Abstract
The novel human coronavirus SARS-CoV-2 has been responsible for a worldwide pandemic. Although media transmission through contaminated surfaces is one of the most recognized ways of transmission, the study on the number and viability of viruses surviving on a surface after leaving the host represents a “blind spot” in current research. In this paper we have reviewed studies on the physical process of droplet evaporation on media surfaces, and analyzed the recent literature related to experiments on the decay of the viral concentration and infectious activity of SARS-CoV-2 and other viruses on those surface and in the air. The huge differences in the risk of media transmission of large saliva and sputum droplets were analyzed in terms of time elapsed. Due to the rapid decrease of water content in the evaporated droplets and the increased concentration of each component, the living environment of the virus tended to deteriorate sharply, and virus concentration plummeted within a few minutes. Although a virus can be detected in a matter of hours, tens of hours, or days, the risk of transmission is negligible compared to when it first left the host. This study suggests that the key to prevention and control is to start from the source, the earlier the better. It is extremely important to develop good public health habits, wear masks, and wash hands frequently. That said, excessive disinfection and sterilization of surfaces during a later period may have adverse effects.
Keywords: SARS-CoV-2, Droplet evaporation, Virus-containing droplets, Transmission risk
1. Introduction
The novel coronavirus SARS-COV-2 has brought about an unprecedented global pandemic. The World Health Organization (WHO) declared the COVID-19 pandemic as the most significant public health emergency that the world has faced in a century [1]. It is considered that there are at least two main routes of transmission of SARS-COV-2 among susceptible people: (1) being in close contact with infected persons or inhaling droplets from patients or asymptomatic patients; (2) touching surfaces contaminated by SARS-COV-2 [2]. The symptoms of this disease may include fever, sore limbs, weakness, sneezing, coughing [3], [4], or no symptoms at all with a greater risk of transmission [5], [6].
Due to the diversity of the cultural backgrounds and customs of social groups, a universal “cough etiquette” does not exist, and some persons may even spit in public places. A large number of droplets are expelled during coughing or sneezing and become the carriers of potential pathogens [7]. The respiratory activities (including breathing, speaking, coughing, etc.) of infected persons will produce virus-containing droplets, which will be larger in size from coughing and sneezing those of normal breathing and speaking [8], [9], [10], quickly settling to the ground or splashing down onto the surface of objects. Droplet size decreases after evaporation, and the droplet nuclei and sputum spots with the virus may be suspended in air due to the movement of people and to air flow [11]. In social life, people may inhale the droplets produced by infected persons during respiratory activities or touch the surfaces of objects contaminated by the droplets. However, when people are exposed to a potentially viral environment or find themselves in contact with contaminated objects, they cannot wash their hands to disinfect them in time, and may then touch their mouths, noses, or eyes unintentionally, which may cause personal infection. Since risk of infection is high when droplets of small size are inhaled in a very short time due to close contact, there exists a blind spot in studies with regard to virus survival on the surface and to the transmission risk of large virus-containing droplets.
The novel coronavirus is an envelope-structure pathogen with a particle size of 60-140 nm [12]. In order to survive, it requires water, nutrients, various salts and acid-base equilibrium conditions, and then to be given the opportunity to enter the host cell nucleus for transcription and proliferation [13]. Respiratory droplets are composed of water and a small amount of non-volatile compounds including sodium chloride, carbohydrate, lipids, protein and microorganisms [14], which provide the physical conditions most suited to survival of the virus. Exhaled virus-containing droplets float briefly in the air and quickly settle to the ground or on the surface of objects within seconds. In the process of air floatation and deposition on the surfaces of objects, droplets will inevitably undergo heat and mass transfer, gradually evaporate, and become concentrated, resulting in major changes in the living environment of the virus [15], [16]. The decrease in the number of viruses and the change of pathogenicity over time are crucial to assessment of the risk of infection.
The droplets generated by human respiratory activities are distributed in a wide size range. According to the literature [8], [9], [10], [17], the particle size of droplets produced by humans in breathing and speaking is usually small, while large droplets above 1000 μm may be generated in violent expiratory activities such as coughing and sneezing. In addition, the size of droplets produced by infected individuals is larger than in healthy individuals. During the exhalation of droplets, individual particles may aggregate, forming large droplets for exhalation [18]. The volume of a droplet is proportional to the cube of the particle size, so the larger the particle size, the larger the droplet volume. Accordingly, the viral load of the droplets will be much higher, and the risk of infection greater.
Recently, researchers [19], [20], [21], [22], [23] detected the viral load of throat swabs and sputum samples obtained from COVID-19 patients (including asymptomatic infections), which was as high as 109 copies/mL at the peak period, indicating that the exhaled droplets contain a large number of viruses. However, the evaporation time of the large particle size virus-containing droplets exhaled by the patient is longer, and the deterioration of the viral living environment is “alleviated”, a factor conducive to the survival of the virus for a longer time, leading to a greater risk of transmission. Therefore, we have focused mainly on the transmission risk of large virus-containing droplets above 500-1000 μm on the media surface.
