Abstract
Background
Adoption of navigated total knee arthroplasty (Nav-TKA) is increasing. However, it has been suggested that a perceived decrease in surgical efficiency and a lack of proven superior functional outcomes associated with Nav-TKA have hindered its widespread adoption.
Methods
The American College of Surgeons National Surgical Quality Improvement Program was queried to identify patients who had undergone TKA with or without navigation between 2012 and 2018. Patients were further subclassified based on the type of navigation used, image-guided or imageless. Multivariate logistic regression was used to compare operative time and 30-day complication rates between conventional TKA (Conv-TKA) and Nav-TKA with and without image guidance.
Results
A total of 316,210 Conv-TKAs and 8554 Nav-TKAs (8270 imageless, 284 image-guided) were identified. Across the study period, the use of Nav-TKA was associated with a 1.5-minute increase in operative time. However, the overall time burden decreased over the study period, and by 2018, the mean operative time for Nav-TKA was 2.4 minutes less than that of Conv-TKA. Compared with Conv-TKA, Nav-TKA was associated with decreased rates of postoperative transfusion and surgical site complications but a similar incidence of systemic thromboembolism.
Conclusions
This is the first large-scale database study to examine the differences in operative time between Conv-TKA and Nav-TKA. The time burden associated with Nav-TKA decreased over the study period and even reversed by 2018. Nav-TKA was associated with lower rates of postoperative transfusion and surgical site complications. Further studies are needed to evaluate the long-term and functional outcomes between conventional and navigated knee arthroplasty techniques.
Keywords: Navigated knee arthroplasty, Navigated arthroplasty outcomes, Navigated arthroplasty trends, Operative time, Postoperative complications
Introduction
Total knee arthroplasty (TKA) is one of the most frequently performed surgeries in the United States [1,2]. The incidence of TKA is rising rapidly, and it is projected that by the year 2030, annual TKA case volume will surpass 3.4 million [[3], [4], [5], [6], [7]]. TKA leads to significant improvements in quality of life for patients suffering from end-stage arthritis, and prosthetic implants have demonstrated remarkable long-term durability [[8], [9], [10], [11], [12]]. Navigated arthroplasty was first introduced nearly 3 decades ago [13]. While utilization of navigated TKA (Nav-TKA) has steadily increased, it has yet to achieve widespread adoption and was used in only 7.0% of all TKA procedures in the United States in 2014 [[14], [15], [16]]. Navigation was initially introduced with the goal of decreasing premature implant wear and increasing implant longevity by improving the accuracy of component placement. To this end, navigated arthroplasty has had mixed success. Although the use of Nav-TKA has been shown to improve radiographic outcomes, a reduction in revision surgery rates and clinical superiority have not been clearly demonstrated [[17], [18], [19], [20]].
Beyond improving the accuracy of component placement, previous work has suggested that, compared with conventional TKA (Conv-TKA), the use of Nav-TKA may be associated with a decreased incidence of select postoperative complications. Two small prospective studies found that the use of Nav-TKA was associated with decreased production of systemic thromboemboli [21,22]. Furthermore, other studies have concluded that patients undergoing Nav-TKA had, on average, decreased intraoperative blood loss, lower rates of transfusion, and decreased risk of superficial wound infection compared with patients undergoing Conv-TKA [23,24].
Despite improvements in component alignment and decreases in select postoperative complications, it has been postulated that one barrier to widespread adoption of navigation is the assumed impact on total operative time [25]. Previous studies have secondarily reported on the impact of navigation on operative time for knee arthroplasty and have produced mixed results, with some reporting that the use of Nav-TKA increases operative time, while other studies found no difference [24,26,27]. Arthroplasty navigation platforms vary in their means of guidance, with some using image-guidance, while others use imageless systems. Within Nav-TKA, specific types of navigation platforms have differing requirements for intraoperative setup and anatomic landmark registration, and thus, the specific type of navigation used may affect the impact on total operative time [28,29].
Nationwide trends show a slow but steady rise in utilization of Nav-TKA, necessitating a better understanding of the impacts on patient outcomes and resource utilization associated with the use of Nav-TKA [14,16]. The primary goal of this study was to compare the mean operative time in cases of Conv-TKA and Nav-TKA using data from a large, nationwide database. The secondary goal was to compare the incidence of postoperative complications within 30 days of Conv-TKA and Nav-TKA. We hypothesize that there will be a decrease in mean operative time for Nav-TKA such that the difference in operative time between Conv-TKA and Nav-TKA will decrease over the course of the study period. Furthermore, in line with previous study, it is expected that Nav-TKA will be associated with decreased incidence of early postoperative complications including blood transfusion and wound complications.
