Abstract
Poverty and threat exposure (TE) predict deficits in emotion regulation (ER). Effective cognitive ER (i.e., reappraisal) may be supported by: (1) cognitive processes implicated in generating and implementing cognitive reappraisal, supported by activation in brain regions involved in cognitive control (e.g., frontal, insular, and parietal cortices) and (2) emotion processing and reactivity, involving identification, encoding, and maintenance of emotional states and related variation in brain activity of regions involved in emotional reactivity (i.e., amygdala). Poverty is associated with deficits in cognitive control, and TE with alterations in emotion processing and reactivity. Our goal was to identify dissociable emotional and cognitive pathways to ER deficits from poverty and TE. Measures of cognitive ability, emotional processing and reactivity, ER, and neural activity during a sadness ER task, were examined from a prospective longitudinal study of youth at risk for depression (n = 139). Both cognitive ability and left anterior insula extending into the frontal operculum activity during a sadness reappraisal task mediated the relationship between poverty and ER. Emotion processing/reactivity didn’t mediate the relationship of TE to ER. Findings support a cognitive pathway from poverty to ER deficits. They also underscore the importance of dissociating mechanisms contributing to ER impairments from adverse early childhood experiences.
Keywords: Emotion regulation, Poverty, Left anterior insula, Language
1. Introduction
One in five children in the US lives below the poverty line and between one in eight and one in four experiences maltreatment including threatening exposures like physical or sexual abuse (Finkelhor et al., 2005, 2013; Koball and Jiang, 2018; Prevention, 2013). Poverty and exposure to threat are associated with negative health consequences throughout the lifetime (Arnow, 2004; McCrory et al., 2011; McLaughlin et al., 2014, 2019). As such, it is imperative to understand the neural and psychological mechanisms through which these experiences confer risk for poor mental health outcomes.
Deficits in effective emotion regulation (ER) may be one mechanism linking both childhood threat exposure (TE) and poverty with risk for psychopathology (Buckner et al., 2003; Johnson et al., 2016; Kim et al., 2013; Liberzon et al., 2015; Lipina and Evers, 2017; Palacios-Barrios and Hanson, 2019). ER is the ability to influence the experience and expression of emotion via both automatic and controlled processes (Gross, 1998). Effective ER is a protective factor against poor health outcomes such as early-onset psychopathology (Aldao et al., 2010; Troy and Mauss, 2011; Yoo et al., 2006). Poverty and TE are both predictors of deficits in ER (Feng et al., 2009; Kim et al., 2013; Kim and Cicchetti, 2010; Kim-Spoon et al., 2013; McLaughlin et al., 2015). Such findings are salient in light of evidence that effective ER buffers against the negative effects of early life adversities such as poverty and TE (Kim-Spoon et al., 2013; Smith et al., 2014). For example, in threat exposed youth, greater ER protected against psychopathology (Kim and Cicchetti, 2010). There is also evidence that cognitive ER is associated with less depression for lower socioeconomic status (SES) but not higher SES individuals, suggesting that ER may be particularly beneficial for individuals from lower SES (Troy et al., 2017).
Thus, interventions that bolster ER could reduce the risk for poor health outcomes in youth exposed to adversity. Until recently, these efforts have been stymied because the literature has lumped together dissociable dimensions of childhood adversity (McLaughlin et al., 2014; Sheridan and McLaughlin, 2014). However, according to the dimensional model of adversity and psychopathology, specific developmental mechanisms link different types of adversity with mental health outcomes and lumping together these dissociable dimensions of adversity may mask distinct targets of intervention (McLaughlin et al., 2014; Sheridan and McLaughlin, 2014).
We will examine two pathways by which poverty and maltreatment, in the form of TE (i.e., exposure to events involving harm or threat of harm to oneself and others), may confer risk for deficits in ER. One potential pathway is from poverty to cognitive deficits that make it difficult to implement cognitive control processes important for ER. A second potential pathway is from TE to alterations in emotional processing and reactivity that make it challenging to modulate emotional reactions. Importantly, we acknowledge that experiences of poverty and TE may co-occur and have attempted to dissociate their effects. Thus, we aim to identify mechanisms through which these early childhood adversities are related to deficits in ER.
1.1. The neural and behavioral underpinnings of ER
Effective ER, specifically cognitive reappraisal of emotion (CER), requires the successful use and integration of cognitive and emotional processes. Effective CER is supported by cognitive processes implicated in generating, maintaining, and implementing a cognitive reframe, and emotional processes implicated in appropriate emotion processing, reactivity, and maintenance of one’s emotional state (Ochsner and Gross, 2008). CER is supported by coordinated activation across brain regions implicated in cognitive control and emotional processing (Buhle et al., 2014; Ochsner and Gross, 2008). During effective CER, dorsal, frontal, and cingulate regions are thought to enhance control over limbic regions implicated in emotional processing (Buhle et al., 2014; Lopez et al., 2018). This process is reflected in greater activation of the cortical regions implicated in cognitive control (dorsal and ventral lateral prefrontal cortex (dlPFC, vlPFC), medial frontal cortex (mFC), dorsal anterior cingulate (dACC), and posterior parietal lobe), and reduced activation in limbic regions implicated in emotional processing like the amygdala (Buhle et al., 2014, 2014; Ochsner and Gross, 2008). Given the coordinated nature of CER, deficits, or alterations in either cognitive or emotional processing will impair effective ER.
1.2. Poverty and ER: the role of cognitive deficits
There is evidence that children from impoverished backgrounds have alterations in the cognitive processes and related neural activity implicated in generating, maintaining, and implementing a cognitive reframe (Merz et al., 2019; Noble et al., 2012, 2015). The effects of poverty on cognitive ability can be seen as early as six-months old; low SES infants demonstrate delays in cognitive flexibility (i.e., cognitive adaptation important in modulating responses to stimuli) (Clearfield and Niman, 2012). Later in childhood and adulthood, poverty is related to reduced executive function skills (Lipina and Evers, 2017), working memory (Farah et al., 2006), and language abilities (Merz et al., 2019). McLaughlin and colleagues suggest that youth reared in poverty may have less cognitively complex and stimulating environments (McLaughlin et al., 2014; Sheridan and McLaughlin, 2014) and that this may be one mechanism through which poverty is associated with poorer cognitive function and alterations in the neural circuits that support cognitive performance (McLaughlin et al., 2014; Sheridan and McLaughlin, 2014).
Importantly, youth facing poverty also demonstrate differences in neural activation in cognitive control brain regions used in ER. Poverty is associated with lower recruitment of left temporal regions during language-related tasks and alterations in the recruitment of the prefrontal cortex (Johnson et al., 2016). Further, lower family income earlier in life predicts reduced activation in frontal/cortical regions such as the dlPFC and vlPFC during ER (Kim et al., 2013; Liberzon et al., 2015). Together, these results suggest that youth exposed to poverty may have blunted activation in brain regions that are thought to enhance control over limbic regions during effective CER.
1.3. Threat exposure and ER: the role of altered emotional processing and reactivity
Exposure to threat in childhood is associated with altered processing of emotional stimuli (e.g., generalization of fear to neutral stimuli), and heightened emotional reactivity (i.e., elevated emotional and neural responses to emotional cues) (Lavi et al., 2019; Pine et al., 2005; Pollak and Tolley-Schell, 2003). These effects are long-lasting; childhood TEs are related to biased attention and increased emotional reactivity toward negatively-valanced emotional stimuli during childhood and adulthood (Dannlowski et al., 2012, 2013; Iffland and Neuner, 2020; McLaughlin et al., 2014, 2019; Pollak, 2008; Sheridan and McLaughlin, 2014). These alterations may be emotion-specific; some studies have reported increased biased attention toward sad faces (Romens and Pollak, 2012) and others have reported decreased attention to angry/threatening stimuli (Pine et al., 2005). It is thought that for TE youth these alterations in emotional reactivity and processing result from insecure early attachments to caregivers that lead to alterations in the encoding of emotional stimuli (Lavi et al., 2019), and that bias toward emotional faces reflects elevated sensitivity to a range of potentially informative emotional cues (Hein and Monk, 2017; McLaughlin et al., 2015).
Neurally, there is robust evidence that threat-exposed youth have heightened neural reactivity to negatively-valanced emotional stimuli in brain regions that support emotional processing, such as the amygdala (McLaughlin et al., 2014, 2019). In a meta-analysis, Hein found robust evidence that childhood maltreatment (including physical and sexual abuse) was associated with increased bilateral amygdala activation to emotional faces in children and adults (Hein and Monk, 2017).
Greater activation in brain regions implicated in emotional reactivity has been observed in threat-exposed youth during CER tasks. In contrast to the blunted cortical activity reported in youth facing poverty, McLaughlin et al. (2015) found maltreated youth had greater activation in limbic brain regions related to emotional processing and reactivity such as the putamen, thalamus, amygdala, and the insula (McLaughlin et al., 2015); of note these youth also had lower parental education. Together, these findings suggest that heightened emotional and limbic activity to negative emotional stimuli may contribute to deficits in ER among threat-exposed youth by making it more challenging for cognitive control processes to effectively downregulate emotion responses to negative-affect eliciting stimuli.
