Abstract
The potential mid-term and long-term consequences after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are as yet unknown. This is the first report of bronchoscopically verified organizing pneumonia as a complication of coronavirus disease 2019 (Covid19). It caused persisting dyspnea, impaired pulmonary function, and radiological abnormalities over 5 weeks after onset of symptoms. While organizing pneumonia frequently requires treatment with systemic corticosteroids, in this case it resolved spontaneously without treatment after 6 weeks. Healthcare professionals should consider organizing pneumonia in patients with persisting respiratory symptoms after Covid19.
Keywords: Interstitial lung disease, Fibrosis, Long Covid, Inflammatory lung disease, Pulmonary infection
Background
The ongoing Covid-19 pandemic causes a huge burden to healthcare providers worldwide [1, 2]. The potential mid-term and long-term clinical consequences for patients after Covid-19 infections are as yet unknown. Radiological studies and clinical courses have indicated possible organizing pneumonia as a consequence of Covid19 pneumonia [3, 4]. Establishing a histological diagnosis of suspected organizing pneumonia is important as it frequently requires treatment with systemic corticosteroids [5]. We report one of the first case of histologically verified organizing pneumonia in a patient with Covid19 pneumonia.
Case description
A 49-year-old previously healthy man had fever up to 39 °C and a dry cough starting on 22 March 2020. Prior to that a family member had experienced similar symptoms after spending time in northern Italy, which was considered the European SARS-CoV‑2 hotspot at that time. A pharyngeal swab for PCR was not obtained since he did not fulfil the case definition during that point of time. His family physician treated him with penicillin.
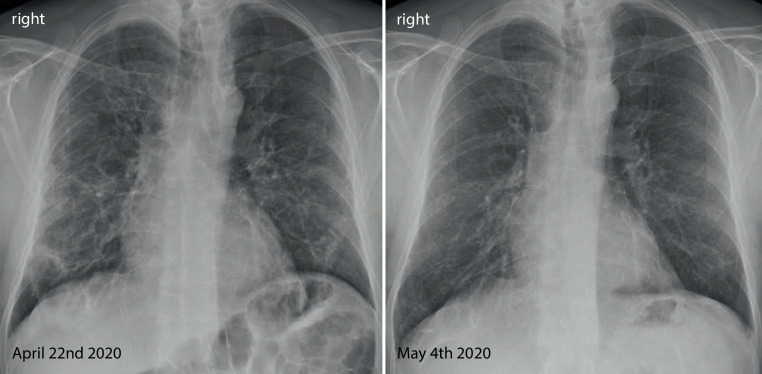
His fever disappeared but he remained substantially short of breath during minor exertion. Suspecting Covid19 he saw a pulmonologist on 15 April. Physical findings were unremarkable and the chest X‑ray showed interstitial opacities with subpleural reticular densities predominantly in the lower fields (Fig. 1). On 20 April the computed tomography (CT) scan showed subpleural patchy ground glass opacities predominantly in the upper lobes (Fig. 2). The lower lobes in addition to ground glass showed arcade-like bands of parenchymal consolidation, peribronchial consolidation and mild bronchiolectasis (Fig. 3). The CT pattern was suggestive of organizing pneumonia. He was referred to the pneumology department for further diagnostic work-up.
Fig. 1.
Posterior-anterior Chest X‑ray from 22 April and 4 May showing substantial spontaneous improvement of the interstitial opacities and reticular densities
Fig. 2.
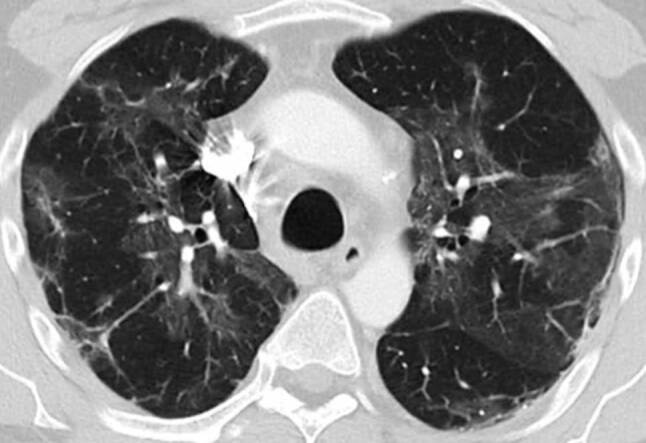
Lung CT scan in the upper lobe 4 weeks after symptom onset showing patchy subpleural ground glass opacities and linear consolidation
Fig. 3.
