Abstract
Regenerative medicine, poised to transform 21st century healthcare, has aspired to enrich care options by bringing cures to patients in need. Science-driven responsible and regulated translation of innovative technology has enabled the launch of previously unimaginable care pathways adopted prudently for select serious diseases and disabilities. The collective resolve to advance the design, manufacture and validity of affordable regenerative solutions aims to democratize such health benefits for all. The objective of this Review is to outline the framework and prerequisites that underpin clinical readiness of regenerative care. Integrated research and development, specialized workforce education and accessible evidence-based practice implementation are at the core of realizing an equitable regenerative medicine vision.
Keywords: : chronic disease, healthcare delivery, innovation, manufacturing, model of care, patient, population, practice, regeneration, regulation, supply chain, workforce
‘Medicine to produce health must examine disease; as music, to create harmony must investigate discord.’ – Plutarch (Πλούταρχος, Greek philosopher, 46–120 AD)
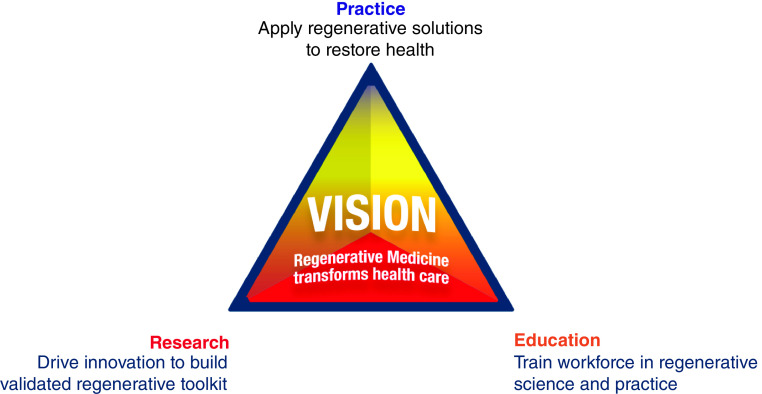
Seeking that words like ‘incurable’ or ‘terminal’ are retired from the dictionary, regeneration (in Greek αναγέννηση) has mesmerized humankind, symbolized by the enduring myth of Prometheus [1]. Anchored by science, the regenerative aspiration has evolved from mythos to realism, gathering credibility. The regenerative ecosystem draws from a discovery-translation-application know-how, patient-ready supply chain, accrued regulatory and clinical experiences and general awareness. Armed with a varied toolkit, ranging from nanotherapies to artificial organs, regenerative science is adept to enrich healthcare systems. Codependent research innovation, workforce education and practice implementation drive the regenerative medicine vision (Figure 1). Regenerative technologies are practice-transformative, offering a disruptive armamentarium that targets normative organ restoration while furthering whole-person care. Clinical development has marshaled innovative therapeutics while integrating a multivalent assessment reflective of standards set by providers, developers, regulators and payers. The emerging landscape announces a holistic evaluation and adoption that integrate the whole patient within a care regime, while underscoring real-life patient needs along with a societal pursuit for health as value. This Review highlights elements contributing to the state of regenerative care readiness. Surveillance from the United States Organ Procurement and Transplantation Network, updated on 12 January 2021, points to 118,618 patients on the transplant waiting list, with every 9 min a new individual added despite nearly 100 transplants performed daily. The gap between organ procurement and patient demand, rendered particularly difficult by the need for chronic immunosuppression, underscores the necessity for alternatives to traditional transplantation [2]. A lack of therapeutic options to address multiorgan disease or anatomical abnormalities imposed by congenital malformation further stresses such pressing need. The regenerative proposition is thus attractive as it provides the therapeutic building blocks required to ultimately reinstate physical and functional integrity in currently insurmountable syndromes.
Figure 1. . Regenerative medicine perspective.
Regenerative medicine is a driver of the healthcare future. Supported by the rigor of research and executed by an educated workforce, regenerative medicine brings innovative and validated cures to the practice, addressing needs of patients and population.
Cause for cure: the regenerative impetus
Progress in science has fundamentally expanded comprehension of health and disease. Success in decoding disease pathogenesis, and the unraveled regenerative biology principles, catalyze the prospect of curative therapies [3]. A deeper understanding of molecular disease culprits has informed the evolution of a healing toolbox, with advances reflected in a specialized vocabulary [4,5]. Technological breakthroughs are transformative, with an anticipated 10% of healthcare reflecting regenerative science contributions. Intended to oppose underlying pathology and rebuild health, curative interventions are disruptive aspiring to go beyond the traditional scope often restricted to disease palliation and symptoms mitigation (Figure 2). Vitally, underlying fundamental science must be responsibly translated into robust, transparent, evidence-based and guidelines-sanctioned best practices, supported by regulatory oversight, quality control and standardized compliance. These prerequisites insure adequate practice implementation and cross-disciplinary success [6]. Regulatory agencies stand to facilitate development and licensure of regenerative therapies with programs and paths designed to accelerate expert review and authorization. Notably, designations by the US FDA of ‘Breakthrough Therapy’ refer to a process designed to expedite the development and review of drugs that are intended to treat a serious condition, while ‘Accelerated Approval’ allows for faster approval of drugs for serious conditions that fill an unmet medical need. Barriers however exist, in particular due to coexistence of unproven therapies jeopardizing the regulatory approval of legitimate regenerative therapies. Prevalence of mixed outcomes in clinical trials and a divergent experience in achieving homogenous, scalable and affordable biotherapeutic solutions in real-world clinical setting are also recognized limitations [7]. The projected roll-out of new care pathways must converge with healthcare systems striving to adopt sustainable, cost-effective options equipped to remedy otherwise nonsustainable trajectories in disease management. There is urgency, intensified by population aging and the burden imposed by degenerative morbidities that encompass end-stage care and high mortality [8].
Figure 2. . Healthcare evolution.
The traditional ‘care’ model, underpinning the medicine of fighting disease and mitigating disease symptoms, is poised to transition into an increasingly ‘curative’ counterpart equipped to rebuild health.