1.4. Separable paths to ER
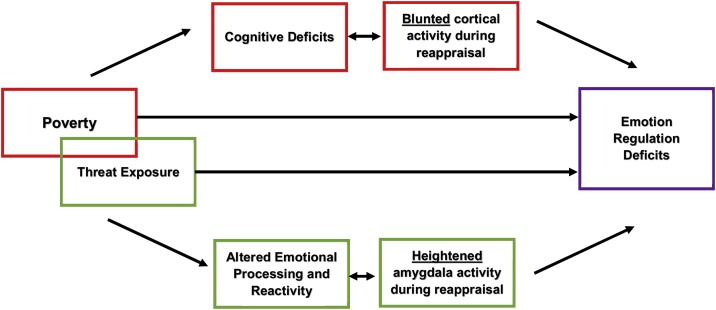
The present study aimed to test the hypothesis that there are dissociable pathways to ER deficits in youth facing poverty versus threat-exposed youth. We hypothesized that youth who have experienced poverty will have lower activity in cognitive control regions during the reappraisal of negative images. In contrast, we hypothesized that TE youth would have heightened emotional reactivity, as reflected in greater amygdala reactivity during both passive viewing and reappraisal of negative images. To test these hypotheses we used an ER fMRI task in which reactivity (viewing of negative images) and reappraisal (reappraisal in response to negative images) are examined. We predicted that the relationship between early poverty and ER deficits would be mediated by cognitive impairments and altered neural activity in cognitive control regions. Further, we predicted that the relationship between TE and ER deficits would be mediated by altered emotion processing and reactivity, as reflected in less accurate labeling of negatively valanced emotional stimuli and heightened amygdala neural activity (Fig. 1).
Fig. 1.
Hypothesized pathways to Emotion Regulation Deficits from Poverty and Threat Exposure.
2. Methods
2.1. Participants
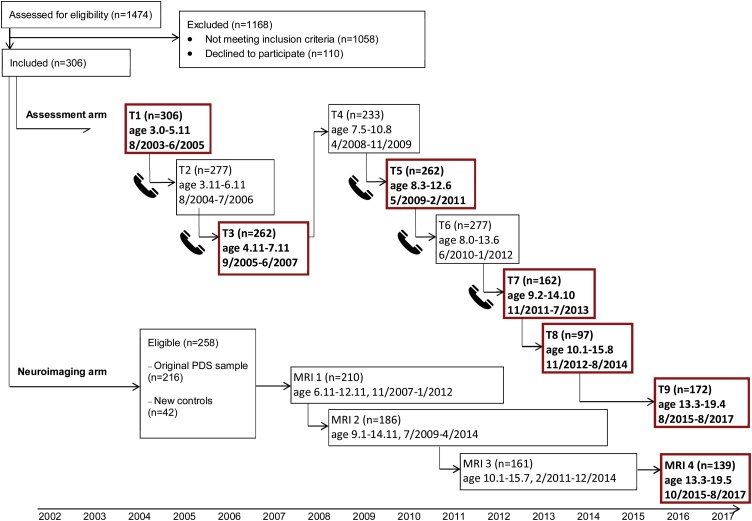
Participants in this study were recruited as part of the Preschool Depression Study (PDS), sampling procedures for which have been previously described (Barch et al., 2016; Belden et al., 2014, 2015; Elsayed et al., 2020; Lopez et al., 2018). Briefly, PDS is an ongoing prospective longitudinal study examining the developmental trajectories of preschool-onset depression. Of note, the PDS oversampled for preschoolers at risk for depression (Luby et al., 2009). All participants in the study have between one and nine assessment waves and between one and four scan waves (Fig. 2). There were 348 participants originally recruited at baseline (ages 3.0–5.11, M = 4.55, SD = 0.81) as part of the full data set, with 210 included at the first wave of imaging (ages 6.11–12.11, M = 10.13, SD = 1.25). From these 210, 171 had behavioral data available at the time of scan four (ages 13.03–19.5, Mean = 16.31, SD = 1.15) when the imaging measure of interest was administered. Given the goals of the study, we focused our analysis on a subset of adolescents from the 171 who had useable imaging data (N = 139) from the most recently completed assessment and scan wave (T9/MRI 4, see Fig. 2). Parents provided written informed consent, whereas children gave either oral or written assent or consent (depending upon age) following study description. Methods were reviewed and approved by the Institutional Review Board at the Washington University School of Medicine (IRB #201,502,094; PDS-III Imaging). Importantly, this project used data from an existing dataset and there were not always direct assessments of all necessary constructs across all time points. As such, we used data where it was available. We aimed to use the earliest possible antecedents and latest possible outcomes for our constructs of interest to best inform any enduring effects of early experience on later outcomes. We will specify this information by assessment. Further, Table 1, Table 2, and 4 provide the number of youth with data for each of the measures.
Fig. 2.
PDS timeline and data collection.
Table 1.
Association of Cognitive Ability and Emotion Processing and Reactivity to Income-to-Needs ratio and Threat Exposure for individuals with Neuroimaging Data.
| Measure | N | Income to Needs (r) | Threat Exposure (TE) (r) | Income to Needs Controlling for TE (r) | TE Controlling for Income to Needs (r) |
|---|---|---|---|---|---|
| Cognitive Measures | |||||
| NIH Toolbox at T9 | |||||
| List Sort (Working Memory) | 135 | 0.33** | 0.04 | 0.39** | 0.33 |
| Pattern Comparison (Processing Speed) | 136 | −0.06 | −0.04 | −0.06 | −0.13 |
| Picture Sequence (Episodic Memory) | 133 | 0.17 | −0.15 | 0.17 | −0.33 |
| Picture Vocabulary (Language) | 135 | 0.49** | −0.11 | 0.48** | 0.04 |
| Flanker (Executive Function & Attention) | 135 | 0.14 | −0.02 | 0.15 | 0.02 |
| Kaufman Brief Intelligence Test (KBIT) at T7-T8 IQ | 48 | 0.66** | −0.18 | 0.65** | −0.04 |
| Emotion Processing and Reactivity | |||||
| Penn Emotion Differentiation at T7-T8 | |||||
| Correct Responses to Sad | 103 | 0.22 | −0.08 | 0.22 | −0.06 |
| Reaction Time for Correct responses to Sad (ms) | 103 | −0.15 | −0.12 | −0.20 | −0.53 |
| Reaction Time for Incorrect responses to Sad (ms) | 103 | 0.09 | −0.07 | 0.07 | −0.15 |
| FACES at T2 | |||||
| Correctly identified Sad Faces | 82 | 0.43*** | −0.23 | 0.38*** | −0.38 |
| Correctly identified Faces | 82 | 0.60*** | −0.17 | 0.55*** | −0.12 |
| Ratio of correct to incorrect for sad faces | 82 | 0.12 | 0.16 | 0.12 | 0.48 |
| FACES at T3 | |||||
| Correctly identified Sad Faces | 82 | 0.06 | −0.11 | 0.04 | −0.27 |
| Correctly identified Faces | 82 | 0.43*** | −0.15 | 0.39*** | −0.16 |
| Ratio of correct to incorrect for sad faces | 82 | 0.06 | 0.07 | 0.09 | 0.22 |
Abbreviations: TE = Threat Exposure, FDR corrected; ***p < .001, **p < .01, * p ≤ .05.
Table 2.
Associations between Income to Needs and Threat Exposure and activation during reappraisal at T9/MRI4.
| Region | X | Y | Z | Beta for Reappraisal M (SE) | Income to Needs (r) | TE (r) |
|---|---|---|---|---|---|---|
| Regions from Diekhof Meta-Analysis | ||||||
| L/R dorsomedial PFC/ACC | −6 | 16 | 58 | 0.56 (0.03) | 0.20* | −0.11 |
| L/R dorsomedial PFC/ACC | 2 | 32 | 44 | 0.31 (0.03) | 0.09 | −0.03 |
| L middle frontal gyrus/inferior frontal sulcus/IFJ | −42 | 18 | 44 | 0.23 (0.03) | 0.14 | −0.08 |
| L middle frontal gyrus/inferior frontal sulcus/IFJ | −42 | 4 | 48 | 0.32 (0.03) | 0.22* | −0.13 |
| R middle frontal gyrus/inferior frontal sulcus | 40 | 22 | 44 | 0.04 (0.02) | −0.08 | 0.11 |
| L inferior frontal gyrus/anterior insula (1) | −50 | 30 | −10 | 0.65 (0.04) | 0.18* | −0.11 |
| L inferior frontal gyrus/anterior insula (2) | −54 | 22 | −2 | 0.57 (0.03) | 0.20* | −0.11 |
| L inferior frontal gyrus/anterior insula (3) | −52 | 42 | −6 | 0.38 (0.02) | 0.10 | −0.02 |
| R inferior frontal gyrus | 50 | 30 | −10 | 0.57 (0.04) | 0.18* | −0.08 |
| L intraparietal cortex | −46 | −66 | 36 | 0.06 (0.03) | 0.03 | 0.09 |
| L intraparietal cortex | −42 | −56 | 38 | 0.09 (0.02) | 0.04 | 0.03 |
| L intraparietal cortex | −38 | −60 | 30 | 0.05 (0.01) | 0.06 | 0.04 |
| R intraparietal cortex | 50 | −58 | 42 | −0.08 (0.03) | −0.08 | 0.08 |
| L inferior temporal sulcus | −60 | −36 | −2 | 0.24 (0.03) | 0.16 | −0.07 |
| L anterior insula/frontal operculum | −38 | 20 | −4 | 0.49 (0.03) | 0.28** | −0.15 |
| R anterior insula/frontal operculum | 46 | 14 | 0 | 0.32 (0.03) | 0.13 | −0.12 |
| L/R VMPFC | 6 | 40 | −22 | 0.25 (0.03) | −0.15 | −0.06 |
| L/R VMPFC | 0 | 38 | −18 | 0.15 (0.03) | −0.02 | −0.06 |
| L middle temporal gyrus | −64 | −4 | −22 | 0.11 (0.01) | 0.08 | −0.05 |
| R front marginal sulcus | 34 | 60 | 8 | 0.10 (0.03) | −0.09 | 0.17 |
| R inferior frontal gyrus | 60 | 26 | 6 | 0.28 (0.02) | 0.05 | −0.10 |
| L ACC | −8 | 28 | 28 | 0.23 (0.02) | 0.14 | −0.05 |
| Roy Amygdala ROIS | ||||||
| Right Centromedial Amygdala | 26 | −9 | −10 | 0.06 (0.01) | −0.17+ | 0.02 |
| Left Centromedial | −22 | −9 | −12 | 0.02 (0.02) | −0.05 | 0.05 |
| R basolateral | 28 | −3 | −26 | 0.09 (0.02) | 0.01 | −0.06 |
| L basolateral | −24 | −5 | −28 | 0.07 (0.02) | 0.004 | −0.10 |
| R superficial | 21 | −4 | −16 | 0.13 (0.02) | 0.05 | −0.14 |
| L superficial | −16 | −5 | −20 | 0.19 (0.03) | 0.02 | 0.03 |
FDR corrected; ***p < .001, **p < .01, * p ≤ .05,+ p = .05, coordinates are MNI.