Material and methods
We used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database to identify patients undergoing Conv-TKA and Nav-TKA between January 1, 2012, and December 31, 2018. NSQIP includes data from over 700 hospitals and is assembled by hospital-appointed, specially trained staff members. The NSQIP database includes data regarding baseline patient demographics, surgical details, and 30-day postoperative outcomes [30]. The data collection process is overseen by a surgeon champion, and an independent review found the overall data reliability to be excellent [31].
Current procedural terminology codes were used to identify patients undergoing Conv-TKA (27447) and Nav-TKA with (0054T, 0055T) or without (20985) image guidance. Thus, 2 overarching cohorts were constructed: one of cases of conventional TKA (Conv-TKA) and one of TKA cases using some form of navigation technology (Nav-TKA). The Nav-TKA cohort was further subdivided into 2 more specific subgroups: one including cases in which image-guided navigation was used (image-guided Nav-TKA) and one in which imageless navigation was used (imageless Nav-TKA). Patient comorbidities and postoperative complications were identified using International Classification of Diseases, 9th revision, (ICD-9) codes for all cases before October 1, 2015, and ICD-10 codes for all cases from October 1, 2015, through the end of the study period.
Patient characteristics collected from the registry included patient age, sex, height, weight, smoking history (within one year), American Society of Anesthesiologists class, medical comorbidities including diabetes, chronic obstructive pulmonary disease, liver disease with ascites, congestive heart failure, hypertension, bleeding disorders, chronic steroid use for a medical condition, disseminated cancer, and dialysis-dependent kidney disease. Body mass index was calculated from each patient’s height and weight. Functional status was defined as the patient’s ability to perform the activities of daily living in 3 categories. These categories included activities of daily living performed independently, in a partially dependent manner, or a completely dependent manner within the 30 days before admission.
Operative time, defined in the NSQIP as time from skin incision to skin closure, was reported in minutes for every case. Data on postoperative medical complications within 30 days were collected. Primary outcomes of the study were operative time, mortality, major complications, venous thromboembolism (VTE; pulmonary embolism [PE] and/or deep vein thrombosis [DVT]), surgical site complication (including superficial infection, deep infection, wound infection, and wound dehiscence), postoperative blood transfusion, sepsis within 48 hours of surgery, and urinary tract infection. Major complications were defined as the occurrence of any of the following: death, postoperative intubation for longer than 48 hours, unplanned intubation, stroke/cerebrovascular accident, DVT, PE, cardiac arrest, myocardial infarction, acute renal failure requiring dialysis, sepsis, septic shock, return to the operating room, wound dehiscence, superficial infection, wound infection, or deep surgical organ/space infection.
Baseline characteristics of patients undergoing TKA were summarized using descriptive statistics. Multivariate regression was used to determine predictors of operative time and postoperative complications. Specific factors adjusted for included patient age, sex, body mass index, comorbidities, and operative time greater than 120 minutes. In the case of operative time, given the significant disparity between cohort sizes, linear terms were included for year of operation to estimate annual trends. Primary analysis compared Conv-TKA to Nav-TKA. Subgroup analysis compared imageless to image-guided Nav-TKA. Standardized odds ratio (OR), 95% confidence intervals, and P values were computed. Patients with missing covariates were excluded from multivariate analysis. Statistical significance was defined as P < .05. Statistical analyses were performed using R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 316,210 Conv-TKAs (97.4%) and 8554 Nav-TKAs (2.6%) were identified over the 7-year study period. Of Nav-TKAs, 8270 were performed with imageless systems (96.7%) and 284 used image-guided platforms (3.3%). Baseline patient characteristics are summarized in Table 1. The Nav-TKA cohort had a significantly higher proportion of white patients (76.6% vs 70.4%, P < .001) and a lower proportion of diabetic patients (16.5% vs 18.2%, P < .001) than the Conv-TKA cohort.
Table 1.
