Abstract
Background
Targeted axillary dissection (TAD), the combination of sentinel lymph node biopsy (SLNB) and targeted lymph node biopsy (TLNB), can reduce the false negative rates of sentinel node biopsy alone dramatically in breast cancer patients, who received neoadjuvant chemotherapy (NAC). However methods for TAD are still under investigation.
Methods
Magseed®, a non-radioactive magnetic marker was used to mark the biopsied positive TLN after NAC. The SLNB with the standard technetium-based method and the selective TLNB with Magseed® localization were performed in 40 patients. The TLNs were identified with the Sentimag® probe and excised in all patients. Specimen x-ray was performed to confirm the Magseed® within the prior to NAC biopsied and clipped lymph node.
Results
The TLN identification rate was 100% (40/40), the SLN identification rate was 82.5% (33/40), the concordance rate between the TLN and the SLN was 65% (26/40). Complications according Magseed® deployment or identification could not be observed.
Conclusion
Magseed® is a reliable and feasible marker for the identification of TLNs after NAC.
Keywords: Targeted axillary dissection, Targeted lymph node biopsy, Magnetic marker
Highlights
-
•
TLNB is an accurate method for the identification of marked lymph nodes after NAC.
-
•
Selective excision of TLNs is feasible.
-
•
Magseed® allows a non-radioactive, wireless marking of TLNs.
1. Introduction
False negative rates of SLNB after NAC vary between 9.1 - 21.1% (ACOSOG Z1071), 6.1–24.3% (SENTINA Trial), and 4.9–18.2% (SN FNAC study) in prospective randomized trials and depend on the number of removed lymph nodes [[1], [2], [3]]. Data from the MD Anderson Cancer Center demonstrated that targeted axillary dissection (TAD), the combination of targeted lymph node biopsy (TLNB) and sentinel lymph node biopsy (SLNB) can reduce false negative rates down to 1.4%, if the clipped nodes (TLNs) and the sentinel lymph nodes (SLNs) were removed [4]. For identification of the clipped node Iodine-125 seed was utilized. Iodine-125 seed is not approved in most European countries for this indication due to strict radiation regulations [5]. In small series several methods for TLNB are described. Methods as wire localization of clipped nodes [[6], [7], [8], [9]], tattooing of positive nodes [[10], [11], [12], [13]], magnetic localization of the clipped nodes [14,15] or radiofrequency identification tags (RFID) [[16], [17], [18]] are under investigation. We report on the feasibility and first results with Magseed® localization of the clipped lymph node after NAC. Few reports in the literature describe the utilization of Magseed® in the breast [19], but there are only rare reports on the use of Magseed® for the identification of lymph nodes in the axilla [14,15].
2. Methods
2.1. Technique
While diagnosis of breast cancer and ultrasound evaluation of the axilla, suspicious or pathologic lymph nodes were core-needle biopsied and then clipped with a HydroMARK® Breast Biopsy Site Marker (Devicor Medical Products, Inc., Cincinnati, OH, USA), which consists of a titanium coil (Titanium Shape 3 or 4) for x-ray visibility within a polymer-hydrogel for ultrasound visibility. This HydroMARK® is ultrasound-visible up to 12 months after deployment. After administration of NAC, on the previous day of surgery, the clipped lymph nodes were marked with the Magseed®, a magnetic marker, which was deployed into or close to the pathologic lymph nodes with a 18ga inserter under ultrasound guidance by the surgeon himself. Magseed® is a non-radioactive tiny metal seed, made of surgical grade stainless steel, 5 mm long and 0.9 mm in diameter, and can be detected with the Sentimag® probe (Magseed®, Sentimag®, Endomagnetics Ltd, Cambridge, United Kingdom), which magnetizes the Magseed® and gives a signal to localize the Magseed® during surgery. Tc-99 injection for SLN localization was performed additionally the previous day of surgery. During breast surgery the SLNB was performed using the Gamma-probe as standard procedure, and the TLNB was performed by using the Sentimag® probe identifying the Magseed® marked TLN. The SLNs and the TLNs were selectively excised. Ex vivo measurments of the hot nodes with the Gamma-probe and the Magseed® containing node with the Sentimag® probe were performed to verify the SLNs and TLNs. Specimen x-ray was performed to identify the clip within the Magseed® marked lymph node. Concordance between TLNs and SLNs was obtained, when the Magseed® and clip containing TLN and the Tc-99 hot SLN matched [Fig. 1, Fig. 2, Fig. 3].