2.2. Inclusion/exclusion from PDS
Participants were excluded from PDS if they had known developmental disabilities, had a diagnosis of autism, schizophrenia, Tourette’s syndrome, experienced a major medical illness known or hypothesized to affect the central nervous systems, a significant neurological illness, were born at 36 weeks gestational age or less, were pregnant, had a history of head injury or loss of consciousness greater than five minutes. Subjects were excluded from the imaging portion if they had intra-ocular metallic objects, cochlear implants, pacemakers, or other electrical, mechanical, or magnet-activated implants or met any condition that makes MRI unsafe such as having certain metals in the body, tattooed eyeliner, or having non-removable piercings.
2.3. Materials and measures
2.3.1. Poverty
2.3.1.1. Income-to-needs ratio
Income-to-needs ratio was operationalized as the caregiver-reported total family income divided by the federal poverty level based on the number of people living in the household (Barch et al., 2016). Data were available from up to 18 waves for each child (Fig. 2). To compute a measure that best characterized early childhood poverty, we entered all values into a multilevel model with random intercept and slope and extracted the intercept and slope from the model for each child to use in subsequent analyses. The final multilevel model suggested that random effects of intercept and slope were highly correlated (r = -0.79) and that there was not meaningful variation across participants in the rate of change of income-to-needs (random effect coefficient = 0.014), thus only the intercept was used in the analyses described below.
2.3.2. Exposure to threat
2.3.2.1. Life events checklist
TE was assessed using two lifetime items from the parent and child reported Life Events Checklist (Gray et al., 2004) at T9. The items asked about lifetime occurrence of physical abuse or sexual abuse. TE was coded as a dichotomous variable with participants considered threat-exposed if either the parent or child reported a lifetime occurrence of either form of abuse.
2.3.3. Emotion regulation
ER was assessed by both parent and child report at T9 (Fig. 2).
2.3.3.1. Parent report
2.3.3.1.1. Emotion Regulation Checklist (ERC)
The ERC is a 24-item parent-report questionnaire assessing youth’s intensity, lability, flexibility, and appropriateness of the child’s positive and negative emotion regulation (Shields and Cicchetti, 1997). It has two subscales: emotion regulation (a = .77, higher scores better regulation) and negative lability (a = .77, higher scores worse dysregulation).
2.3.3.2. Child report
2.3.3.2.1. Children’s emotion management scale (CEMS)
The CEMS (a = .71) is a 30-item child-report questionnaire assessing the likelihood of a child to engage in inhibition, dysregulated expression, or coping for the emotions of anger, sadness, or worry (Zeman et al., 2001).
2.3.3.2.2. Cognitive Emotion Regulation Questionnaire – kid (CERQ-K)
The CERQ-K (a = .88) is a 36-item child-report questionnaire assessing children’s tendencies to engage in a variety of adaptive (i.e., Acceptance, Positive Refocusing, Refocus on Planning, Positive Reappraisal, Putting it into Perspective) or maladaptive (i.e., Self-Blame, Rumination, Catastrophizing, Other-Blame) ER strategies (Jermann et al., 2006).
2.3.3.2.3. Child response style questionnaire (CRSQ)
The CRSQ (a = .82) is a 25-item self-report questionnaire assessing the ER strategies youth use in response to sadness including scales for rumination, distraction, and problem solving (Abela et al., 2000).
2.3.3.3. ER factors
Principal component analyses (PCA) were performed on ER variables to reduce data dimensionality of the emotion regulation data. Details have been previously described (Elsayed et al., 2020). Briefly, a PCA of the ER data returned two factors. One corresponded to the youth’s tendencies to engage in ER skills thought to be adaptive (i.e., tendency to engage in adaptive ER skills) and one to skills thought to be maladaptive (i.e. tendency to engage in maladaptive ER skills). Given the ERC scales did not specifically load onto either factor, we also examined ERC negative lability and emotion regulation as more general indices of the efficacy of emotion regulation versus the use of specific skills (Elsayed et al., 2020).
2.3.4. Cognitive assessment
Youth completed the Kaufman Brief Intelligence Test twice during the study at T7 (ages 9.2–14.10, M = 9.50, SD = 0.84) and T8 (ages 10.1–15.8, M = 10.17, SD = 0.89). The KBIT is a highly reliable measure of IQ across the lifespan, with most estimates placing reliability in the 0.80 to 0.90 range (Bain and Jaspers, 2010). Youth also completed a subset of the NIH Toolbox cognitive measures at T9 (Weintraub et al., 2013) (Fig. 2). The age-corrected scores from each of the five task domains were examined for the NIH Toolbox, with higher scores indicating better performance. Intraclass correlation coefficients NIH Toolbox have ranged from 0.78 to 0.90 in previous literature (Weintraub et al., 2013).
2.3.4.1. Kaufman brief intelligence test (KBIT)
The KBIT assesses verbal and nonverbal intelligence (Kaufman, 1990). Average scores for the verbal, non-verbal, and composite subscales from school age were calculated by averaging scores from two-time points (T7, T8) during childhood (Fig. 2).
2.3.4.2. Toolbox picture sequence memory test (TPSMT)
The TPSMT was used to assess episodic memory. Participants were presented with pictures depicting activities or events that could occur in a particular setting (i.e., working on a farm) (Weintraub et al., 2013). After being shown the pictures in order, the pictures appear in scrambled order on the screen, and they attempt to arrange them in the correct order on the screen. Participants are given multiple trials with the same set of pictures.
2.3.4.3. Toolbox list sorting working memory test (TLSWMT)
The TLSWMT was used to assess working memory. Participants were presented with a variant of the letter-number sequencing test uses pictures rather than words or letters (Weintraub et al., 2013). Participants are presented with a series of pictures of animals or foods of different sizes, accompanied by the name presented auditorily by an iPad, and asked to repeat the items back in order from smallest to largest. The TLSWMT starts with a single category (i.e., animals). Participants are presented with a two-item list, and if they get it correct, the next trial increases to three items, and so on. Participants have two opportunities to provide a correct answer at each list length and continue to the next length if they get at least one of the trials correct. Participants then progress to the next phase, where the trials interleave two different categories (i.e., animals and food). The participant is asked to first organize and repeat back the items for one category (i.e., animals) and then the other.
2.3.4.4. Toolbox flanker task (TFT)
The TFT was used to assess selective attention in participants and is a variant of the Eriksen Flanker task (Eriksen and Eriksen, 1974) that was adapted from the Attention Network Task (Fan et al., 2002; Rueda et al., 2004). There are four flanking arrows (two on the outer left and two on the outer right) that are all facing the same way, either left or right. The middle arrow is then either facing the same way (congruent trial) or a different way (incongruent trial). Participants push a button to indicate whether the middle arrow is facing left or right. Scoring is based both on speed and accuracy.
2.3.4.5. Toolbox pattern comparison processing speed test (TPCPST)
The TPCPTS was used to assess processing speed and was modeled on the Pattern Comparison Task developed by Salthouse (Salthouse et al., 1991). Participants are shown two pictures and asked to determine whether the pictures are the same or not. The score is based on how many items they can complete correctly in a specific amount of time.
2.3.4.6. Toolbox picture vocabulary task (TPVT)
The TPVT was used to assess verbal IQ and is a variant on the Peabody Picture Vocabulary Test (PPTV) (Gershon et al., 2014). Participants hear audio files of words and are shown four pictures in a square, one that depicts the concept, idea, or object referenced by the auditorily presented words. The participant is asked to touch the picture that matches the word.
2.3.5. Emotion processing and reactivity
Emotion processing and reactivity were assessed through two different measures at different timepoints in childhood.
2.3.5.1. Penn emotion differentiation
Participants completed the computerized 40-item version of the Penn Emotion Differentiation averaged across both T7 and T8 to assess emotion recognition (Fig. 2) (Erwin et al., 1992). The task involved selecting the more intense facial expression of emotion based on 40 pairs of happy and sad faces shown one pair at a time. We examined correct responses to sad faces. To assess reactivity to sad faces, we also examined reaction time for responding to sad faces. This task has been used before with acceptable reliability (Moore et al., 2015).
2.3.5.2. Facial affect comprehension evaluation (FACES)
The FACES is a 38-item task that assesses the child’s ability to recognize and verbally label seven different emotions from facial expressions and was administered at T1, T2, T3 (Fig. 2) (Mrakotsky and Luby, 2000). Stimuli consisted of 38 colour photographs of male and female adults and children displaying seven different emotions (i.e., happiness, sadness, anger, fear, surprise, disgust, and shame). The child received one point for every correct emotion. Each emotion is treated as its own subscale by summing together all items probing for that emotion. We calculated the correct number of responses for sad faces and the correct number of responses to all faces. To assess reactivity to sad faces, we also calculated a ratio of correct to incorrect responses for sad faces at T2 and T3 (Fig. 2 and Table 1).