In their study of virus survival on the surface of objects, Thomas et al. [24] evaluated the survival of the human influenza virus on banknotes, while Van Doremalen et al. [25] evaluated the stability of SARS-CoV-2 and SARS-CoV-1 in air and on four common surfaces (copper, cardboard, stainless steel and plastic). The results showed that while virus concentration on the surface decreased gradually with the passage of time, a very small amount of virus could be detected after several hours or even a few days on the surface. The virus may be resuspended in the air from the contaminated surface and also from the floor surface [11]. We are cognizant of the fact that there is some risk of infection when touching a contaminated surface or being exposed to the secondary dust of the virus. However, the number and viability of the viruses at this time are different from those in the process leaving the host. The existing literature does not clearly define or treat these two moments as equivalent.
In this paper we review the experimental results of the physical process of droplet evaporation on the media surface and analyze the experimental research literature on the decay of the virus concentration and infectious activity of SARS-CoV-2 and other viruses on the surface of media and in the air, over time. The risk of media transmission of large virus-containing mucosalivary droplets and the risk of airborne transmission of secondary dust from surface salivary spots have been analyzed. Numerous experimental data were used to determine the actual situation of virus survival, and the transmission risk of large virus-containing droplets was considered with regard to temporality. These findings may serve as references for epidemic prevention and control measures based on the difference of transmission risk over time.
2. Physical process of surface droplet evaporation and transmission risk
2.1. Surface droplet evaporation and the concentration process
The large-size mucosalivary droplets in a patient's exhaled virus-containing droplets float briefly through the air and then quickly settle to the ground or onto object surfaces. The exhaled droplets are composed of about 98.2% water and 1.8% non-volatile solid compounds [26]. During surface evaporation, the water in the droplet gradually decreases, while the concentration of other compounds increases, which affects the number of viruses in the droplet and pathogenicity. An experimental study on the physical process of droplet evaporation can predict the time of its occurrence and provide a basis for the prevention and control of the risk of transmission of virus-containing droplets on the media surface. Due to the restrictions of experimental conditions involving liquid extraction devices, measuring instruments and observation equipment, the evaporation time of liquid droplets with a diameter of less than 1000 μm is very short. In the relevant evaporation experiments, the particle size of liquid droplets is mostly within the range of 1000-4000 μm, which can cover the physical evaporation process of large saliva and sputum droplets on the surface.
During the evaporation process, droplet diameter decreases according to the D2 law, that is, the diameter squared changes linearly with time. Moreover, the higher the temperature, the higher the airflow velocity and the lower the relative humidity, the faster the droplet evaporation speed and the shorter the evaporation time [27], [28]. The survival of pathogens in droplets is affected by the droplet composition, and droplet evaporation is consequently crucial to the survival of viruses in droplets and to the resultant risk of infection [15], [16]. Ma et al. [28] studied the evaporation process of a single liquid droplet using the pendant droplet method, and they found that particle size decreased slowly in the early stage, and decreased more quickly in the later stage, as droplet diameter decreased. Shao [29] likewise studied the convective evaporation process of multi-component liquid droplets using the pendant droplet method, and demonstrated that the higher the ambient temperature, the higher the evaporation rate, and the faster the droplet diameter decreases. Zhang [30] used an analytical balance to record the mass loss of a droplet and studied the change of particle size over time in the evaporation process of pure water, NaCl solution and bacterial liquid on the hydrophobic surface. Liu et al. [17] predicted that the size of droplet nuclei formed after evaporation of saliva droplets was 32% of the original diameter, which was similar to the maximum residual size in the classic Duguid study [31], [32]. Vejerano et al. [33] carried out evaporation experiments on three component droplets containing NaCl, mucin and water (3 C) and human saliva droplets (HS). The results showed that droplet evaporation are affected by relative humidity. The higher the humidity, the less likely the droplet would be to dry out, and the longer the droplet could exist on the surface.
Fig. 1 shows the relationship between particle size and time, based on published evaporation experiments. Due to the limitations of the test method, the final droplet diameter may not reach the equilibrium diameter. Since researchers have provided data on droplet mass change during evaporation, this paper converts droplet mass into approximate droplet size according to pure water density. It can be seen in the figure that whatever the droplet type, initial particle size environmental temperature and humidity conditions, the trend in time of the particle size change during the droplet evaporation process is similar. Droplet size decreases slowly in the early stage of evaporation, and then, as evaporation speed accelerates, droplet size decreases more quickly with the passage of time. Moreover, the larger the initial droplet diameter, the slower the evaporation, and the longer the time it takes to reach the equilibrium diameter. However, due to the difference of temperature and humidity in these experimental conditions, evaporation time is not affected by initial particle size alone. The higher the ambient temperature and the lower the relative humidity during the experiment, the shorter the time required for droplet evaporation and drying. The loss of viability of pathogens after leaving the host is largely determined by the environmental conditions surrounding the parasitic droplets. During droplet evaporation, the concentration of nutrient, salt and acid and alkali change constantly, thereby destroying the living environment of the pathogens and leading to the inactivation of pathogens in the droplet [34], [35]. In these evaporation experiments, the initial droplet size approximated 1130-3150 μm, and the corresponding droplet volume was about 0.75-16.36 μL. This size is similar to the virus suspension volume inoculated on the surface during the subsequent phase of surface survival of the viral droplet, which can better explain the transmission risk of large saliva and sputum droplets.
Fig. 1.