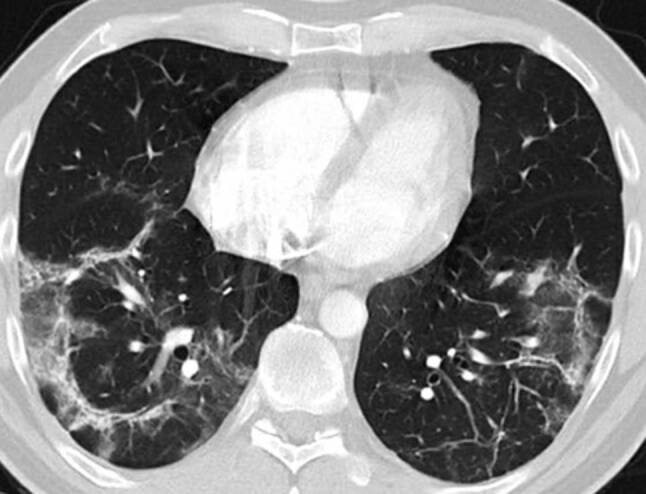
Lung CT scan in the lower lobes showing ground glass, arcade-like bands of parenchymal consolidation, peribronchial consolidation and mild bronchiolectasis
On 22 April pulmonary function tests showed a borderline restrictive ventilatory defect (total lung capacity 82%) without evidence of airflow obstruction. Diffusion capacity of the lung for carbon monoxide (DLCO) was reduced (59%) and blood gases showed mild hypoxemia with substantial hypocapnia (arterial partial pressure of oxygen [paO2] 65 mm Hg, arterial partial pressure of carbon dioxide [paCO2] 28 mm Hg, alveolararterial difference in partial pressure of oxygen [AaDO2] 51 mm Hg). Blood tests were normal apart from mildly elevated alanine aminotransferase and gamma-glutamyl transferase. The SARS-CoV‑2 PCR from a nasopharyngeal swab was negative. Serum neutralizing antibodies against SARS-CoV‑2 were positive proving the suspected Covid19 infection.
On 27 April bronchoscopy was performed with the patient under general anesthesia. Endobronchial findings were normal. Bronchoalveolar lavage from the middle lobe showed 41% alveolar macrophages and 59% lymphocytes. T cells were minimally elevated and the CD4/CD8 ratio was normal. Activated T cells and natural-killer-like T cells were substantially elevated (19% and 25% of lymphocytes, respectively). Bacterial culture and SARS-CoV‑2 PCR from the lavage were negative. Cytology obtained by endobronchial ultrasound-guided biopsy of a mildly enlarged subcarinal lymph node showed normal lymphocytes. Lung histology obtained by fluoroscopy-guided transbronchial biopsy from the right lower lobe demonstrated granulation tissue in the alveoli and bronchioles, typical of organizing pneumonia (Fig. 4).
Fig. 4.