2.4. Brain analyses
2.4.1. Task explanation
A version of this task has been described in detail elsewhere (Belden et al., 2014, 2015). Briefly, following the pre-scan training procedure to ensure that children understood how to use reappraisal in response to negative stimuli children were instructed to either passively view sad or neutral images, or to decrease their experience of negative emotions in response to viewing sad images. They were taught to do this using cognitive reappraisal strategies like imagining a good outcome to the image. At the start of each trial, participants fixated on a cross for 500 milliseconds (ms). Following, participants were told to either view or try to decrease their experienced emotion for 2000 ms. Finally, participants were presented with a photo (i.e., neutral or sad) for an 8000 ms interval. Following each picture, children were prompted to answer the question, “How do you feel?”. Children had four seconds to rate their negative affect on a scale from one to four. Responses were made on a four-button box (see supplemental results for details). After the affect-rating period, the word “RELAX” appeared on the screen for four to eight seconds. The combination of neutral and sad photographs with just view versus regulate instructions resulted in three conditions: view neutral (non-emotional photo), view sad (sadness without reappraisal) and reappraise sad (reappraise while viewing a sad photo).
2.4.2. Image acquisition
Data were collected on a Siemens PRISMA 3 T scanner with a 32-channel head coil. Participants completed T1- and T2-weighted structural scans (0.8 mm (mm)3) in addition to approximately 19 min of task-based blood level-dependent (BOLD) scanning across four scans. Task-based scans were acquired using a T2*-weighted multiband EPI sequence (Multiband [MB] = 7, 72 axial slices per volume, 2.4 mm isotropic voxels, TE = 33.1 ms, TR = 720 ms, FOV = 216 mm, flip = 52˚).
2.4.3. fMRI analyses
fMRI data were run through the Human Connectome Project minimal preprocessing pipelines (Barch et al., 2013; Glasser et al., 2013) (see supplement). Neuroimaging data from 10 individuals were excluded bringing the total neuroimaging sample size to 139. Of these 10 individuals, four had missing information on one or more runs, three or more had a root mean square values greater than 0.20 for more than two run runs of the study, one had missing structural data, one had unusable motion data, and one had a root mean square values greater than 0.20 for one run and missing data for another run.
2.4.4. Statistical analyses of brain
We computed general linear models (GLM) for each individual using an event-related design analysis in AFNI. We estimated the hemodynamic response function for each condition (i.e., view sad, view neutral, reappraise sad) and for the rating period (not examined, but used to account for variance appropriately). Within the GLM, a hemodynamic response shape was assumed using an eight-second boxcar function convolved with a hemodynamic response function. This produced parameter estimates for each stimulus type relative to baseline fixation; these estimates were used in all subsequent statistical analyses. These individual-level estimates of blood oxygen level-dependent (BOLD) activity for each condition were submitted to group-level random-effects models.
We analyzed the data in two ways. First, we used nested models (nested within an individual) with BOLD response as the dependent variable, condition as a factor (view neutral, view sad, reappraise sad), and either poverty or TE as continuous predictors and examined interactions between condition and either poverty or TE (Table S3). When there were significant interactions, we followed up with posthoc tests that examined the relationships of either poverty or TE with each of the individual conditions to determine the source of the interaction (Table S4). As can be seen in Table S3, maltreatment was not associated with neural activity.
Second, we examined the relationships of poverty and TE to standard contrasts of conditions (i.e., view sad > neutral trials, reappraise sad > view sad, reappraise sad > view neutral). When significant (Table S5), we conducted follow up analyses to examine which condition(s) continued to show a significant association with poverty and/or TE (supplemental results) (Belden et al., 2014, 2015). An examination of both of these sets of results indicated that all but one of the significant relationships were being driven by associations to activity during the reappraise sad condition (Table S3, S4, supplemental text). Thus to simplify presentation of the findings, below we present results from only associations to the reappraise sad condition. Importantly to identify which brain regions to include in subsequent analyses we examined results from both the posthoc analyses (Table S4) as well as correlations between income-to-needs and neural activation during reappraisal as indicated by the posthoc analyses (Table 3). Ultimately given the similarity of the results we examined brain regions that significantly correlated with income-to-needs (Table 3).
Table 3.
Emotional Regulation Measures Relationship to Poverty and Threat Exposure.
| Measure | N | Income to Needs (r) | TE (r) | Income to Needs Controlling for TE (r) | TE Controlling for Income to Needs (r) |
|---|---|---|---|---|---|
| Emotion Regulation Checklist (ERC) at T9 | |||||
| Emotion Regulation | 137 | 0.36 *** | −0.26* | 0.29** | −0.56* |
| Lability/Negativity | 137 | −0.31* | 0.33** | −0.26* | 0.81* |
| Emotion Regulation Factors at T9 | |||||
| Tendency to Engage in Adaptive ER strategies | 137 | 0.26** | −0.17 | 0.20* | −0.30 |
| Tendency to Engage in Maladaptive ER strategies | 137 | −0.16 | 0.24* | −0.10 | 0.57* |
Abbreviations: ER = Emotion Regulation, TE = Threat Exposure; FDR corrected; ***p < .001, **p < .01, * p ≤ .05.
2.4.5. Whole brain analyses to establish task validity
To establish that the task conditions elicited the expected brain activity based on previous studies, we conducted whole-brain voxel-by-voxel analyses, using the ANOVA models described above, with a corrected whole-brain false-positive rate of p < .05 (voxel-level p-value = .005 and cluster size of 93) (Supplement Tables S12-S14).
2.4.6. A priori ROI analyses
To test the hypothesis regarding localized functional changes we used ROI analyses. We used bilateral ROIs for three subdivisions of the amygdala, namely, centromedial, laterobasal, and superficial, from Roy et al. (Roy et al., 2009) (Table 3). For cognitive control regions, we focused on the regions implicated in cognitive down-regulation of negative emotion from a meta-analysis (Diekhof et al., 2011) (Table 3). We used the published MNI coordinates to create spherical ROIs that were 10 mm in diameter. Beta values from each ROI were extracted for each participant and entered into analyses described below.
2.4.7. Analytical procedures
To test the hypothesis that cognitive impairments and altered neural activity in cognitive control regions mediate the relationship between early poverty (operationalized as income-to-needs) and ER deficits, and to test the hypothesis that altered emotion processing and reactivity would mediate the relationship between TE and ER deficits, we began first by ensuring that proposed mediators correlated with both income-to-needs and TE (Table 1,2). To do this we used correlations (point-biserial for threat exposure and Pearson’s for income-to-needs) with each of the proposed emotional, cognitive and neural mediators (Table 1, Table 2). In our second step we examined whether income-to-needs was associated with our proposed ER outcomes (Table 3). In our third step, we examined if mediators, found to found to be related to income-to-needs, correlated with measures of self-or-parent-reported ER (Table 4). For each of these sets of tests, we corrected for multiple comparisons using FDR correction at p < .05 across the class of mediators (i.e., neural, cognitive, or emotional).
Table 4.
Relationships between Cognitive and Processing and Reactivity Variables with Emotion Regulation.
| Measure | N | ERC: Emotion Regulation at T9 | ERC: Lability/ Negativity at T9 (r) | Adaptive Emotion Regulation (r) | Maladaptive Emotion Regulation (r) |
|---|---|---|---|---|---|
| Cognitive Measures | |||||
| NIH Toolbox - List Sort (Working Memory) at T9 | 135 | 0.10 | −0.10 | 0.04 | −0.02 |
| NIH Toolbox - Picture Vocabulary (Language) at T9 | 135 | 0.34*** | −0.34** | 0.10 | −0.01 |
| Kaufman Brief Intelligence Test (KBIT) – IQ at T7 – T8 | 48 | 0.36* | −0.35* | 0.26 | −0.10 |
| Emotion Processing and Reactivity | |||||
| FACES – Correctly identified Sad Faces at T2 | 110 | 0.12 | −0.27* | 0.13 | −0.12 |
| FACES – Correctly identified Faces at T2 | 110 | 0.26* | −0.39*** | 0.13 | −0.14 |
| Brain Activation During Reappraisal | |||||
| L/R dorsomedial PFC/ACC | 137 | 0.23* | −0.21* | 0.19 | −0.16 |
| L middle frontal gyrus/inferior frontal sulcus/IFJ | 137 | 0.27** | −0.27** | 0.12 | −0.11 |
| L inferior frontal gyrus/anterior insula (2) | 137 | 0.34*** | −0.21* | 0.15 | −0.05 |
| L inferior frontal gyrus/anterior insula (3) | 137 | 0.37*** | −0.23* | 0.16 | −0.14 |
| R inferior frontal gyrus | 137 | 0.23* | −0.21* | 0.16 | −0.04 |
| L anterior insula/frontal operculum | 137 | 0.36*** | −0.24* | 0.25** | −0.12 |
Abbreviations: ERC = Emotion Regulation Checklist, TE = Threat Exposure; FDR corrected; ***p < .001, **p < .01, * p ≤ .05.
After identifying which mediators correlated with either income-to-needs or TE, we moved onto conducting mediations with lavaan 0.6–7 (Rosseel, 2012) in R with 1000 bootstrapped standard errors and bias-corrected 95 % confidence intervals. When p-values and bias corrected 95 % confidence intervals differed (due to the nature of the resampling process), we chose to use p-values to identify significant effects as Type I error is found to be somewhat high for bias-corrected bootstrapped confidence intervals in the context of indirect effects (Biesanz et al., 2010) and in order to be most conservative with our results. Of note, each of these mediations were conducted using only the cognitive and neural variables that were significantly related to both income-to-needs and ER, because no mediators were found to be associated to TE. Therefore, the cognitive variables included in the mediation analyses were the NIH picture vocabulary and the KBIT total IQ (Table 1); the neural variables were left dmPFC (LDMPFC), left anterior insula (LAI), left anterior insula frontal operculum (LAI – frontal operculum), left middle frontal gyri (LMFG), and right inferior frontal gyrus (RIFG) BOLD signal to reappraisal (Table 2); the ER variables were the ERC ER, ERC Lability/Negativity, Adaptive ER strategies (Table 3).
