Abstract
Background/objective
Spinal arachnoid cysts (SAC) are intradural lesions, which may provoke a compression of the spinal cord and roots. Endoscopic techniques are increasingly used to minimize the surgical access and the postoperative scar tissue. Shunts may also represent an option. The aim of this paper is to illustrate the technique of endoscopic-assisted fenestration and positioning of a cysto-peritoneal diversion in a thoracic SAC using a flexible endoscope and to perform a systematic literature review on this subject.
Material and methods
We reported our case and we performed a review of the literature, searching for all the adult cases of Type III SACs in English language treated through endoscopic procedures.
Results
We found 5 articles matching our search criteria and we included 9 adult patients in our analysis. Six patients were females and the most common localization was the thoracic spine. Six patients underwent selective laminectomies followed by endoscopic fenestration without cyst wall resection. Three patients had a percutaneous endoscopic inspection of the cyst and in two cases a cysto-subarachnoid shunt space was performed. Improvement of pre-operative neurological deficit was reported in six patients, no patients experienced clinical deterioration. The mean follow-up was 22 months and no progression or recurrence was reported.
Conclusion
The implementation of endoscopy allows a minimally invasive treatments with good visualization of cyst anatomy and precise shunt positioning under real-time guidance. Endoscopy is technically demanding but it can offer similar clinical outcomes when compared to microscopic procedures with a limited rate of post-operative complications.
The long-term risk of recurrence should be established by prospective studies.
Keywords: Spinal arachnoid cyst, Endoscopy, Outcome, Shunt, Surgery
Spinal arachnoid cyst, Endoscopy, Outcome, Shunt, Surgery.
1. Introduction
Spinal arachnoid cysts (SACs) are rare diverticula of the arachnoid membrane of the spinal cord described first by Magendie in 1842 [1]. They comprise about 1–3% of all primary spinal space-occupying lesions [2]. From a histological point of view, spinal cysts are classified according to Nabors et al. as extradural without involvement of spinal nerve root fiber (Type I); extradural cysts with spinal nerve root fiber involvement (Type II) and intradural cysts (Type III) [3]. SACs are located most commonly in the thoracic spine leading to compression of the spinal cord and most commonly they are located dorsal to the spinal cord [3, 4]. Their pathogenesis remains uncertain. Primary spinal arachnoid cysts could originate from diverticula of the septum posticum or from ectopic arachnoid granulations [5, 6] while secondary SAC may result from several conditions such as hemorrhage, trauma and infection or they may develop after surgical procedures involving the spinal cord [3, 6, 7, 8, 9]. In secondary SACs, arachnoiditis seems to be the key in the process, as fibrosis and adhesions in the subarachnoid space impede the circulation of cerebrospinal fluid [10].
SACs may result in spinal cord compression requiring surgical treatment [4, 11]. Traditionally, multiple level laminectomies are performed to expose the SAC craniocaudally, with microsurgical release of arachnoid trabeculations and cyst diversion in the subdural space and/or the peritoneal cavity [4, 5, 11]. SACs located in the thoracic spine may require complex posterolateral approaches with consistent risk of spinal cord injury and postoperative deficit [4, 11]. Furthermore, multiple level laminectomies may result in progressive kyphotic deformity thus requiring fixation [12]. To reduce the surgical risks, minimal invasive techniques have been described for SACs fenestration, such as endoscopic assisted procedures [10, 13, 14]. Placement of a cysto-peritoneal shunt also represents a valuable option of treatment [15, 16].
In this paper, we illustrate the technique of endoscopic-assisted fenestration and cysto-peritoneal diversion of a SAC located in the thoracic spine using a flexible endoscope and we discuss the surgical outcomes on the subject after a systematic literature review.