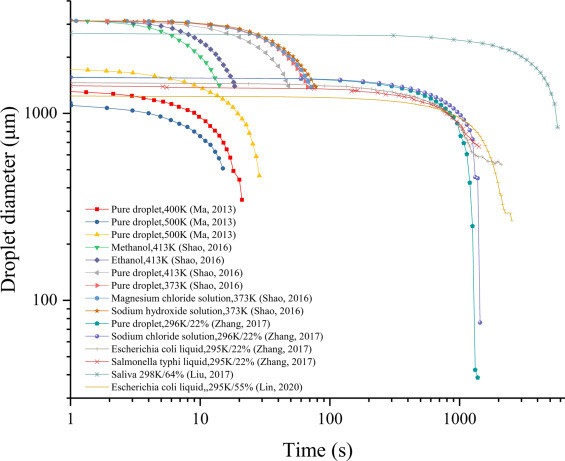
Particle size changes with time during droplet evaporation [17], [28], [29], [30], [35].
2.2. Characteristics of mucosalivary droplets
Human respiratory activities generate droplets with different particle size distributions, and those exhaled by patients contain viruses and other pathogens. Assuming that the pathogens are uniformly distributed in the droplets, the larger the particle size, the higher the load of the pathogen. As concerns risk of transmission, researchers have conducted studies on the quantity and particle size distribution of exhaled droplets [8], [9], [10], [36]. Chen et al. [36] tested the number of exhaled droplet particles in both coughing and speaking, and found there were more particles produced during coughing than during speaking. Lindsley et al. [8] compared the number and size distribution of exhaled droplet particles between influenza patients and healthy individuals, and found that the droplets generated by influenza patients and the number of exhaled particles was larger than in healthy individuals. Morawska et al. [9] tested droplet size distribution in human breathing, speaking and sneezing. It was observed that the number of particles produced by sneezing in different respiratory activities was the highest, while the number of droplets produced by respiration was the lowest. Bourouiba et al. [10] studied the number and particle size distribution of droplets produced by violent respiratory activities such as coughing and sneezing. In their experiment, one droplet with a particle size of 2000 μm was detected, and its volume was larger than the sum of all the small particles.
Fig. 2 shows the distribution of droplets with different particle sizes generated by human respiratory activities. It can be observed that the number of small-sized droplets is greater than that of large-sized droplets, and that those generated by different respiratory activities are distributed in a wide size range. The particle size of droplets exhaled in breathing and speaking generally does not exceed 1000 μm, while coughing, sneezing and other violent respiratory activities can produce droplets with larger particle size. The differences between the test results of different researchers underscores the complexity of the droplet quantities and particle size distribution in respiratory activities. Since the volume of a spherical particle is proportional to the cube of its diameter, the volume of a 100 μm virus-containing droplet is equivalent to millions of droplets of 1 μm diameter. Although the number of large particle size droplets is often relatively small, their volume is large and they carry proportionately more viruses. The large droplets evaporate slowly and take longer to dry, which is conducive to virus survival. There is consequently a much greater risk of transmission once large saliva and sputum droplets have been touched or inhaled by susceptible individuals.
Fig. 2.
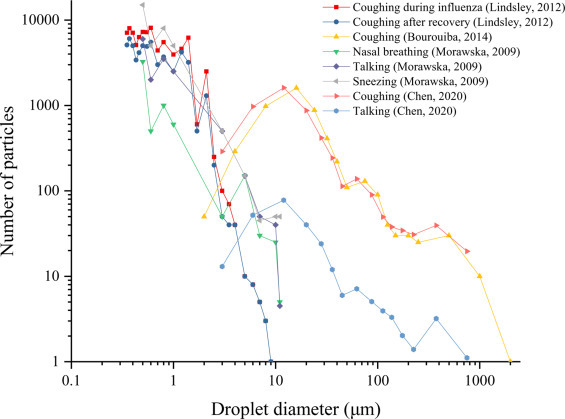
The number of droplets with different particle sizes produced by various respiratory activities [8], [9], [10], [36].
2.3. Viral load in clinical samples and transmission risk of mucosalivary droplets
SARS-CoV-2 is mainly transmitted by the respiratory droplets produced by an infected person's sneezing and coughing that directly reach another person's nose, mouth or eyes, or contaminate surfaces that are touched by susceptible individuals. Researchers [19], [20], [21], [22], [23] have detected the viral load of patients’ nose and throat swabs, sputum samples and stool samples, etc., and monitored their physical condition and treatment effects. As a result, test data on patients’ viral load can help to establish the risk differential from the source of virus transmission.
Using throat swab, Cao et al. [19] detected the SARS-CoV-2 viral load of 199 patients, of whom 99 were treated with lopinavir–ritonavir, while the remaining 100 were treated with standard care, their objective being to evaluate the effect of the drug on the patients’ clinical treatment. Pan et al. [23] examined the throat swabs, sputum, urine and stool samples of two patients, and showed that the viral load of throat swabs and sputum samples peaked at around 5-6 days after symptom onset. This pattern of change in viral load is distinct from that observed in patients with SARS, which normally peaked at around 10 days after onset [37]. In addition, Zou et al. [21] monitored SARS-CoV-2 viral loads in upper respiratory samples obtained from 18 patients in Zhuhai, Guangdong, China, and their results showed that high viral load was detected soon after symptom onset, and that the viral load detected in the nose was higher than that detected in the throat.