Each of these mediations were done in three steps. First, we evaluated mediations from income-to-needs to ER via cognitive variables (Table 5). This enabled us to directly test the hypothesis that cognitive impairments would mediate the relationship between early poverty and ER deficits. Second, we evaluated mediations from income-to-needs to ER via just neural correlates of ER (Table 6). This enabled us to directly test the hypothesis that altered neural activity in cognitive control regions would mediate the relationship between early poverty and ER deficits. This resulted in four mediations with cognitive variables, and eleven mediations with neural variables.
Table 5.
Direct and Indirect pathway from income to needs ratio to deficits in emotion regulation from cognitive measures.
| Direct Effect |
Indirect Effect |
|||||||
|---|---|---|---|---|---|---|---|---|
| ß (se) | z | CI | p | ß (se) | z | CI | p | |
| NIH Picture Vocabulary (Language) at T9 | ||||||||
| ERC: Emotion Regulation | 0.98 (0.44) | 2.22 | 0.09, 1.85 | 0.03 | 0.50 (0.23) | 2.18 | 0.11, 1.03 | 0.03* |
| ERC: Lability/ Negativity | −1.45 (0.76) | −3.31 | −2.94, 0.09 | 0.05 | −0.88 (0.42) | −2.09 | −1.86, −0.21 | 0.04* |
| Kaufman Brief Intelligence Test at T7 and T8 | ||||||||
| ERC: Emotion Regulation | 0.17 (0.81) | 0.21 | −1.60, 1.61 | 0.83 | 0.95 (0.65) | 1.48 | −0.15, 2.43 | 0.14 |
| ERC: Lability/ Negativity | 0.17 (0.78) | 0.22 | −1.43, 1.66 | 0.83 | 0.95 (0.61) | 1.58 | −0.10, 2.34 | 0.12 |
Abbreviations: ERC = Emotion Regulation Checklist; ***p < .001, **p < .01, * p ≤ .05.
Table 6.
Direct and Indirect pathway from income to needs ratio to deficits in emotion regulation from Neural Activation during Reappraise Sad.
| Direct Effect (with mediators in model) |
Indirect Effect (mediation) |
|||||||
|---|---|---|---|---|---|---|---|---|
| ß (se) | z | CI | p | ß (se) | z | CI | p | |
| L/R dorsomedial PFC/ACC (-6, 16, 58) | ||||||||
| ERC: Emotion Regulation | 1.37 (0.31) | 4.44 | 0.77, 1.95 | <0.001** | 0.14 (0.08) | 1.69 | 0.01, 0.32 | 0.091 |
| ERC: Lability/Negativity | −2.01 (0.61) | −3.33 | −3.23, −0.85 | 0.001** | −0.21 (0.15) | −1.4 | −0.55, 0.02 | 0.161 |
| L middle frontal gyrus/inferior frontal sulcus/IFJ (−42, 4, 48) | ||||||||
| ERC: Emotion Regulation | 1.33 (0.31) | 4.27 | 0.72, 1.90 | <0.001** | 0.19 (0.10) | 1.95 | 0.04, 0.42 | 0.051 |
| ERC: Lability/Negativity | −1.90 (0.61) | −3.14 | −3.14, −0.71 | 0.002** | −0.32 (0.19) | −1.74 | −0.74, −0.06 | 0.083 |
| L inferior frontal gyrus/anterior insula (-54, 22, -2) | ||||||||
| ERC: Emotion Regulation | 1.23 (0.32) | 3.81 | 0.61 (1.86) | <0.001** | 0.25 (0.13) | 1.9 | 0.04, 0.54 | 0.051 |
| ERC: Lability/Negativity | −2.07 (0.68) | −3.03 | −3.43, −0.72 | 0.002** | −0.26 (0.18) | −1.4 | −0.70, −0.01 | 0.16 |
| L inferior frontal gyrus/anterior insula (-50, 30, -10) | ||||||||
| ERC: Emotion Regulation | 1.30 (0.31) | 4.14 | 0.68, 1.87 | <0.001** | 0.22 (0.12) | 1.76 | 0.03, 0.52 | 0.079 |
| ERC: Lability/Negativity | −2.01 (0.62) | −3.24 | −3.26, −0.85 | 0.001** | −0.21 (0.17) | −1.27 | −0.61, 0.01 | 0.205 |
| R inferior frontal gyrus (50, 30, -10) | ||||||||
| ERC: Emotion Regulation | 1.39 (0.32) | 4.3 | 0.72, 1.98 | <0.001** | 0.13 (0.09) | 1.46 | −0.001, 0.34 | 0.14 |
| ERC: Lability/Negativity | −2.02 (0.60) | −3.34 | −3.20, −0.85 | 0.001** | −0.21 (0.17) | −1.22 | −0.62, 0.02 | 0.22 |
| Left anterior insula/ frontal operculum activity (-38, 20, -4) | ||||||||
| ERC: Emotion Regulation | 1.19 (0.31) | 3.85 | 0.57, 1.76 | <0.001** | 0.33 (0.13) | 2.57 | 0.13, 0.63 | 0.010* |
| ERC: Lability/Negativity | −1.88 (0.63) | −2.99 | −3.13, −0.68 | 0.003** | −0.34 (0.18) | −1.9 | −0.73, −0.05 | 0.058 |
| Adaptative Emotion Regulation | 0.19 (0.07) | 2.84 | 0.05, 0.33 | 0.005** | 0.05 (0.02) | 2.02 | 0.01, 0.10 | 0.043* |
***p < .001, **p < .01, * p ≤ .05.
Finally, we evaluated multiple mediations from income-to-needs to ER from both cognitive and neural measures simultaneously (Table 7, Table 8). We only conducted multiple mediation in cases when the cognitive and neural variables were related to one another (Table S8). This resulted in four total tests of multiple mediation. All multiple mediations were conducted in Mplus with 1000 bootstrapped standard errors and confidence intervals (Muthén & Muthén, 2010) and included sex and age as covariates for both the direct and indirect paths. Except for mediations using the KBIT total IQ score, all mediations were cross-sectional in nature. We did not correct for multiple comparisons in the multiple mediations as we had taken an extremely conservative approach in identifying potential mediators and corrected for multiple comparisons in every step prior (i.e., when identifying variables related to income-to-needs, when examining associations between income-to-needs and mediators, when examining relationships between income to needs and outcomes variables).
Table 7.
Direct and Indirect Pathway from Language ability and Anterior Insula Frontal Operculum (-38, 20, -4) activity to Emotion Regulation.
| Direct Effect (with mediators in model) |
Indirect Effect (mediation) |
|||||
|---|---|---|---|---|---|---|
| β (S.E.) | CI | p | β (S.E) | CI | p | |
| ERC: Lability/Negativity | ||||||
| Income to Needs -> Language Ability | −0.12 (0.06) | −0.20, −0.03 | 0.03 | |||
| Income to Needs -> Left Anterior Insula Frontal Operculum | −0.04 (0.02) | −0.08, 0.00 | 0.10 | |||
| Income to needs -> Language Ability -> Left Anterior Insula | −0.01 (0.01) | −0.02, 0.01 | 0.57 | |||
| Income to Needs | −0.15 (0.10) | −0.31,0.01 | 0.13 | |||
| ERC: Emotion Regulation | ||||||
| Income to Needs -> Language Ability | 0.10 (0.05) * | 0.02, 0.19 | 0.047 | |||
| Income to Needs -> Left Anterior Insula Frontal Operculum | 0.07 (0.03) * | 0.02, 0.12 | 0.03 | |||
| Income to needs -> Language Ability -> Left Anterior Insula Frontal Operculum | 0.01 (0.01) | −0.01, 0.03 | 0.53 | |||
| Income to Needs | 0.18 (0.10) | 0.02, 0.34 | 0.06 | |||
***p < .001, **p < .01, * p ≤ .05.
Table 8.
Direct and Indirect Pathway from Language ability and Left Middle Frontal Gyrus (-42, 4, 48) activity to Emotion Regulation.
| Direct Effect (with mediators in model) |
Indirect Effect (mediation) |
|||||
|---|---|---|---|---|---|---|
| β (S.E.) | CI | p | β (S.E) | CI | p | |
| ERC: Lability/Negativity | ||||||
| Income to Needs -> Language Ability | −0.11 (0.05) | −0.19, −0.02 | 0.048* | |||
| Income to Needs -> Left Middle Frontal Gyrus | −0.03 (0.02) | −0.06, 0.01 | 0.21 | |||
| Income to needs -> Language Ability -> Left Middle Frontal Gyrus | −0.02(0.01) | −0.03, 0.00 | 0.20 | |||
| Income to Needs | −0.16 (0.10) | −0.32, -0.01 | 0.08 | −0.15(0.06) | −0.24, −0.06 | 0.008 |
| ERC: Emotion Regulation | ||||||
| Income to Needs -> Language Ability | 0.10 (0.06) | 0.01, 0.19 | 0.08 | |||
| Income to Needs -> Left Middle Frontal Gyrus | 0.02(0.02) | −0.01, 0.06 | 0.20 | |||
| Income to needs -> Language Ability -> Left Middle Frontal Gyrus | 0.02 (0.01) | −0.00, 0.03 | 0.19 | |||
| Income to Needs | 0.22 (0.10) | 0.06, 0.38 | 0.02 | 0.13 (0.06) | 0.05, 0.22 | 0.01 |
***p < .001, * *p < .01, * p ≤ .05.
3. Results
3.1. Demographic characteristics
Youth with or without imaging data did not differ in sex, ethnicity, age at T1, T2 or T3, in the prevalence of TE or income-to-needs ratio (Table S1). At T9/MRI4, youth who had imaging data were slightly older than youth who did not have imaging data (Table S1).
