Abstract
Smoking, heavy alcohol drinking and drug abuse are detrimental lifestyle factors leading to loss of million years of healthy life annually. One of the major health complications caused by these substances is the development of cardiovascular diseases (CVD), which accounts for a significant proportion of substance-induced death. Smoking and excessive alcohol consumption are related to the higher risk of acute myocardial infarction. Similarly, opioid addiction, as one of the most commonly used substances worldwide, is associated with cardiac events such as ischemia and myocardial infarction (MI). As supported by many studies, coronary artery disease (CAD) is considered as a major cause for substance-induced cardiac events. Nonetheless, over the last three decades, a growing body of evidence indicates that a significant proportion of substance-induced cardiac ischemia or MI cases, do not manifest any signs of CAD. In the absence of CAD, the coronary microvascular dysfunction is believed to be the main underlying reason for CVD. To date, comprehensive literature reviews have been published on the clinicopathology of CAD caused by smoking and opioids, as well as macrovascular pathological features of the alcoholic cardiomyopathy. However, to the best of our knowledge there is no review article about the impact of these substances on the coronary microvascular network. Therefore, the present review will focus on the current understanding of the pathophysiological alterations in the coronary microcirculation triggered by smoking, alcohol and opioids.
Keywords: Smoking, Alcohol, Opioid, Coronary microvascular dysfunction, Ischemic heart disease, Myocardial infarction
Background
Different studies have shown that 20% to 50% of angina patients undergoing coronary angiography have normal or near normal arteries, (non-obstructive coronary disease) [1–9]. These groups of patients were conventionally diagnosed as Cardiac Syndrome X. Today it is demonstrated that a large proportion of these patients have coronary microvascular dysfunction (CMD) [3]. CMD has gained more attention over the last 15 years as a cardiac cause of morbidity and as critical as CAD. CMD diagnosed patients display high rates of hospitalization for unstable angina, myocardial infarction (MI) and heart failure [7, 10, 11]. Pathophysiology studies suggest that CMD develops by: (1) structural alterations including the remodeling of microvessels or microcircular rarefaction; or (2) functional abnormalities such as spasm or impaired dilatory function in endothelial or vascular smooth muscle cell (VSMC). The underlying mechanisms are the dysregulation of the related hormonal, metabolic or neurosympathetic (neural tone) stimuli [12–14]. CMD can occur as a primary condition in patients with no obstructive coronary disease or can exist in the setting of diffuse and focal epicardial coronary disease [15] or occur as a consequence of acute MI or other cardiac events leading to damage in coronary microcirculation (e.g. percutaneous coronary intervention). In case CMD and epicardial problems exists side by side, the diagnosis of CMD is challenging but clinically important for determining the prognosis of ischemic and angina patients. For example, diagnosis of CMD can explain why symptoms persist in some CAD patients following percutaneous coronary interventions (PCI) [15–17]. CMD diagnosis is often challenging and not as well established as diagnosis of CAD. Yet no imaging method is available to visualize vessels smaller than 500 µm directly. Therefore, only indirect measurements of microvascular function are practiced in clinical settings today. Commonly, coronary flow reserve (CFR) is used as an indirect indicator of coronary microvascular function in the absence of CAD (normal or near normal angiography) [18–22]. CFR represents the capacity of coronary circulation to increase coronary blood flow (CBF) from basal levels to maximum in response to a vasodilatory stimulus. Therefore, CFR is proportional to blood flow in epicardial vessels plus microvasculature [23]. The reduced coronary flow reserve (CFR) manifested in CMD patients with no obstructive CAD (determined by normal angiography), is due to an impaired coronary vasodilation of microvasculature at higher demanding conditions of myocardium [23, 24].
Today, coronary microvascular function can be measured using invasive angiographic methods such as Doppler-tipped coronary guidewire and wire-based thermodilution techniques. Additionally, non-invasive imaging technologies such as positron emission tomography (PET) cardiac magnetic resonance imaging (CMR) or transthoracic Doppler echocardiography of the left anterior descending coronary artery are applied to measure indexes of coronary microvascular function. Therefore, one complexity for diagnosis of CMD is that it requires technologies that may not be widely available [14, 25–27]. CMD is associated with conventional cardiac risk factors such as smoking, aging, obesity, diabetes mellitus, and hypertension [18, 28]. However, growing number of publications point to the significant contribution of non-traditional risk factors in CMD development, including substance abuse (e.g., alcohol, opioids, cocaine). Here, we overview and highlight on the clinical and pathophysiological effects of substance abuse i.e., smoking, alcohol and opioids on CMD, with an aim to bring this topic to the attention of more researchers in the field.
