Abstract
The chromosome 22q11.2 deletion syndrome (22q11DS) is associated with impaired cognitive functions and increased risk for schizophrenia spectrum disorders. Speech and language deficits are prominent in the syndrome with evidence of decline anteceding emergence of psychosis. Idiopathic schizophrenia is also associated with language deficits and decline from premorbid levels. There is a paucity of data examining language function in children with 22q11DS followed longitudinally and assessed for clinical risk for psychosis. We examined the association between early language measures and current psychosis spectrum (PS) status in 166 individuals with 22q11DS. Participants were administered the Pre-School Language Scale and the Clinical Evaluation of Language Fundamentals and age appropriate IQ tests. The Structured Interview for Prodromal Syndromes (SIPS) assessed current PS symptoms. We found that performance on all measures administered begining in infancy declined with age, and males performed more poorly on core and receptive language measures. For language assessment later in childhood, poorer performance was consistently associated with current PS status. Furthermore, steeper age decline was associated with psychosis across language measures and marginally for FSIQ. These findings suggest that while early childhood language testing is useful in characterizing performance decline in individuals with 22q11DS, it does not robustly differentiate those with current PS from those without. However, language testing in the school age population can help identify individuals with 22q11DS who are at risk for psychosis. Such data are needed for elucidating a lifespan trajectory for affected individuals and may help understand pathways to psychosis applicable to the general population.
Keywords: 22q11.2 deletion syndrome, language, psychosis spectrum, cognitive development
1. INTRODUCTION
Precision medicine envisions the identification of mechanisms linking molecular markers with later onset of disorders. This mission can be facilitated by a gene-first approach, investigating copy number variations (CNVs) that confer increased vulnerability for a disorder. Schizophrenia spectrum disorders commonly emerge in late adolescence and early adulthood, and longitudinal studies starting from early childhood are needed to identify vulnerability markers. The study of early manifestations of “at risk” for psychosis has received significant effort in help-seeking young people (Cannon et al., 2016; Fusar-Poli et al., 2017) and in community samples (Calkins et al., 2014; Jones et al., 2016). A complementary approach entails examining the neurogenetic 22q11.2 Deletion Syndrome (22q11DS). The 22q11DS confers about a 25 fold increase in incidence of schizophrenia spectrum disorders, compared to the 1% in the general population (Bassett et al., 2005; Green et al., 2009; Schneider et al., 2014). It therefore provides a unique opportunity to assess the emergence of psychosis with sufficient power to identify potential biomarkers. Furthermore, most affected individuals are ascertained early and followed clinically, allowing longitudinal prospective research to examine predictors of outcome across the lifespan.
Individuals with 22q11DS vary in the range and severity of associated medical, developmental and neuropsychiatric conditions (McDonald-McGinn et al., 2015; Tang, Sunny X. & Gur, 2018). Many have congenital anomalies, medical co-morbidities, developmental delay, cognitive deficits, and learning disabilities. Neuropsychiatric conditions include anxiety, attention deficit, and autism spectrum disorders. Importantly, the pattern and timing of emergence of psychosis features resembles that of idiopathic samples (Tang & Gur, 2018). For example, as in idiopathic schizophrenia (Bearden et al., 2000; Reichenberg et al., 2005), a decrease in verbal IQ, in addition to symptoms of anxiety and depression, can precede the onset of psychosis (Gothelf et al., 2007; Tang, S. X. et al., 2014; Tang, S. X. et al., 2017; Tang, Sunny X. et al., 2014; Tang, Sunny X. et al., 2017; Vorstman et al., 2015).
Establishing a neurocognitive signature for early detection of psychosis risk in 22q11DS is challenging because speech and language disorders are evident in almost all patients. Studies of language development show receptive and expressive language delays or disorders (Persson, Laakso, Edwardsson, Lindblom, & Hartelius, 2017; Scherer, D’Antonio, & Rodgers, 2001; Solot et al., 2000, 2011, 2019). Impairments include delay in emergence of early language and ongoing difficulties with language throughout childhood and adolescence. In the early years, expressive language delay is often greater than receptive language or that predicted by overall cognitive function (Gerdes, Solot, Wang, McDonald-McGinn, & Zackai, 2001; Solot et al., 2001). There is a decline in language performance from pre-school to school-aged period, with language scores dropping from 77 on the Preschool Language Scale-3 (PLS-3), to 65.5 on the Clinical Evaluation of Language Fundamentals - R (CELF-R) (Solot et al., 2001). Children continue to demonstrate deficits in all language domains especially evident in poor organization, poor narrative cohesion, and low complexity of referential language (Solot et al., 2001; Persson et al., 2006; Van den Heuvel et al., 2017).
Language disturbance is common in idiopathic schizophrenia as well, and relates to negative symptoms, thought disorder, and impaired functioning (Andreasen & Grove, 1986). Deficits are evident in syntactic complexity and semantic coherence (Bora, Yalincetin, Akdede, & Alptekin, 2019). Abnormalities are most clinically evident in language production, but deficits in higher-order comprehension have also been noted (Kuperberg, 2010). Impairment in several linguistic domains have been identified in individuals with psychosis, including syntax, semantics, cohesion and use of metaphors (Roche, Creed, MacMahon, Brennan, & Clarke, 2014). Reduced complexity is observed even in the earliest stages of the condition (Thomas et al., 1996) and subtle disturbances are noted in individuals at clinical high risk for schizophrenia (Corcoran et al., 2018; DeVylder et al., 2014; Gooding, Ott, Roberts, & Erlenmeyer-Kimling, 2013). Furthermore, speech and language in first onset psychosis differentiate among people with schizophrenia, mania, and controls (Thomas et al., 1996).