3.2. Prevalence of early childhood adversity
In this sample, 23.74 % of the sample had an intercept of poverty that was at or below the poverty line, 17.27 % were within two times the poverty line and the remainder (57.1 %) were above these cut-offs. Twenty youth endorsed an experience of physical or sexual abuse.
3.3. ER fMRI task validation
ROI analyses results were consistent with previous work from our group using this task(Belden et al., 2014, 2015) and with the meta-analysis results (Diekhof et al., 2011), showing significantly greater activation in the CER portion of the task as compared to passive viewing conditions in the vast majority of cortical regions (Table S5). Further, whole-brain voxel-by-voxel analyses results showed a similar pattern, including greater activation of the during the reappraise sad as compared to passive viewing condition across a host of cognitive control regions (supplemental results, Tables S12-S14).
3.4. The relationship among income to needs, TE, and potential mediators
3.4.1. Income-to-needs and cognitive abilities
As predicted, higher income-to-needs was related to greater working memory ability at T9/MRI4, enhanced language ability at T9/MRI4, and higher total IQ during early adolescence (Table 1), with these associations remaining when controlling for threat exposures. Threat exposures were not associated with any indices of cognitive ability (Table 1).
3.4.2. TE and emotion processing and reactivity
Contrary to prediction, TE was not related to any measure of emotion processing or reactivity. However, higher income-to-needs was related to greater ability to differentiate sad faces from other emotional faces during early or late childhood and overall correct facial emotion identification, even after controlling for the influence of TE (Table 1, Table S2).
3.5. Relationships of income-to-needs and TE to neural activity during ER task
ROI analyses revealed that there was no association between TE or income-to-needs with activation while passively viewing neutral photos (Tables S4, S6) or passively viewing sad images (Table S7). However, greater income-to-needs was associated with greater activation in cortical brain regions (LDMPFC, LMFG, RIFG, LAI, LAI-frontal operculum), but not in the amygdala, during reappraisal of sad images (Table 2, Table S4). TE was not related to activation in any brain regions during reappraisal of sad images (Table 2, Table S3).
3.6. Relationship between income-to-Needs, TE, and emotion regulation
As hypothesized, higher income-to-needs predicted higher positive successful emotion regulation on the ERC, lower levels of dysregulated lability on the ERC Lability/Negativity subscale, and higher tendency to engage in adaptive ER skills; all of these results remained significant after accounting for the influence of TE (Table 3). TE was associated with less successful ER on the ERC, higher ERC dysregulated negative affect, and a greater tendency to engage in more maladaptive ER skills. These associations also remained significant after controlling for income-to-needs (Table 2).
3.7. Relationship between mediators (Neural activity, cognitive ability, emotion processing) and emotion regulation
The analyses described above provided evidence as to which cognitive, emotion reactivity, emotion regulation, and neural activation measures were related to income-to-needs and/or TE. We next asked whether any of the potential mediators (i.e., cognitive, emotional, or neural) that were related to either income-to-needs or TE were also related to any of the ER measures also shown to be related to income-to-needs or TE. As shown in Table 4, both better language ability at T9/MRI4 and higher IQ in childhood were related to better emotion regulation and less lability on the ERC. Higher correct identification of sad faces in early childhood was related to less lability on the ERC, and overall better identification of facial emotion was related to both better emotion regulation and reduced lability on the ERC. Further, activation during reappraisal in all of the brain regions, related to income-to-needs, were also related to better ER and less lability on the ERC. Further, activation in the LAI-frontal operculum was associated with higher adaptive ER skills. In examining which mediators where related to each other, we found that activation in the LMFG and LAI-frontal operculum during reappraise sad were related to greater better language ability at T9/MRI4 (Table S8).
3.8. Testing mediations
3.8.1. Cognition as a mediator between income-to-needs and ER
Language ability at the time of scan partially mediated the relationship from higher income-to-needs to less lability on the ERC, with a similar finding for better ER on the ERC (Table 5). Correlations revealed that income-to-needs was related to measures of emotional reactivity, although this was not predicted. Thus an exploratory approach was taken, and we examined if the relationship between income-to-needs and some indices of ER were mediated by emotional reactivity (Tables S9-S11). We did find some evidence that facial recognition during early childhood mediated the relationship between income-to-needs and lability on the ERC. These were not tested in the multiple mediations because emotion variables did not correlate with neural activity during the CER task.
3.8.2. Neural activity as a mediator between income-to-Needs and ER
As shown in Table 6, activity in the LAI-frontal operculum during reappraisal of sad images partially mediated the relationship between income-to-needs and ERC ER. Activity in the LAI-frontal operculum during reappraisal of sad images also mediated the relationship between income-to-needs and tendency to engage in more adaptive ER skills, with a similar trend for both the left anterior insula and the left middle frontal gyrus. There were no significant mediations by brain activity for the relationship between income-to-needs and ERC lability, though there was a trend for both the left middle frontal gyrus and left anterior insula - frontal operculum.
3.9. Testing multiple mediation: cognition and neural variables as additive mediators between income to needs and emotion regulation
3.9.1. Language ability and left anterior insula frontal operculum as mediators to emotion regulation
As shown in Table 7, language ability was again the only significant indirect path mediating the relationship between income-to-needs and ERC lability. However, both language ability and left anterior insula- frontal operculum activity were significant independent indirect mediators of the relationships between income-to-needs and ERC ER; the serial indirect pathway from language ability through brain activity was not significant.
3.9.2. Language ability and middle frontal gyrus activity as mediators to emotion regulation
As shown in Table 8, similar to the results above, the only significant mediator in the path from income-to-needs to either ERC lability or ER was through language ability, with no significant indirect effects involving inferior frontal gyrus once language ability was in the model (Table 8, Fig. 3).
Fig. 3.
Mechanisms mediating the relationship between Poverty and Emotion Regulation.
4. Discussion
The goal of the current study was to dissociate two potential pathways to ER deficits: one via cognitive processing among impoverished youth and one via emotional processing and reactivity in threat-exposed youth. The current findings provide novel evidence supporting a pathway involving impaired cognitive processing (decreased language ability) as well as blunted neural activation in the left anterior insula which extends into the adjacent inferior frontal operculum (LAI frontal operculum), during cognitive reappraisal, as independent putative mechanisms through which poverty is associated with decreased ER ability.
Consistent with our hypotheses, we found that lower income-to-needs and TE were both related to deficits in ER, particularly deficits in adaptive ER and managing lability. With regard to cognitive control and emotional reactivity, as hypothesized, greater language ability, higher total IQ in adolescence, and increased ability to correctly identify emotional faces in early childhood were all positively related to enhanced parent-reported ability to engage in positive ER and decreased child lability. Further, in support of our hypotheses, we found that poverty, but not TE, was related to reduced cognitive ability in the domains of working memory, language ability, and total IQ in early adolescence. These findings are consistent with evidence that poverty is related to deficits across a host of cognitive domains, especially in working memory and language ability (Noble et al., 2007; Raizada and Kishiyama, 2010). Contrary to our hypothesis we did not find that TE was related to a reduced ability to correctly identify sad and emotional faces in childhood. We did, however, find evidence that poverty was related to reduced ability to correctly identify sad and emotional faces in childhood.
We found that poverty, but not TE, was related to reduced recruitment of cortical regions (i.e., LDMPFC, LAI, LAI-FO, RIFG, LMFG) during reappraisal of sad images. With regard to activation in brain regions typically associated with cognitive control, our results mirrored those obtained from the cognitive measures. More explicitly, we found that greater recruitment of cortical regions (LDMPFC, LMFG, RIFG, LAI, LAI-frontal operculum) during reappraisal of sad images was associated with enhanced parent-reported ER. We also found that increased activation in the LAI extending to the frontal opercula of the insula during reappraisal of sad stimuli was related to enhanced child-reported tendency to engage in adaptive ER strategies in the sample. These findings are consistent with previous literature, which reports that in the contrast of reappraisal vs. maintain, lower family income at age nine predicted reduced activation in the insula (Kim et al., 2013). It is worth noting that our neural activation in this paper is measured during purely reappraise and not during the standard contrast of reappraise sad > view sad or view neutral. Our reasoning for why our analyses were done this way can be found in statistical analyses of brain section above and reflects that there is something about the cognitive demands of reappraisal, specifically, and not of looking at rich complex or emotional stimuli that differs as a function of childhood poverty.
Furthermore, this finding adds support for the role of the dorsal anterior insula in high-level cognitive and attentional processes (Nelson et al., 2010), as compared to more posterior or ventral portions of the insula that may be more involved in affective integration. Our results might seem to contrast with results that TE is related to greater insula activity (McLaughlin et al., 2014), and increased insula response among impoverished children (Liberzon et al., 2015). However, both Mclaughlin and Liberzon’s results were in a more ventral/posterior insula region and thus may reflect a different function than the one captured by our more dorsal anterior insula findings. Our results also provide further support for the conceptualization of this slightly less dorsal and anterior portion of the insula spanning into the adjacent inferior frontal operculum in interoception-related activities and supports it’s engagement in activities such as rating or observing one’s own internal state (Zaki et al., 2012), like what would be required during active ER.