2. Material and methods
An electronic literature search in PubMed/MEDLINE, Scopus, Cochrane, and Embase was conducted for articles published between January 1990 and the end of December 2019. The search was conducted using the free text terms “spinal arachnoid cyst” and “endoscopy”. The literature search strategy was designed to identify all articles in English describing the use of endoscopic techniques in the treatment of adult patients with Type III SACs. Articles in another language and pediatric cases were excluded during our first screening. Papers dealing with Type I and Type II SAC treated via endoscopic techniques were also excluded from this work since their pathophysiology is different. Thereafter, studies focused on the usage of endoscopic techniques for other pathologies such as spinal tumors, spinal cord herniation, chronic pain or non-spinal pathologies were excluded during the eligibility assessment phase. The reference lists in all of the identified relevant articles were manually screened for additional relevant references.
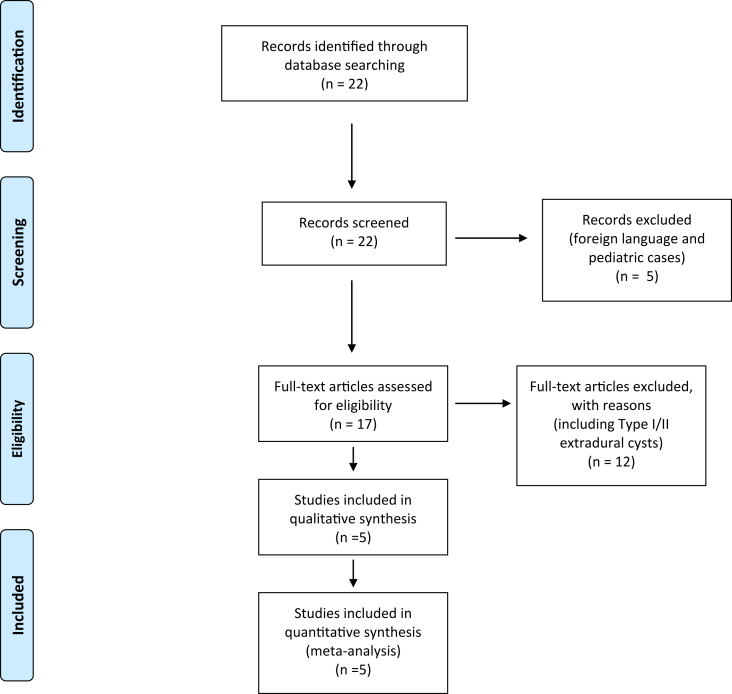
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [17] were implemented for this study and the flow chart details the selection process (Figure 1).
Figure 1.
This flow chart illustrates the selection of the pertinent articles during the literature analysis. Initially 22 articles were identified and only 5 were retained for our final analysis.
3. Results
3.1. Our case
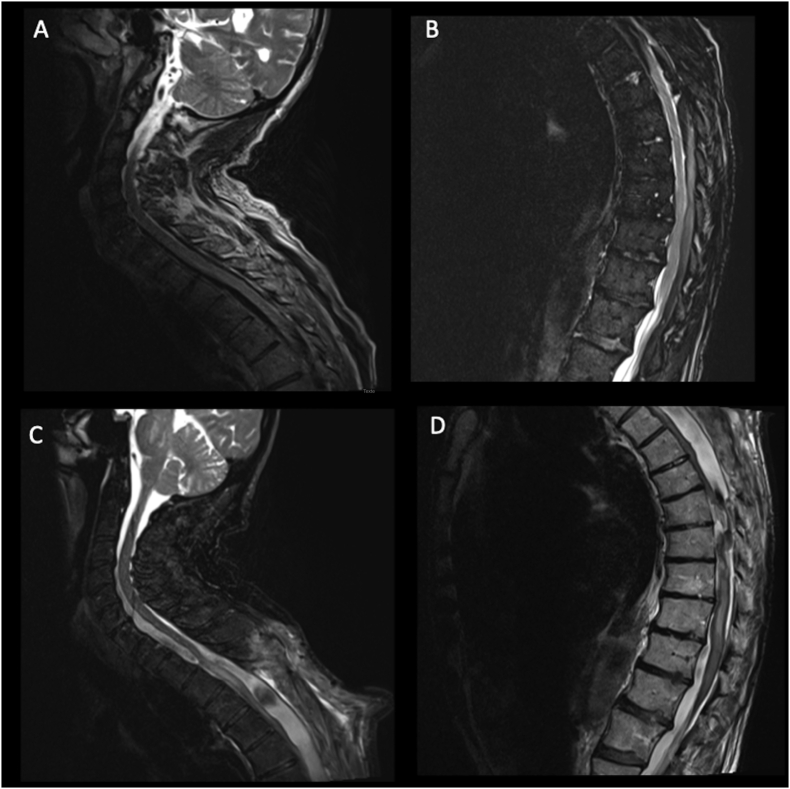
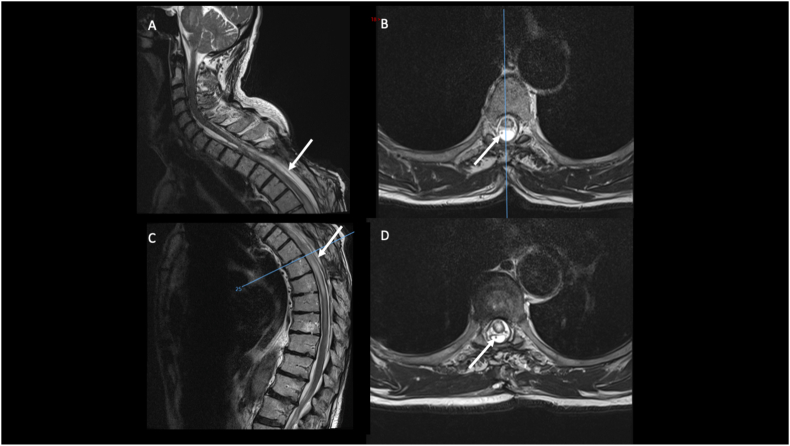
A 79-years old patient presented with a progressive dorsal myelopathy (Nurick grade 3) with a T4 sensitive level. Twelve months earlier he had undergone an evacuation of a C7 to T12 acute spinal subdural hematoma through a posterior dorsal laminectomy from T3 to T6 (Figure 2A and B). The patient was being treated with Acenocumarol for atrial fibrillation and therefore the hematoma was