We have examined brain-behavior measures, including psychopathology (Niarchou et al., 2017; Tang et al., 2014; Yi et al., 2015), neurocognition (Gur et al., 2014; Yi et al., 2015) and neuroimaging (Schmitt, J. E. et al., 2014; Schmitt, J. Eric et al., 2015) in youth with 22q11DS focusing on psychosis (Tang et al., 2017; Tang et al., 2017). Our studies have included individuals age 8 and older. As these participants were previously evaluated at CHOP, we set out to examine the association between early language and cognitive development and psychosis. We hypothesized that the performance of youth with psychosis spectrum features on language tests obtained earlier in childhood are impaired compared to those with no current psychosis spectrum symptoms.
2. MATERIALS AND METHODS
2.1. Sample
Participants were drawn from the collaborative prospective Brain-Behavior study of the Children’s Hospital of Philadelphia (CHOP) “22q and You Center” and the University of Pennsylvania Brain-Behavior Laboratory. All 22q11.2 deletions were confirmed in participants by multiplex ligation-dependent probe amplification in the laboratory of BSE (Jalali et al., 2008). The 166 individuals included in the analysis are an intersection of those currently enrolled in the Brain-Behavior study (n=493) and those who were previously evaluated clinically for language and cognitive development at CHOP (n=898). The recruitment for the brain-behavior study was based on consecutive presentations to the “22q and You Center” in patients who met study criteria (Gur et al., 2014): age > 8 years, stable health, IQ>70. The Institutional Review Boards of the University of Pennsylvania and CHOP approved all studies. Informed consent/assent was obtained from each participant and accompanying parent for those < 18. Sample characteristics are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics of Sample, by Language Test (PLS vs. CELF) and Psychosis Spectrum Status
| PLS | CELF | ||||||
|---|---|---|---|---|---|---|---|
| PS Status | PS Status | ||||||
| Variable | Full Sample | Full Group | Non-PS | PS | Full Group | Non-PS | PS |
| N | 166 | 102 | 44 | 58 | 64 | 17 | 47 |
| Age (mo) (SD) | 71.0 (47.3) | 40.3 (21.3) | 44.0 (22.9) | 37.5 (19.8) | 119.9 (34.4) | 120.9 (35.6) | 119.6 (34.3) |
| Prop. White | 0.88 | 0.89 | 0.98 | 0.83 | 0.86 | 0.94 | 0.83 |
| Prop. Female | 0.39 | 0.34 | 0.32 | 0.36 | 0.47 | 0.53 | 0.45 |
| GAF (SD) | 61.1 (15.3) | 61.6 (14.5) | 67.0 (16.5) | 57.7 (11.6) | 60.3 (16.6) | 74.1 (14.0) | 55.0 (14.3) |
| Receptive Lan. (SD) | 78.6 (13.3) | 79.9 (13.1) | 81.6 (13.1) | 78.6 (13.0) | 75.9 (13.7) | 82.6 (12.5) | 73.3 (13.3) |
| Expressive Lan. (SD) | 73.8 (14.6) | 73.5 (13.9) | 72.9 (13.3) | 74.0 (14.4) | 74.2 (16.0) | 82.1 (17.2) | 71.0 (14.5) |
| Core Lan. (SD) | 73.5 (14.1) | 73.2 (13.0) | 74.0 (12.6) | 72.6 (13.3) | 74.0 (15.8) | 81.6 (14.8) | 71.3 (15.4) |
| VIQ (SD) | 82.2 (14.5) | 83.2 (15.7) | 86.0 (13.9) | 80.2 (17.5) | 81.6 (13.9) | 86.7 (9.5) | 79.5 (15.0) |
| PIQ (SD) | 69.6 (14.2) | 67.7 (14.9) | 69.3 (15.9) | 66.6 (14.2) | 73.3 (12.2) | 74.1 (13.1) | 73.0 (12.1) |
| FSIQ (SD) | 75.0 (13.7) | 73.9 (14.6) | 75.2 (14.7) | 72.9 (14.6) | 76.8 (11.9) | 82.0 (11.3) | 74.7 (11.6) |
| SIPS Total (SD) | 26.3 (19.0) | 24.4 (16.3) | 15.6 (13.9) | 30.9 (14.8) | 29.3 (22.6) | 15.2 (15.4) | 34.3 (22.7) |
Note. PLS = Pre-School Language Scale; CELF = Clinical Evaluation of Language Fundamentals; PS = psychosis spectrum; mo = months; SD = standard deviation; Prop = proportion; GAF=Global Assessment of Function; Lan = language; VIQ =Verbal Intelligence Quotient; PIQ = Performance IQ; FSIQ = full-scale IQ; SIPS = Structured Interview for Prodromal Syndromes.
2.2. Neuropsychiatric assessment
Psychiatric evaluation was conducted by highly trained assessors under the supervision of faculty, as part of the collaborative brain-behavior study, and included direct assessment of the individuals with 22q11DS, collateral information from parents and review of records (Tang et al., 2014; Tang et al., 2017; Tang et al., 2014; Tang et al., 2017; Tang & Gur, 2018; Yi et al., 2015). A computerized adaptation of the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al., 1997) was administered and the assessment incorporated a timeline of life events, demographics, medical history, social and treatment histories, and psychopathology evaluation. K-SADS sections assessed DSM-IV mood, and substance related disorders, and ADHD. The complete Structured Interview for Prodromal Syndromes (SIPS) (McGlashan, Walsh, & Woods, 2010) was administered to evaluate threshold and subthreshold psychotic symptoms. A consensus conference reviewed the obtained data and assigned a Psychosis Spectrum (PS) status, or lack thereof, based on criteria previously detailed (Tang et al., 2014; Tang et al., 2017; Tang et al., 2014; Tang et al., 2017). Briefly, the 19 items on the Scale of Prodromal Symptoms (SOPS) were rated according to standardized anchors on a 7-point scale (0=absent to 6=severe and psychotic/extreme. Only symptoms occurring in the last six months were considered in the following domains: positive (5 items), negative (6 items), disorganization (4 items) and general (4 items) symptoms subscales (McGlashan et al., 2010; Miller et al., 2003). The presence of subthreshold psychotic symptoms was based on summing up items’ ratings of positive, negative and disorganization subscales (Tang et al., 2014; Tang et al., 2014; Weisman et al., 2017). The SIPS Global Assessment of Functioning Scale (GAF) was included as part of the assessment. If the participant had more than one clinical research assessment (i.e. multiple time points), his/her most recent time point was used.