Catalyzing new pathways: addressing unmet needs
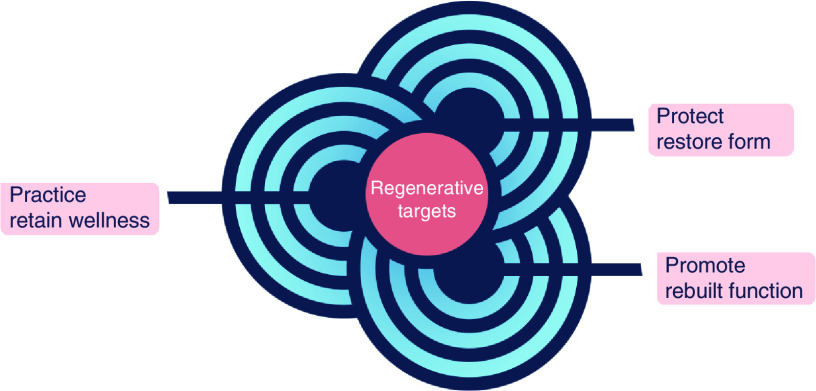
Healthy aging is a global priority. The elderly population presents with a high prevalence of chronic diseases [9]. In this context, the 21st Century Cures Act has helped advance first-in-class medical innovations [10]. Enabled by scientific breakthroughs, and buttressed by regulatory rigor, bioethics considerations, quality control/quality assurance and clinical/public health scrutiny, a number of regenerative biotherapy pipelines have been developed. As a case in point, advances in cellular engineering and cell-based technology have opened a curative perspective in oncology, enabled by introduction of gene editing and immunotherapies [11]. Bolstered by the ability to modify the immune system, through engineered expression of chimeric antigen receptors or through priming with tumor antigens, on-demand regenerative immunity against blood cancers is achievable, transforming cancer care [12]. New regenerative immunotherapies are available in the management of lymphoma and leukemia [13]. In parallel, personalized treatments that leverage programming of the patient’s own immune system are exploited in urology to seek out and destroy prostate malignancy, with reported survival benefit [14]. Regulatory approvals, pioneered in Europe, have also been achieved in other areas of need with regenerative choices made available for corneal healing in ophthalmology addressing limbal stem cell deficit after ocular burns [15], or for complex fistula repair in gastroenterology and surgery with favorable outcomes documented in Crohn’s disease [16]. Cartilage‐derived chondrocytes for cartilage repair, and mesenchymal stem cells in graft‐versus‐host disease, reveal a further growth of registered therapies [17]. Optimization of stem cell therapy is another area of emphasis, exemplified in advanced multinational clinical trials utilizing cardioreparative cells developed for mending failing hearts [18]. Growth of human organoids has also gathered interest, illustrated with the scale-up of in vitro generated liver buds into authentic tissue enabled by in vivo orthotopic transplantation onto native liver parenchyma or by ex vivo biofabrication using 3D bioprinting of liver-like self-organized tissue [19]. Complementing the manufacture of functional organoids, cross-cutting biotechnologies, including nanomedicine and materials science, have expanded regenerative approaches to include exosome factories, biopotentiated biomaterials and/or biografts for enhanced neo-organogenic and organotypic engineering [20–24]. Notably, the concept of the human body used as a natural bioreactor has been advanced reflecting on the capacity of scaffolds to be repopulated by the recipient’s stem cells after implantation, as for example in in vivo tissue engineering of bones or airways [25,26]. In parallel, off-the-shelf noncellular biologics are increasingly realized, rendering point-of-care regenerative medicine feasible and no longer limited to highly specialized tertiary care centers. Clinically, regenerative interventions utilized as bridging interventions to reverse multiorgan syndromes can serve to advance current standards of care as viable therapeutic options. Regardless of the nature of the regenerative biotherapy, a common denominator in establishing a healing platform is the reliance on the body’s innate regenerative aptitude leveraging and boosting endogenous restorative and repair mechanisms [27,28]. Agnostic to the applied therapeutic modality, the central regenerative medicine objective embodies the prevent-protect-promote paradigm aiming to retain wellness, restore form and rebuild function, and ultimately afford a prospect in extending disease-free life (Figure 3). Seeking for the patient to reclaim health, the regenerative perspective is applicable across a spectrum of diseases, directed at transforming the healthcare horizon [29].
Figure 3. . Regenerative medicine aspiration.
Conceived to be agnostic to the therapeutic modality utilized, the overarching regenerative medicine aspiration embodies the ‘P3’ paradigm encompassing the ‘prevent-protect-promote’ attributes directed to retain wellness, restore form and rebuild function, ultimately reimagining the healthcare horizon.
Workforce readiness: educated proficiency
The quest in adopting regenerative solutions has exposed an education breach as regenerative modalities are underemphasized in undergraduate, graduate and postgraduate training. Scarce are the physicians, nurses, therapists or biomedical engineers that have been educated in the regenerative acumen. A systematic introduction of content during medical/paramedical and/or scientific/technical training is needed. To ensure workforce readiness, assimilating newly acquired knowledge should encompass proficiency in regenerative principles and practices, as highlighted in emerging patient-centric educational prototypes [30]. Launched as portals of new knowledge, learner proficiency is instilled using the ‘from the patient to the patient’ model employing a pedagogy that covers the spectrum from innovation to implementation. Specific teachings concerning advanced technologies, including complex areas such as artificial organs, may be of particular interest in master/doctorate degree-granting tracks. Trained, specialized workforce must be able to distinguish approved options from unproven counterparts. Foundational and applied competencies are essential in order to offer trusted, best care practices. The contribution of the healthcare profession to (pre)clinical evaluation, patient (reported) experiences and practice optimization are areas of growth [31]. There is a pressing need for programs designed to instruct core competencies in patient selection, delivery of regenerative treatments, management of potential side effects and responsible use [32]. Contrasting the direct-to-consumer marketing, legitimate regenerative medicine practitioners will differentiate as a trusted source of care trained to offer proven treatments. Inclusion of online learning platforms for accelerated digital content distribution will expand the pools of learners catalyzing broader connectivity across the science and medicine community [33]. Notably, the regenerative network is to promote shared knowledge and evidence-based practice, and should encompass the education of providers and patients/public alike.
Supply readiness: from protocol to product
Reliable uptake of the regenerative portfolio into practice mandates an advanced supply chain. Validated fundamental research protocols and ensuing standard operating procedures, such as for stem cell and extracellular vesicle production, gene and tissue engineering, scaffold and biomaterial generation, molecular imaging, are initiating product/process development steps in the chain [34–38]. Guidelines for biofabrication are integral for compliant translation of research-grade technologies into reproducible clinical-grade counterparts [39]. The suitability for scale-up and standardized high-quality output are primordial obstacles linked to difficulties in achieving and maintaining consistent technology industrialization in generating patient-ready products [40]. To date, scaled procurement has been primarily confined for indications in regenerative hematology/oncology/transplant and musculoskeletal medicine/surgery, employing a narrow range of product types [41]. Cost of goods, raw material supply, product stability, grade and modularity of clean rooms, scalability, automation, process control and general process robustness or failure rate, are all areas that need to be further addressed as a broader and diversified product assortment becomes available [42]. Notably, the increase in the number and scope of specialties adopting regenerative products imposes that advanced biotherapy supply chains are equipped with flexible tools and decision-support processes aimed at a quality-controlled production and delivery of qualified products tailored to patient specifications. In parallel, the growth of the pool of potential candidate recipients necessitates technical and mechanical advancements combined with cutting-edge informatics architecture to support and manage the logistics of patient sample collection, scaled manufacturing, product delivery to provider/patient, timely clinical use and longitudinal follow-up. Both decentralized and centralized production systems have served the regenerative sector, although this distinction is projected to evolve with launch of hybrid models and versatile supply chain capabilities [43]. In a globalized and competitive world where margins are under pressure, supply chain optimization is critical. It includes an optimal use of resources, modular construction of cleanrooms for ease to expand or diversify in scope, ability to reconfigure or relocate, outsourcing of nonvalue add activities, institutionalization of just-in-time systems and progressive investments in communication technology. While operating business paradigms can increase efficiency and improve system responsiveness, the supply chain risk profile remains a point of vulnerability. This is commonly due to insufficient risk mitigation systems in place, creating an unprotected exposure to variations in demand or supply. Positioning supply chain management as integral in ensuring robust regenerative medicine readiness highlights areas amenable to optimization and in turn value creation.