Importantly, we found that lower activation in some of these cortical regions (LAI-frontal operculum, LMFG) was also related to lower language ability, lower parent-reported ER ability, and more poverty. These results are consistent with previous findings of lower frontal recruitment during reappraisal in individuals with lower income-to-needs(Kim et al., 2013). Failure of amygdala regulation by cortical regions may explain the relationship between reduced activation of regions involved in cognitive control and ER ability(Kim et al., 2013). We did not find evidence for increased amygdala activity associated with lower income-to-needs but did find reduced activation of cognitive regions. As such, emotion regulation deficits in impoverished youth may be related to dampened recruitment of cognitive resources that may not necessarily reflect a failure of amygdala regulation, as seen in previous work (Kim et al., 2013). Notably, the stimuli in our task were of sad images, but we anticipate that greater income would predict enhanced recruitment of cognitive control regions across negative emotions because regions such as the LDMPFC, LMFG, LAI, and LAI-frontal operculum function for general cognitive control and not in response to specific emotions (Ochsner et al., 2004; Ochsner and Gross, 2008).
Contrary to our hypotheses, we found no evidence that TE was related to heightened activity in brain regions implicated in a) emotional reactivity to sad images, b) processing during reappraisal of sad images, or c) during passive viewing of sad images. These findings are contrary to a robust literature demonstrating heightened reactivity to arousing negative emotions (e.g., disgust and fear) in multiple salience network nodes among threat-exposed youth (Dannlowski et al., 2012; Hein and Monk, 2017; McLaughlin et al., 2014, 2015). However, these findings must be carefully interpreted as only 20 individuals in this sample reported TE, and a posthoc power analysis revealed low power to identify effects of TE on emotional phenomena. Further, it is important to consider that our regulation task involved sadness. Sadness is a low arousal negative emotion that does not contain directly relevant information about novel threats in the same way as high arousing negative emotions such as disgust and fear (Belden et al., 2014). Thus, a lack of heightened reactivity to sad images in TE youth in this sample could suggest that TE might confer specific risks to ER deficits to highly salient fearful, threatening faces. This may not be the case for less arousing emotional faces such as sadness, that may contain less information about potential threats in the environment. Such a hypothesis is consistent with findings that TE individuals show no difference in activation to viewing sad images when compared to non-maltreated individuals (Hart et al., 2018).
Our mediation analyses also provided evidence consistent with our hypotheses about cognitive pathways contributing to the relationship between poverty and ER deficits. We found that lower language ability, as well as lower activation in the LAI extending into the frontal operculum during reappraisal, partially mediated the relationship between poverty and greater parent-reported lability. These also partially mediated the relationship between poverty and poorer parent-reported emotion regulation ability. These findings are interesting in light of work by Noble et al. that suggests that language ability mediates the association between SES and executive function (Noble et al., 2005) and between SES and visuospatial skills, memory, and working memory (Noble et al., 2012). This supports the proposition that language skill predicts childhood emotional and behavioral problems, and that this relationship may be mediated by children’s self-regulation and emotion understanding skills (Salmon et al., 2016). Furthermore, language ability may be particularly important in the process of emotion regulation (Cole et al., 2010), potentially by contributing to specific functions such as self-monitoring or verbal reappraisal.
Concerning the anterior insula, our findings converge with Silverman and colleagues report that low SES individuals show lower activity in the insula in anticipation of unpleasant stimuli (Silverman et al., 2009). Silverman et al. relate their findings to results that individuals with PTSD similarly show lower activity in the insula in anticipation of an unpleasant stimulus. They speculate that poverty is a chronic stressor and offer the idea that the reduction in anterior insula activity may reflect a failure to engage in internal preparation for upcoming negative stimuli (Silverman et al., 2009; Simmons, 2011). Together, our findings that income-to-needs predicts emotional lability as well poor ER ability, and that this is mediated by reduced activation of the LAI during reappraisal, may indicate that impoverished youth fail to engage internal preparation processes that afford adequate reactions to unpleasant events.
Limitations of the current study include that the original study sample was oversampled for preschoolers with symptoms of depression, which may limit the generalizability of these findings. Furthermore, we used bilateral ROIs as specified in a metanalyses by Roy and colleagues however, these subdivisions are as large as 2.4 mm and therefore there may be bleed over between amygdala subdivisions. Additionally, the relationships in the mediation model may be bi-directional as our mediations were cross-sectional in nature. Multiple waves of imaging data starting earlier in development are necessary to adequately test directionality. Future studies with such designs and more detailed assessments of the correlates of poverty, such as nutrition, microbial composition, and parental engagement are needed to elucidate the mechanisms of risk. Regarding TE, as previously mentioned, only twenty individuals in this sample reported TE. However, recruitment, and maintenance, of youth with TE is a problem across a host of studies (Hussey et al., 2006; Kinard, 2001. Furthermore, the use of a dichotomous variable for childhood TE may limit the ability to detect differences by type of TE (Iffland and Neuner, 2020). In addition, information on timing and duration of TE was not collected as part of this project, and thus differential associations based on chronicity and severity of TE to emotion regulation outcomes cannot be inferred from this data and continue to be an important target for research.
5. Conclusions
We believe these findings -- that language ability and left anterior insula extending into the frontal operculum activity, mediate the relationship between poverty and deficits in ER -- can inform preventive interventions for youth facing poverty and suggest that language ability may be a specific target of early intervention. Language provides a method to communicate needs and understand the emotional lives of self and others (Cole et al., 2010). Thus, deficits in language ability in youth may limit their ability to understand and communicate their emotional experiences. The potential importance of language-based ER interventions is underscored by literature highlighting the relationships between language competence, social competence, and behavioral/emotional regulation in preschool-aged children (Izard et al., 2016). This finding, when replicated, would support public policies and programs that enhance language education in youth from impoverished backgrounds as a means to support effective ER and potentially reduce negative mental health outcomes.
Funding
This study was supported by grants R01MH064769-06 and R01 MH098454 and NSF DGE-1745038.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank the Preschool Depression Study participants and their families.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100952.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abela J.R., Rochon A., Vanderbilt E. 2000. The Children’s Response Styles Questionnaire. Unpublished Questionnaire. [Google Scholar]
- Aldao A., Nolen-Hoeksema S., Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Arnow B.A. 2004. Relationships Between Childhood Maltreatment, Adult Health and Psychiatric Outcomes, and Medical Utilization; p. 6. [PubMed] [Google Scholar]
- Bain S.K., Jaspers K.E. Test Review: Review of Kaufman Brief Intelligence Test, Second Edition: Kaufman, A. S., & Kaufman, N. L. (2004). Kaufman Brief Intelligence Test, Second Edition. Bloomington, MN: Pearson, Inc. J. Psychoeduc. Assess. 2010;28(2):167–174. doi: 10.1177/0734282909348217. [DOI] [Google Scholar]
- Barch D.M., Burgess G.C., Harms M.P., Petersen S.E., Schlaggar B.L., Corbetta M., Glasser M.F., Curtiss S., Dixit S., Feldt C., Nolan D., Bryant E., Hartley T., Footer O., Bjork J.M., Poldrack R., Smith S., Johansen-Berg H., Snyder A.Z., Van Essen D.C. Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D., Pagliaccio D., Belden A., Harms M.P., Gaffrey M., Sylvester C.M., Tillman R., Luby J. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am. J. Psychiatry. 2016;173(6):625–634. doi: 10.1176/appi.ajp.2015.15081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden A.C., Luby J.L., Pagliaccio D., Barch D.M. Neural activation associated with the cognitive emotion regulation of sadness in healthy children. Dev. Cogn. Neurosci. 2014;9:136–147. doi: 10.1016/j.dcn.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden A.C., Pagliaccio D., Murphy E.R., Luby J.L., Barch D.M. Neural activation during cognitive emotion regulation in previously depressed compared to healthy children: evidence of specific alterations. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(9):771–781. doi: 10.1016/j.jaac.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J.C., Mezzacappa E., Beardslee W.R. Characteristics of resilient youths living in poverty: the role of self-regulatory processes. Dev. Psychopathol. 2003;15(01):139–162. doi: 10.1017/s0954579403000087. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Weber J., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearfield M.W., Niman L.C. SES affects infant cognitive flexibility. Infant Behav. Dev. 2012;35(1):29–35. doi: 10.1016/j.infbeh.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Cole P.M., Armstrong L.M., Pemberton C.K. 2010. The Role of Language in the Development of Emotion Regulation. [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Kugel H., Huber F., Stuhrmann A., Redlich R., Grotegerd D., Dohm K., Sehlmeyer C., Konrad C., Baune B.T., Arolt V., Heindel W., Zwitserlood P., Suslow T. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala: amygdalar Emotion Processing Bias. Hum. Brain Mapp. 2013;34(11):2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Elsayed N.M., Vogel A.C., Luby J.L., Barch D.M. Labelling emotional stimuli in early childhood predicts neural and behavioral indicators of emotion regulation in late adolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020 doi: 10.1016/j.bpsc.2020.08.018. S2451902220302573. [DOI] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16(1):143–149. [Google Scholar]