Baseline demographics of patients undergoing conventional TKA (Conv-TKA) and navigated TKA (Nav-TKA).
| Patient characteristic | Conv-TKA |
Nav-TKA |
P value |
|---|---|---|---|
| n = 316,210 | n = 8554 | ||
| Sex, Female | 61.1% (194,917) | 61.1% (5227) | .32 |
| Age | |||
| 0-50 | 3.5% (10,959) | 3.2% (276) | .55 |
| 50-65 | 36.3% (114,785) | 36.8% (3149) | |
| 65-80 | 51.1% (161,426) | 50.9% (4350) | |
| 80+ | 9.2% (29,040) | 9.1% (779) | |
| Race | |||
| White | 70.4% (222,657) | 76.6% (6556) | <.001 |
| Hispanic | 5.1% (16,168) | 5.1% (434) | |
| Black | 7.6% (23,875) | 5.8% (492) | |
| Asian | 2.1% (6627) | 1.3% (110) | |
| Other | 14.8% (46,883) | 11.2% (962) | |
| BMI | |||
| Mean | 33.0 | 32.8 | <.001 |
| ASA | |||
| 1 | 1.9% (6084) | 1.3% (108) | <.001 |
| 2 | 48.8% (154,263) | 49.0% (4187) | |
| 3 | 47.6% (150,253) | 48.1% (4110) | |
| 4+ | 1.7% (5257) | 1.7% (142) | |
| Functional status | |||
| Independent | 98.8% (310,911) | 99.2% (8484) | .003 |
| Partially dependent | 1.1% (3499) | 0.7% (62) | |
| Totally dependent | <0.1% (133) | <0.1% (3) | |
| Medical comorbidities | |||
| Smoker | 8.3% (26,225) | 7.8% (671) | .14 |
| Diabetes | 18.2% (57,433) | 16.5% (1409) | <.001 |
| HTN | 64.7% (204,669) | 66.5% (5689) | .001 |
| COPD | 3.5% (10,929) | 3.8% (323) | .12 |
| CHF | 0.3% (942) | 0.3% (24) | .85 |
| Dialysis | 0.2% (513) | 0.1% (8) | .15 |
| Steroids | 3.6% (11,269) | 3.1% (269) | .042 |
| Bleeding disorder | 2.1% (6650) | 2% (170) | .49 |
ASA, American Society of Anesthesiologists; BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HTN, hypertension; TKA, total knee arthroplasty.
Over the study period, the mean operative time was 91.8 ± 37.1 minutes for Conv-TKA and 93.5 ± 34.3 minutes for Nav-TKA. Within Nav-TKA, the mean operative time was 93.1 ± 34.1 minutes for imageless Nav-TKA and 104.8 ± 33.2 minutes for image-guided Nav-TKA (Table 2). For all groups, the mean operative time decreased over the study period. From 2012 to 2018, operative time decreased by 5.4%, 7.5%, and 32.1% for Conv-TKA, imageless Nav-TKA, and image-guided Nav-TKA, respectively.
Table 2.
Mean operative time, in minutes, for all cases of conventional TKA (Conv-TKA) and navigated TKA (Nav-TKA) by intervention.
| Cohort | N | Mean | SD | IQR |
|---|---|---|---|---|
| Conv-TKA | 316,210 | 91.8 | 37.1 | (68, 107) |
| Nav-TKA | 8554 | 93.5 | 34.3 | (72, 107) |
| Imageless | 8270 | 93.1 | 34.1 | (72, 106) |
| Image-guided | 284 | 104.8 | 33.2 | (79, 127) |
IQR, interquartile range; SD, standard deviation; TKA, total knee arthroplasty.