Delivery readiness: a regenerative medicine-augmented model of care
Responding to practice needs, deployment of a regenerative medicine enriched model of care is predicated on the validity and utility of regenerative products and associated interventions (Figure 4). Transition from research and development into a ready-to-use service line is favored by alignment with clinical priorities and recognized or anticipated patient needs. The changeover from an exploratory pipeline into a full-fledge care pathway must respect, beyond regulatory endorsements, available implementation capacity and expertise readouts of the state of technological, translational and clinical readiness. Notably, adoption of a regenerative solution is grounded on a value-added proposition, advancing intended outcomes beyond the extent of current management options. A comprehensive regenerative medicine-augmented model of care would thus entail a standardized delivery of clinical-grade biotherapies supported by supply capabilities that integrate sourcing and manufacturing with patient delivery. Access can be coordinated through designated patient portals, serving to facilitate on-site or distance patient intake. Patient portals offer assimilated education programs, regenerative workup services, clinical trial enrolment, procurement of patient biomaterials, referral to specialty clinics, delivery of regenerative interventions and follow-up steps that benefit from integration within individualized care plans [44]. The collection, preservation, processing and manufacture of clinical-grade biospecimens offer a prospect for companion diagnostic and therapeutic twin use, a trend utilized in precision medicine based care [45,46]. The build-out of such ‘bio-insurance’ platforms must conform in consistency, uniformity and stability to regulatory guidelines. Care delivery is realized in both in-patient and out-patient settings, and relies on dedicated infrastructures, such as regenerative multispecialty therapeutic suites. Multimodal imaging and advanced visualization technologies are powerful complements to in-suite capabilities, including proximal access to on-site manufacturing and processing of biotherapeutics. Delivery protocols are developed in conjunction with care regimens ensuring incorporation of regenerative technologies within charted management plans. Outcomes are tracked to inform clinical decision-making and enable iterative care refinement.
Figure 4. . Regenerative care readiness.

Driven by unmet patient needs and enabled by a maturing technological and translational, new services lines are realized across regenerative medicine-intense care pathways. A permissive ecosystem, encompassing patient/public awareness, regulatory approval and care access/affordability, underpins long-term success in advancing a curative model of care.
Mixed outcomes: impediment to adoption readiness
Interindividual variability in response to regenerative treatments is commonly observed. Mixed outcomes reflect an uncertain mode of action, and are compounded by a limited long-term clinical experience. Development programs have therefore placed emphasis on decoding the attributes that define regenerative responsiveness. The capacity for repair is innate to the active ingredient of the biologics, and is (co)dependent on the disease substrate inherent to the treated recipient. To mitigate intrinsic variability, quality control standards are tested or have been put in place to certify the regenerative fitness of a biotherapeutics. Still, therapy is rarely tailored to the recipient due to the assumption of a uniform patient phenotype. Yet, among multifactorial contributors that segregate responders from nonresponders, the makeup of the recipient along with the severity of the underlying disease, co-morbidities, vulnerability to adverse effects, adherence to treatment regimen and/or social factors have all been incriminated [47]. Genetic variance, for example, impacts both individual disease risk and therapeutic outcome, as exemplified in non-responding or hyporesponding patients carrying pathogenic variants, contrasting regenerative therapy benefit achieved in noncarriers [48]. Similarly, disease severity, reflected in the degree of pathological organ remodeling, should be considered in patient inclusion or exclusion. Those with limited, as well as those with most exaggerated, remodeling are apparently less likely to respond to stem cell therapy, requiring adjustment of therapeutic goals matched to the individual recipient profile and respective disease state [49–51]. Multiparametric assessment, ideally performed preintervention as part of a regenerative workup, is needed to fortify clinical decision-making. Efforts to standardize effectiveness and homogenize outcomes consist of implementing phenotype-based patient selection, optimize biomarker-enforced intervention targeting the disease substrate and preferably utilize an augmented intelligence-powered approach in support of quality control of regenerative products, clinical testing and care delivery [47]. Criteria developed in successful clinical applications, in turn, provide useful blueprints for next-generation therapies [52].
Stakeholders readiness: synergy of purpose
Regenerative medicine development relies on diverse stakeholders. These include patients, families, biomedical innovators, healthcare providers, industry developers, government regulators, public and private insurers and payers. It is vital that readiness advances in tandem with protection of vulnerable patients from risks posed by unproven interventions [53]. Codevelopment partnerships thus aim to combine strengths and eliminate deficiencies, and evolve centered on technological knowledge, prospect for commercialization, regulatory and/or clinical expertise, and financing. Risk-sharing synergies, and unison of purpose, engender unique resources, build new infrastructures and eliminate obstacles to foster progress. A just system of resource and product distribution is a prerequisite for regenerative therapies to responsibly deliver on their potential [54]. Past regulatory approval and supply chain execution, a sustainable practice uptake mandates a market advantage over existing choices paired with an adequate level of coverage. Lasting success depends on securing reimbursement objectively supported by health technology assessment analysis and pharmaco-economic evaluation [55,56]. Regulatory designation impacts how payers construe a therapy to determine compensation, thereby fast-tracking or conversely slowing market access upon achieved market clearance. Definitive therapies that lessen the need for costly options should in principle provide considerable savings, and be prioritized as cost-effective, value-added alternatives [57]. Legal frameworks, including those that allow conditional and provisional approval, are expected to streamline provision to patients. Sustaining the utmost safety and efficacy rigor is essential while ensuring regulatory coordination that transcends geographic boundaries and multinational idiosyncrasies. The global experience accumulated to date underscores the need to alleviate evidence deficiencies that may endanger long-term market success in order to secure sought after market sustainability [58,59]. The advanced therapy medicinal products refer to a designation that encompasses diverse therapeutic strata including cell therapy, gene therapy and tissue-engineered products. In the European Union, where this designation is increasingly used, 14 advanced therapy medicinal products have received marketing authorization [59]. However, disappointing market performance, exemplified with five products, has led to notable withdrawals associated with shortcomings in the assembled clinical datasets, and the necessity for greater commitment postapproval to consistently ensure market performance [59]. Within a macroenvironment in which treatment failure risk is real, and where price protection is integral in contracting health services, the pricing strategy must be cautiously ascertained [60]. To align payments with care models, fee-for-value models with data-driven measurable outcomes that consider total cost of care will likely be favored [61]. Elevated pricing produces resistance or even opposition among payers, consumers, legislators and policy makers, and although early consideration may focus on individual benefit, favorable cost–benefit analysis at wider society level is decisive in defining long-term success. Ultimately, it is upon the community of practice to diffuse innovation broadly as a means to prevent socioeconomic divides, augment access and safeguard from healthcare disparities. In principle, biotherapies manufactured and delivered at a low cost of goods, as for example shelf-ready cell-free products stable at room temperature, would enable broader and diverse access, including in environments frequently deprived from advanced innovation.