attributed to daily anticoagulant therapy. On twelve-month follow-up, a spine MRI revealed signs of myelomalacia associated with a syrinx formation and a dorsal SAC extending from T3 to T10 and compressing the spinal cord (Figure 2C and D). The myelopathy was attributed to the developing syrinx and he was initially treated with a lumbo-peritoneal shunt at another hospital. The patient showed no improvement after this procedure. A new spine MRI showed the persistence of his large dorsal SAC. Because of the progressive dorsal myelopathy, we decided to perform a SAC fenestration with placement of a cysto-peritoneal shunt under endoscopic guidance in order to prevent cyst enlargement. A STRATA® valve (NSC Valve, Medtronic) was used and the opening pressure was 0.5. The patient improved in the postoperative period (Nurick grade 2). He was discharged on day 5 to a rehabilitation center. Immediate post-operative MRI showed that the drain spanned craniocaudally the SAC. At last follow-up (30 months), the patient's myelopathy continued to improve and he was able to ambulate with one cane. Spine MRI showed a radiological improvement of the SAC with no compression of the spinal cord (Figure 3). The syringomyelia, however, remained radiologically stable.
Figure 2.
Spinal sagittal T2-weighted MRI illustrating the preoperative status of our patient (A and B): an anterior and posterior subdural hematoma starting from the lower cervical spine (A) and going down to T12 (B) was detected and responsible for a flaccid paraparesis. The patient was operated for the evacuation of this symptomatic hematoma and a dorsal laminectomy wad performed for the microsurgical evacuation of the collection. In the postoperative period the patient presented a progressive neurological deterioration with a follow-up MRI showing the development of a large posterior SAC, with an important mass effect on the spinal cord (C, cervical and D, dorsal).
Figure 3.