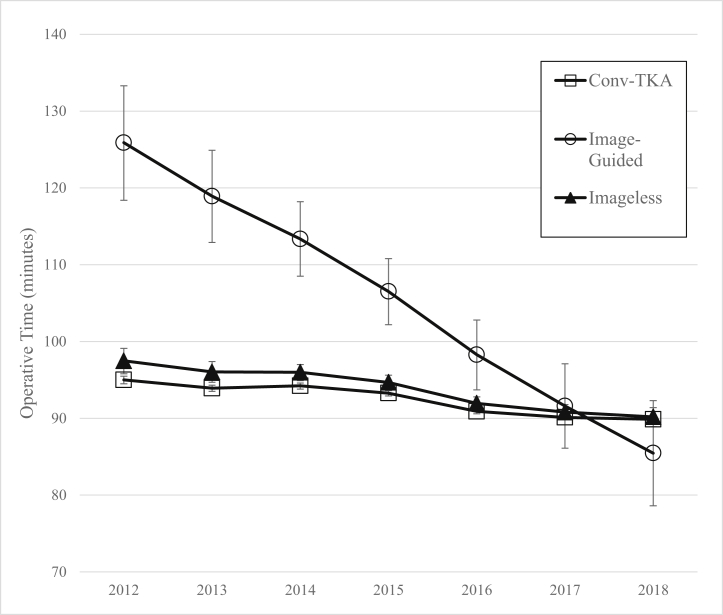
Multivariate regression analysis revealed that the use of Nav-TKA was associated with a significant, but not substantial, 1.5-minute increase in operative time compared with Conv-TKA (P < .001). Throughout the study period, the use of image-guided Nav-TKA was associated with an 11.8-minute increase in operative time over both Conv-TKA and imageless Nav-TKA (P < .001). Compared with Conv-TKA, the use of imageless Nav-TKA was associated with a 1.15-minute increase in operative time (P = .004). By 2018, the mean operative time for all Nav-TKA was 2.4 minutes shorter than that of Conv-TKA (P = .002). Furthermore, by 2018, there was no significant difference between operative time for image-guided and imageless Nav-TKA (difference 2.6 minutes, P = .43). Results of a linear fit model demonstrated that relative to operative time for Conv-TKA, over the study period, mean operative time for image-guided and imageless Nav-TKA decreased by 5.9 minutes annually (P < .001) and 0.4 minutes annually (P = .08), respectively (Fig. 1).
Figure 1.
Linear fit model depicting trends in mean operative time, in minutes, for image-guided and imageless navigated TKA, relative to conventional TKA (Conv-TKA), over the study period.
Postoperative complications are summarized in Table 3. On bivariate analysis, Conv-TKA was associated with a significantly lower incidence of urinary tract infection (P = .01), PE (P = .01), and superficial infection (P = .03). Upon multivariate analysis, controlling for both patient factors and operative time (Table 4), compared with patients undergoing Conv-TKA, patients undergoing Nav-TKA were less likely to suffer surgical site complications (OR, 0.76; P = .032). Patients undergoing Nav-TKA were also less likely to require a transfusion (OR, 0.78; P < .001) than patients undergoing Conv-TKA (Table 4). However, there was no significant difference with regard to the incidence of major complications (OR, 0.92; P = .209) or specifically VTE (OR, 0.88; P = .76) between the Conv-TKA and Nav-TKA groups. Subgroup analysis showed no difference in the rates of major complications, VTE, transfusion, or surgical site complications between cases using image-guided vs imageless Nav-TKA.
Table 3.
Incidence of postoperative complications after conventional TKA (Conv-TKA) and navigated TKA (Nav-TKA).
| Complication | Conv-TKA | Nav-TKA | P value |
|---|---|---|---|
| Death | 0.1% (318) | 0.11% (9) | 1.00 |
| Unplanned readmit | 1.69% (5348) | 1.47% (126) | .14 |
| Return to OR | 1.15% (3649) | 1.13% (97) | .91 |
| Reintubation | 0.14% (437) | 0.13% (11) | .93 |
| Postop intubation >48 hrs | 0.06% (194) | 0.08% (7) | .60 |
| Cardiac arrest | 0.08% (253) | 0.02% (2) | .10 |
| MI | 0.2% (628) | 0.16% (14) | .55 |
| CVA | 0.08% (251) | 0.07% (6) | .92 |
| Sepsis | 0.19% (600) | 0.16% (14) | .67 |
| Septic shock | 0.05% (168) | 0.01% (1) | .16 |
| PNA | 0.32% (1019) | 0.27% (23) | .45 |
| AKI | 0.11% (342) | 0.07% (6) | .37 |
| Dialysis | 0.06% (174) | 0.05% (4) | .93 |
| UTI | 0.77% (2422) | 0.53% (45) | .01 |
| VTE | |||
| PE | 0.55% (1735) | 0.34% (29) | .01 |
| DVT | 0.78% (2470) | 0.82% (70) | .75 |
| Blood transfusion | 3.91% (12,375) | 3.66% (313) | .26 |
| Surgical site complications | |||
| Wound dehiscence | 0.21% (658) | 0.13% (11) | .14 |
| Infection, superficial | 0.53% (1675) | 0.35% (30) | .03 |
| Infection, wound | 0.12% (394) | 0.08% (7) | .34 |
| Infection, deep | 0.19% (593) | 0.2% (17) | .91 |
AKI, acute kidney injury; CVA, cerebrovascular accident; DVT, deep vein thrombosis; MI, myocardial infarction; OR, odds ratio; PE, pulmonary embolism; PNA, pneumonia; TKA, total knee arthroplasty; UTI, urinary tract infection; VTE, venous thromboembolism.