Conclusion
The increased longevity of the global population reflects a coveted success for humanity, yet the accompanying pandemic of chronic degenerative diseases poses a formidable challenge for which existing solutions remain insufficient [62–66]. Addressing the healthspan–lifespan divide is a key objective for regenerative medicine, empowered by the fundamental understanding of targetable disease causes and the growth of a technology-driven curative toolbox (Figure 5). The enticing concept of rebuilding health to inverse causal pathology, as opposed to a battle to palliate disease symptomatology, has emerged as an exemplar of the reach of medical sciences reflecting healthcare evolving from reactive to proactive [67]. The technological concentration maximizing use of various stem cell types is expanding to embrace next-generation biotherapeutics [68,69]. These comprise, in the context of the body as a genuine bioreactor, antibodies, cytokines and growth factors (with proangiogenic, chemoattractant, immunomodulatory properties), biopotentiated 3D-printed matrices and biomimetics, cytoengineered products, gene-encoded therapy, nanodrugs and exosome technologies, the later aimed to hone in on the active ingredient of repair [70–74]. Alongside, the notion that a curative intervention may alter the economics of disease management is appealing easing the cost liability linked to care of a population vulnerable to, and seriously debilitated by, protracted degenerative disease. To properly attend to patients seeking regenerative solutions, a number of requirements must be met (Figure 6). Foremost, clinical adoption must ensure the innovation stringency with responsible translation of an evidence-based, regulatory authority-sanctioned, regenerative therapeutics portfolio that demonstrates validity (safety and efficacy) and utility (verified long-term outcomes) [75–77]. The rise of clinics marketing unproven therapies is a departure from responsible translation, cofounds the regulatory approval process and jeopardizes adoption of proven therapies. So that a viable regenerative medicine-powered model of care, with adequate supply chain support, advanced access and added therapeutic value, can be envisaged – comprehensive evidence during therapeutic development and postmarket launch must be collected and communicated through rigorous regulatory and surveillance processes. To this end, the healthcare profession must be educated to achieve proficiency for responsible delivery of regenerative care, meeting ethical norms [78]. To ensure bioethical standards, participants in clinical trials and biobank repository donors must be acknowledged for their contributions toward the enablement of clinical risk–benefit assessment [79]. Ethical and social issues related to biopreservation of stem cells for future regenerative therapies are a point of attention in order to counter ‘hype’ and promote realistic ‘hope.’ Emphasis is placed on medical validation of regenerative interventions and their influence on public understanding, the impact of public trust on for-profit cell preservation ventures, and the logistical issues pertinent to collection and governance including ownership and dispositional authority, informed consent and access, and withdrawal and nonpayment [80]. Moreover, objective harmonization of regenerative therapeutic properties, along with the automation and standardization of product manufacturing, are critical components in achieving a scalable, clinical-grade biotherapy gamut that is homogeneous and affordable [81,82]. Notably, personalized/precision therapies provide a range of challenges in terms of manufacturing, supply chain, clinical adoption/application and affordability. For example, autologous and allogeneic therapy-dependent care pathways will differ as wider scale roll-out proceeds. Decision-support processes needed for quality-controlled production and delivery, invigorated by an informatics empowered management of complex logistics, can reduce points of vulnerability and, in sequence, may contribute to a value-added proposition. However, mixed outcomes in the response to regenerative treatments remain a major impediment to practice adoption, as multifactorial contributors segregate responders from nonresponders. Conventional doctrines that apply in orthodox drug development may not be adaptable to the regenerative pharmacy, reflecting the contrast between drugs as chemicals versus drugs as biologics. Customary medicinal products feature defined mechanisms of action and delineated processes of absorption, distribution, metabolism and excretion. In contrast, the understanding of emergent biotherapeutics displaying polyvalent modes of action and divergent pharmacodynamic and pharmacokinetic profiles has been limited, and is in part related to the inherent variability of autologous biologics manufactured with patients serving as their own donors. Closer understanding of the dose–exposure–response trinity is needed to inform dose and schedule selection [83]. Achieving an accurate preintervention forecast of likely responsive recipients is an area of priority [84]. Patient access is another dimension needed to be expanded, in tandem with care plan integration. In helping to ensure access for patients, clinical development programs should generate, in addition to clinical data, the health economic datasets necessary to support the value proposition of an emerging therapeutic product [85]. Postlaunch, pharmacovigilance-based evidence can help overcome challenges around product uncertainty at launch and reduce market delays, while promoting equity and access of patients to innovative therapies [86]. Overall, in an environment of limited healthcare resources and increased scrutiny over the real value proposition, it is judicious to incorporate an economic conjecture early in the product development process that fosters market access and facilitates long-term market viability [86], while underscoring equitable regenerative care.
Figure 5. . Regenerative medicine-catalyzed rebalancing.
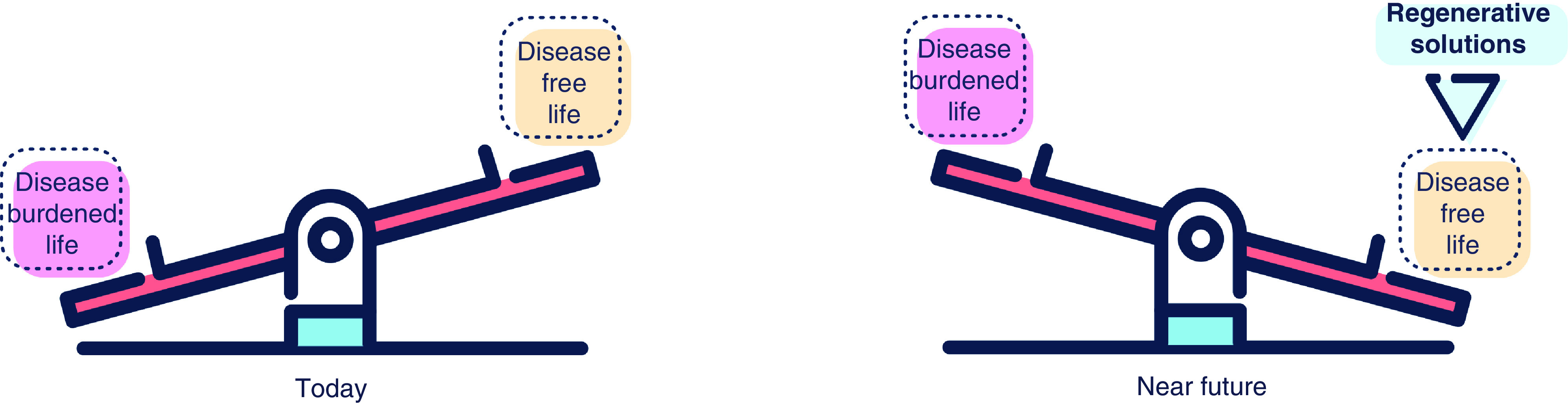
To reconcile the misbalance between a disease burdened as opposed to a disease-free life is the purpose of regenerative medicine.