Sagittal (A and C) and axial (B and D) views of T2-weighted MRI showing the postoperative results after the endoscopic opening of the cyst and the positioning of the cysto-peritoneal drain (arrow) under endoscopic-assistance. The patient experienced a neurological improvement and the follow-up images confirmed the reduction in size of the SAC with a relief of the mass effect on the spinal cord.
3.2. Surgical procedure (Video)
Supplementary video related to this article can be found at https://doi.org/10.1016/j.heliyon.2021.e06736
The following is the supplementary data related to this article:
The patient was placed in ventral decubitus on a Wilson frame. Following intraoperative radioscopy, two small 3cm incisions were performed on the cranio-caudal edges of the old incision. The dural sac was exposed and the SAC was sharply fenestrated and drained at these levels. A flexible endoscope (Boston Scientific, LithoVue™) was inserted at the cephalic extremity of the cyst through a small dural opening with an arachnoid blade and the entire cyst anatomy was visualized. Through a dural opening at the caudal extremity of the cyst, a 2mm forceps was inserted through the working channel of the flexible endoscope and we opened all the trabeculations within the cyst under endoscopic control.
A shunt was then introduced under endoscopic guidance from the caudal to the cephalic extremity of the cavity. The distal part of the shunt was then connected with the already existing valve and then connected with the peritoneal drain left in place.
Watertight closure of the dural margins at both the cephalic and caudal cavity incisions was performed, and the suture was then reinforced using muscle graft and fibrin glue. The surgical time was about 3 h and the blood loss was negligible.
Informed consent was obtained from the patient.
3.3. Results of the literature search
The literature search process is illustrated in the flow chart in Figure 1.
Only studies including adult patients with Type III spinal arachnoid cysts treated with endoscopic techniques were included in our final analysis. A total of 22 articles were identified by our primary search. After exclusion of 5 articles (not in English or focused on a pediatric cohort), 17 articles were screened on the basis of title and abstract. Among them, 12 articles were excluded because they were not focused on our above mentioned pre-defined endpoints.
A total of five articles were then included in our analysis [11, 15, 16, 18, 19], including nine adult patients with Type III SAC treated through endoscopic techniques (Table 1). One study reported on lysis of adhesions in 12 patients with SACs using a Karl Storz flexibile endoscope [20] However, the authors did not report their patients’ characteristics and it was not included in the final analysis.
Table 1.
The epidemiologic, clinical and radiological characteristics of the patients included in our analysis are here summarized, along with the outcomes of the follow-up. Our case is added to the cases described in literature at the end of this table.
| Author | Age/Sex | Localization of the SAC | Etiology | Treatment | Clinical Outcomes | Recurrence | Follow up |
|---|---|---|---|---|---|---|---|
| Tanaka et al., 1997 [18] | 49/F | T1-T6 | Aneurysmal Subarachnoid hemorrhage | Percutaneous endoscopic fenestration and diversion in subarachnoid space without cystic wall resection | Improvement of neurological symptoms | None | 15 months |
| Eguchi et al., 1999 [19] | 62/F | T5-T10 | Idiopathic | Preoperative endoscopic percutaneous observation by lumbar puncture followed by microsurgical resection | N/A | N/A | N/A |
| Guest et al., 2005 [16] | N/A/M | T11-L3 | s/p ependymoma resection 10 years earlier | Trial of percutaneous, endoscopic external drainage | Neurologically unchanged | None | N/A |
| Endo et al., 2010 [11] | 74/M | T8-T9 | Idiopathic | Selective laminectomies + Endoscopic inspection/fenestration without cyst wall removal | Improvement of neurological symptoms | None | Mean 115 months |
| 27/F | T3-T10 | Meningitis | |||||
| 32/M | C7-T2 | Idiopathic | |||||
| 45/F | T2-T8 | Idiopathic | |||||
| 57/F | T5-T8 | Epidural hematoma | |||||
| Tan et al., 2019 [15] | 76/F | T4-T12 | Spontaneous subdural hematoma due to anticoagulation | Selective laminectomy and endoscopic fenestration and placement of a Spinal Cysto-Subarachnoid Shunt | N/A | N/A | N/A |
| Our case | 79/M | T3-T10 | Spontaneous subdural hematoma due to anticoagulation | Endoscopic fenestration and placement of cysto-peritoneal shunt | Myelopathy improvement | None | 30 months |
From our review, 6 patients were females and the mean age of presentation was 52.8 years (range 27–76 years). The most common localization was the thoracic spine (7 patients). In one patient, SAC was localized in the thoracolumbar spine, and in one patient it spanned in the cervicothoracic spine. The etiology was idiopathic (4 patients), secondary to a hemorrhagic event such as a subdural or epidural hematoma and subarachnoid hemorrhage (3 patients), post-infectious (1 patient) and secondary to tumor resection surgery (1 patient) (Table 1).