Table 4.
Multivariate regression assessing for risk factors for major complication, transfusion, venous thromboembolism (VTE), and wound complications after TKA.
| Complication | Conv-TKA | Nav-TKA |
P value | |
|---|---|---|---|---|
| OR | CI | |||
| Major complications | Ref | 0.92 | (0.80-1.05) | .209 |
| Transfusion | Ref | 0.78 | (0.70-0.88) | <.001 |
| VTE | Ref | 0.88 | (0.71-1.08) | .76 |
| Surgical site complication | Ref | 0.76 | (0.59-0.98) | .032 |
Major complications include death, on ventilator more than 48 hours, unplanned intubation, stroke/cerebrovascular accident, deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac arrest, myocardial infarction, acute renal failure requiring dialysis, sepsis, septic shock, return to the operating room, wound dehiscence, superficial infection, wound infection, deep surgical organ/space infection. VTE includes both PE and DVT. Surgical site complications include deep infection, wound infection, superficial infection, or wound dehiscence. Additional factors controlled for include age, sex, body mass index, American Society of Anesthesiologists class, total odds ratio time, race, and medical comorbidities including smoking status, presence of diabetes, hypertension, chronic obstructive pulmonary disease, congestive heart failure, dialysis-dependence, chronic steroid use, or a bleeding disorder.
Discussion
The goal of this study was to analyze trends in operative time for Conv-TKA and Nav-TKA and to compare the incidence of early postoperative complications. Specific attention was paid to potential differences between image-guided and imageless Nav-TKA. Consistent with our hypothesis, our findings indicate that the time burden associated with the use of Nav-TKA has decreased from 2012 to 2018 and that Nav-TKA is associated with decreased risk for select early postoperative complications. Furthermore, we found no significant differences in the incidence of postoperative complications between image-guided and imageless Nav-TKA.
Over the study period, compared with Conv-TKA, Nav-TKA was associated with prolonged operative time; however, this difference was rather unsubstantial, at just 1.5 minutes. Furthermore, the time burden associated with the use of navigation decreased over the study period, and by 2018, the mean operative time for Nav-TKA was actually shorter than that for Conv-TKA. While previous studies have produced mixed results regarding the impact of navigation on operative time, to the authors’ knowledge, this is the first large study to primarily focus on assessing trends in operative time for Conv-TKA and Nav-TKA over multiple years [[26], [27], [28]]. A study by Grau et al. found that after adoption of a new system of Nav-TKA, both the average and maximum operative time decreased with experience [29]. Furthermore, they found that the greatest decrease in operative time occurred within the first 40 cases, suggesting that the inflection point of the surgeons’ learning curve occurs relatively rapidly [29].
Along with other studies which have shown increasing utilization of Nav-TKA, our findings suggest that as surgeons familiarize themselves with navigation platforms, they may achieve similar, if not superior, levels of efficiency when performing Nav-TKA [16]. Furthermore, we found an especially pronounced decline in mean operative time for image-guided Nav-TKA, such that by 2018, the difference in operative time between image-guided and imageless Nav-TKA was no longer significant. This suggests that, regardless of the specific means of guidance, navigation can be incorporated into a surgeon’s practice, and following a brief acclimation period, it can be done so without sacrificing operative efficiency.
Contrary to previous small randomized studies, our findings indicate that, compared with Conv-TKA, Nav-TKA is not associated with a significantly decreased risk for systemic thromboembolism (OR, 0.88; P = .76). In a prospective, randomized trial of 24 patients by Kalairajah et al., it was demonstrated that patients undergoing Conv-TKA had significantly increased production of microemboli, as seen by transcranial ultrasound, compared with patients undergoing Nav-TKA [32]. A similar study by Malhotra et al. used transesophageal echocardiography to monitor the right atrium for passage of microemboli during Conv-TKA and Nav-TKA carried out in 57 patients. They similarly found that the use of conventional alignment jigs was associated with increased incidence of systemic microemboli [22]. Furthermore, both studies found that the time of greatest microemboli production occurred as the femoral alignment rod was introduced into the intramedullary canal, supporting the hypothesis that pressurization of the intramedullary canal underlies the production of microemboli. While these studies put forth compelling evidence that the use of Conv-TKA increases the risk of systemic thromboembolism, neither demonstrated that microemboli formation led to clinically significant thromboembolic events. Despite the decrease in theoretical risk of VTE after Nav-TKA, our findings are in agreement with the work of Liodakis et al. and provide updated evidence to suggest that there is no significant difference in the incidence of clinically evident VTE after Conv-TKA and Nav-TKA [24].