Fig. 3 shows the viral load test results from patients infected with SARS-CoV-2, where A, B, and C represent the viral load of nasal swabs, throat swabs, and sputum samples, respectively. It can be observed that the viral load of patients changed with the number of days after symptom onset in a similar pattern. Higher in the early stage, the viral load gradually decreased in the later stage. After certain patients’ treatment and recovery, the virus was no longer detected in the samples. Although patients’ SARS-CoV-2 viral load showed some differences, it generally reached its peak (up to 109 copies/mL) about 4-6 days after symptom onset. There is a correspondingly elevated risk of transmission in the early stages after onset of the symptoms. The average viral loads of the nasal swabs, throat swabs and sputum samples were 1.66 × 103 copies/mL, 3.1 × 103 copies/mL, and 1.07 × 105 copies/mL, respectively. The large mucosalivary droplets produced by violent respiratory activities such as coughing may indeed lead to heightened risk of transmission. Droplets generated from patient respiratory activities are distributed in a wide size range and contain different amounts of viruses. As for the large virus-containing droplets produced by coughing and other violent respiratory activities, they quickly settle on the surface of objects after a short drift in the air. The heat and mass transfer between the droplets and the environment leads to gradual evaporation of the droplets, which further changes the virus's living environment. Inactivation of the virus over time after leaving the host is a major determinant of the risk of transmission.
Fig. 3.
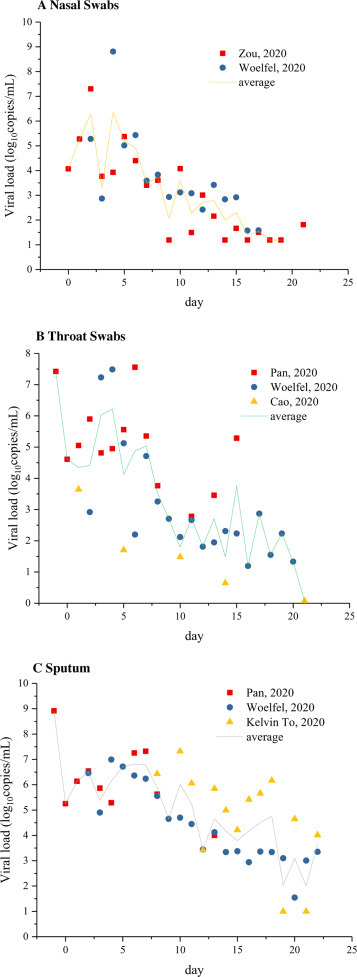
Viral load of SARS-CoV-2 in infected patients [19], [20], [21], [22], [23].
3. Virus survival in mucosalivary droplets: An experiment
3.1. Virus stability in air
A patient exhales virus-containing droplets with different particle size distributions. The droplets are initially sprayed into the air, and those with smaller particle size spread in the air, gradually evaporating and even becoming droplet nuclei in the process of transmission. Droplets of large size (e.g. salivary droplets) float briefly in the air and quickly settle to the ground or the surface of objects. The virus may be resuspended from the contaminated surface to the air due to people's movements. of. It leaves the host as the patient (including asymptomatic patients) breathes, talks, coughs, or sneezes, drifting in the air and sinking to surfaces. The new parasitic environment poses a serious challenge to its survival. In this section, we will review the survival after exhalation of viruses and other pathogens of the virus-containing droplets in the air.
In a survival experiment concerning viruses and other pathogens in the air, an initial concentration of microbial solution is sprayed into an aerosol chamber or rotating drum under certain environmental (temperature and humidity conditions) through a nebulizer. Samples are taken at different time nodes after atomization to test microbial concentration at that moment, the objective being to determine the survival conditions of viruses and other pathogens in the air after different periods of time. Sattar et al. [38] used a 6-jet Collison nebulizer to spray a Klebsiella pneumoniae soil load into an aerosol chamber. After about 210 minutes, the bacteria were no longer detected. Sattar et al. [39] assessed rate of biological decay at different time nodes after staphylococcus aureus was sprayed into a car in a level 3 biosafety containment facility. Pyankov et al. [40] sprayed a MERS-COV isolate HCOV-EMC/2012 virus-containing suspension into a Rotating Aerosol Chamber, and sampled the aerosol from the chamber at 0, 5, 15, 30 and 60 min after aerosolization to monitor viable virus concentration. Van Doremalen et al. [25] detected the virus stability of SARS-CoV-2 and SARS-COV-1 virus in air within 3 hours at 65% relative humidity (RH) and 21-23 °C. Zhao et al. [41] sprayed 5 ml of Gumboro vaccine virus suspension into an aerosol chamber with a Walther Pilot spray head, and the inactivation rate was high in the first few seconds or minutes after aerosolization, followed by a lower inactivation rate. Table 1 shows the main conditions and parameters of the survival experiments for different pathogens in the air.
Table 1.