Search strategy
In this literature review, a systematic search strategy was performed in electronic scientific databases PUBMED, Medline, Google Scholar using advanced search and all combinations of search terms. Search terms were selected based on the entry terms suggested by Medical Subject Headings (MeSH). Title and abstracts were first screened based on relevance to the subject and the study was selected to be included in the review after assessing the full text for eligibility and relevance. Additionally, the reference and citing publication lists from the retrieved articles were checked to identify further relevant studies. The search time limit was since 1980 to date. We included only studies published in English. Our search terms include: (heart, coronary); (percutaneous coronary intervention, percutaneous coronary, revascularization, reperfusion, angioplasty); (smoking, tobacco, cigars, cigarette); (microvessel, Microvasculature, microvascular network, microvascular, microcirculation, small vessels, capillary, arteriole, angiogenesis, intimal proliferation, intimal neoplasia, neointimal, microvascular obstruction, MVO, coronary flow reserve, CFR, index of microvasculatory resistance, IMR); (Ethanol, alcohol, ethyl, EtOH, alcoholic, drinking, wine); (opioid, opiate, opium, morphine, amphetamine, methadone, analgesics) and (ischemia, infarction, MI).
Impact of smoking on coronary microvasculature
Impact of smoking on coronary microvasculature and stable CMD
Smoking pattern impact on the coronary microvascular function
Epidemiological and case–control studies demonstrated chronic long-term smoking as one of the predictors of CMD in asymptomatic individuals [29–32] the non-obstructive coronary ischemic patients (no CAD) (stable CMD) [33, 34] as well as patients with vasospastic Angina [35] or CAD [36] background. These studies rely on the measured CFR and its stimulus-induced changes, due to unavailability of a direct technique to assess the coronary microvasculature status in vivo. Compared to long-term effects, the short-term chronic smoking assessed in healthy young smokers (with no evidence of CAD) did not affect the myocardial blood flow at resting conditions. However, smokers displayed a lower CFR in response to stress [29, 31].
Acute smoking can also exert negative impact on coronary microvascular function (in habitual smokers or non-smokers). Park et al. conducted a study on healthy young smokers and non-smokers, comparing CFR and the coronary vascular resistance index (CVRI) after a 4-h period of smoking abstinence. No significant difference between smokers and non-smokers was observed. However, after consumption of only two cigarettes in the smoking group, a considerable decline in CFR and an increase in CVRI were observed in the smokers [37].
On the other hand, the acute CFR declining effect was shown to be equivalent upon light (containing 0.6 mg nicotine, 8 mg tar, 9 mg carbon monoxide) and regular cigarette smoking (containing 0.9 mg nicotine, = 12 mg tar, 12 mg carbon monoxide) [38, 39]. On the contrary, one group found enhanced acute effect of high nicotine content cigarettes on CFR compared to the low content ones, showing a dose-dependent contribution of nicotine component of cigarettes in microvascular damaging effects of smoking [40].
Multiple studies have implicated passive smoking as a significant risk factor in CHD, being associated with higher rate of morbidity and mortality and poor outcome in CHD and acute coronary syndrome patients [41–49]. Additionally, an impairment of microvascular function and reactivity was supported by other studies indicating passive smoke exposure as a risk factor in CMD development [50, 51]. On the other hand, passive smoking is associated with lower odds ratio of smoking cessation in CHD patients [52, 53]. This is important due to the fact that smoking cessation is considered as a major preventive measure and management strategy to reduce mortality risk among CHD patients [54, 55] as well as patients with coronary microvascular dysfunction [56, 57].
Based on current information on the effects of different patterns of smoking on CMD (light vs. regular cigarette; active vs. passive smoking), evidence does not support one pattern over the other, emphasizing on the detrimental effects of smoking nevertheless. However, the limited number of studies on smoking-connected CMD, and particularly the small population size in most of these studies, necessitates conduction of large-scale research on this subject in the future.