In our analysis, we found that patients undergoing Nav-TKA were at significantly lower risk of requiring blood transfusions. Several previous studies have concluded that the use of navigation is associated with significantly decreased blood loss [23,[32], [33], [34]]. Furthermore, the clinical relevance of this difference has been underscored in a study by Liodakis et al., which found that patients undergoing Nav-TKA were significantly less likely to require postoperative transfusion [24]. While the reasons for increased blood loss after Conv-TKA are likely numerous, the use of intramedullary alignment jigs is almost certainly a contributing factor. The femoral intramedullary canal can be a source of substantial blood loss during TKA, and sparing its violation is likely to significantly affect blood loss and the subsequent need for transfusion [35].
Little is known about how Conv-TKA and Nav-TKA compare with regard to wound complication rates, although it has been reported that patients undergoing Nav-TKA had a lower incidence of superficial surgical site infection than patients undergoing Conv-TKA [24]. In our study, we found lower rates of surgical site complications, including deep and superficial surgical site infection, wound infection, and wound dehiscence in the Nav-TKA group. It has been suggested that this difference is likely attributable to the use of smaller incisions, decreased need for soft tissue release, and because Nav-TKA forgoes the need for intramedullary femoral guidance, all of which would contribute to decreased blood loss and subsequent wound drainage [23,35]. Wound complications are significant risk factors for the development of periprosthetic joint infection, a tremendously devastating complication of TKA [36]. As such, in reducing the incidence of postoperative wound complications, the adoption of Nav-TKA may represent a contributory measure in the attempts to minimize the incidence of periprosthetic joint infection.
We acknowledge that there are limitations to this study. While large, administrative databases provide researchers with tremendous statistical power, there are inherent limitations including potential errors in coding and data entry. The NSQIP database restricts follow-up for all patients to 30 days postoperatively and is not intended to be a nationally representative sample. However, NSQIP uniquely provides information on operative time, and as we primarily sought to understand the impact of navigation on operative time in TKA, we felt as though sacrificing longer term follow-up and a nationally representative dataset were justifiable tradeoffs. Furthermore, while our results show an overall trend toward decreased operative time with the use of Nav-TKA, we were unable to control for surgeons’ personal experience with navigation. Therefore, we can only comment on trends in operative time in aggregate, and our results may not necessarily reflect any one surgeon’s experience with Nav-TKA. As this study includes data from multiple institutions, there is also potential for variation in perioperative procedures which may have influenced operative time and early postoperative complications independent of the type of navigation used for TKA. Finally, higher annual procedure volume at both the surgeon and hospital level has been demonstrated to be associated with lower rates of adverse outcomes after total joint arthroplasty [37]. The use of navigated arthroplasty platforms is more common at higher volume arthroplasty centers, and as such, it is possible that decreased postoperative complication rates in the setting of Nav-TKA may be only partially attributable to the type of component alignment system used [16]. Despite the limitations of this study, to our knowledge, this is the first large investigation to report on trends in operative time for conventional and navigated TKA while specifically addressing potential differences between image-guided and imageless navigation systems.
Conclusions
Although previously associated with significantly increased operative time, largely driven by the use of image-guided navigation systems, the average operative time for Nav-TKA is now shorter than that for Conv-TKA. While adoption of new technologies, such as navigation, is likely to result in short-lived increases in operative time, surgeons can expect to quickly acclimate and achieve similar, if not superior, efficiency when adopting Nav-TKA. Our data suggest that surgeons can expect to reap the benefits of Nav-TKA, including decreased incidence of select early postoperative complications, without sacrificing operative efficiency. However, further study is needed to better understand the impact of Nav-TKA on long-term patient outcomes and the costs and resource-utilization associated with adopting this type of technology.
Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interestsOn behalf of all authors, the following were reported: Paid consultant for a company or supplier (The following conflicts were disclosed): Zimmer Biomet, Smith & Nephew. Stock or stock options in a company or supplier (The following conflicts were disclosed): Radlink, Inc. Research support from a company or supplier as a principal investigator (The following conflicts were disclosed): Zimmer Biomet. Medical/Orthopedic publications editorial/governing board (The following conflicts were disclosed): Journal of Arthroplasty, Arthroplasty Today. Board member/committee appointments for a society (The following conflicts were disclosed): American Association of Hip and Knee Surgeons (Evidence Based Medicine Committee).