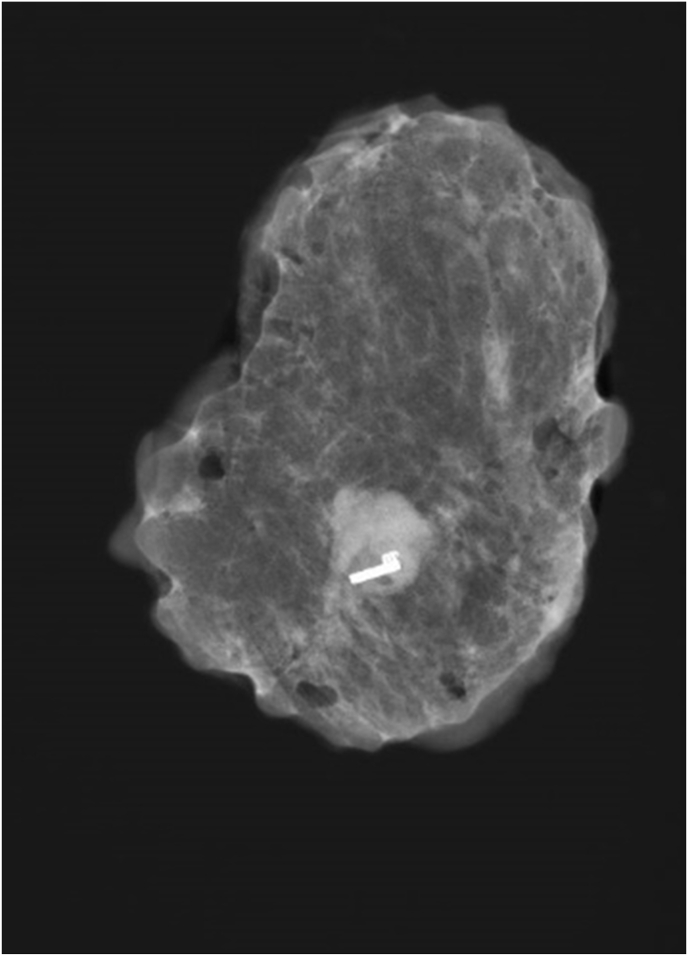
Fig. 1.
One Magseed® marks one clipped lymph node.
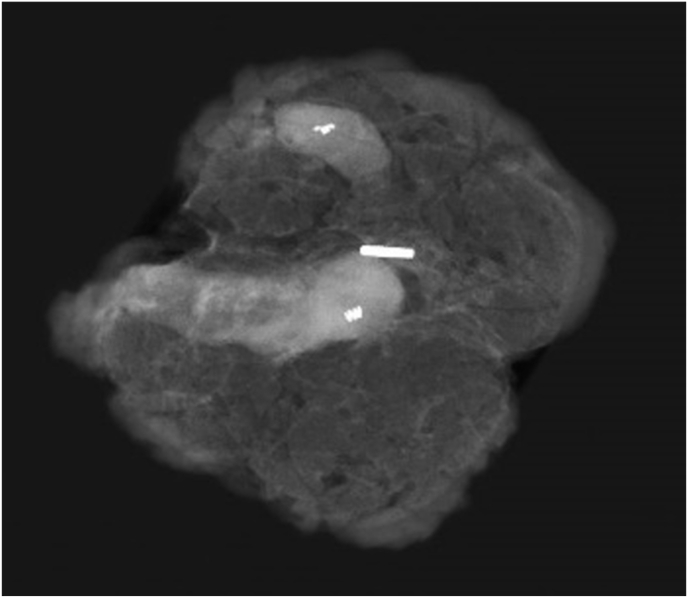
Fig. 2.
One Magseed® marks two clipped lymph nodes.
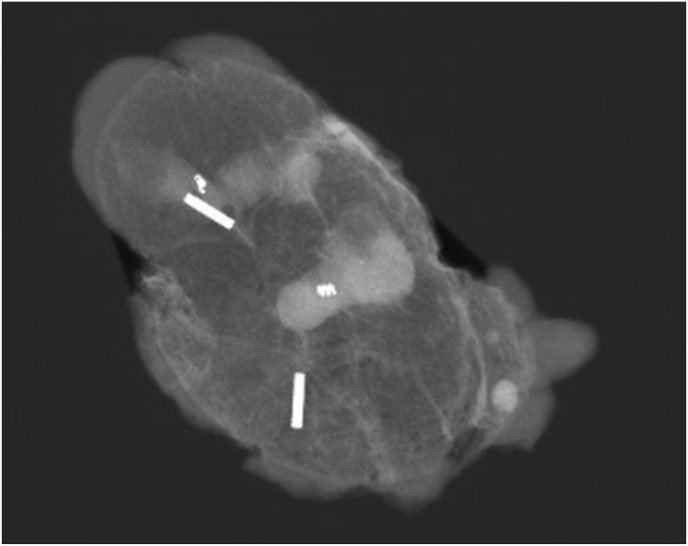
Fig. 3.
