Highlights
-
•
Mechanism of action of Shikonin against PEL.
-
•
Therapeutic potential of Shikonin as an alternative herbal medicine.
-
•
Interconnectivity among cellular stress, apoptosis pathways (extrinsic and intrinsic) and Shikonin.
Keywords: Apoptosis, ROS, Primary effusion lymphoma (PEL), Shikonin
A Commentary on:
Induction of apoptosis by Shikonin through ROS-mediated intrinsic and extrinsic apoptotic pathways in primary effusion lymphoma
By Alam, M.M., Kariya, R., Boonnate, P., Kawaguchi, A., Okada, S. (2021)
Translational Oncology 14 (2021) 101006
doi: 10.1016/j.tranon.2020.101006
Primary effusion lymphoma (PEL) is a rare disease belonging to the large B-cell lymphoma. It is characterized by an increased neoplastic effusion in the patients’ body cavities (pleural space, peritoneal cavity, pericardium) without detectable tumor masses [1]. PEL occurrence is frequently associated with immunocompromised states such as HIV/AIDS and human herpes virus-8 (HHV-8). Despite the effective use of antiretroviral therapy against HIV/AIDS's related lymphomas, an optimal treatment regimen for PEL cases does not yet exist. Additionally, methotrexate, cycophosphospahmide, doxorubicin, vincristine, and prednisolone (CHOP) based chemotherapeutic options have been tried for PEL with systemic toxicity. Therefore, there is a current need to understand PEL disease biology with the goal of advancing possible therapeutics and exploring alternative treatment options.
Cell death is an essential biological process required to maintain normal cellular homeostasis. Apoptosis is a form of programmed cell death that is primarily mediated by two pathways: a caspase-dependent and a caspase-independent approach. The caspase-dependent pathway is further divided into intrinsic and extrinsic pathways with the intrinsic apoptotic pathway typically triggered in the absence of growth factors, hypoxia, DNA damage, and irradiation-like conditions leading to the activation of a tumor suppressor protein p53 [2]. The activation of p53 further leads to elevated expression of cell cycle inhibitory proteins: p21 (gene: CDKN1A) and p45 (gene: SKP2). This signaling cascade results in the interruption of the cell cycle directly allowing the repair of genomic damage before entering S phase or mitosis. If DNA damage cannot be repaired, p53 induces pro-apoptotic protein (Bax, Bak) expression which triggers programmed cell death [2]. Ensuing cellular apoptotic stress, there is a conformational change at the C-terminal end of the Bax protein. This conformational change helps Bax adhere to the outer mitochondrial membrane. The interaction of Bax and the mitochondrial membrane leads to membrane punctures resulting in the release of cytochrome-c, apoptosis-inducing factor (AIF) and endonuclease G (EndoG) [2]. The release of cytochrome-c from mitochondria into cytosol cannot be reversed. Cytochrome-c further stimulates the production of reactive oxygen species (ROS) [2]. The elevated ROS level in the cytoplasm can damage cellular components and induce oxidative stress. Alternatively, the extrinsic apoptotic pathway involves tumor necrosis factor-α (TNF-α) or Fas ligand (FasL) and their cognate receptors like TNF-R1 receptor. Subsequently, the activated receptor allows the assembly of a cytoplasmic multiprotein complex called DISK (Death-Inducing Signaling Complex) [3]. This complex initiates the apoptotic cascade primarily by activation of procaspase-8 through FADD proteins [3]. The activation of caspase-3, −6, and −7 by proteolytic cleavage of caspase-8 induces the cell's self-destruction by cleaving essential substrates for the maintenance of cell life. The apoptosis signaling is also facilitated by the release of ATP or other nucleotides through a channel protein called PANX1. Activated caspases-3, and 7 use PANX1 protein to mediate their apoptotic activities.