2.3. Speech and language evaluation
Patients were seen by a single licensed, certified speech-language pathologist, experienced in test administration and 22q11.2 DS. Age appropriate, standard measures were used. The Pre-School Language Scale (PLS) versions 3–5 was administered to children aged 4 months to 7.6 years. The PLS measures early language development, assessing a broad range of receptive and expressive language skills. The Clinical Evaluation of Language Fundamentals (CELF) version R-5 was administered to children aged 5.5 to 17.3 years. The CELF is designed to identify children who lack the basic foundations of form (syntax) and content (semantics) of language understanding and use. Both of these tests provide a score for receptive and expressive language and derive a composite score from the two subtests, with a mean of 100 and a standard deviation of 15. The mean interval between language test and diagnosis was 121.2 months (10.1 years).
Language data were augmented with developmental psychological testing conducted within two years of the language evaluation. Testing was administered by psychologists with expertise assessing youth with 22q11DS. Standard psychological instruments were used. The Bayley Test of Infant Development (versions 2, 3) was administered to children ages 4 to 55 months. This test is designed to identify children with cognitive and motor delays. Children aged 32 to 84 months were administered the Wechsler Pre-School and Primary Scales of Intelligence (WPPSI) versions R, III, IV. The WPPSI measures cognitive development in preschoolers and young children, ages 2.6 – 7.7 years. There are three main index scores: Verbal, Performance, and Full Scale IQ. Children ages (6–18 years) received the Wechsler Intelligence Test for Children, (WISC; versions R, III, IV). For the current analysis, Verbal IQ was used as a comparison score with the language measures. Table 1 details the sample size for each language measure obtained, age at assessment, demographics, IQ estimate available, and the total SIPS scores. The timing and extent of the clinical evaluation commonly related to referral and scheduling constraints. Notably, there were no differences in any of the measures between the larger sample examined clinically and the subsample enrolled later in the Brain-Behavior study and presented here.
2.4. Data analysis
To test the effects of age, sex, and psychosis spectrum (PS) symptoms on cognitive performance, we used repeated-measures mixed models, first including only main effects, and subsequently adding an interaction term between age and PS. The sample was split into two groups based on age. The younger group (mean age = 40.3 months; min = 4; max = 115) was administered the PLS language tests plus the WISC, Bayley, and WPPSI. The older group (mean age = 119.9 months; min = 66; max = 208) received only the CELF language tests and the WISC.
3. RESULTS
Table 2 shows the results of the repeated-measures mixed models in the younger (PLS) sample, separated by PS status. All language tests show a significant decline in performance with age, regardless of sex or PS status (i.e. no significant interaction with age), and males showed significantly worse performance (main effect) on Core and Receptive language. Finally, while relationships of IQ with age, sex, and psychosis were mostly non-significant, there was a significant positive association of age with PIQ.
Table 2.
Mixed Model Results Predicting Cognitive Scores with Sex, Age, Psychosis Status, and Age-by-Psychosis Interaction* in the PLS Sample.
| Dependent Variable | Predictor | Std. Coef. | t | p-value |
|---|---|---|---|---|
| Core | Age | −0.58 | −3.9 | <0.0005 |
| Psychosis | −0.14 | −0.8 | 0.431 | |
| Male Sex | 0.39 | 2.1 | 0.034 | |
| Age*Psychosis | 0.13 | 0.5 | 0.638 | |
| Receptive | Age | −0.46 | −3.0 | 0.003 |
| Psychosis | −0.24 | −1.3 | 0.196 | |
| Male Sex | 0.52 | 2.8 | 0.007 | |
| Age*Psychosis | 0.35 | 1.2 | 0.219 | |
| Expressive | Age | −0.65 | −4.2 | <0.0005 |
| Psychosis | −0.04 | −0.2 | 0.814 | |
| Male Sex | 0.36 | 1.9 | 0.055 | |
| Age*Psychosis | 0.01 | 0.0 | 0.965 | |
| VIQ | Age | 0.27 | 1.2 | 0.389 |
| Psychosis | −0.11 | −0.3 | 0.787 | |
| Male Sex | 0.12 | 0.3 | 0.776 | |
| Age*Psychosis | 0.46 | 0.6 | 0.585 | |
| PIQ | Age | 0.64 | 2.5 | 0.014 |
| Psychosis | −0.02 | −0.1 | 0.951 | |
| Male Sex | 0.11 | 0.4 | 0.691 | |
| Age*Psychosis | −0.13 | −0.3 | 0.747 | |
| FSIQ | Age | 0.32 | 1.3 | 0.200 |
| Psychosis | 0.03 | 0.1 | 0.902 | |
| Male Sex | 0.16 | 0.6 | 0.527 | |
| Age*Psychosis | 0.32 | 0.9 | 0.392 |
Note.
interactions were tested in a separate model including all effects, so the main effects reported here are for a model without the interaction; PLS=Pre-School Language Scale; Std = standardized; Coef = coefficient; VIQ = verbal IQ; FSIQ = full-scale IQ; significant (p < 0.05) effects bolded.
Table 3 shows the results of the repeated-measures mixed models in the older (CELF) sample, separated by PS status. Core and Expressive language tests were significantly lower for PS participants than for non-PS, and Expressive language showed a significant increase with age (main effect). Receptive language showed a significant age-by-PS interaction, whereby non-PS participants showed improvement in Receptive language score across time and the PS participants showed worsening. Finally, FSIQ was significantly lower for PS participants than for non-PS (main effect).
Table 3.
