDR patients with diabetic macular oedema. Acta Ophthalmol. 2018;96(3):e377-e385. doi: 10.1111/aos.13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399-2405. doi: 10.2337/dc10-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger A, Sheidow T, Cruess AF, Arbour JD, Courseau A-S, de Takacsy F. Efficacy/safety of ranibizumab monotherapy or with laser versus laser monotherapy in DME. Can J Ophthalmol. 2015;50(3):209-216. doi: 10.1016/j.jcjo.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 40.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. ; RESTORE study group . The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615-625. doi: 10.1016/j.ophtha.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 41.Ishibashi T, Li X, Koh A, et al. ; REVEAL Study Group . The REVEAL Study. Ophthalmology. 2015;122(7):1402-1415. doi: 10.1016/j.ophtha.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 42.Nguyen QD, Brown DM, Marcus DM, et al. ; RISE and RIDE Research Group . Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 43.Tan MH, McAllister IL, Gillies ME, et al. Randomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusion. Am J Ophthalmol. 2014;157(1):237-247.e1. doi: 10.1016/j.ajo.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 44.Tufail A, Patel PJ, Egan C, et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ. 2010;340(jun09 4):c2459-c2459. [DOI] [PubMed]
- 45.Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121(12):2473-2481. doi: 10.1016/j.ophtha.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 46.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078-1086.e2. doi: 10.1016/j.ophtha.2010.03.045 [DOI] [PubMed] [Google Scholar]
- 47.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. ; CLARITY Study Group . Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi: 10.1016/S0140-6736(17)31193-5 [DOI] [PubMed] [Google Scholar]
- 48.Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119(5):1024-1032. doi: 10.1016/j.ophtha.2012.01.042 [DOI] [PubMed] [Google Scholar]
- 49.Do DV, Schmidt-Erfurth U, Gonzalez VH, et al. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118(9):1819-1826. doi: 10.1016/j.ophtha.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 50.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015;122(3):538-544. doi: 10.1016/j.ophtha.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 51.Wei W, Weisberger A, Zhu L, Cheng Y, Liu C; BLOSSOM Study Group . Efficacy and safety of ranibizumab in Asian patients with branch retinal vein occlusion: results from the randomized BLOSSOM Study. Ophthalmol Retina. 2020;4(1):57-66. doi: 10.1016/j.oret.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 52.Hattenbach L-O, Feltgen N, Bertelmann T, et al. ; COMRADE-B Study Group . Head-to-head comparison of ranibizumab PRN versus single-dose dexamethasone for branch retinal vein occlusion (COMRADE-B). Acta Ophthalmol. 2018;96(1):e10-e18. doi: 10.1111/aos.13381 [DOI] [PubMed] [Google Scholar]
- 53.Hoerauf H, Feltgen N, Weiss C, et al. ; COMRADE-C Study Group . Clinical Efficacy and Safety of Ranibizumab Versus Dexamethasone for Central Retinal Vein Occlusion (COMRADE C): A European Label Study. Am J Ophthalmol. 2016;169:258-267. doi: 10.1016/j.ajo.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 54.Filho JAR, Messias A, Almeida FPP, et al. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol. 2011;89(7):e567-e572. doi: 10.1111/j.1755-3768.2011.02184.x [DOI] [PubMed] [Google Scholar]
- 55.Comyn O, Sivaprasad S, Peto T, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). Am J Ophthalmol. 2014;157(5):960-970. doi: 10.1016/j.ajo.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 56.Nguyen QD, Shah SM, Heier JS, et al. ; READ-2 Study Group . Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116(11):2175-81.e1. doi: 10.1016/j.ophtha.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 57.Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol. 2013;97(3):278-284. doi: 10.1136/bjophthalmol-2012-301504 [DOI] [PubMed] [Google Scholar]
- 58.Xu Y, Tan CS. Safety and complications of intravitreal injections performed in an Asian population in Singapore. Int Ophthalmol. 2017;37(2):325-332. doi: 10.1007/s10792-016-0241-4 [DOI] [PubMed] [Google Scholar]
- 59.Sangroongruangsri S, Chaikledkaew U, Kumluang S, et al. Real-world safety of intravitreal bevacizumab and ranibizumab treatments for retinal diseases in Thailand: a prospective observational study. Clin Drug Investig. 2018;38(9):853-865. doi: 10.1007/s40261-018-0678-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanhart J, Comaneshter DS, Freier-Dror Y, Vinker S. Mortality associated with bevacizumab intravitreal injections in age-related macular degeneration patients after acute myocardial infarct: a retrospective population-based survival analysis. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):651-663. doi: 10.1007/s00417-018-3917-9 [DOI] [PubMed] [Google Scholar]