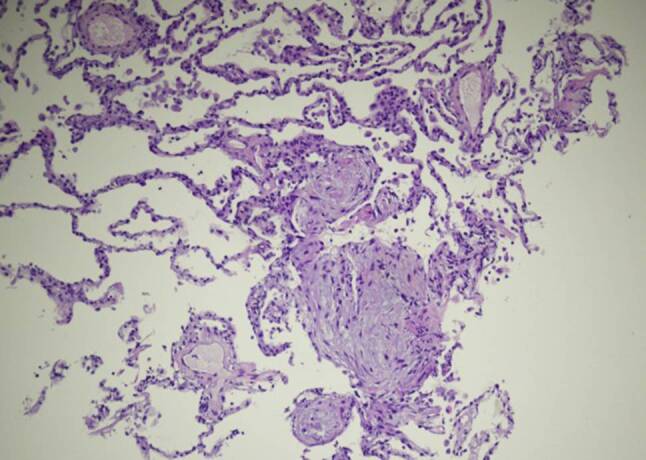
Lung histology demonstrating micropolypoid buds of pale, myxoid granulation tissue in the alveoli. These granulation areas are known as Masson bodies, protruding into the alveoli and bronchioles (hematoxylin and eosin × 100)
At the follow-up visit on 4 May originally intended to discuss potential treatment options following histological verification of organizing pneumonia, the patient reported substantial spontaneous improvements in well-being and dyspnea, prompting repeated functional assessments. Compared to previous testing lung volumes were normalized (TLC 98%) and gas exchange improved (DLCO 70%, paO2 81 mm Hg, paCO2 29 mm Hg, AaDO2 33 mm Hg). Chest X‑ray findings were also substantially improved (Fig. 1). Given the patients current clinical and functional status consensual agreement was made not to treat with systemic corticosteroids. Follow-up by chest X‑ray and pulmonary function tests were scheduled. The final diagnosis was organizing pneumonia following Covid19.
Discussion
Radiological changes during Covid19 pneumonia peak around 10 days after onset of symptoms and gradually decrease thereafter [6–8]. Ground glass opacities are predominant upon onset of symptoms and progressively transform into multifocal consolidation with septal thickening [9–11]. This radiomorphological course is indicative for an evolution towards organizing pneumonia, which is a common response to lung injury [12].
Organizing pneumonia is characterized by proliferation of granulation tissue in the alveoli or alveolar ducts, with or without obliteration of distal bronchioles [5]. It can occur without an apparent cause (cryptogenic organizing pneumonia) or as a consequence of viral infections including influenza, severe acute respiratory syndrome coronavirus 1 (CoV-1) and Middle East respiratory syndrome [13–20] as well and many other underlying causes [5]. Clinical and radiological features point towards the diagnosis, which is verified by surgical or transbronchial lung biopsy [21, 22]. The clinical and radiological course of organizing pneumonia is highly variable, ranging from mild with spontaneous remission to progressive and relapsing [22]. Patients with organizing pneumonia frequently require treatment with systemic corticosteroids. So far organizing pneumonia following Covid19 has been suspected on a radiological basis and has been found in post-mortem studies [23, 24]. One case report described organizing pneumonia following Covid19 diagnosed by thoracoscopic lung biopsy [25]. To our knowledge this is the first short report of bronchoscopically verified organizing pneumonia following Covid19.
Prognosis and response to treatment in organizing pneumonia following Covid19 is so far unknown. Since corticosteroid use might be associated with increased mortality in patients with acute coronavirus pneumonia [26] histological verification of suspected organizing pneumonia seems mandatory.
Conclusion
This is one of the first reports of organizing pneumonia as a complication of Covid19. It was the cause of persisting dyspnea, impaired pulmonary function, and radiological abnormalities 5 weeks after onset of symptoms. It improved spontaneously without treatment 6 weeks after the first symptoms. Healthcare professionals should consider organizing pneumonia in patients with persisting respiratory symptoms after Covid19.
Acknowledgments
Acknowledgements
The authors thank Michael Schleicher and Dr. Lim for language editing.
Author Contribution
G.-C. Funk, B. Thaler, C. Nell and G. Rainer wrote the case report. W. Pokieser contributed histology. A. Valipour reviewed and critically checked the text. G.-C. Funk is the guarantor of the paper, taking responsibility for the integrity of the work as a whole from inception to published article.
Funding
Open access funding provided by Medical University of Vienna.
Declarations
Conflict of interest
G.-C. Funk, C. Nell, W. Pokieser, B. Thaler, G. Rainer and A. Valipour report no conflict of interest and have read and approved the submission.
Ethical standards
The patient gave informed consent for publication of this case. Anonymity is ascertained. Ethics committee approval was waived.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Georg-Christian Funk, Email: georg-christian.funk@gesundheitsverbund.at.