Mixed Model Results Predicting Cognitive Scores with Sex, Age, Psychosis Status, and Age-by-Psychosis Interaction* in the CELF Sample.
| Dependent Variable | Predictor | Std. Coef. | t | p-value |
|---|---|---|---|---|
| Core | Age | −0.01 | −0.1 | 0.935 |
| Psychosis | −0.70 | −2.8 | 0.006 | |
| Male Sex | 0.16 | 0.6 | 0.518 | |
| Age*Psychosis | −0.39 | −1.8 | 0.083 | |
| Receptive | Age | 0.03 | 0.2 | 0.836 |
| Psychosis | −0.50 | −1.9 | 0.061 | |
| Male Sex | −0.09 | −0.3 | 0.738 | |
| Age*Psychosis | −0.68 | −2.3 | 0.029 | |
| Expressive | Age | 0.30 | 2.8 | 0.010 |
| Psychosis | −0.71 | −2.7 | 0.010 | |
| Male Sex | −0.13 | −0.5 | 0.615 | |
| Age*Psychosis | −0.25 | −1.1 | 0.270 | |
| VIQ | Age | −0.23 | −1.1 | 0.276 |
| Psychosis | −0.55 | −1.7 | 0.090 | |
| Male Sex | −0.35 | −1.1 | 0.260 | |
| Age*Psychosis | −0.45 | −1.0 | 0.318 | |
| PIQ | Age | −0.13 | −0.7 | 0.486 |
| Psychosis | −0.22 | −0.8 | 0.435 | |
| Male Sex | −0.15 | −0.6 | 0.567 | |
| Age*Psychosis | −0.39 | −1.0 | 0.339 | |
| FSIQ** | Age | −0.27 | −1.6 | 0.106 |
| Psychosis | −0.48 | −2.0 | 0.046 | |
| Male Sex | −0.12 | −0.5 | 0.602 | |
| Age*Psychosis | −0.23 | −0.6 | 0.522 |
Note.
interactions were tested in a separate model including all effects, so the main effects reported here are for a model without the interaction; CELF=Clinical Evaluation of Language Fundamentals; Std = standardized; Coef = coefficient; VIQ = verbal IQ; FSIQ = full-scale IQ; significant (p < 0.05) effects bolded;
due to non-convergence across all mixed model optimizers (likely due to too few complete longitudinal observations), the FSIQ model was estimated as a general linear model.
Figures 1, 2, and 3 show the key language-related results (from Tables 2 and 3). There is a clear pattern across language tests whereby the younger (PLS) participants show worsening of language skills across time whereas the performance of the older (CELF) participants across time depends on their PS status. Specifically, language skills of non-PS participants consistently improve over time, while the PS participants’ skills worsen (or stay the same, in the case of Expressive language). It is also worth noting that, although the PS-by-age interaction was not found for the younger (PLS) participants, there is a clear trend across Figures 1–3 whereby PS participants’ language scores decrease at a faster rate than those of non-PS participants. This suggests that the null interaction results for the younger sample is likely due to insufficient power.
Figure 1.
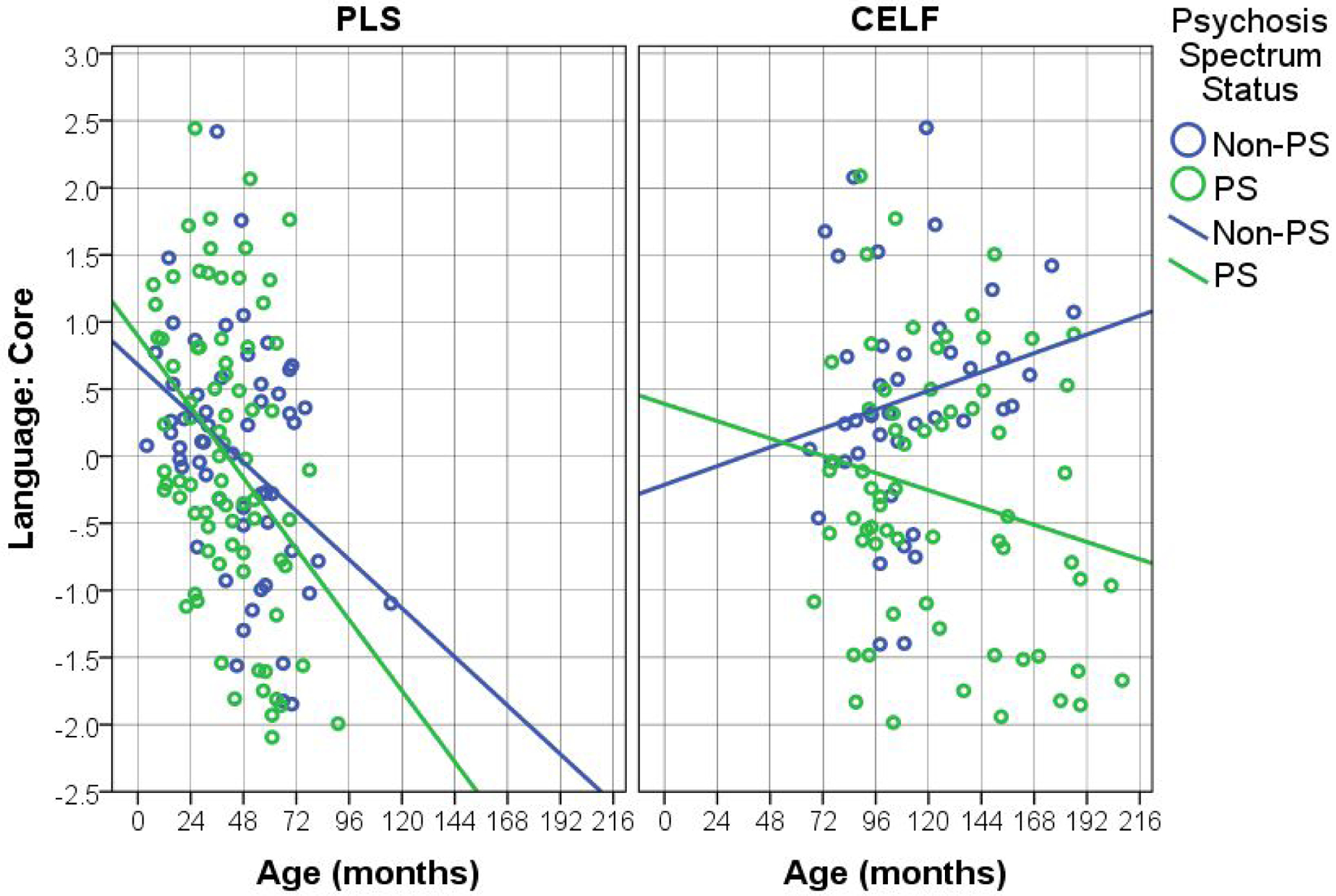
Effects of Age on Core Language, by Test Type (Pre-School Language Scale – PLS; Clinical Evaluation of Language Fundamentals - CELF) and Psychosis Spectrum Status (PS).