Two Magseed®s mark two clipped lymph nodes.
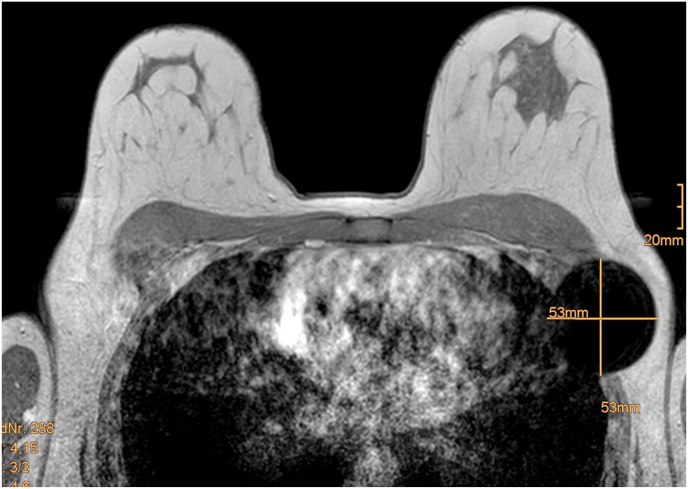
In most cases Magseed® deployment was performed after NAC, but currently, since Magseed® is approved for long-term insertion, we deploy the Magseed® immediately after core-needle biopsy of the lymph node and leave it inside during NAC until surgery, except for patients who need MRI for further evaluation. Even if Magseed® is licensed for MRI investigation (MRI conditional - 1.5T and 3.0T), an evaluation of an area of 5 cm diameter around the Magseed® is definitely impossible (Fig. 4). due to an extinction phenomenon.
Fig. 4.
Extinction artefact of Magseed® in the MRI.
2.2. Patients
Forty patients (primary tumor stage cT1-cT4, pN1), median age 52 years (range 29–81), scheduled for NAC were included in the study. Suspicious axillary lymph nodes were biopsied and clipped with a HydroMark®. In 2 patients (2/40) immediate Magseed® insertion was performed. In 38/40 patients the clipped nodes were marked with the Magseed® under ultrasound guidance after NAC, the previous day of surgery. In 30/40 patients one Magseed® marked one TLN, in 10/40 patients two Magseed®s marked two different TLNs.
Breast surgery and TAD was performed in TLN-negative patients, ALND additionally was performed in TLN-positive patients. ALND was not performed in patients with negative TLNs.
2.3. Data analysis
Data were analyzed for identification rates and concordance rates, as well as the pathologic results of the Magseed® marked targeted lymph nodes in comparison to the Technetium-marked sentinel lymph nodes. Problems with the procedure of HydroMark® clip-placement or Magseed® placement were also documented and analyzed.
3. Results
3.1. Identification rate
TAD with Tc99 and Magseed® was performed in 40 patients [Table 1]. The identification and selective excision of the Magseed® marked lymph nodes was successful in all patients. The TLN identification rate was 100% (40/40). The Magseed® marked TLN could be identified with the Sentimag® probe and excised in every patient. The specimen x-ray verified the Magseed® within the clipped node in every patient. The mean distance between clip and Magseed® was 3.1 mm (min 0.0 mm, max 14.0 mm).
Table 1.
Characteristics of patients.
| n (%) | |
|---|---|
| Number of Patients | 40 (100) |
| Clinical T-stage | |
| T1 | 11 (27.5) |
| T2 | 18 (45.0) |
| T3 | 5 (12.5) |
| T4 | 6 (15.0) |
| Pathological N-stage before NAC | |
| pN1 | 40 (100) |
| Tumorbiology | |
| Triple-negative | 12 (30.0) |
| ER neg/PR neg/Her2 pos | 12 (30.0) |
| ER pos/PR pos/Her2 pos | 6 (15.0) |
| Luminal B - like | 9 (22.5) |
| Luminal A - like | 1 (2.5) |
| Type of Surgery | |
| BCS | 29 (72.5) |
| NSM + DTI | 8 (20.0) |
| Mastectomy | 3 (7.5) |
| Lymph Node Localization | |
| 1 Clip | 26 (68.4) |
| 2 Clips | 10 (26.3) |
| 3 Clips | 2 (5.3) |
| 1 Magseed® | 30 (75.0) |
| 2 Magseed®s | 10 (25.0) |
ER Estrogen-Receptor, PR Progesteron-Receptor, BCS Breast Conserving Surgery, NSM Nipple-Sparing Mastectomy, DTI Direct-to-Implant Reconstruction.