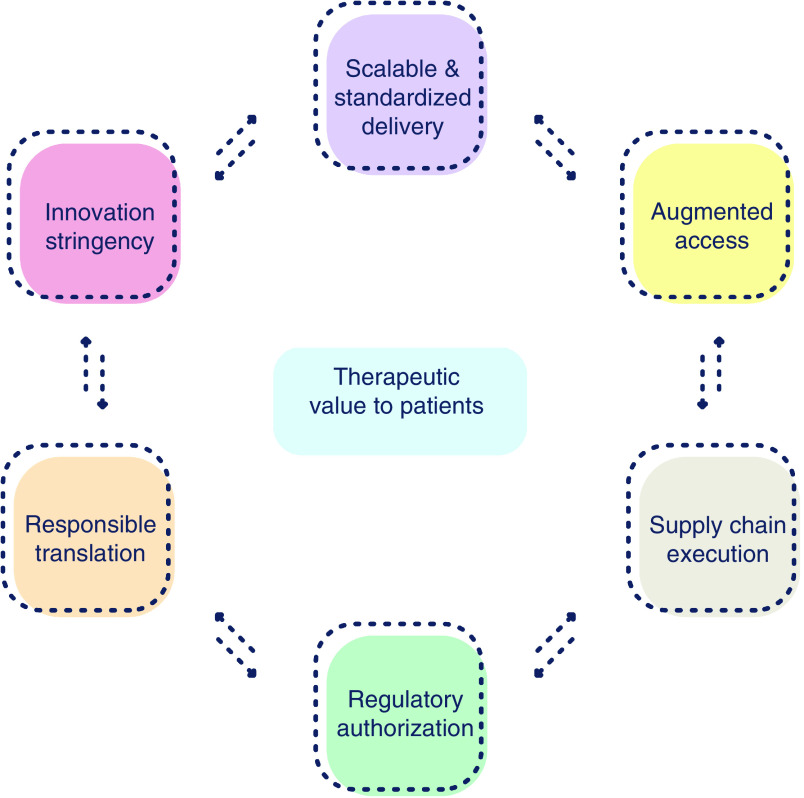
Figure 6. . Practice uptake.
Adoption by practice requires confluence of complementary levers. These range from innovation stringency, responsible translation and regulatory harmonization to supply chain implementation, advanced access and scalable/standardize delivery, with the express purpose of ensuring therapeutic value.
Future perspective
The near future for regenerative science and medicine seems favorable and inspirational. The engine of discovery is a fundamental driver of innovation, exemplified by the decoding of molecular and epigenetic mechanisms underpinning stem cell and acellular applications, demystifying tissue degeneration and regeneration, capturing the immune response in tissue healing, leveraging gene editing to alter disease progression, employing tissue engineering to bioengineer novel therapies, to name a few [87–92]. Anticipated to contribute toward global solutions, the audacious goals of tomorrow’s regenerative vision include equity and equality of care [93]. Science-driven perspectives are founded on the increasingly precise decrypting of the disease pathobiology fabric, guiding an ever growing specificity in target identification and/or patient(s) selection [94–98]. Deciphering the molecular intimacy of biological diversity and resolving the mechanisms underpinning regenerative processes will usher the very next wave of sophistication in regenerative protocols anchored on desired therapeutic attributes [99–104]. The means to achieve optimization of the regenerative toolbox, coupled with a proactive failure-safe selection of patients that have high likelihood of responding, will be considered transformative. Iterative translational know-how will continue to evolve, with newly acquired knowledge steadily applied to advance clinical-grade biomanufacturing and quality control developed for the express purpose of securing a reliable, cost-effective, supply chain fit to meet the needs of diverse real-world scenarios. Responsible, yet seamless, integration of innovative regenerative medicine into care lines will be paramount, with curative toolkits embedded and integrated across specialties [105–107]. This technology-enabled, big data-supported, patient-centric enrichment will provide the medicine of tomorrow with a healthcare prospective suitable in addressing unmet needs of vulnerable individuals and populations [108]. The long-term success will critically depend on the ability of the ecosystem as a whole, namely developers, manufacturers, providers and insurers, to deliver regulated regenerative products that are achieving increased homogeneity, standardization and scalability, underscoring a needed maturation of the regenerative industry and its lexicon [109]. Adoption of approved, and practice guidelines implemented, regenerative therapies must be made available through accessible and affordable management plans built to ensure healthcare equity, and the opportunity for all to achieve their full healthcare potential [110]. A precondition for responsible regenerative care relies on a just resource distribution, today and in the future, here and beyond. Viable uptake will mandate a market advantage, with lasting success dependent on cost–effectiveness and population-validated data-driven reimbursements. Finally, in order for regenerative medicine to truly benefit the patients who needs it most, the sociotechnical landscape must be conceived as a global, shared endeavor [111–115]. As long as the development of regenerative care comes from unison of forces recognizing and confronting global challenges, including unprecedented disease outbreaks, it is poised to contribute to the upcoming offerings of reliable and globally applicable solutions achieving the ultimate objective – that of ‘regeneration without borders.’
Executive summary.
Cause for cures: the regenerative impetus
Decoding disease pathogenesis has catalyzed the prospect of curative therapies.
Disruptive regenerative technologies aim at reversal of underlying pathology.
Evidence-based, regulated practices are prerequisites for clinical adoption.
Insufficient homogeneity, scalability and affordability impede uptake.
Roll-out of care pathways converges with unmet needs of an aging population.
Catalyzing new pathways: addressing unmet needs
Regenerative biotherapies are an area of growth.
Innovative technologies have expanded the regenerative armamentarium.
Boosting innate healing aptitude underlies therapeutic regeneration.
Regulatory approval achieved in areas of need, advancing in particular cancer care.
The regenerative lens aspires for the patient to reclaim health.
Workforce readiness: educated proficiency
Paucity of trained physicians, nurses and therapists in regenerative modalities.
Education readiness requires proficiency in regenerative principles and practices.
Competencies in patient selection, treatment delivery and side effect management.
Trained workforce must distinguish approved options for responsible regenerative care.