Figure 2.
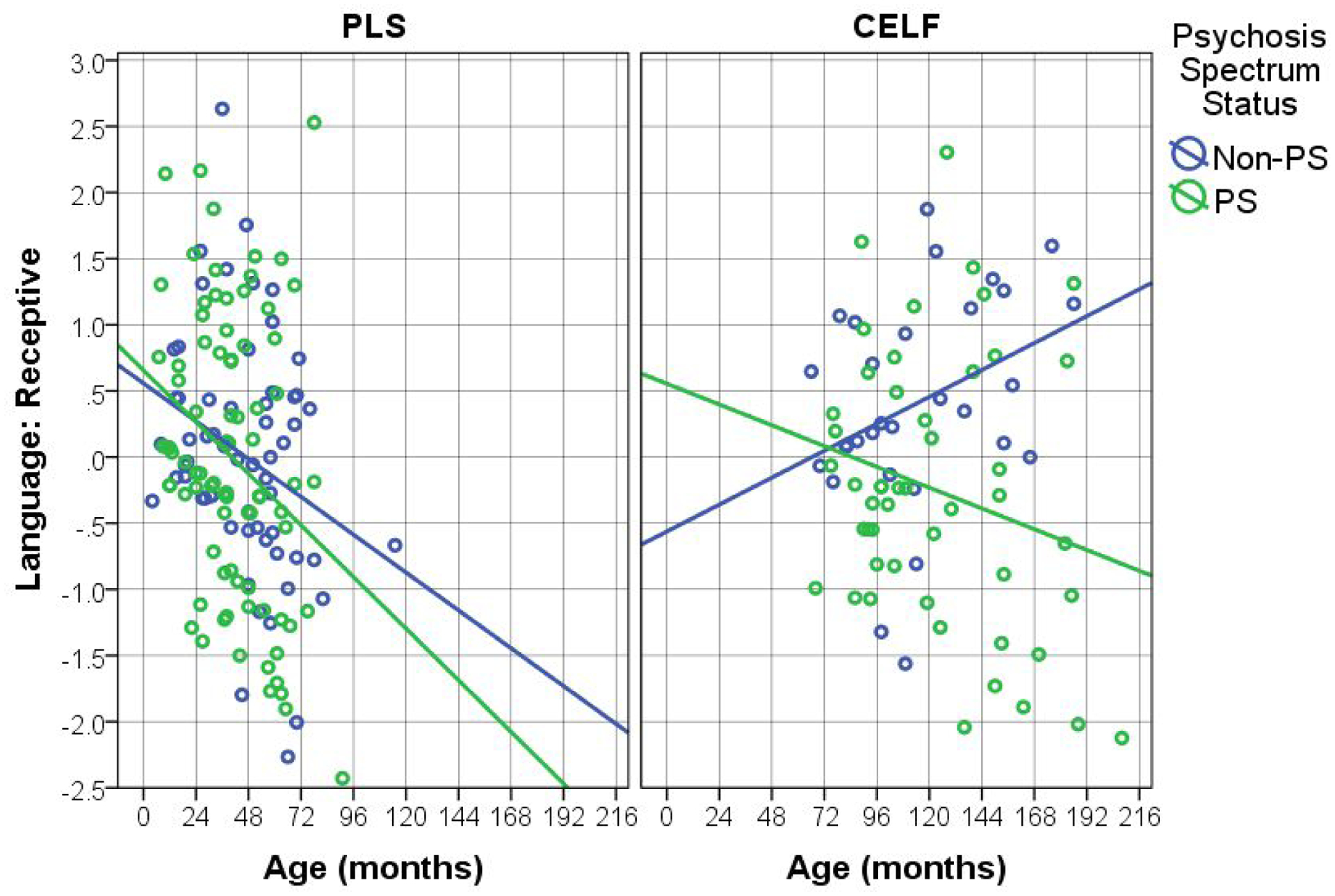
Effects of Age on Receptive Language, by Test Type (Pre-School Language Scale – PLS; Clinical Evaluation of Language Fundamentals - CELF) and Psychosis Spectrum Status (PS).
Figure 3.