Appendix A. Supplementary data
References
- 1.Fingar K.R., Stocks C., Weiss A.J., Steiner C.A. Healthcare cost and utilization project (HCUP) statistical briefs. Agency for Healthcare Research and Quality (US); Rockville (MD): 2006. Most frequent operating room procedures performed in U.S. Hospitals, 2003–2012: statistical brief #186. [PubMed] [Google Scholar]
- 2.Elixhauser A., Andrews R.M. Profile of inpatient operating room procedures in US hospitals in 2007. Arch Surg (Chicago, Ill : 1960) 2010;145(12):1201. doi: 10.1001/archsurg.2010.269. [DOI] [PubMed] [Google Scholar]
- 3.Bashinskaya B., Zimmerman R.M., Walcott B.P., Antoci V. Arthroplasty utilization in the United States is predicted by age-specific population groups. ISRN Orthop. 2012;2012 doi: 10.5402/2012/185938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz S.M., Ong K.L., Lau E., Bozic K.J. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 5.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 7.Inacio M.C.S., Paxton E.W., Graves S.E., Namba R.S., Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797. doi: 10.1016/j.joca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Sinicrope B.J., Feher A.W., Bhimani S.J. Increased survivorship of cementless versus cemented TKA in the morbidly obese. A minimum 5-year follow-up. J Arthroplasty. 2019;34(2):309. doi: 10.1016/j.arth.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Hopley C.D., Crossett L.S., Chen A.F. Long-term clinical outcomes and survivorship after total knee arthroplasty using a rotating platform knee prosthesis: a meta-analysis. J Arthroplasty. 2013;28(1):68. doi: 10.1016/j.arth.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 10.van der List J.P., Sheng D.L., Kleeblad L.J., Chawla H., Pearle A.D. Outcomes of cementless unicompartmental and total knee arthroplasty: a systematic review. Knee. 2017;24(3):497. doi: 10.1016/j.knee.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Bae D.K., Song S.J., Park M.J., Eoh J.H., Song J.H., Park C.H. Twenty-year survival analysis in total knee arthroplasty by a single surgeon. J Arthroplasty. 2012;27(7):1297. doi: 10.1016/j.arth.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Montonen E., Laaksonen I., Matilainen M. What is the long-term survivorship of cruciate-retaining TKA in the Finnish registry? Clin Orthop Relat Res. 2018;476(6):1205. doi: 10.1007/s11999.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee S., Cherian J.J., Elmallah R.K., Jauregui J.J., Pierce T.P., Mont M.A. Robotic-assisted knee arthroplasty. Expert Rev Med Dev. 2015;12(6):727. doi: 10.1586/17434440.2015.1086264. [DOI] [PubMed] [Google Scholar]
- 14.Antonios J.K., Korber S., Sivasundaram L. Trends in computer navigation and robotic assistance for total knee arthroplasty in the United States: an analysis of patient and hospital factors. Arthroplast Today. 2019;5(1):88. doi: 10.1016/j.artd.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretti V. National trends and peri-operative outcomes for navigated versus unnavigated total knee arthroplasty. Orthop Proc. 2018;96(Supplement 11) [Google Scholar]
- 16.Boylan M., Suchman K., Vigdorchik J., Slover J., Bosco J. Technology-Assisted hip and knee arthroplasties: an analysis of utilization trends. J Arthroplasty. 2018;33(4):1019. doi: 10.1016/j.arth.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Hsu R.W., Hsu W.H., Shen W.J., Hsu W.B., Chang S.H. Comparison of computer-assisted navigation and conventional instrumentation for bilateral total knee arthroplasty: the outcomes at mid-term follow-up. Medicine. 2019;98(47):e18083. doi: 10.1097/MD.0000000000018083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beal M.D., Delagramaticas D., Fitz D. Improving outcomes in total knee arthroplasty-do navigation or customized implants have a role? J Orthop Surg Res. 2016;11(1):60. doi: 10.1186/s13018-016-0396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamora L.A., Humphreys K.J., Watt A.M., Forel D., Cameron A.L. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22. doi: 10.1111/j.1445-2197.2012.06255.x. [DOI] [PubMed] [Google Scholar]
- 20.de Steiger R.N., Liu Y.L., Graves S.E. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635. doi: 10.2106/JBJS.M.01496. [DOI] [PubMed] [Google Scholar]