- Erwin R.J., Gur R.C., Gur R.E., Skolnick B., Mawhinney-Hee M., Smailis J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Res. 1992;42(3):231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Farah M.J., Shera D.M., Savage J.H., Betancourt L., Giannetta J.M., Brodsky N.L., Malmud E.K., Hurt H. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Feng X., Keenan K., Hipwell A.E., Henneberger A.K., Rischall M.S., Butch J., Coyne C., Boeldt D., Hinze A.K., Babinski D.E. Longitudinal associations between emotion regulation and depression in preadolescent girls: moderation by the caregiving environment. Dev. Psychol. 2009;45(3):798–808. doi: 10.1037/a0014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D., Ormrod R., Turner H., Hamby S.L. The victimization of children and youth: a comprehensive, national survey. Child Maltreat. 2005;10(1):5–25. doi: 10.1177/1077559504271287. [DOI] [PubMed] [Google Scholar]
- Finkelhor D., Turner H.A., Shattuck A., Hamby S.L. Violence, crime, and abuse exposure in a national sample of children and youth: an update. JAMA Pediatr. 2013;167(7):614. doi: 10.1001/jamapediatrics.2013.42. [DOI] [PubMed] [Google Scholar]
- Gershon R.C., Cook K.F., Mungas D., Manly J.J., Slotkin J., Beaumont J.L., Weintraub S. Language measures of the NIH toolbox cognition battery. J. Int. Neuropsychol. Soc. 2014;20(6):642–651. doi: 10.1017/S1355617714000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.J., Litz B.T., Hsu J.L., Lombardo T.W. Psychometric properties of the life events checklist. Assessment. 2004;11(4):330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- Gross J.J. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 1998;2(3):271–299. doi: 10.1037/1089-2680.2.3.271. [DOI] [Google Scholar]
- Hart H., Lim L., Mehta M.A., Simmons A., Mirza K.A.H., Rubia K. Altered fear processing in adolescents with a history of severe childhood maltreatment: an fMRI study. Psychol. Med. 2018;48(7):1092–1101. doi: 10.1017/S0033291716003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein T.C., Monk C.S. Research Review: neural response to threat in children, adolescents, and adults after child maltreatment – a quantitative meta-analysis. J. Child Psychol. Psychiatry. 2017;58(3):222–230. doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- Hussey J.M., Chang J.J., Kotch J.B. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. PEDIATRICS. 2006;118(3):933–942. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- Iffland B., Neuner F. Varying cognitive scars—differential associations between types of childhood maltreatment and facial emotion processing. Front. Psychol. 2020;11:732. doi: 10.3389/fpsyg.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard C., Fine S., Schultz D., Mostow A., Ackerman B., Youngstrom E. Emotion knowledge as a predictor of social behavior and academic competence in children at risk. Psychol. Sci. 2016 doi: 10.1111/1467-9280.00304. [DOI] [PubMed] [Google Scholar]
- Jermann F., Van der Linden M., d’Acremont M., Zermatten A. Cognitive emotion regulation questionnaire (CERQ) Eur. J. Psychol. Assess. 2006;22(2):126–131. doi: 10.1027/1015-5759.22.2.126. [DOI] [Google Scholar]
- Johnson S.B., Riis J.L., Noble K.G. State of the art review: poverty and the developing brain. PEDIATRICS. 2016;137(4) doi: 10.1542/peds.2015-3075. e20153075–e20153075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.S. AGS, American Guidance Service; Circle Pines, MN: 1990. Kaufman Brief Intelligence Test: KBIT. [Google Scholar]
- Kim J., Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. J. Child Psychol. Psychiatry. 2010;51(6):706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Evans G.W., Angstadt M., Ho S.S., Sripada C.S., Swain J.E., Liberzon I., Phan K.L. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. 2013 doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J., Cicchetti D., Rogosch F.A. A longitudinal study of emotion regulation, emotion lability-negativity, and internalizing symptomatology in maltreated and nonmaltreated children. Child Dev. 2013;84(2):512–527. doi: 10.1111/j.1467-8624.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinard E.M. Recruiting Participants for Child Abuse Research: What Does It Take? J. Fam. Violence. 2001;16(3):219–236. doi: 10.1023/A:1011170914965. [DOI] [Google Scholar]
- Koball H., Jiang Y. 2018. Basic Facts About Low-income Children: Children Under 9 Years, 2016. [DOI] [Google Scholar]
- Lavi I., Katz L.F., Ozer E.J., Gross J.J. Emotion reactivity and regulation in maltreated children: a meta-analysis. Child Dev. 2019;0(0) doi: 10.1111/cdev.13272. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Ma S.T., Okada G., Shaun Ho S., Swain J.E., Evans G.W. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc. Cogn. Affect. Neurosci. 2015;10(11):1596–1606. doi: 10.1093/scan/nsv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina S.J., Evers K. Neuroscience of childhood poverty: evidence of impacts and mechanisms as vehicles of dialog with ethics. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez K.C., Luby J.L., Belden A.C., Barch D.M. Emotion dysregulation and functional connectivity in children with and without a history of major depressive disorder. Cogn. Affect. Behav. Neurosci. 2018;18(2):232–248. doi: 10.3758/s13415-018-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Si X., Belden A.C., Tandon M., Spitznagel E. Preschool depression: homotypic continuity and course over 24 MonthsPreschool depression. JAMA Psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E., De Brito S.A., Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front. Psychiatry. 2011;2 doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Peverill M., Gold A.L., Alves S., Sheridan M.A. Child maltreatment and neural systems underlying emotion regulation. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., DeCross S.N., Jovanovic T., Tottenham N. Mechanisms linking childhood adversity with psychopathology: learning as an intervention target. Behav. Res. Ther. 2019;118:101–109. doi: 10.1016/j.brat.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz E.C., Wiltshire C.A., Noble K.G. Socioeconomic inequality and the developing brain: spotlight on language and executive function. Child Dev. Perspect. 2019;13(1):15–20. [Google Scholar]
- Moore T.M., Reise S.P., Gur R.E., Hakonarson H., Gur R.C. Psychometric properties of the penn computerized neurocognitive battery. Neuropsychology. 2015;29(2):235–246. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrakotsky C., Luby J.L. Vienna University and Washington University; Vienna/St. Louis (MO): 2000. The Facial Affect Comprehension Evaluation (FACE): a Test for Emotion Perception and Emotion Recognition in the Preschool Age. [Google Scholar]
- Nelson S.M., Dosenbach N.U.F., Cohen A.L., Wheeler M.E., Schlaggar B.L., Petersen S.E. Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 2010;214(5–6):669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Norman M.F., Farah M.J. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Schork N.J., Murray S.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kennedy D.N., Van Zijl P. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr. Dir. Psychol. Sci. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D.E., Gross J.J. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Palacios-Barrios E.E., Hanson J.L. Poverty and self-regulation: connecting psychosocial processes, neurobiology, and the risk for psychopathology. Compr. Psychiatry. 2019;90:52–64. doi: 10.1016/j.comppsych.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Mogg K., Bradley B.P., Montgomery L., Monk C.S., McClure E., Guyer A.E., Ernst M., Charney D.S., Kaufman J. Attention Bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am. J. Psychiatry. 2005;162(2):291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Pollak S.D. Mechanisms linking early experience and the emergence of emotions: illustrations from the study of maltreated children. Curr. Dir. Psychol. Sci. 2008;17(6):370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S.D., Tolley-Schell S.A. Selective attention to facial emotion in physically abused children. J. Abnorm. Psychol. 2003;112(3):323–338. doi: 10.1037/0021-843X.112.3.323. [DOI] [PubMed] [Google Scholar]
- Prevention, C. for D. C. and . 2013. Child Maltreatment Facts at a Glance 2013. Retrieved from CDC Injury Prevention and Control: www.Cdc.Gov/Violenceprevention/Childmaltreatment/Datasources. Html. [Google Scholar]
- Raizada R.D.S., Kishiyama M.M. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front. Hum. Neurosci. 2010;4 doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens S.E., Pollak S.D. Emotion regulation predicts attention bias in maltreated children at-risk for depression. J. Child Psychol. Psychiatry. 2012;53(2):120–127. doi: 10.1111/j.1469-7610.2011.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Salmon K., O’Kearney R., Reese E., Fortune C.-A. The role of language skill in child psychopathology: implications for intervention in the early years. Clin. Child Fam. Psychol. Rev. 2016;19(4):352–367. doi: 10.1007/s10567-016-0214-1. [DOI] [PubMed] [Google Scholar]
- Salthouse T.A., Babcock R.L., Shaw R.J. Effects of adult age on structural and operational capacities in working memory. Psychol. Aging. 1991;6(1):118. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., McLaughlin K.A. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn. Sci. (Regul. Ed.) 2014;18(11):580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Cicchetti D. Emotion regulation among school-age children: the development and validation of a new criterion Q-sort scale. Dev. Psychol. 1997;33(6):906–916. doi: 10.1037/0012-1649.33.6.906. [DOI] [PubMed] [Google Scholar]
- Silverman M.E., Muennig P., Liu X., Rosen Z., Goldstein M.A. The impact of socioeconomic status on the neural substrates associated with pleasure. Open Neuroimag. J. 2009;3:58–63. doi: 10.2174/1874440000903010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons O.S. Lost in transition: the implications of social capital for Higher Education Access. Notre Dame Law Rev. 2011;87(1):205–252. [Google Scholar]
- Smith A.L., Cross D., Winkler J., Jovanovic T., Bradley B. Emotional dysregulation and negative affect mediate the relationship between maternal history of child maltreatment and maternal child abuse potential. J. Fam. Violence. 2014;29(5):483–494. doi: 10.1007/s10896-014-9606-5. [DOI] [Google Scholar]
- Troy A.S., Mauss I.B. 2011. Chapter Resilience in the Face of Stress: Emotion 2 Regulation as a Protective Factor; p. 15. [Google Scholar]
- Troy A.S., Ford B.Q., McRae K., Zarolia P., Mauss I. Change the things you can: emotion regulation is more beneficial for people from lower than from higher socioeconomic status. Emotion (Washington, D.C.) 2017;17(1):141–154. doi: 10.1037/emo0000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Carlozzi N.E., Slotkin J., Blitz D., Wallner-Allen K. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.H., Matsumoto D., LeRoux J.A. The influence of emotion recognition and emotion regulation on intercultural adjustment. Int. J. Intercult. Relat. 2006;30(3):345–363. doi: 10.1016/j.ijintrel.2005.08.006. [DOI] [Google Scholar]
- Zaki J., Davis J.I., Ochsner K.N. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62(1):493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman J., Shipman K., Penza-Clyve S. Development and initial validation of the children’s sadness management scale. J. Nonverbal Behav. 2001;25(3):187–205. doi: 10.1023/A:1010623226626. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.