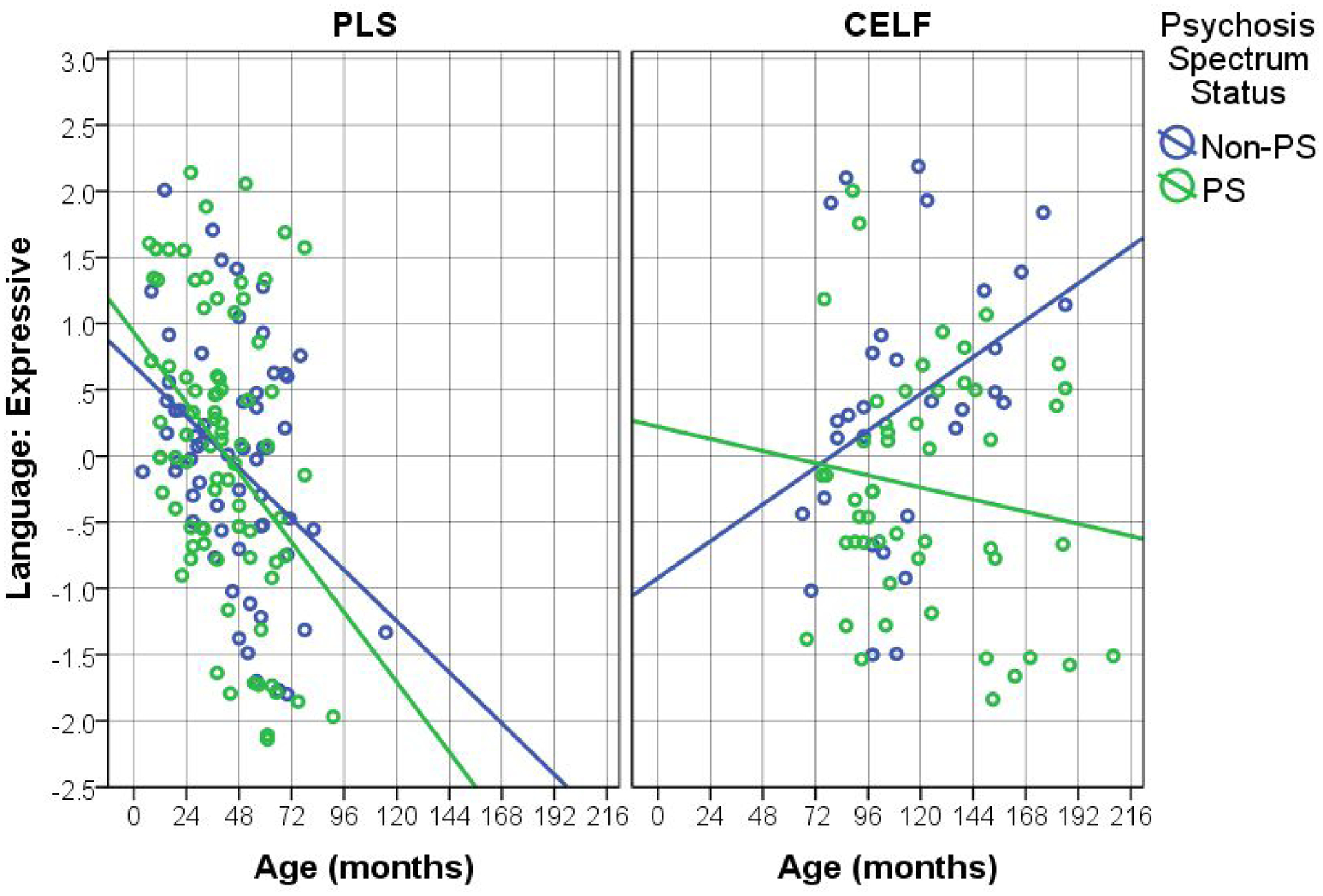
Effects of Age on Expressive Language, by Test Type (Pre-School Language Scale – PLS; Clinical Evaluation of Language Fundamentals - CELF) and Psychosis Spectrum (PS) Status.
DISCUSSION
In this study we examined whether behavioral measures of development administered in early years are associated with psychosis spectrum symptoms evident later. We found that for language measures administered to preschoolers, all showed decline with age while IQ was stable. Males performed more poorly on Core and Receptive language, with a trend for Expressive language measures. For language related assessment later in childhood, we found poorer performance consistently associated with later psychosis features and greater age decline associated with psychosis in Core, Receptive and Expressive language as well as FSIQ. Thus, language performance deficits and language decline with age are markers of 22q11DS vulnerability to psychosis.
Language capacities are key cognitive domains that are impaired in several neurodevelopmental disorders. The current study capitalized on available systematic assessments of development, language and cognition in an informative neurogenetic condition associated with occurrence of psychosis. In the subsample that years later enrolled in our study of brain and behavior, several findings emerged by examining this archival data. As in previous reports on 22q11DS, we observed receptive and expressive language delays (Solot et al., 2019; Swillen, Vogels, Devriendt, & Fryns, 2000; Van Den Heuvel et al., 2017) with a significant decline in performance from the pre-school through the school-aged period. Thus, language deficits persist across domains and the pattern of language related deficits is consistent with previous literature on 22q11DS.
Against this background, the group that at followup exhibited psychosis spectrum symptoms showed a distinct trajectory of greater decline in language abilities compared to individuals with 22q11DS without current psychosis spectrum features. This effect can be seen in language performance starting at school age. Thus, early deficits in language development in children with 22q11DS portend greater vulnerability to psychosis at later ages. Language development has been examined in typically developing children and across neurodevelopmental disorders and has been linked to brain maturation and abnormalities observed in MRI studies (Weiss-Croft & Baldeweg, 2015). 22q11DS is characterized by significant structural and functional brain aberrations consistent with abnormal maturation (Mattiaccio et al., 2018; Padula et al., 2018; Roalf et al., 2017; Schmitt et al., 2015). The present results show that deficits in language performance may relate to a pathway to psychosis and therefore efforts at amelioration likely address a core feature of the disorder.
As psychosis commonly emerges in adolescence and early adulthood, there is a clear need for longitudinal studies examining common measures in children across the developmental span. Our unique sample demonstrates the value of such measures to potentially identify vulnerable subpopulations. Notably, the same pattern of findings has been documented in idiopathic schizophrenia spectrum disorders, with subtle developmental deficits, including language (Corcoran et al., 2018; DeVylder et al., 2014). The sex differences we found of poorer language skills in pre-school males is likewise consistent with findings in non-deleted samples and other neurodevelopmental disorders (Etchell et al., 2018). Similarly, the lower level of functioning measured in the GAF (Table 1), evident in the PS groups, accords with non-deleted samples (Fusar-Poli et al., 2015).