Six patients underwent selective laminectomies followed by endoscopic fenestration without cyst wall resection [11, 15]. Three patients had a percutaneous endoscopic inspection of the cyst [16, 18, 19]. In two of these patients an endoscopic fenestration and diversion in the subarachnoid space was performed without cyst wall removal [18] and in the other case a radical microsurgical resection was carried out [19]. Lastly, a trial of external drainage placement under endoscopic guidance for 3 days was performed in the last patient [16].
None of the authors reported deterioration of symptoms post-operatively. Improvement of pre-operative neurological deficit was reported in six patients. In one patient, the neurological symptoms remained stable. In two patients, the neurological status was not reported. The follow-up varied between 15 and 115 months and no cyst progression or recurrence was reported.
In our case, a cysto-peritoneal shunt was placed spanning cranio-caudally the cyst following endoscopic fenestration. Post-operative imaging revealed a good placement of the catheter. There were no surgical complications and radiologically the SAC was reduced in size at 30-month follow-up.
4. Discussion
SACs are uncommon entities but they may cause neurological symptoms, secondary to spinal cord or nerve roots compression [3, 4]. The most common presenting symptoms are back pain (92%), gait disturbance (80%), motor weakness (80%), dysesthesia of the lower limb (64%), paresis (80%), and fine motor skill disturbance (68%) [2, 4, 9]. According to Fam et al, the most common clinical findings are signs of dorsal and cervical myelopathy, in 37% and 32% of cases respectively [4].
Surgical treatment is indicated for patients with myelopathy or refractory radicular pain due to SAC enlargement [4, 5, 11]. In most cases, SACs span multiple levels thus requiring multilevel laminectomies to ensure cyst fenestration or resection [21]. Cyst fenestration aims at creating a communication between the cystic cavity and the subarachnoid space [4, 11, 14, 22]. Despite successful cyst fenestration, the cyst progression is reported to occur in between 10 % and 60 % [4, 9, 14]. To reduce the risk of cyst recurrence, some authors propose a complete cyst wall resection [4, 19]. However, this procedure is technically demanding because SACs are often adherent to the spinal cord or nerve roots and it may be associated with new neurological deficits in the postoperative period [11, 23]. Furthermore, multilevel laminectomies increase the rate of postoperative complications, such as infections, hemorrhage and spinal instability [9]. Interlaminar fenestrations or laminoplasty with small dural openings on the cranial and caudal edges can be proposed as alternative surgical technique [2].
Percutaneous drainage or the positioning of cysto-subarachnoid or cysto-peritoneal shunts are alternative procedures [10, 15, 16] and they may be useful in recurrent arachnoid cysts [14, 23, 24]. The implementations of shunt procedures in SAC is poorly discussed in the literature and remains controversial due to fear of shunt failure and short term benefit [24, 25, 26].
According to our literature analysis, none of the patients undergoing endoscopic fenestration and diversion in the subarachnoid space without cyst wall resection showed any signs of progression on follow-up [11, 19, 26]. In endoscopic-assisted cases, selective laminectomies are performed at the cranial and caudal end of the cyst followed by two small dural openings to introduce the endoscope and obtain CSF diversion in the subarachnoid space [11]. Alternatively, an initial approach in the middle of the cyst can be performed to safely manipulate the endoscope in both cranial and caudal directions [20]. The use of the flexible endoscope is reported is reported to be a safe and effective procedure [11, 16, 19, 20, 26, 27].