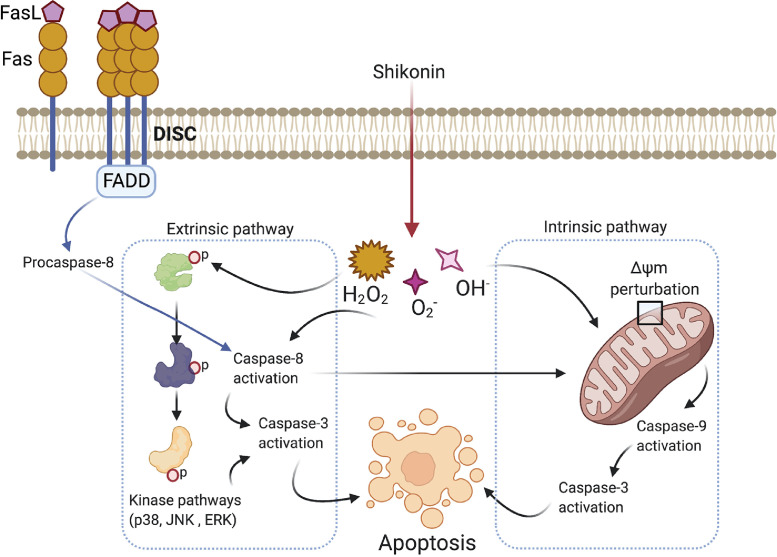
Shikonin (SHK) is a Chinese herbal medicine frequently used for its anti-inflammatory and wound healing effects. SHK is derived from Lithospermum erythrorhizon roots. This has also been shown to inhibit human immunodeficiency virus (HIV) type-1 (HIV-1) and cancer cell proliferation [4] by affecting various signaling pathways (MAPK, PI3K/AKT, STAT3) and through activating DNA fragmentation through apoptosis [5], [6]. Repurposing SHK for the treatment of leukemia has been reported previously [7]. Alam et al. [8] for the first time reported the use of SHK and its therapeutic potential against PEL in vitro and in vivo model systems. This study employed 5 human PEL cell lines: BCBL-1, BC-1, BC-3, TY-1, GTO, and PEL-xenograft mouse model to show the anti-tumor effects of SHK against PEL. Based on the findings, SHK specifically induces apoptosis in the PEL cell lines and it is mediated through caspase-8. As reported in the study, caspase-8 activation is in part due to the loss of mitochondrial ΔΨm. However, there is a possibility that the classical DISK based extrinsic pathway activation might also be involved in the caspase-8 activation. Furthermore, caspase-3 activation is also reported post SHK treatment. Additionally, SHK disrupts mitochondrial function by causing the loss of ΔΨm and activation of caspase-9. This mechanism indicates that SHK activates both the extrinsic and intrinsic apoptosis pathways to kill the PEL cells (Fig. 1).
Fig. 1.
Schematic depicting the involvement of extrinsic and intrinsic apoptosis pathways in SHK mediated cell death on PEL cells. The extrinsic pathway is triggered by kinases (p38, JNK, ERK) mediated caspase-3 activation. Additionally, caspase-8 could also activate caspase-3 independent of the kinases mediated activation and this route is also considered as the extrinsic pathway of activation. This pathway is primarily controlled by a multiprotein complex called DISK downstream of Fas receptor. Mitochondrial membrane potential perturbation leads to the intrinsic pathway activation. This pathway activates caspase-9 and caspase-3 inducing apoptosis.
The authors have also examined the role of JNK signaling in the induction of SHK based apoptosis in PEL cells. SHK was shown to elevate the phosphorylation of p38, ERK, and JNK in PEL cells. Furthermore, JNK inhibitor treatment rescued the PEL cells from SHK mediated apoptotic effects. These results suggest a kinase mediated signaling mechanism in SHK induced apoptosis. Interestingly, major survival pathways such as NF-kB, PI3K were not affected by SHK treatment. STAT3 activation was significantly inhibited by SHK treatment and SHK induced PEL cell death potentially uses JNK mediated extrinsic apoptosis pathway (Fig. 1).
The authors showed an intracellular ROS accumulation upon SHK treatment in PEL cells. Based on pan-caspase inhibitor treatment experiments, ROS generation was a proximal and initiating event of JNK as well as caspase-3 activation in SHK-induced apoptosis of PEL cells. The tumor inhibitory effects of SHK were also replicated in the PEL xenograft model where a significant reduction in spleen size was observed post treatment. Overall this study provides evidence of the involvement of both intrinsic and extrinsic apoptotic pathways in SHK's anti-PEL effects (Fig. 1).