- 61.Reibaldi M, Fallico M, Avitabile T, et al. Risk of death associated with intravitreal anti–vascular endothelial growth factor therapy: a systematic review and meta-analysis. JAMA Ophthalmol. 2019;138(1):50-57. doi: 10.1001/jamaophthalmol.2019.4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmucker C, Ehlken C, Agostini HT, et al. A safety review and meta-analyses of bevacizumab and ranibizumab: off-label versus gold standard. PLoS One. 2012;7(8):e42701. doi: 10.1371/journal.pone.0042701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christoforidis JB, Briley K, Binzel K, et al. Systemic biodistribution and intravitreal pharmacokinetic properties of bevacizumab, ranibizumab, and aflibercept in a nonhuman primate model. Invest Ophthalmol Vis Sci. 2017;58(13):5636-5645. doi: 10.1167/iovs.17-22431 [DOI] [PubMed] [Google Scholar]
- 64.Maguire MG, Shaffer J, Ying G, et al. ; Bevacizumab-Ranibizumab International Trials Group . Serious adverse events with bevacizumab or ranibizumab for age-related macular degeneration: meta-analysis of individual patient data. Ophthalmol Retina. 2017;1(5):375-381. doi: 10.1016/j.oret.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maloney MH, Payne SR, Herrin J, Sangaralingham LR, Shah ND, Barkmeier AJ. Risk of systemic adverse events after intravitreal bevacizumab, ranibizumab, and aflibercept in routine clinical practice. Ophthalmology. 2021;128(3):417-424. doi: 10.1016/j.ophtha.2020.07.062 [DOI] [PubMed] [Google Scholar]
- 66.Pham B, Thomas SM, Lillie E, et al. Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis. BMJ Open. 2019;9(5):e022031. doi: 10.1136/bmjopen-2018-022031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731-1739. doi: 10.1016/j.ophtha.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 68.Brown DM, Nguyen QD, Marcus DM, et al. ; RIDE and RISE Research Group . Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022. doi: 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 69.Zarbin MA, Francom S, Grzeschik S, et al. Systemic safety in ranibizumab-treated patients with neovascular age-related macular degeneration: a patient-level pooled analysis. Ophthalmol Retina. 2018;2(11):1087-1096. doi: 10.1016/j.oret.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 70.Zarbin MA, Dunger-Baldauf C, Haskova Z, et al. Vascular safety of ranibizumab in patients with diabetic macular edema: a pooled analysis of patient-level data from randomized clinical trials. JAMA Ophthalmol. 2017;135(5):424-431. doi: 10.1001/jamaophthalmol.2017.0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005;112(4):540-547. doi: 10.1016/j.ophtha.2004.10.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Network of Anti-VEGF Comparisons (Anti-VEGF vs Control, Anti-VEGF vs Another Anti-VEGF and Dose or Regimen Comparison for the Same Anti-VEGF) for the 25 Included AMD Studies
eFigure 2. Network of Anti-VEGF Comparisons (Anti-VEGF vs Control, Anti-VEGF vs Another Anti-VEGF and Dose Regimen Comparison for the Same Anti-VEGF) for the 23 Included DME/PDR Studies
eFigure 3. Network of Anti-VEGF Comparisons (Anti-VEGF vs Control, Anti-VEGF vs Another Anti-VEGF and Dose Regimen Comparison for the Same Anti-VEGF) for the 17 Included RVO Studies
eFigure 4. Risk of Bias Summary: Review Authors' Judgements About Each Risk of Bias Item for Each Included Study
eFigure 5. Funnel Plot for the Major Cardiovascular Events (APTC Criteria) Outcome, Comparison Anti-VEGF vs Control
eFigure 6. Funnel Plot for Total Mortality Outcome, Comparison Anti-VEGF vs Control
eTable 1. Search strategy for Medline and Embase
eTable 2. Characteristics of Included Studies, Population and Intervention
eTable 3. Methodology and Systemic Safety of Included Studies
eTable 4. Summary Statistics of Anti-VEGF Treatments (Aflibercept, Bevacizumab, Ranibizumab) Versus Control Comparisons for Primary and Secondary Outcomes, and Sub-group Analyses
eReferences
eTable 5. Sensitivity Analysis for Primary Outcomes by Changing Methods and Models
eTable 6. Funnel Plot Asymmetry Tests (With Continuity Correction if Necessary) for Primary Outcomes
eTable 7. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence Table for Primary Outcomes and Non-Ocular Hemorrhages
eTable 8. Summary Statistics of Aflibercept vs Ranibizumab, Aflibercept vs Bevacizumab, Bevacizumab vs Ranibizumab Comparison for Primary and Secondary Outcomes
eTable 9. Summary Statistics Of Between Doses (Ranibizumab 0.5 mg vs 2 mg; 0.3 mg vs 0.5 mg and Aflibercept 0.5 mg vs 2.0 mg) Comparisons for Primary and Secondary Outcomes
eTable 10. Summary Statistics of Anti-VEGF Drugs (Aflibercept, Bevacizumab, Ranibizumab) As Needed (PRN) or Treat and Extend (TE) Regimens vs Monthly Regimens Comparisons for Primary And Secondary Outcomes