Endo et al compared the outcomes obtained in patients treated with endoscopic assisted procedures versus traditional microsurgical technique, namely multi-level laminectomies and radical cyst resection [11]. In the endoscopic group, the number of levels of laminectomy were fewer and operative times were shorter. Also, the blood loss was less in the endoscopic group but the difference was not significant. The shorter extent of laminectomies in the endoscopy group could explain the lower rate of kyphotic deformity in this group (0% versus 40% in the microsurgical group respectively). The authors reported no cyst recurrence regardless of the surgical technique applied.
Schmutzer et al. [2] treated 72 patients with idiopathic (88.9%), post-hemorrhagic (8.3%), and post-inflammatory (2.8%) Type III SAC with traditional open techniques [2]. Their follow up period was 44.8 ± 60.0 months and the authors reported nine patients (12.5%) undergoing a second surgery for recurrence, wound infection or hematoma formation [2]. The association between the etiology of SAC and the surgical difficulty or clinical outcomes is not clear [2] and it could be the subject for larger dedicated studies. Having said that, it remains difficult to make any direct comparison between open and endoscopic techniques. Endoscopic techniques are relatively new in the neurosurgical practice for spinal disorders and therefore the follow up period is shorter.
Independent of the surgical technique performed, less satisfactory outcomes were observed in patients with long duration of symptoms and in patients with large SAC extending more than 5 vertebrae [11]. Notably, in the endoscopic group here reported, no cases of neurological deterioration were reported.
Although endoscopic approaches are less invasive and are associated with lower risk of complications such as wound infection, hematoma formation, post kyphotic deformity and recurrence, endoscopic techniques for spine pathologies require a substantial learning period before adequate performance can be achieved [28, 29].
A limitation of our analysis is that the few pertinent studies were heterogeneous. Thus, it is difficult to draw conclusions regarding the clinical outcomes in patients treated for Type III SAC using endoscopic techniques. SAC are rare pathologies and further larger prospective studies should be performed in order to establish the optimal management and to clarify the long-term recurrence rate and clinical outcomes.
5. Conclusion
Our literature review revealed a paucity of studies describing the surgical results of endoscopic-assisted techniques for patients with SACs. Large symptomatic spinal arachnoid cysts remain challenging to treat. During the last decade endoscopic fenestration coupled or not with shunt placement opened the way for minimally invasive treatments. We believe that the implementation of endoscopy for the treatment of SACs allows good and accurate visualization of cysts’ anatomy with precise shunt placement under real time endoscopic guidance. Less invasive endoscopic approaches may be more technically demanding but they might offer similar clinical outcomes with less post-operative complications. The clinical outcomes and long-term risk of recurrence should be established by further prospective studies.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Magendie F. Méquignon-Marvis fils; 1842. Recherches physiologiques et cliniques sur le liquide céphalorachidien ou cérébro-spinal. [Google Scholar]