Our study has several limitations. The ascertainment method for the brain-behavior study limited the sample of 22q11DS to individuals with IQ≥70. This limitation may have attenuated effects but is unlikely to have generated them. Another limitation is that the IQ-related tests were not administered concomitantly with the language tests (PLS and CELF). The clinical evaluation was conducted by the same professional adhering to standard procedures, yet scheduling and burden to patients and family had to be considered. We focus in the current study on earlier obtained language parameters in relation to psychosis features later in life. Participants also received a computerized neurocognitive assessment that can be examined in future analysis. Finally, advances in linguistics can be incorporated into future measures and studies.
5. CONCLUSIONS
Early language testing is useful in characterizing cognitive development and can establish the pattern and progression of deficits associated with neurogenetic and neuropsychiatric disorders. By examining the intersection of these disorders, the present study demonstrates that language testing at school age can not only identify deficits associated with 22q11DS, but also differentiate individuals at risk for psychosis spectrum symptoms.
ACKNOWLEDGEMENTS
This study was funded by National Institute of Health (NIH) grants MH087626; MH087636, MH119737, and MH119738. Additional support was provided by the Lifespan Brain Institute, Children’s Hospital of Philadelphia and Penn Medicine, University of Pennsylvania. We thank the participants and their families, the teams at the 22q and You center at CHOP and in Neuropsychiatry, the Department of Psychiatry, Penn Medicine, University of Pennsylvania.
Footnotes
CONFLICT OF INTEREST
All listed authors have no financial disclosures.
REFERENCES
- Andreasen NC, & Grove WM (1986). Thought, language, and communication in schizophrenia: Diagnosis and prognosis. Schizophrenia Bulletin, 12(3), 348–359. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, & Gatzoulis MA (2005). Clinical features of 78 adults with 22q11 deletion syndrome. American Journal of Medical Genetics Part A, 138(4), 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Rosso IM, Hollister JM, Sanchez LE, Hadley T, & Cannon TD (2000). A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophrenia Bulletin, 26(2), 395–410. [DOI] [PubMed] [Google Scholar]
- Bora E, Yalincetin B, Akdede BB, & Alptekin K (2019). Neurocognitive and linguistic correlates of positive and negative formal thought disorder: A meta-analysis. Schizophrenia Research, 209:2–11. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, … Gur RE (2014). The psychosis spectrum in a young US community sample: Findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry, 13(3), 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, … Kattan MW (2016). An individualized risk calculator for research in prodromal psychosis. American Journal of Psychiatry, 173(10), 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Carrillo F, Fernández Slezak D, Bedi G, Klim C, Javitt DC, … Cecchi GA (2018). Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry, 17(1), 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVylder JE, Muchomba FM, Gill KE, Ben-David S, Walder DJ, Malaspina D, & Corcoran CM (2014). Symptom trajectories and psychosis onset in a clinical high-risk cohort: The relevance of subthreshold thought disorder. Schizophrenia Research, 159(2–3), 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchell A, Adhikari A, Weinberg LS, Choo AL, Garnett EO, Chow HM, & Chang S (2018). A systematic literature review of sex differences in childhood language and brain development. Neuropsychologia, 114, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, De Micheli A, Cappucciati M, Rutigliano G, Davies C, Ramella-Cravaro V, … McGuire P (2017). Diagnostic and prognostic significance of DSM-5 attenuated psychosis syndrome in services for individuals at ultra high risk for psychosis. Schizophrenia Bulletin, 44(2), 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Rocchetti M, Sardella A, Avila A, Brandizzi M, Caverzasi E, … McGuire P (2015). Disorder, not just state of risk: Meta-analysis of functioning and quality of life in people at high risk of psychosis. The British Journal of Psychiatry, 207(3), 198–206. [DOI] [PubMed] [Google Scholar]
- Gerdes M, Solot C, Wang PP, McDonald-McGinn DM, & Zackai EH (2001). Taking advantage of early diagnosis: Preschool children with the 22q11. 2 deletion. Genetics in Medicine, 3(1), 40. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Ott SL, Roberts SA, & Erlenmeyer-Kimling L (2013). Thought disorder in mid-childhood as a predictor of adulthood diagnostic outcome: Findings from the new york high-risk project. Psychological Medicine, 43(5), 1003–1012. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, … Reiss AL (2007). Risk factors for the emergence of psychotic disorders in adolescents with 22q11. 2 deletion syndrome. American Journal of Psychiatry, 164(4), 663–669. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, … Eliez S (2009). Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11. 2 deletion) syndrome. Journal of the American Academy of Child & Adolescent Psychiatry, 48(11), 1060–1068. [DOI] [PubMed] [Google Scholar]
- Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D, … Gur RC (2014). Neurocognitive development in 22q11. 2 deletion syndrome: Comparison with youth having developmental delay and medical comorbidities. Molecular Psychiatry, 19(11), 1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali GR, Vorstman J, Errami AB, Vijzelaar R, Biegel J, Shaikh T, & Emanuel BS (2008). Detailed analysis of 22q11. 2 with a high density MLPA probe set. Human Mutation, 29(3), 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, … Jones PB (2016). Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry, 73(3), 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR (2010). Language in schizophrenia part 1: An introduction. Language and Linguistics Compass, 4(8), 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiaccio LM, Coman IL, Thompson CA, Fremont WP, Antshel KM, & Kates WR (2018). Frontal dysconnectivity in 22q11. 2 deletion syndrome: An atlas-based functional connectivity analysis. Behavioral and Brain Functions, 14(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, … Bassett AS (2015). 22q11. 2 deletion syndrome. Nature Reviews Disease Primers, 1, 15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Walsh B, & Woods S (2010). The psychosis-risk syndrome: Handbook for diagnosis and follow-up Oxford University Press. [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, & McFarlane W (2003). Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms. Schizophrenia Bulletin, 29, 703–715. [DOI] [PubMed] [Google Scholar]