The SLN identification rate was 82.5% (33/40). The identification of SLNs with Tc-99 was unsuccessful in 7 patients receiving NAC (7/40; 17.5%).
3.2. Concordance rate
The concordance rate between TLN and SLN was 65.0% (26/40). In 18/26 patients both TLN and SLN were negative, and in 8/26 patients both TLN and SLN were positive. In 7/40 patients the TLN was not concordant with the SLN, in 2 patients the TLN was positive and the SLN was negative, in 5 patients TLNs and SLNs were negative. In 7/40 patients the TLN could be identified and excised but the SLN could not be identified [Table 2]. In 3 of those 7 patients the TLNs were positive and in 4 patients the TLNs were negative.
Table 2.
Concordance rate between TLN and SLN according identification.
| SLN = TLN | SLN ≠ TLN | SLN not identified | Total | |
|---|---|---|---|---|
| TLN = SLN | 26 | 26 | ||
| TLN ≠ SLN | 7 | 7 | ||
| TLN identified | 7 | 7 | ||
| Total | 40 |
TLN Targeted Lymph Node, SLN Sentinel Lymph Node.
In 13/40 patients the TLN was still positive after NACT. In 5/13 TLN positive patients, the SLN was either negative (2/13) or could not be identified (3/13). In 4/27 TLN negative patients, the SLN could not be identified [Table 3]. There was no patient with negative TLN but positive SLN.
Table 3.
Concordance rate between TLN and SLN according pathological result.
| SLN negative | SLN positive | SLN not id | Total | |
|---|---|---|---|---|
| TLN negative | 23 | 0 | 4 | 27 |
| TLN positive | 2 | 8 | 3 | 13 |
| Total | 25 | 8 | 7 | 40 |
TLN Targeted Lymph Node, SLN Sentinel Lymph Node.
However with SLN biopsy alone the primarily positive lymph node could not be identified in 17.5% (7/40) of patients, or identified a non-TLN in 17.5% (7/40) patients, totalling in 35.0% (14/40) patients.
The number of TLNs excised was 1 TLN in 30 patients, 2 TLNs in 8 patients and 3 TLNs in 2 patients. The number of SLNs excised was 1 SLN in 22 patients, 2 SLNs in 6 patients, 3 SLNs in 3 patients and more than 3 SLNs in 2 patients. In 7 patients the SLN could not be identified.
3.3. Complications
The clip could be placed directly into the marked lymph node, or very close to the lymph node without difficulty. The mean distance between the HydroMark® and the Magseed® was 3.1 mm, with a minimum zero mm, the Magseed® was directly overlying the HydroMark®, and a maximum of 14 mm. There were no complications according placement of the HydroMark® nor of the Magseed®, as hematoma or bleeding due to vessel injury. Misplacement of the Magseed® could not be observed.
4. Discussion
SLN identification rates after NAC vary between 93.8% in the ACOSOG Z1071 trial and 80.1% in Arm C of the SENTINA trial, and 87.6% in the SN FNAC study, if a dual mapping method was used. Identification rates with a single method blue dye alone or radiolabelled technique alone drop to 78.6%.
In our study the identification rate of the TLN with Magseed® and Sentimag® was 100%, and the SLN-identification rate was 82.5%.
False-negative rates could not be calculated as we did not perform ALND in TLN-negative patients any longer. But as all TLNs were confirmed positive before NAC and all TLNs could be identified and excised, all negative TLNs were true negative TLNs. All patients, with positive lymph nodes prior to NAC were scheduled for postoperative radiotherapy of the axilla, according to our protocol.
The Magseed® was easy to deploy under ultrasound guidance, and the TLN was easy to localize with the Sentimag® probe. If there were two Magseed®s in two TLNs they could be discriminated simply by angulation of the probe.