Expanded pools of learners catalyze connectivity across communities of practice.
Supply readiness: from protocol to product
Challenges in translating a research grade technology into a clinical grade product.
Success determined by cost, supply, stability, scalability, automation and failure rate.
Decision-support processes needed for quality-controlled production and delivery.
Informatics architecture required to manage complex logistics.
Points of vulnerability can constrain translation or lead to value creation.
Delivery readiness: a regenerative medicine-augmented model of care
Regenerative medicine model of care predicated on clinical validity and clinical utility.
Build-out of service lines mandates technological/translational/clinical readiness.
Regenerative products and services must achieve a value-added proposition.
Coordinated access via multifunctional patient portals for seamless care plan integration.
Delivery realized in in/out-patient settings and outcomes tracked for improvement.
Mixed outcomes: impediment to adoption readiness
Outcome heterogeneity is a major obstacle to practice adoption.
Multifactorial contributors segregate responders from nonresponders.
Genetic makeup and disease severity considered for individualization of regimens.
Multiparametric assessment needed to guide clinical decision-making.
Standardized effectiveness/homogenized outcomes are clinical priorities.
Stakeholders readiness: synergy of purpose
Ecosystem involves patients, innovators, providers, developers, regulators and insurers.
Technology, commercial, regulatory, financial codevelopment risk-sharing.
Prerequisite for responsible regenerative care relies on just resource distribution.
Viable uptake mandates a market advantage over available options.
Lasting success depends on data-driven reimbursement.
Acknowledgments
The authors acknowledge Adam Price-Evans (a former employee of Future Science Group, was the Commissioning Editor for Regenerative Medicine at the time of writing) for his invaluable discussion and input. A Terzic holds the Marriott Family Professorship in Cardiovascular Diseases Research, and is Michael S. and Mary Sue Shannon Director of the Mayo Clinic Center for Regenerative Medicine.
Footnotes
Author contributions
S Yamada, A Behfar and A Terzic were responsible for the conception and design, data acquisition, analysis and interpretation, drafting the work, final approval of the manuscript, financial and administrative support, and agreement to be accountable for all aspects of the work.
Financial & competing interests disclosure
The authors are supported by the National Institutes of Health (R01 HL134664), Marriott Family Foundation, Van Cleve Cardiac Regenerative Medicine Program, Michael S. and Mary Sue Shannon Family, and Center for Regenerative Medicine at Mayo Clinic. S Yamada, A Behfar and A Terzic are coinventors on regenerative sciences related intellectual property disclosed to Mayo Clinic. Previously, Mayo Clinic has administered research grants from Celyad. Mayo Clinic, A Behfar and A Terzic have interests in Rion LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Terzic A, Nelson TJ. Regenerative medicine primer. Mayo Clin. Proc. 88(7), 766–775 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Edgar L, Pu T, Porter B et al. Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 107(7), 793–800 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Waldman SA, Terzic A. Bioinnovation Enterprise: an engine driving breakthrough therapies. Clin. Pharmacol. Ther. 99(1), 8–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regenerative Medicine and RegMedNet. The glossary for advanced therapies. Regen. Med. 15(12s), 1–170 (2020).33624525 [Google Scholar]
- 5.International Society for Stem Cell Research. Core concepts in stem cell biology: syllabus and learning guide. version 1, 1–49 (2020). http://www.isscr.org
- 6.Cossu G, Fears R, Griffin G, Ter Meulen V. Regenerative medicine: challenges and opportunities. Lancet 395(10239), 1746–1747 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Behfar A, Terzic A. Regeneration for all: an odyssey in biotherapy. Eur. Heart J. 40(13), 1033–1035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terzic A, Harper CM Jr, Gores GJ, Pfenning MA. Regenerative medicine blueprint. Stem Cells Dev. 22(Suppl. 1), 20–24 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Terzic A, Waldman SA. Chronic diseases: the emerging pandemic. Clin. Transl. Sci. 4(3), 225–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Congress. 21st Century Cures Act Public Law. 114–255 (2016).
- 11.Liu E, Marin D, Banerjee P et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382(6), 545–553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June CH, Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379(1), 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Regenerative immunotherapy highlighted in the context of cancer care.
- 13.Beyar-Katz O, Gill S. Advances in chimeric antigen receptor T cells. Curr. Opin. Hematol. 27(6), 368–377 (2020). [DOI] [PubMed] [Google Scholar]
- 14.McKay RR, Hafron JM, Ferro C et al. A retrospective observational analysis of overall survival with sipuleucel-T in medicare beneficiaries treated for advanced prostate cancer. Adv. Ther. 37(12), 4910–4929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini G, Ardigò D, Milazzo G et al. Navigating market authorization: the path Holoclar took to become the first stem cell product approved in the European Union. Stem Cells Transl. Med. 7(1), 146–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panés J, García-Olmo D, Van Assche G et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology 154(5), 1334–1342.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Saris DBF, de Windt TS, Vonk LA, Krych AJ, Terzic A. Regenerative musculoskeletal care: ensuring practice implementation. Clin. Pharmacol. Ther. 103(1), 50–53 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Bartunek J, Terzic A, Davison BA et al. Cardiopoietic stem cell therapy in ischaemic heart failure: long‐term clinical outcomes. ESC Heart Fail. 7(6), 3345–3354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagi Y, Nakayama K, Taguchi T et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci. Rep. 7(1), 14085 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Rabbani CC, Gao H et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582(7812), 399–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • New inroads of evolving regenerative technology for functional restoration.
- 21.Ibrahim AGE, Li C, Rogers R et al. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat. Biomed. Eng. 3(9), 695–705 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34(3), 312–319 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Martinod E, Chouahnia K, Radu DM et al. Feasibility of bioengineered tracheal and bronchial reconstruction using stented aortic matrices. JAMA 319(21), 2212–2222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magalhaes RS, Williams JK, Yoo KW, Yoo JJ, Atala A. A tissue-engineered uterus supports live births in rabbits. Nat. Biotechnol. 38(11), 1280–1287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs: the bone bioreactor. Proc. Natl Acad. Sci. USA. 102(32), 11450–11455 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinod E, Paquet J, Dutau H et al. In vivo tissue engineering of human airways. Ann. Thorac. Surg. 103(5), 1631–1640 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Service RF. Tissue engineering: technique uses body as ’bioreactor’ to grow new bone. Science 309(5735), 683 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Wells JM, Watt FM. Diverse mechanisms for endogenous regeneration and repair in mammalian organs. Nature 557(7705), 322–328 (2018). [DOI] [PubMed] [Google Scholar]; • Innate mechanisms of regeneration and repair are presented.