Main conditions and parameters of pathogen survival experiment in air.
| Year | Atomization device | Environmental parameters | Pathogen | The initial concentration | Initial sampling concentration | Reference |
|---|---|---|---|---|---|---|
| 2020 | A 3-jet Collison nebulizer | T = 21-23°C RH = 65% |
SARS-CoV-2 | 4.49 log10TCID50/L | 3.5 log10TCID50/L | van Doremalen et al., 2020 [25] |
| SARS-CoV-1 | 5.94 log10TCID50/L | 4.3 log10TCID50/L | ||||
| 2018 | A 3-jet Collison nebulizer | T = 25°C RH = 79% |
MERS-CoV | 6.1 log10TCID50/mL | 3.22 log10TCID50/mL | Pyankov et al., 2018 [40] |
| T = 38°C RH = 24% |
MERS-CoV | 6.1 log10TCID50/mL | 2.81 log10TCID50/mL | |||
| 2017 | A Collison nebulizer | T = 22 ± 1°C RH = 50 ± 5% |
S. aureus | 6.1 log10CFU/m3 | 4.37 log10CFU/m3 | Sattar et al., 2017 [39] |
| 2016 | A 6-jet Collison nebulizer | T = 20 ± 2°C RH = 50 ± 5% |
K. pneumoniae | 4.61 log10CFU/m3 | 4.17 log10CFU/m3 | Sattar et al., 2016 [38] |
| 2011 | A Walther Pilot spray head | T = 10°C RH = 40% |
Gumboro Vaccine Virus | 6.5 log10TCID50/mL | 2.86 log10TCID50/mL | Zhao et al., 2011 [41] |
| T = 10°C RH = 70% |
Gumboro Vaccine Virus | 6.5 log10TCID50/mL | 4.48 log10TCID50/mL | |||
| T = 20°C RH = 40% |
Gumboro Vaccine Virus | 6.5 log10TCID50/mL | 2.16 log10TCID50/mL | |||
| T = 20°C RH = 70% |
Gumboro Vaccine Virus | 6.5 log10TCID50/mL | 2.84 log10TCID50/mL |
The results of the tests on the survival of klebsiella pneumoniae and staphylococcus aureus are shown in Fig. 4 . Initial sampling was carried out immediately after the suspension aerosolization was completed. The sampling time was 2 minutes at each time node, and sample concentration represented the average concentration during the period. Initial concentration at the end of aerosolization is highlighted in the figure. It can be observed that the concentration of pathogens in both groups decreased sharply after aerosolization and the first sampling. Klebsiella pneumoniae decreased from an initial nebulization concentration of 4.61 log10CFU/m3 to 4.17 log10CFU/m3 (equivalent to 10% virus inactivation) and, for staphylococcus aureus, from 6.1 log10CFU/m3 to 4.37 log10CFU/m3 (equivalent to 28.4% viral inactivation). After which, the logarithmic concentration of pathogens in the air decayed linearly with time; about 50% of the pathogens were inactive after 60 min.
Fig. 4.
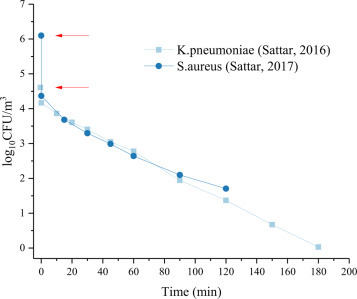
Biologic decay of Staphylococcus aureus and Klebsiella pneumoniae in air [38], [39].
Fig. 5 compares the survival of different types of viruses in air. (A) is the MERS and Gumboro Vaccine Virus presented by the authors [40], [41] in units of virus concentration of TICD50/mL; (B) the viability of SARS and SARS-CoV-2 in air postulated by the author [25] in terms of TICD50/L air (translated as per L air). While the survival time of different kinds of viruses in the air is somewhat divergent, the trend concerning virus survival in air is similar. Within a few minutes after the end of atomization and initial sampling, virus concentration decreased sharply and the virus inactivation rate was very high. In Figure 5A, the virus decreased from an initial concentration of 6-7 log10TCID50/mL to 3-5 log10TCID50/mL. In Fig. 5B, the SARS virus was reduced from 5.94 log10TCID50/L to 4.3 log10TCID50/L, which is equivalent to 27.6% virus inactivation; SARS-CoV-2 virus went down from 4.49 log10TCID50/L to 3.5 log10TCID50/L, which is equivalent to 22% virus inactivation. This was followed by a lower inactivation rate. In addition, virus survival is related initial droplet size and virus concentration. When the atomized particle size in the experiment was small, the virus survived in the air for a shorter time. However, when the droplets atomized into the air featured a larger particle size, the viral load in the droplets was higher and it took longer for the droplets to evaporate completely. As a result, the deterioration of the viral living environment was “alleviated”, and the virus could still be detected after a longer lapse of time. Indoor environmental parameters affect droplet evaporation, leading to the difference in virus survival. Setting When indoor environmental parameters are most adverse to virus survival, the risk of infection will be correspondingly reduced. It bears mentioning that different sample detection methods (immediate detection or post-incubation) may lead to significant differences in the results. As shown in the experiment in Fig. 5 (B), the virus concentration is significantly higher than in Fig. 5 (A) after 180 minutes. The fundamental reason is that the samples had been incubated for 18 to 20 hours. Therefore, accurate understanding of the factors conditioning experimental results potently contributes to objective judgment of the attendant risks.
Fig. 5.
Viability of virus in air. (A) The virus concentration unit is given as TCID50/mL. GV, Gumboro Vaccine Virus [40], [41]; (B) The virus concentration unit is given as TCID50/L air [25].