- 2.Schmutzer M., Tonn J.C., Zausinger S. Spinal intradural extramedullary arachnoid cysts in adults-operative therapy and clinical outcome. Acta Neurochir. 2019 doi: 10.1007/s00701-019-04156-0. [DOI] [PubMed] [Google Scholar]
- 3.Nabors M.W., Pait T.G., Byrd E.B., Karim N.O., Davis D.O., Kobrine A.I., Rizzoli H.V. Updated assessment and current classification of spinal meningeal cysts. J. Neurosurg. 1988;68(3):366–377. doi: 10.3171/jns.1988.68.3.0366. [DOI] [PubMed] [Google Scholar]
- 4.Fam M.D., Woodroffe R.W., Helland L., Noeller J., Dahdaleh N.S., Menezes A.H., Hitchon P.W. Spinal arachnoid cysts in adults: diagnosis and management. A single-center experience. J. Neurosurg. Spine. 2018:1–9. doi: 10.3171/2018.5.SPINE1820. [DOI] [PubMed] [Google Scholar]
- 5.Fortuna A., Mercuri S. Intradural spinal cysts. Acta Neurochir. 1983;68(3-4):289–314. doi: 10.1007/BF01401186. [DOI] [PubMed] [Google Scholar]
- 6.Nath P.C., Mishra S.S., Deo R.C., Satapathy M.C. Intradural spinal arachnoid cyst: a long-term postlaminectomy complication: a case report and review of the literature. World Neurosurg. 2016;85 doi: 10.1016/j.wneu.2015.09.058. 367 e361-364. [DOI] [PubMed] [Google Scholar]
- 7.Kriss T.C., Kriss V.M. Symptomatic spinal intradural arachnoid cyst development after lumbar myelography. Case report and review of the literature. Spine. 1997;22(5):568–572. doi: 10.1097/00007632-199703010-00023. [DOI] [PubMed] [Google Scholar]
- 8.Mauer U.M., Gottschalk A., Kunz U., Schulz C. Arachnoscopy: a special application of spinal intradural endoscopy. Neurosurg. Focus. 2011;30(4):E7. doi: 10.3171/2011.1.FOCUS10291. [DOI] [PubMed] [Google Scholar]
- 9.Mohindra S., Gupta R., Bal A. Intra-dural spinal arachnoid cysts: a short series of 10 patients. Br. J. Neurosurg. 2010;24(6):679–683. doi: 10.3109/02688697.2010.504052. [DOI] [PubMed] [Google Scholar]
- 10.Basaran R., Kaksi M., Efendioglu M., Onoz M., Balkuv E., Kaner T. Spinal arachnoid cyst associated with arachnoiditis following subarachnoid haemorrhage in adult patients: a case report and literature review. Br. J. Neurosurg. 2015;29(2):285–289. doi: 10.3109/02688697.2014.976175. [DOI] [PubMed] [Google Scholar]
- 11.Endo T., Takahashi T., Jokura H., Tominaga T. Surgical treatment of spinal intradural arachnoid cysts using endoscopy. J. Neurosurg. Spine. 2010;12(6):641–646. doi: 10.3171/2009.12.SPINE09577. [DOI] [PubMed] [Google Scholar]
- 12.Funao H., Nakamura M., Hosogane N., Watanabe K., Tsuji T., Ishii K., Kamata M., Toyama Y., Chiba K., Matsumoto M. Surgical treatment of spinal extradural arachnoid cysts in the thoracolumbar spine. Neurosurgery. 2012;71(2):278–284. doi: 10.1227/NEU.0b013e318257bf74. discussion 284. [DOI] [PubMed] [Google Scholar]
- 13.Stevens J.M., Kendall B.E., Davis C., Crockard H.A. Percutaneous insertion of the spinal end of a cysto-peritoneal shunt as definitive treatment to relieve cord compression from a spinal arachnoid cyst. Neuroradiology. 1987;29(2):190–195. doi: 10.1007/BF00327548. [DOI] [PubMed] [Google Scholar]
- 14.Pradilla G., Jallo G. Arachnoid cysts: case series and review of the literature. Neurosurg. Focus. 2007;22(2):E7. doi: 10.3171/foc.2007.22.2.7. [DOI] [PubMed] [Google Scholar]
- 15.Tan D.C.H., Vaughan K.A., Koeck H. Endoscopic-assisted spinal arachnoiditis adhesiolysis and placement of a spinal cysto-subarachnoid shunt. World Neurosurg. 2019;131:43–46. doi: 10.1016/j.wneu.2019.07.160. [DOI] [PubMed] [Google Scholar]