- 29.Hargraves IG, Behfar A, Foxen JL, Montori VM, Terzic A. Towards regeneration: the evolution of medicine from fighting to building. BMJ 361, k1586 (2018). [Google Scholar]
- 30.Wyles SP, Terzic A. Building the regenerative medicine workforce of the future: an educational imperative. Regen. Med. 14(7), 613–615 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Chlan LL, Tofthagen C, Terzic A. The regenerative horizon: opportunities for nursing research and practice. J. Nurs. Scholarsh. 51(6), 651–660 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyles SP, Hayden RE, Meyer FB, Terzic A. Regenerative medicine curriculum for next-generation physicians. NPJ Regen. Med. 4, 3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyles SP, Meyer FB, Hayden R, Scarisbrick I, Terzic A. Digital regenerative medicine and surgery pedagogy for virtual learning in the time of COVID-19. Regen. Med. 15(8), 1937–1941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma A, McKeithan WL, Serrano R et al. Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat. Protoc. 13(12), 3018–3041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronaldson-Bouchard K, Yeager K, Teles D et al. Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat. Protoc. 14(10), 2781–2817 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tysoe OC, Justin AW, Brevini T et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 14(6), 1884–1925 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Jacob F, Ming GL, Song H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat. Protoc. 15(12), 4000–4033 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Bansal A, Pandey MK, Yamada S et al. [89Zr]Zr-DBN labeled cardiopoietic stem cells proficient for heart failure. Nucl. Med. Biol. 90-91, 23–30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanicola HW, Stewart CE, Mueller M et al. Guidelines for establishing a 3-D printing biofabrication laboratory. Biotechnol. Adv. 45, 107652 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Terzic A, Pfenning MA, Gores GJ, Harper CM Jr. Regenerative medicine build-out. Stem Cells Transl. Med. 4(12), 1373–1379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Prototype for institutional regenerative medicine readiness.
- 41.Nelson TJ, Behfar A, Yamada S, Martinez-Fernandez A, Terzic A. Stem cell platforms for regenerative medicine. Clin. Transl. Sci. 2(3), 222–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunsberger J, Harrysson O, Shirwaiker R et al. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Transl. Med. 4(2), 130–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunsberger J, Simon C, Zylberberg C et al. Improving patient outcomes with regenerative medicine: How the Regenerative Medicine Manufacturing Society plans to move the needle forward in cell manufacturing, standards, 3D bioprinting, artificial intelligence-enabled automation, education, and training. Stem Cells Transl. Med. 9(7), 728–733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith C, Martin-Lillie C, Higano JD et al. Challenging misinformation and engaging patients: characterizing a regenerative medicine consult service. Regen. Med. 15(3), 1427–1440 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terzic A, Edwards BS, McKee KC, Nelson TJ. Regenerative medicine: a reality of stem cell technology. Minn. Med. 94(5), 44–47 (2011). [PubMed] [Google Scholar]
- 46.Waldman SA, Terzic A. Companion diagnostics at the intersection of personalized medicine and healthcare delivery. Biomark. Med. 9(1), 1–3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada S, Jeon R, Garmany A, Behfar B, Terzic A. Screening for regenerative therapy responders in heart failure. Biomark. Med. (Epub ahead of print) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rieger AC, Myerburg RJ, Florea V et al. Genetic determinants of responsiveness to mesenchymal stem cell injections in non-ischemic dilated cardiomyopathy. EBioMedicine 48, 377–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartunek J, Terzic A, Behfar A, Wijns W. Clinical experience with regenerative therapy in heart failure: advancing care with cardiopoietic stem cell interventions. Circ. Res. 122(10), 1344–1346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frljak S, Poglajen G, Zemljic G et al. Larger end-diastolic volume associates with response to cell therapy in patients with non-ischemic dilated cardiomyopathy. Mayo Clin. Proc. 95(10), 2125–2133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada S, Arrell DK, Rosenow CS, Bartunek J, Behfar A, Terzic A. Ventricular remodeling in ischemic heart failure stratifies responders to stem cell therapy. Stem Cells Transl. Med. 9(1), 74–79 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Luca M, Aiuti A, Cossu G et al. Advances in stem cell research and therapeutic development. Nat. Cell Biol. 21, 801–811 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Lomax GP, Torres A, Millan MT. Regulated, reliable, and reputable: protect patients with uniform standards for stem cell treatments. Stem Cells Transl. Med. 9(5), 547–553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardner J. Distributive justice and regenerative medicine. Regen. Med. 12(7), 865–874 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Cho E, Yoo SL, Kang Y, Lee JH. Reimbursement and pricing of regenerative medicine in South Korea: key factors for achieving reimbursement. Regen. Med. 15(4), 1550–1560 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Coyle D, Durand-Zaleski I, Farrington J et al. HTA methodology and value frameworks for evaluation and policy making for cell and gene therapies. Eur. J. Health Econ. 21(9), 1421–1437 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Thavorn K, Van Katwyk S, Krahn M et al. Value of mesenchymal stem cell therapy for patients with septic shock: an early health economic evaluation. Int. J. Technol. Assess. Health Care. 36(5), 525–532 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Marks P, Gottlieb S. Balancing safety and innovation for cell-based regenerative medicine. N. Engl. J. Med. 378(10), 954–959 (2018). [DOI] [PubMed] [Google Scholar]; • The US FDA perspective on regenerative medicine regulatory path.