Caroline Nell, Email: caroline.nell@gesundheitsverbund.at.
Wolfgang Pokieser, Email: wolfgang.pokieser@gesundheitsverbund.at.
Birgit Thaler, Email: birgit.thaler@gesundheitsverbund.at.
Gernot Rainer, Email: gernot.rainer@gmx.at.
Arschang Valipour, Email: arschang.valipour@gesundheitsverbund.at.
References
- 1.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Xu J, Li H, Cao BA. Novel Coronavirus (COVID-19) outbreak: a call for action. Chest. 2020;157(4):e99–e101. doi: 10.1016/j.chest.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hani C, Trieu NH, Saab I, et al. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kory P, Kanne JP. SARS-CoV‑2 organising pneumonia: ‘Has there been a widespread failure to identify and treat this prevalent condition in COVID-19? BMJ Open Respir Res. 2020 doi: 10.1136/bmjresp-2020-000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006;28(2):422–446. doi: 10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]
- 6.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel Coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L, Han R, Ai T, et al. Serial quantitative chest CT assessment of COVID-19: deep-learning approach. Radiology. 2020;2(2):e200075. doi: 10.1148/ryct.2020200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Chest. 2020;158(1):106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;1:7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296(2):E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua F, Armstrong-James D, Desai SR, et al. The role of CT in case ascertainment and management of COVID-19 pneumonia in the UK: insights from high-incidence regions. Lancet Respir Med. 2020;8(5):438–440. doi: 10.1016/S2213-2600(20)30132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33(7):1951–1975. doi: 10.1148/rg.337130057. [DOI] [PubMed] [Google Scholar]
- 13.Asai N, Yokoi T, Nishiyama N, et al. Secondary organizing pneumonia following viral pneumonia caused by severe influenza B: a case report and literature reviews. BMC Infect Dis. 2017;17(1):572. doi: 10.1186/s12879-017-2677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He H, Wang H, Li X, Tang X, Sun B, Tong Z. Successful management of refractory respiratory failure caused by avian influenza H7N9 and secondary organizing pneumonia: a case report and literature review. BMC Infect Dis. 2019;19(1):671. doi: 10.1186/s12879-019-4306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tse GM, To KF, Chan PK, et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J Clin Pathol. 2004;57(3):260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao CH, Wu MZ, Chen CL, et al. Evolution of pulmonary pathology in severe acute respiratory syndrome. J Formos Med Assoc. 2005;104(2):75–81. [PubMed] [Google Scholar]
- 17.Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18(1):1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai RQ, Feng XD, Gu YY, et al. Pathological changes of lungs in patients with severity acute respiratory syndrome. Zhonghua Bing Li Xue Za Zhi. 2004;33(4):354–357. [PubMed] [Google Scholar]
- 19.Kim I, Lee JE, Kim K-H, Lee S, Lee K, Mok JH. Successful treatment of suspected organizing pneumonia in a patient with Middle East respiratory syndrome coronavirus infection: a case report. J Thorac Dis. 2016;8(10):E1190–E1194. doi: 10.21037/jtd.2016.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 21.King TEJ. Organizing pneumonia. In: Schwarz MK, King TE Jr, editors. Interstitial lung disease. 5. Shelton: People’s Medical Publishing House; 2011. p. 981. [Google Scholar]
- 22.Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 23.Okamori S, Lee H, Kondo Y, et al. Coronavirus disease 2019-associated rapidly progressive organizing pneumonia with fibrotic feature: Two case reports. Medicine. 2020;99(35):e21804–e21804. doi: 10.1097/MD.0000000000021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flikweert AW, Grootenboers MJJH, Yick DCY, et al. Late histopathologic characteristics of critically ill COVID-19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae I‑G, Hong K‑W, Yang JW, Moon K, Duk JK, Ju S, et al. Persistent pneumonic consolidations due to secondary organizing pneumonia in a patient recovering from COVID-19 pneumonia: A case report. research square. 2020 doi: 10.21203/rs.3.rs-37580/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]