3.2. Virus stability on surfaces
To accurately define the transmission risk of SARS-CoV-2 virus deposited on the surface of objects through infected patients’ sneezing and coughing, the virus surface survival experiment is an important support. Surface survival experiments are generally conducted by setting a given volume of a virus-containing droplet onto the surface of an object in a specific temperature and humidity environment, by sampling at different time nodes, and by testing virus concentration at the different times by the plaque method or the PCR technique. Although researchers’ environmental parameters, virus types and inoculation surfaces may vary, their respective experiments reliably assess virus survival on the surface of objects. Chan et al. [42] titrated the SARS virus with high concentrations (107 TCID50/mL) on smooth plastic surfaces, and TCID50 was determined according to Reed and the Muench method. They found that virus concentration gradually decreased over time, and that the virus could not be detected after the 5th day. Duan et al. [43] used the SARS coronavirus strain CoV-P9 to study virus stability on the surface of commonly used materials in a mimic environment. Casanova et al. [44] evaluated the survival of the SARS virus on environmental surfaces and strove to determine how survival is affected by environmental variables. Van Doremalen et al. [25] titrated 50 μL of virus suspension on four common surfaces (copper, cardboard, stainless steel and plastic), and compared the stability of SARS-CoV-2 and SARS viruses on each one of them. Thomas et al. [24] evaluated the survival of influenza virus on banknotes. Tests showed that HRV2 and HRV37 survived on paper currency for 2 days and 5 days, respectively.
Fig. 6 illustrates the viability of the SARS-CoV-2 and SARS viruses on different surfaces over time. It can be observed that the initial concentration of SARS-CoV-2 and SARS virus suspensions on each surface approximates 107 TCID50/mL. The first sampling was conducted immediately after inoculation on the surface of the object, and the virus number quickly decayed to 103-4 TCID50/mL, while the virus concentration decreased by 3log10, which is equivalent to the rapid inactivation of most viruses within a few seconds or minutes after the virus left the host and settled on the surface of an object. With increased deposition time on the surface, the number of viruses decreased gradually. There are some differences in the virus decay rate for different media surfaces; viable SARS-CoV-2 virus can be detected after 4 hours on a copper surface and 24 hours on cardboard. Although the presence of viruses can be detected for hours or even days, their number and pathogenicity becomes negligible compared to when they were deposited to the surface, and the risk of transmission is greatly reduced. As illustrated in Figure 6, experimental results concerning droplets on surfaces show that in the evaporation of droplets with a particle size of 1.2 mm, it does not take more than 30 min for equilibrium diameter to be reached. During the process, the concentration of various salts in the droplets increases rapidly, the balance of acid and base is broken, and the living environment of the virus deteriorates rapidly, which is the fundamental reason for the massive decline of the virus. It bears mentioning that in the different experiments, the concentrations of viruses sampled on the surface at each time node were all measured after incubation under the same conditions, which represented the relative activity and reproductive ability of the viruses in the droplets deposited on the surface at different times.
Fig. 6.
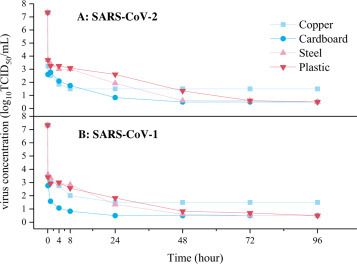
Viability of SARS-CoV-1 and SARS-CoV-2 on different surfaces [25].
The volume of a virus droplet may vary widely between different researchers conducting surface-survival experiments. Similarly, bad habits of coughing, sneezing or spitting may result in large differences in the volume of droplets deposited on the surface, presenting different risks of transmission when exposed to susceptible populations at different time points. Fig. 7 extracts different experimental data, and compares change of virus concentration over time when 50 μL [25], 20 μL [45] and 1 μL [45] virus suspensions were respectively titrated on copper surface. While the former contained SARS-CoV-2 virus, the latter contained Human Coronavirus 229E at 21 °C and 30%-40% relative humidity (RH). The equivalent diameter of the titrated droplet volume ranged from 1240-4570 μm. Concentration of the virus decays more rapidly when the amount of titrated droplets on the surface is smaller. Within a few minutes, the concentration of viruses in a 1 μL droplet drops by 4 orders of magnitude (equivalent to 99.99% virus inactivation). It takes 40 to 50 minutes for the virus concentration in a 20 μL droplet to drop by 4 orders of magnitude, while the concentration of viruses in a 50 μL droplet decreases more slowly, close to the experimental results in Fig. 1. This shows that due to droplet evaporation, water volume decreases rapidly, while the concentration of each chemical component increases, leading to deterioration of the virus's living environment and to substantial virus inactivation. To conclude, the difference in virus inactivation between droplets of different volumes is conditioned by the rate of the above-mentioned deterioration
Fig. 7.
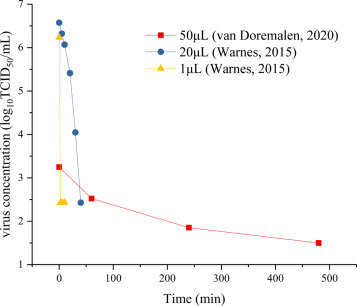
Virus inactivation in droplets of different volumes on a copper surface [25], [45].