Although the study provides an understanding of the MOA behind SHK's effects on PEL cells, further investigations will be necessary to fully understand the translational effects on PEL. Firstly, the authors have explored a subset of known survival pathways involved in SHK inhibitory effects on PEL cells. It will be informative if the following future studies could be performed; Given JNK signaling is involved in SHK induced apoptosis, it will be interesting to implement a global phosphoproteomics approach to unlock a system-level understanding of other kinase mediators involved in the inhibitory effects of SHK. A global phosphoproteomics study would provide a comprehensive view of cross-talk between different kinase networks in PEL cells after SHK treatment. In the past, a comprehensive phospho-tyrosine signaling study was employed to understand leukemia initiating mutated granulocyte colony stimulating factor signaling [9]. Secondly, the interesting link between ROS and JNK signaling downstream of SHK requires in-depth understanding by future experiments. The authors provide some insights using experimental findings of the perturbation in the ΔΨm. One area where follow up studies could provide further cellular insights would be in understanding how elevated ROS is affecting the mitochondrial membrane integrity (by focusing on functional deficiency in MOMP or mitochondrial permeability transition (MPT)) after SHK treatment. Additionally, the mitochondrial ROS source needs to be explored in connection with SHK induced apoptosis. The cellular glutathione pool acts as a ROS scavenger and wards off the apoptosis initiated by increased ROS level [10]. Therefore, a future study aiming to study the cellular ROS homeostasis and investigate a correlation between SHK induced increased ROS and glutathione pool would be informative. It is appreciated that mitochondrial membrane integrity serves as a deciding factor on whether cells go through apoptosis. It will also be interesting to understand if there is any involvement of mitophagy in SHK treated PEL cells. Even though p53 is not mutated with PEL cases, it is a universal sensor of genotoxic stress in the cells. Therefore, a future study describing SHK's effects on p53 protein would be insightful in understanding of the cellular signaling network of PEL cells. Most of the mechanistic studies shown in the paper, are based on a single cell line (GTO). Therefore, it is suggested to validate the mechanistic findings in the other related PEL cell lines in the future study. Lastly, in vivo studies only show a single set of experiment using PEL xenograft mouse model's spleen weight. Future experiments using colony forming unit (CFU) showing the anti-PEL effects at progenitor cells would be instrumental in understanding how SHK inhibits PEL cells. Additionally, this would provide a window of understanding of SHK's MOA at the hematopoiesis level.
Taken together, this is an interesting study describing the involvement of extrinsic as well as intrinsic pathways in the apoptotic cell death induced by SHK in PEL in vitro and in vivo models. The new findings provide unique insights into the mechanism of action of Chinese herbal medicine: SHK against rare disease PEL and significantly contribute to the understanding of PEL biology. Based on the in vitro and in vivo studies, SHK does exhibits anti-tumor effects. However, to fully validate the therapeutic potential as well as efficacy of this herbal medicine, a clinical trial is warranted where it could be tested with or without existing chemotherapeutic options for PEL patients. The clinical trial-based studies would provide avenues to understand as well as evaluate whether SHK could be used primarily or as a combination therapy along with the available standard of care. Overall, the study provides a unique avenue where an existing therapeutic entity could be repurposed as a possible treatment option for a rare disease like PEL.
CRediT author statement
Pankaj Dwivedi: Conceptualization, writing original draft preparation, Visualization, Reviewing and Editing.
The figure was prepared using Biorender.com.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The author is thankful to Donald Kirkpatrick for critical reading, edits, insightful comments throughout the preparation of this commentary.
Funding
Not applicable.
References
- 1.Shimada K., Hayakawa F., Kiyoi H. Biology and management of primary effusion lymphoma. Blood. 2018;132(18):1879–1888. doi: 10.1182/blood-2018-03-791426. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A., O'Connor L., Dixit V.M. Apoptosis signaling. Ann. Rev. Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A., Dixit V.M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Chen X., Yang L., Zhang N., Turpin J.A., Buckheit R.W., Osterling C., Oppenheim J.J., Zack Howard O.M. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2003;47(9):2810–2816. doi: 10.1128/AAC.47.9.2810-2816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn J., Won M., Choi J.H., Kim Y.S., Jung C.R., Im D.S., Kyun M.L., Lee K., Song K.B., Chung K.S. Reactive oxygen species-mediated activation of the Akt/ASK1/p38 signaling cascade and p21(Cip1) downregulation are required for Shikonin-induced apoptosis. Apotosis. 2013;18:870–881. doi: 10.1007/s10495-013-0835-5. [DOI] [PubMed] [Google Scholar]
- 6.Lu D., Qian J., Li W., Feng Q., Pan S., Zhang S. Beta-hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncol. Lett. 2015;10:3434–3442. doi: 10.3892/ol.2015.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao X., Yu C.R., Li W.H. Induction of apoptosis by Shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogeneous leukemia (CML) cells. Cell Res. 2008;18:879–888. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]
- 8.Alam M.M., Kariya R., Boonnate P., Kawaguchi A., Okada S. Induction of apoptosis by Shikonin through ROS-mediated intrinsic and extrinsic apoptotic pathways in primary effusion lymphoma. Transl. Oncol. 2021;14 doi: 10.1016/j.tranon.2020.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwivedi P., Muench D., Wagner M., Azam M., Grimes H.L., Greis K.D. Time resolved quantitative phospho-tyrosine analysis reveals Bruton's Tyrosine kinase mediated signaling downstream of the mutated granulocyte-colony stimulating factor receptors. Leukemia. 2019;33(1):75–87. doi: 10.1038/s41375-018-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon H.U., Haj-Yehia A., Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]