There are two other studies published on Magseed® localization for TLNs with an excellent result. Heather Greenwood [14], reported on an identification-rate of 97% (37/38 patients). 30/35 (86%) patients received NAC. In one patient the Magseed® marked lymph node was identified and retrieved, but this was not the clipped node. Possibly the clip was lost or the clipped node was missed. Janine Simons [15] reported on an identification-rate of 100% for the Magseed® marked lymph nodes. The magnetic seed and the clip were in the same specimen in 49/50 (98%) patients. The excised TLN matched the SLN in 80% (40/50) patients.
Other methods for TLN localization as Iodine-125, described by Caudle et al. [4] or the MARI procedure, described by Donker et al. [5] underly strict radiation regulations and therefore are limited in general application. Further localization techniques with wire-localization, tattooing with charcoal injection, or radiofrequency identification tags are published.
The results of wire-localization of TLNs are varying. Hartmann et al. [7] published on wire localization of clipped nodes after NAC and concluded this technique not to be suitable for clinical practice. In 30 clipped lymph nodes wire localization was possible in 24/30 (80%), and the clipped node identification rate was 17/24 (70.8%). In 9/30 patients (30%) the clipped node removal was not confirmed by intraoperative radiologic examination. Balasubramanian et al. [8] reported on an identification rate of 92% in 23/25 patients with wire-localization. Otherwise, García-Novoa et al. [9] reported on an identification rate of 100% in 42/42 patients.
Several studies on lymph node tattooing have been published recently. Goyal et al. [10] reported a surprisingly low identification rate of 64% in patients after NAC. Although numbers were low (22 patients after NAC) in only 4 patients SLNB and identification and excision of the tattooed node was performed. In most cases ALND was performed immediately, what lowers dramatically the validity of this technique for TLNB after NAC. Allweis et al. identified 60/63 (95.2%) of the tattooed lymph nodes. The concordance rate between tattooed and hot nodes was 80% (40/50 patients) [11]. Natsiopoulos et al. described an identification rate of 94.6% (71/75 patients) [12]. Correspondence between tattooed nodes and SLNs could be observed in 75.3% (53/70 patients). Patel et al. described an identification rate of 100% in 47/47 of lymph node tattooed patients after NAC [13].
Laws et al. [18] used several methods for clipped node localization, as RFIDs in 43 patients and Magseed® in 12 patients. The failure rate was 25% with Magseed® localization. The TLN did not contain the clipped node in all 3 cases. We could assume that either the HydroMark® or the Magseed® was not deployed correctly.
The ground-breaking paper by Caudle et al. with the combination of TLNB and SLNB resulting in false-negative rates of 1.4%, and the results of the discussed studies for TLNB can be outthought to use TLNB only after NAC, without the need of SLNB, as TLNB can provide a 100% identification rate of primarily positive axillary lymph nodes. Costs and side-effects (no radioactive tracer, no blue dye), as well as surgery planning, can be reduced and optimized by the omission of SLNB. TLN negative patients after NAC can be spared ALND, as is already standard of care in some breast centers, TLN positive patients also could be spared ALND, in analogy to ACOSOG Z 0011, to give radiotherapy to the breast and the axilla and the regional lymph nodes plus additional systemic treatment according to tumor biology.
Simons et al. [20] recently published a survey on management practices in node positive patients after NAC with a wide variety in management practices. Further clinical trials are under investigation to determine the optimal surgical approach for patients with positive lymph nodes and NAC, with the goal to replace SLNB by TLNB and to omit ALND for patients who may not benefit from axillary clearance. Prospective trials as the TAXIS Trial or the AXSANA/EUBREAST Trial are on the way to give further insights in those questions.
5. Conclusion
The Magseed® marking of lymph nodes allows an exact identification and selective excision of the marked lymph nodes. The TLNs could be identified and selectively excised in all patients after NAC, in those who converted from node-positive to node negative as well as in those who stayed positive after NAC.
Declaration of competing interest
None of the authors has to disclose any commercial interest in the subject of the study, and there was no financial or material support.