4. Discussion and conclusions
COVID-19 patients’ respiratory activities (including breathing, speaking, coughing, etc.) produce virus-containing droplets with different particle size distributions, and violent respiratory activities such as coughing or sneezing can lead to larger-sized droplets. Although the proportion of large-sized droplets in the different exhaled droplets is small, the cumulative volume of large-sized droplets is huge, and the number of viruses contained in large-sized droplets is pronouncedly larger than in small-sized droplets. In the nose and throat swabs of COVID-19 patients, the amount of viruses contained in their secretions is relatively high, reaching a peak of 109 copies/mL in a few days, with a high viral load. The large, exhaled droplets contain more viruses, and present a greater risk of disease. From the instant when the patient exhales the virus-containing droplets, the virus concentration drops rapidly as they enter the air. The small particle droplets are suspended in the air, and virus concentration gradually decreases with the passage of time. However, large droplets travel through the air for a short time after exhalation, and while the size of the large droplets decreases slowly in the initial stage, without having a fatal impact on the virus’ living environment, they quickly settle to the ground or on the surface of nearby objects.
As regards large virus-containing droplets that quickly settle to the ground or splash onto the surface of objects, experimental study on the survival of viruses on the media surfaces shows that within a few seconds or minutes, virus concentration declines by about 3-4 orders of magnitude after the droplet falls onto the surface. Experimental results suggest that virus concentration changes after the exhaled droplets settle to the ground or to the surface of objects, and that the viral living environment deteriorates rapidly after the virus leaves the host and settles on the surfaces of nearby objects, and virus concentration decreases significantly. Although 1 in 10,000 or 1 in a million viruses on the surface can remain active for hours to days compared to the initial stage, their number and pathogenicity are greatly reduced. In fact, the highest risk of media transmission occurs when the surface is exposed to susceptible persons shortly after being contaminated by infected droplets. As time goes by, virus concentration decreases gradually, and the risk drops accordingly, becoming small or even insignificant after a few days or even a few hours. It also bears mentioning that the larger the exhaled droplets, the larger the volume of the virus-containing droplets deposited on the surface of an object, which is equivalent to the evaporation of large particle size virus-containing droplets on the surface of an object. The inactivation rate of the virus on the object surface slows down significantly, and the deterioration of the viral living environment is “alleviated”. As a result, large virus-containing droplets exhaled by violent respiratory activities such as coughing and sneezing increase the risk of transmission. These results further suggest that prevention of media transmission over time should be undertaken as early as possible.
The large saliva and sputum droplets deposited on the surface of objects and clothing will evaporate and dry for several minutes and go on to form droplet nuclei or spots. Due to the movement of people or the flow of air, the virus may be resuspended into the air, possibly entailing a further risk of infection if inhaled by susceptible people. The field measurement results in Wuhan Fangcang Hospital showed that the highest concentration of SARS-CoV-2 virus in the dressing room was due to resuspension of the virus deposited on the surface of the medical's staff protective apparel [11]. However, the risk of transmission in secondary dust still depends on the point in time when it is inhaled by susceptible people, so prevention of infection should also be contemplated from the time axis standpoint, and carried out as soon as possible. It behoove us to be attentive the locations where patients or asymptomatic patients often appear; that said in places where there are few patients or that remain unoccupied for tens of minutes or hours, the risk of transmission is very low.
The virus loads of patients or asymptomatic infected patients have shown that the virus concentration released by the source of infection varies pronouncedly at different times, at times reaching 109 copies/mL, and that it does so quite randomly. Once as normal social activities are resumed, it is difficult to prevent the spread of the virus. Experiments on aerosol virus-containing droplets have shown that the concentration of viruses in the droplets declines rapidly, and that the earlier the exposure to the virus, the greater the risk of infection of susceptible people. That is why, first of all, social distancing is so important. Secondly, during a severe epidemic it is necessary during normal social activities to wear a mask, which can not only prevent the inhalation of droplets that have just left the contagious patient, but also to the greatest possible extent preclude a potential impact on other individuals. Hence, it is more considerate and of much lower social cost to wear a mask than to be grounded at home or banned from social activities.
Surface survival experiments of virus-containing droplets have shown that concentration of the viruses declines sharply with time due to the rapid deterioration of the viral living environment, and that over 99% of the virus are inactivated within a few minutes in normally sized saliva and sputum droplets. Taken together, these observations suggest that novel coronavirus transmission risk can be effectively reduced when practicing good coughing etiquette and maintaining good sanitary habits such as no spitting, frequent hand washing and no eye rubbing, the objective being to reduce the sources of infection. Secondly, surfaces should be frequently cleaned with disinfectant in areas where people are active and tend to congregate, the objective being to remove potential sources of infection. On the other hand, surface survival experiments remind us that in low-risk public spaces, especially those protected by patient isolation, close contact tracing and body temperature measurement during epidemic outbreak, the negative effects of excessive surface disinfection through the use of chemicals may far outweigh the positive effects on epidemic prevention and control. In brief, disinfection and sterilization of outdoor urban roads and facilities is not necessary; even though these surfaces may indeed be contaminated with viruses, the likelihood of human contact is very low, and transmission risk over time is close to negligible.
The primary transmission route of COVID-19 consists in inhaling droplets produced by an infected person and circulating in the air or in touching virus-containing droplets deposited on the surface of objects. According to the results of survival experiments dedicated to virus-containing droplets in the air and on a surface, and given the mechanisms of virus survival and the specificities of propagation environments, it has been revealed that when virus transmission risk is analyzed over time after the virus has left the host, the focus and eventual difficulties of prevention and control can be accurately assessed and determined. Effective scientifically validated prevention and control strategies can be communicated the public to avoid human-induced panic and rapidly restore routine social and economic activities.