- Niarchou M, Moore TM, Tang SX, Calkins ME, McDonald-McGuinn DM, Zackai EH, … Gur RE (2017). The dimensional structure of psychopathology in 22q11. 2 deletion syndrome. Journal of Psychiatric Research, 92, 124–131. [DOI] [PubMed] [Google Scholar]
- Padula MC, Schaer M, Armando M, Sandini C, Zöller D, Scariati E, … Eliez S (2018). Cortical morphology development in patients with 22q11. 2 deletion syndrome at ultra-high risk of psychosis. Psychological Medicine, 48(14), 2375–2383. [DOI] [PubMed] [Google Scholar]
- Persson C, Laakso K, Edwardsson H, Lindblom J, & Hartelius L (2017). Signs of dysarthria in adults with 22q11. 2 deletion syndrome. American Journal of Medical Genetics Part A, 173(3), 618–626. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, … Harvey PD (2005). Elaboration on premorbid intellectual performance in schizophrenia: Premorbid intellectual decline and risk for schizophrenia. Archives of General Psychiatry, 62(12), 1297–1304. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Schmitt JE, Vandekar SN, Satterthwaite TD, Shinohara RT, Ruparel K, … Gur RE (2017). White matter microstructural deficits in 22q11. 2 deletion syndrome. Psychiatry Research: Neuroimaging, 268, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche E, Creed L, MacMahon D, Brennan D, & Clarke M (2014). The epidemiology and associated phenomenology of formal thought disorder: A systematic review. Schizophrenia Bulletin, 41(4), 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer NJ, D’Antonio LL, & Rodgers JR (2001). Profiles of communication disorder in children with velocardiofacial syndrome: Comparison to children with down syndrome. Genetics in Medicine, 3(1), 72. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Yi JJ, Roalf DR, Loevner LA, Ruparel K, Whinna D, … Gur RE (2014). Incidental radiologic findings in the 22q11. 2 deletion syndrome. American Journal of Neuroradiology, 35(11), 2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR, … Gur RE (2015). Aberrant cortical morphometry in the 22q11. 2 deletion syndrome. Biological Psychiatry, 78(2), 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Bassett AS, Chow EW, Fung WLA, van den Bree MBM, … Eliez S (2014). Psychiatric disorders from childhood to adulthood in 22q11. 2 deletion syndrome: Results from the international consortium on brain and behavior in 22q11. 2 deletion syndrome. American Journal of Psychiatry, 171(6), 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solot CB, Gerdes M, Kirschner RE, McDonald-McGinn DM, Moss E, Woodin, Wang PP (2001). Communication issues in 22q11. 2 deletion syndrome: Children at risk. Genetics in Medicine, 3(1), 67. [DOI] [PubMed] [Google Scholar]
- Solot CB, Knightly C, Handler SD, Gerdes M, McDonald-McGinn DM, Moss E, … Driscoll DA (2000). Communication disorders in the 22Q11. 2 microdeletion syndrome. Journal of Communication Disorders, 33(3), 187–204. [DOI] [PubMed] [Google Scholar]
- Solot CB, Sell D, Mayne A, Baylis AL, Persson C, Jackson O, & McDonald-McGinn DM (2019). Speech-language disorders in 22q11. 2 deletion syndrome: Best practices for diagnosis and management. American Journal of Speech-Language Pathology, , 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Vogels A, Devriendt K, & Fryns J (2000). Chromosome 22q11 deletion syndrome: Update and review of the clinical features, cognitive behavioral spectrum, and psychiatric complications. American Journal of Medical Genetics, 97(2), 128–135. [DOI] [PubMed] [Google Scholar]
- Tang SX, Moore TM, Calkins ME, Yi JJ, McDonald-McGinn DM, Zackai EH, . . Gur RE (2017). Emergent, remitted and persistent psychosis-spectrum symptoms in 22q11. 2 deletion syndrome. Translational Psychiatry, 7(7), e1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC, … Gur RC (2014). Psychiatric disorders in 22q11. 2 deletion syndrome are prevalent but undertreated. Psychological Medicine, 44(6), 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, & Gur RE (2018). Longitudinal perspectives on the psychosis spectrum in 22q11. 2 deletion syndrome. American Journal of Medical Genetics Part A, 176(10), 2192–2202. [DOI] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Moore TM, Calkins ME, Kohler CG, Whinna DA, … Gur RE (2014). Subthreshold psychotic symptoms in 22q11. 2 deletion syndrome. Journal of the American Academy of Child & Adolescent Psychiatry, 53(9), 99–1000. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Moore TM, Calkins ME, James JY, Savitt A, Kohler CG, … Gur RE (2017). The psychosis spectrum in 22q11. 2 deletion syndrome is comparable to that of nondeleted youths. Biological Psychiatry, 82(1), 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Kearney G, Napier E, Ellis E, Leudar I, & Johnston M (1996). Speech and language in first onset psychosis differences between people with schizophrenia, mania, and controls. The British Journal of Psychiatry, 168(3), 337–343. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel E, ReuterskiöLd C, Solot C, Manders E, Swillen A, & Zink I (2017). Referential communication abilities in children with 22q11. 2 deletion syndrome. International Journal of Speech-Language Pathology, 19(5), 490–502. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, … Bassett AS (2015). Cognitive decline preceding the onset of psychosis in patients with 22q11. 2 deletion syndrome. JAMA Psychiatry, 72(4), 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Guri Y, Gur RE, McDonald-McGinn DM, Calkins ME, Tang SX, … Gothelf D (2017). Subthreshold psychosis in 22q11. 2 deletion syndrome: Multisite naturalistic study. Schizophrenia Bulletin, 43(5), 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss-Croft LJ, & Baldeweg T (2015). Maturation of language networks in children: A systematic review of 22 years of functional MRI. NeuroImage, 123, 269–281. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Calkins ME, Tang SX, Kohler CG, McDonald-McGinn DM, Zackai EH, . . Gur RE (2015). Impact of psychiatric comorbidity and cognitive deficit on function in 22q11. 2 deletion syndrome. The Journal of Clinical Psychiatry, 76(10), 1262. [DOI] [PubMed] [Google Scholar]