- 21.Kalairajah Y., Cossey A.J., Verrall G.M., Ludbrook G., Spriggins A.J. Are systemic emboli reduced in computer-assisted knee surgery?: a prospective, randomised, clinical trial. J Bone Joint Surg Br. 2006;88(2):198. doi: 10.1302/0301-620X.88B2.16906. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra R., Singla A., Lekha C. A prospective randomized study to compare systemic emboli using the computer-assisted and conventional techniques of total knee arthroplasty. J Bone Joint Surg Am. 2015;97(11):889. doi: 10.2106/JBJS.N.00783. [DOI] [PubMed] [Google Scholar]
- 23.Millar N.L., Deakin A.H., Millar L.L., Kinnimonth A.W., Picard F. Blood loss following total knee replacement in the morbidly obese: effects of computer navigation. Knee. 2011;18(2):108. doi: 10.1016/j.knee.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Liodakis E., Antoniou J., Zukor D.J., Huk O.L., Epure L.M., Bergeron S.G. Navigated vs conventional total knee arthroplasty: is there a difference in the rate of respiratory complications and transfusions? J Arthroplasty. 2016;31(10):2273. doi: 10.1016/j.arth.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Clayton A.W., Cherian J.J., Banerjee S. Does the use of navigation in total knee arthroplasty affect outcomes? J Knee Surg. 2014;27(3):171. doi: 10.1055/s-0034-1374814. [DOI] [PubMed] [Google Scholar]
- 26.Kinney M.C., Cidambi K.R., Severns D.L., Gonzales F.B. Comparison of the iAssist handheld guidance system to conventional instruments for mechanical Axis restoration in total knee arthroplasty. J Arthroplasty. 2018;33(1):61. doi: 10.1016/j.arth.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Ajwani S.H., Jones M., Jarratt J.W., Shepard G.J., Ryan W.G. Computer assisted versus conventional total knee replacement: a comparison of tourniquet time, blood loss and length of stay. Knee. 2012;19(5):606. doi: 10.1016/j.knee.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Harvie P., Sloan K., Beaver R.J. Three-dimensional component alignment and functional outcome in computer-navigated total knee arthroplasty: a prospective, randomized study comparing two navigation systems. J Arthroplasty. 2011;26(8):1285. doi: 10.1016/j.arth.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Grau L., Lingamfelter M., Ponzio D. Robotic arm assisted total knee arthroplasty workflow optimization, operative times and learning curve. Arthroplast Today. 2019;5(4):465. doi: 10.1016/j.artd.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.About ACS NSQIP. https://www.facs.org/quality-programs/acs-nsqip/about [accessed 30.06.20]
- 31.Shiloach M., Frencher S.K., Jr., Steeger J.E. Toward robust information: data quality and inter-rater reliability in the American College of surgeons national surgical quality improvement Program. J Am Coll Surg. 2010;210(1):6. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Kalairajah Y., Simpson D., Cossey A.J., Verrall G.M., Spriggins A.J. Blood loss after total knee replacement: effects of computer-assisted surgery. J Bone Joint Surg Br. 2005;87(11):1480. doi: 10.1302/0301-620X.87B11.16474. [DOI] [PubMed] [Google Scholar]
- 33.Conteduca F., Massai F., Iorio R., Zanzotto E., Luzon D., Ferretti A. Blood loss in computer-assisted mobile bearing total knee arthroplasty. A comparison of computer-assisted surgery with a conventional technique. Int Orthop. 2009;33(6):1609. doi: 10.1007/s00264-008-0651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinarejos P., Corrales M., Matamalas A., Bisbe E., Cáceres E. Computer-assisted surgery can reduce blood loss after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2009;17(4):356. doi: 10.1007/s00167-008-0683-y. [DOI] [PubMed] [Google Scholar]
- 35.Ko P.S., Tio M.K., Tang Y.K., Tsang W.L., Lam J.J. Sealing the intramedullary femoral canal with autologous bone plug in total knee arthroplasty. J Arthroplasty. 2003;18(1):6. doi: 10.1054/arth.2003.50001. [DOI] [PubMed] [Google Scholar]
- 36.Illingworth K.D., Mihalko W.M., Parvizi J. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50. doi: 10.2106/JBJS.L.00596. [DOI] [PubMed] [Google Scholar]
- 37.Bozic K.J., Maselli J., Pekow P.S., Lindenauer P.K., Vail T.P., Auerbach A.D. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643. doi: 10.2106/JBJS.I.01477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.