Funding
This work was supported by the National Natural Science Foundation of China (51778382), and the National Key R&D Program of China (2016YFC0700400).
Authors’ contributions
Luyao Guo & Min Wang: Data curation, Writing- Original draft preparation.
Li Zhang: Supervision.
Ning Mao: Reviewing and Editing.
Congkang An: Reviewing and Editing.
Luting Xu: Reviewing and Editing.
Enshen Long: Conceptualization, Methodology.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.WHO. 2020. Coronavirus disease 2019 (COVID-19) Situation Report, 51. [https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?.sfvrsn=1ba62e57_10] [Google Scholar]
- 2.CDC. 2019. How COVID-19 spreads, 2020. [https://www.cdc.gov/coronavirus/-ncov/about/transmission.html] [Google Scholar]
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan X., Chen D., Xia Y., et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothe C., Schunk M., Sothmann P., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ Int. 2020 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsley W.G., Pearce T.A., Hudnall J.B., et al. Quantity and size distribution of Cough-Generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morawska L., Johnson G.R., Ristovski Z.D., et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40:256–269. [Google Scholar]
- 10.Bourouiba L., Dehandschoewercker E., Bush J.W.M. Violent expiratory events: On coughing and sneezing. J Fluid Mech. 2014;745:537–563. [Google Scholar]
- 11.Liu Y., Ning Z., Chen Y., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhu N., Zhang D., Wang W., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortorici M.A., Walls A.C., Lang Y., et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redrow J., Mao S., Celik I., Posada J.A., Feng Z. Modeling the evaporation and dispersion of airborne sputum droplets expelled from a human cough. Build Environ. 2011;46:2042–2051. [Google Scholar]
- 15.Weber T.P., Stilianakis N.I. Inactivation of influenza A viruses in the environment and modes of transmission: A critical review. J Infection. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metz J.A., Finn A. Influenza and humidity - Why a bit more damp may be good for you! J Infection. 2015;711:S54–S58. doi: 10.1016/j.jinf.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu L., Wei J., Li Y., Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27:179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- 18.Gralton J., Tovey E., McLaws M., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: A review. J Infection. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao B., Wang Y., Wen D. A trial of Lopinavir-Ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woelfel R., Corman V.M., Guggemos W., et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv. 2020 [Google Scholar]
- 21.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.To K.K., Tsang O.T., Leung W., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Y., Zhang D., Yang P., Poon L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas Y., Vogel G., Wunderli W., et al. Survival of influenza virus on banknotes. Appl Environ Microb. 2008;74:3002–3007. doi: 10.1128/AEM.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. medRxiv. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: Emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei J., Li Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build Environ. 2015;93:86–96. [Google Scholar]
- 28.Ma L., Qiu X., Wang J., et al. Experimental research on single droplet evaporation factors. Modern Chemical Industry. 2013 [In Chinese] [Google Scholar]
- 29.Shao B. Experiment Study on the Evaporation of Multicomponent Droplet in Airflow. Chongqing University. 2016 [In Chinese] [Google Scholar]
- 30.Zhang X. Study on Evaporation Characteristics Impact of Indoor Microbial Aerosol. Xi‘an University of Architecture and Technology. 2017 [In Chinese] [Google Scholar]
- 31.Duguid J.P. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinburgh Med J. 1945;52:385–401. [PMC free article] [PubMed] [Google Scholar]
- 32.Duguid J.P. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg (Lond) 1946;44:471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vejerano E.P., Marr L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J R Soc Interface. 2018:15. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces?. A systematic review. BMC Infect Dis. 2006:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin K., Marr L.C. Humidity-Dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environ Sci Technol. 2020;54:1024–1032. doi: 10.1021/acs.est.9b04959. [DOI] [PubMed] [Google Scholar]
- 36.Chen W., Zhang N., Wei J., Yen H., Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020:106859. doi: 10.1016/j.buildenv.2022.109166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.JSM P. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet. 2003:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sattar S.A., Kibbee R.J., Zargar B., Wright K.E., Rubino J.R., Ijaz M.K. Decontamination of indoor air to reduce the risk of airborne infections: Studies on survival and inactivation of airborne pathogens using an aerobiology chamber. Am J Infect Control. 2016;44:E177–E182. doi: 10.1016/j.ajic.2016.03.067. [DOI] [PubMed] [Google Scholar]
- 39.Sattar S.A., Zargar B., Wright K.E., Rubino J.R., Ijaz M.K. Airborne Pathogens inside Automobiles for Domestic Use: Assessing In-Car Air Decontamination Devices Using Staphylococcus aureus as the Challenge Bacterium. Appl Environ Microb. 2017:83. doi: 10.1128/AEM.00258-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y., Aarnink A.J.A., Dijkman R., Fabri T., de Jong M.C.M., Koerkamp P.W.G.G. Effects of temperature, relative humidity, absolute humidity, and evaporation potential on survival of airborne gumboro vaccine virus. Appl Environ Microb. 2012;78:1048–1054. doi: 10.1128/AEM.06477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan K.H., Peiris J.S.M., Lam S.Y. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Advances in Virology. 2011:7. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan S., Zhao X., Wen R., et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomedical and environmental sciences: BES. 2003:246–255. [PubMed] [Google Scholar]
- 44.Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Applied & Environmental Microbiology. 2010:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. Mbio. 2015;6:e1615–e1697. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