References
- 1.Boughey J.C., Suman V.J., Mittendorf E.A. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. J Am Med Assoc. 2013;310(14):1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuehn T., Bauerfeind I., Fehm T. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 3.Boileau J.F., Poirier B., Basik M. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–264. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 4.Caudle A.S., Yang W.T., Krishnamurthy S., Mittendorf E.A., Black D.M. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–1078. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donker M., Straver M.E., Wesseling J. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–382. doi: 10.1097/SLA.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 6.Flores-Funes D., Aguilar-Jiménez J., Martínez-Gályez M. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: preliminary results. Surg Oncol. 2019;30:52–57. doi: 10.1016/j.suronc.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann S., Reimer T., Gerber B. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018;44(9):1307–1311. doi: 10.1016/j.ejso.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian R., Morgan C., Shaari E. Wire guided localisation for targeted axillary node dissection is accurate in axillary staging in node positive breast cancer following neoadjuvant chemotherapy. Eur J Surg Oncol. 2020;46(6):1028–1033. doi: 10.1016/j.ejso.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 9.García-Novoa A., Acea-Nebril B., Díaz Carballada C. Combining wire localization of clipped nodes with sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer: preliminary results from a prospective study. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-08925-5. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Goyal A., Puri S., Marshall A., Valassiadou K., Hoosein M.M., Carmichael A.R., Erdelyi G., Sharma N., Dunn J., York J. A multicentric prospective feasibility study of carbon dye tattooing of biopsied axillary node and surgical localisation in breast cancer patients. Breast Canc Res Treat. 2020 doi: 10.1007/s10549-020-05961-3. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Allweis T.M., Menes T., Rotbart N. Ultrasound guided tattoing of axillary lymph nodes in breast cancer patients prior to neoadjuvant therapy, and identification of tattooed nodes at the time of surgery. Eur J Surg Oncol. 2020;46(6):1041–1045. doi: 10.1016/j.ejso.2019.11.501. [DOI] [PubMed] [Google Scholar]
- 12.Natsiopoulos I., Intzes S., Liappis T. Axillary lymph node tattooing and targeted axillary dissection in breast cancer patients who presented as cN+ before neoadjuvant chemotherapy and became cN0 after treatment. Clin Breast Canc. 2019;19(3):208–215. doi: 10.1016/j.clbc.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Patel R., MacKerricher W., Tsai J. Pretreatment tattoo marking of suspicious axillary lymph nodes: reliability and correlation with sentinel lymph node. Ann Surg Oncol. 2019;26(8):2452–2458. doi: 10.1245/s10434-019-07419-3. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood H.I., Wong J.M., Mukhtar R.A. Feasibility of magnetic seeds for preoperative localization of axillary lymph nodes in breast cancer treatment. AJR Am J Roentgenol. 2019;213(4):953–957. doi: 10.2214/AJR.19.21378. [DOI] [PubMed] [Google Scholar]
- 15.Simons J.M., Scoggins M.E., Kuerer H.M. Prospective registry trial assessing the use of magnetic seeds to locate clipped nodes after neoadjuvant chemotherapy for breast cancer patiens. Ann Surg Oncol. 2021 doi: 10.1245/s10434-020-09542-y. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Taback B., Jadeja P., Ha R. Enhanced axillary evaluation using reflector-guided sentinel lymph node biopsy: a prospective feasibility study and comparison with conventional lymphatic mapping techniques. Clin Breast Canc. 2018;18(5):e869–e874. doi: 10.1016/j.clbc.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Sun J., Henry D.A., Carr M.J. Feasibility of axillary lymph node localization and excision using radar reflector localization. Clin Breast Canc. 2020;(20):30206–30208. doi: 10.1016/j.clbc.2020.08.001. S1526-8209. [DOI] [PubMed] [Google Scholar]
- 18.Laws A., Dillon K., Kelly B.N., Kantor O., Hughes K.S., Gadd M.A., Smith B.L., Lamb L.R., Specht M. Node-positive patients treated with neoadjuvant chemotherapy can be spared axillary lymph node dissection with wireless non-radioactive localizers. Ann Surg Oncol. 2020;27(12):4819–4827. doi: 10.1245/s10434-020-08902-y. [DOI] [PubMed] [Google Scholar]
- 19.Gera R., Tayeh S., Al-Reefy S., Mokbel K. Evolving role of Magseed in wireless localization of breast lesions: systematic review and pooled analysis of 1559 procedures. Anticancer Res. 2020;40(4):1809–1815. doi: 10.21873/anticanres.14135. [DOI] [PubMed] [Google Scholar]
- 20.Simons J.M., Maaskant-Braat A.J.G., Luiten E.J.T. Patterns of axillary staging and management in clinically node positive breast cancer patients treated with neoadjuvant systemic therapy: results of a survey amongst breast cancer specialists. Eur J Surg Oncol. 2020;46(1):53–58. doi: 10.1016/j.ejso.2019.08.012. [DOI] [PubMed] [Google Scholar]