- 59.Elsallab M, Bravery CA, Kurtz A, Abou-El-Enein M. Mitigating deficiencies in evidence during regulatory assessments of advanced therapies: a comparative study with other biologicals. Mol. Ther. Methods Clin. Dev. 18, 269–279 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bandeiras C, Hwa AJ, Cabral JMS, Ferreira FC, Finkelstein SN, Gabbay RA. Economics of beta-cell replacement therapy. Curr. Diab. Rep. 19(9), 75 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Patel NA. Fee-for-value in the pharmaceutical industry: a policy framework applying data science to negotiate drug prices. J. Law Biosci. 4(1), 205–215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature 561(7721), 45–56 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Tchkonia T, Kirkland JL. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA 320(13), 1319–1320 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Olshansky SJ. From lifespan to healthspan. JAMA 320(13), 1323–1324 (2018). [DOI] [PubMed] [Google Scholar]
- 65.van Deursen JM. Senolytic therapies for healthy longevity. Science 364(6441), 636–637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roth GA, Mensah GA, Johnson CO et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76(25), 2982–3021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waldman SA, Terzic A. Health care evolves from reactive to proactive. Clin. Pharmacol. Ther. 105(1), 10–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson TJ, Behfar A, Terzic A. Strategies for therapeutic repair: the R3 regenerative medicine paradigm. Clin. Transl. Sci. 1(2), 168–171 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamanaka S. Pluripotent stem cell-based cell therapy: promise and challenges. Cell Stem Cell. 27(4), 523–531 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Huang RL, Kobayashi E, Liu K, Li Q, Huang RL. Bone graft prefabrication following the in vivo bioreactor principle. EBioMedicine. 12, 43–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffman T, Khademhosseini A, Langer R. Chasing the paradigm: clinical translation of 25 years of tissue engineering. Tissue Eng. Part A. 25(9-10), 679–687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20(2), 101–124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gensler M, Leikeim A, Möllmann M et al. 3D printing of bioreactors in tissue engineering: a generalised approach. PLoS ONE 15(11), e0242615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fuchs E, Blau HM. Tissue stem cells: architects of their niches. Cell Stem Cell. 27(4), 532–556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marks PW, Hahn S. Identifying the risks of unproven regenerative medicine therapies. JAMA 324(3), 241–242 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Matsushita S, Tachibana K, Kusakabe T, Hirayama R, Tsutsumi Y, Kondoh M. The roadmap to approval under Japan’s two-track regulatory system: comparing six regenerative medical products. Cell Stem Cell. 27(4), 515–518 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Nagai S. Flexible and expedited regulatory review processes for innovative medicines and regenerative medical products in the US, the EU, and Japan. Int. J. Mol. Sci. 20(15), 3801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wyles SP, Morie DD, Paradise CR, Meyer FB, Hayden RE, Terzic A. Emerging workforce readiness in regenerative healthcare. Regen. Med. (2021) (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.King NMP, Bishop CE. How should physicians help patients understand unknowns of nanoparticle-based medicines? AMA. J. Ethics. 21(4), E324–E331 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Master Z, Crowley AP, Smith C, Wigle D, Terzic A, Sharp RR. Stem cell preservation for regenerative therapies: ethical and governance considerations for the health care sector. NPJ Regen. Med. 5(1), 23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karanu F, Ott L, Webster DA, Stehno-Bittel L. Improved harmonization of critical characterization assays across cell therapies. Regen. Med. 15(5), 1661–1678 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Doulgkeroglou MN, Di Nubila A, Niessing B et al. Automation, monitoring, and standardization of cell product manufacturing. Front. Bioeng. Biotechnol. 8, 811 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terzic A, Behfar A, Filippatos G. Clinical development plan for regenerative therapy in heart failure. Eur. J. Heart Fail. 18(2), 142–144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terzic A, Behfar A. Posology for regenerative therapy. Circ. Res. 121(11), 1213–1215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Driscoll D, Farnia S, Kefalas P, Maziarz RT. The high cost of high tech medicine: planning ahead for market access. Stem Cells Transl. Med. 6(8), 1723–1729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonçalves E. Advanced therapy medicinal products: value judgement and ethical evaluation in health technology assessment. Eur. J. Health Econ. 21(3), 311–320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Llorens-Bobadilla E, Chell JM, Le Merre P et al. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science 370(6512), eabb8795 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Yokota T, McCourt J, Ma F et al. Type V collagen in scar tissue regulates the size of scar after heart injury. Cell 182(3), 545–562.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim E, Choi S, Kang B et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 588(7839), 664–669 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Nikolaev M, Mitrofanova O, Broguiere N et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585(7826), 574–578 (2020). [DOI] [PubMed] [Google Scholar]
- 91.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584(7822), 535–546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doudna JA. The promise and challenge of therapeutic genome editing. Nature 578(7794), 229–236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terzic A, Leask F. Regenerative outlook: offering global solutions for equity of care. Regen. Med. 15(11), 2249–2252 (2020). [DOI] [PubMed] [Google Scholar]
- 94.Blau HM, Daley GQ. Stem cells in the treatment of disease. N. Engl. J. Med. 380(18), 1748–1760 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Fioretta ES, Motta SE, Lintas V et al. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 18(2), 92–116 (2021). [DOI] [PubMed] [Google Scholar]
- 96.Wu JC, Garg P, Yoshida Y et al. Towards precision medicine with human iPSCs for cardiac channelopathies. Circ. Res. 125(6), 653–658 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schaum N, Lehallier B, Hahn O et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583(7817), 596–602 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rajewsky N, Almouzni G, Gorski SA et al. LifeTime and improving European healthcare through cell-based interceptive medicine. Nature 587(7834), 377–386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.West MD, Sternberg H, Labat I et al. Toward a unified theory of aging and regeneration. Regen. Med. 14(9), 867–886 (2019). [DOI] [PubMed] [Google Scholar]; • Intersections unifying aging biology and regeneration.
- 100.Correa-Gallegos D, Jiang D, Christ S et al. Patch repair of deep wounds by mobilized fascia. Nature 576(7786), 287–292 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Hirose K, Payumo AY, Cutie S et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 364(6436), 184–188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Ouyang JF, Rossello FJ et al. Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature 586(7827), 101–107 (2020). [DOI] [PubMed] [Google Scholar]; • Dissection of regenerative programs at cell fate level.
- 103.Arrell DK, Rosenow CS, Yamada S, Behfar A, Terzic A. Cardiopoietic stem cell therapy restores infarction-altered cardiac proteome. NPJ Regen. Med. 5, 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Becker SM, Wright CB. Update on the status and impact of the National Eye Institute audacious goals initiative for regenerative medicine. J. Ocul. Pharmacol. Ther. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Webster A. Regenerative medicine and responsible research and innovation: proposals for a responsible acceleration to the clinic. Regen. Med. 12(7), 853–864 (2017). [DOI] [PubMed] [Google Scholar]; • Perspective on responsible acceleration of regenerative medicine.
- 106.Gardner J, Webster A, Barry J. Anticipating the clinical adoption of regenerative medicine: building institutional readiness in the UK. Regen. Med. 13(1), 29–39 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Shapiro SA, Smith CG, Arthurs JR, Master Z. Preparing regenerative therapies for clinical application: proposals for responsible translation. Regen. Med. 14(2), 77–84 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Waldman SA, Terzic A. Process improvement for maximized therapeutic innovation outcome. Clin. Pharmacol. Ther. 103(1), 8–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terzic A. Regenerative medicine lexicon. Regen. Med. 15(12), 2325–2328 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Evans MK. Health equity - are we finally on the edge of a new frontier? N. Engl. J. Med. 383(11), 997–999 (2020). [DOI] [PubMed] [Google Scholar]
- 111.Gardner J, Higham R, Faulkner A, Webster A. Promissory identities: sociotechnical representations & innovation in regenerative medicine. Soc. Sci. Med. 174, 70–78 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Gostin LO, DeBartolo MC, Katz R. The global health law trilogy: towards a safer, healthier, and fairer world. Lancet 390(10105), 1918–1926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fernández-Avilés F, Sanz-Ruiz R, Climent AM et al. Global position paper on cardiovascular regenerative medicine. Eur. Heart J. 38(33), 2532–2546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Behfar A, Terzic A. Make regeneration great again; stronger together. Eur. Heart J. 38(15), 1094–1095 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Ramezankhani R, Torabi S, Minaei N et al. Two decades of global progress in authorized advanced therapy medicinal products: an emerging revolution in therapeutic strategies. Front. Cell. Dev. Biol. 8, 547653 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]