- 16.Guest J.D., Silbert L., Casas C.E. Use of percutaneous endoscopy to place syringopleural or cystoperitoneal cerebrospinal fluid shunts: technical note. J. Neurosurg. Spine. 2005;2(4):498–504. doi: 10.3171/spi.2005.2.4.0498. [DOI] [PubMed] [Google Scholar]
- 17.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., Group P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T., Sakamoto T., Koyama T., Watanabe K., Tanaka T., Murata K. Endoscopic treatment of symptomatic spinal subarachnoid cysts. AJR Am. J. Roentgenol. 1997;169(6):1719–1720. doi: 10.2214/ajr.169.6.9393196. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi T., Tamaki N., Kurata H. Endoscopy of the spinal cord: cadaveric study and clinical experience. Minim. Invasive Neurosurg.: MIN. 1999;42(3):146–151. doi: 10.1055/s-2008-1053388. [DOI] [PubMed] [Google Scholar]
- 20.Kashcheev A.A., Arestov S.O., Gushcha A.O. Flexible endoscopy in surgical treatment of spinal adhesive arachnoiditis and arachnoid cysts. Zhurnal voprosy neirokhirurgii imeni N N Burdenko. 2013;77(5):44–54. discussion 54-45. [PubMed] [Google Scholar]
- 21.Kizilay Z., Yilmaz A., Ozkul A., Ismailoglu O. Cervicothoracic arachnoid cyst causing cervical myelopathy: a case report. Open access Macedonian J. Med. Sci. 2015;3(1):135–138. doi: 10.3889/oamjms.2015.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonoff E.T., Lopez W.O., de Oliveira Y.S., Lara N.A., Teixeira M.J. Endoscopic approaches to the spinal cord. Acta Neurochir. Suppl. 2011;108:75–84. doi: 10.1007/978-3-211-99370-5_12. [DOI] [PubMed] [Google Scholar]
- 23.Jensen F., Knudsen V., Troelsen S. Recurrent intraspinal arachnoid cyst treated with a shunt procedure. Acta Neurochir. 1977;39(1-2):127–129. doi: 10.1007/BF01405250. [DOI] [PubMed] [Google Scholar]
- 24.Hirai T., Kato T., Kawabata S., Enomoto M., Tomizawa S., Yoshii T., Sakaki K., Shinomiya K., Okawa A. Adhesive arachnoiditis with extensive syringomyelia and giant arachnoid cyst after spinal and epidural anesthesia: a case report. Spine. 2012;37(3):E195–198. doi: 10.1097/BRS.0b013e31822ba817. [DOI] [PubMed] [Google Scholar]
- 25.Griessenauer C.J., Bauer D.F., Moore T.A., 2nd, Pritchard P.R., Hadley M.N. Surgical manifestations of thoracic arachnoid pathology: series of 28 cases. J. Neurosurg. Spine. 2014;20(1):30–40. doi: 10.3171/2013.9.SPINE1323. [DOI] [PubMed] [Google Scholar]
- 26.Eneling J., Bostrom S., Rossitti S. Subarachnoid hemorrhage-associated arachnoiditis and syringomyelia. Clin. Neuroradiol. 2012;22(2):169–173. doi: 10.1007/s00062-011-0082-5. [DOI] [PubMed] [Google Scholar]
- 27.Chern J.J., Gordon A.S., Naftel R.P., Tubbs R.S., Oakes W.J., Wellons J.C., 3rd Intradural spinal endoscopy in children. J. Neurosurg. Pediatr. 2011;8(1):107–111. doi: 10.3171/2011.4.PEDS10533. [DOI] [PubMed] [Google Scholar]
- 28.Ao S., Wu J., Tang Y., Zhang C., Li J., Zheng W., Zhou Y. Percutaneous endoscopic lumbar discectomy assisted by O-Arm-Based navigation improves the learning curve. BioMed Res. Int. 2019;2019:6509409. doi: 10.1155/2019/6509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S.M., Kim H.J., Kim G.U., Choi M.H., Chang B.S., Lee C.K., Yeom J.S. Learning curve for lumbar decompressive laminectomy in biportal endoscopic spinal surgery using the cumulative summation test for learning curve. World Neurosurg. 2019;122:e1007–e1013. doi: 10.1016/j.wneu.2018.10.197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.