Mechanistic studies on the effects of smoking on coronary microvasculature in human subjects
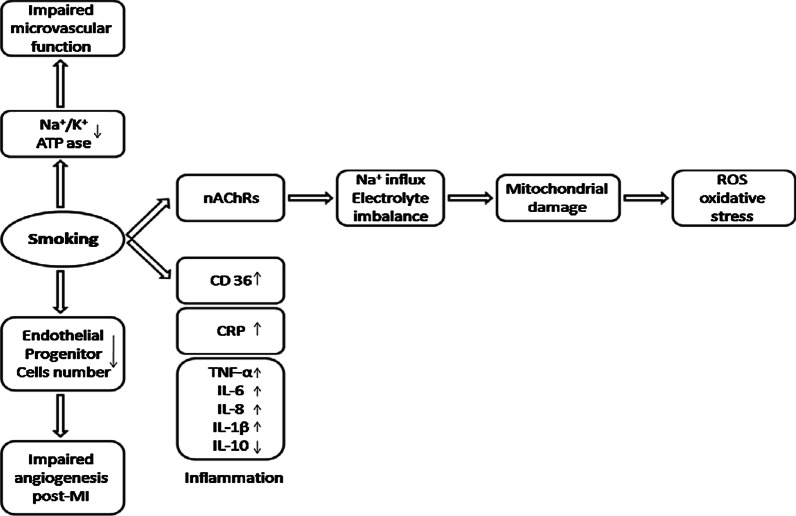
Evidence indicates that smoking affects coronary microvasculature via altering endothelial cells. Impaired coronary microvascular function in healthy young chronic smokers was observed under cold stress as an endothelium-dependent stimuli [29, 31] whereas dipyridamole which acts through endothelium-independent mechanisms, failed to affect microvascular function in healthy smokers [29]. The main mechanisms underlined in the literature for the smoking-induced vascular endothelial cell damage are oxidative stress, inflammation, impaired Na+/K+ ATPase function (Fig. 1). Cigarette smoke contains several radical or non-radical oxidants including superoxide radicals (.O2−), hydroxyl radicals (.OH), and peroxides (ROOH). Therefore, smoking can induce oxidative stress directly via its content and cause damage to coronary microvascular cells [58, 59]. It was shown that vitamin C as a potent antioxidant has been demonstrated to normalize the impaired coronary microvascular function in chronic smokers, whereas it did not alter CFR in non-smokers at all [32]. In addition to the oxidative stress, smoking is considered as a potent pro-inflammatory factor in cardiac pathology. The reciprocal stimulatory crosstalk between inflammation and oxidative stress has been widely discussed in the literature [60].
Fig. 1.
Pathophysiology of smoking-triggered coronary microvascular damage. Summary of the underlying mechanisms of coronary microvascular damage caused by smoking
Multiple studies have linked chronic inflammatory disorders such as lupus, rheumatoid arthritis, and inflammatory bowel disease with higher risk of developing CMD [61–63]. Others confirmed this result finding a significant correlation between systemic inflammatory markers (mostly plasma CRP) and risk of CMD [64–66]. Recently, Schroder et al. assessed the differential blood protein profile of CMD patients and healthy volunteers. Interestingly, the main differentially expressed protein biomarkers they found to be associated with CMD were several pro-inflammatory factors, related to the TNF-α-IL-6-CRP pathway [66]. Smoking is conventionally considered to induce inflammation based on studies who rely on self-reports on smoking status [67–70]. Recently, the National Health Survey in Korea conducted on 8655 men and 10,432 women reported a systemic pro-inflammatory effect of smoking. This study investigated the dose-dependent effect of cigarette smoking based on the cotinine concentration in the urine, on the systemic inflammation measured by leukocyte count [71]. In addition, they found higher plasma levels of inflammatory markers TNF-α, IL-6, IL-8 and IL-1β in current smokers, while the anti-inflammatory marker IL-10 showed reduction [58].
Rooks et al. measured CFR and different inflammatory markers (e.g., interleukin-6 and C-reactive protein), and oxidative status indicators (e.g., plasma hydroperoxides and the glutathione oxidation (GSSG/GSH ratio)) in healthy smoker and non-smoker twin couples. After adjusting the CFR levels to the inflammatory and oxidative stress indices, the declined level of CFR in smokers retained; which implicates the contribution of other underlying mechanisms to this difference, other than inflammation and ROS [30]. One such mechanism may be smoking-induced downregulation of ATPases [72]. Impaired microvascular reactivity and vasodilation in chronic smokers have been suggested to be caused at least partially because of the down-regulation of Na+/K+ ATPase in coronary microcirculatory endothelial cells in smokers. Since, Quabain as an inhibitor of Na+/K+ ATPase, that normally induces vasodilation in coronary microcirculation, failed to act on chronic smokers' microcirculation [72].
Mechanistic ex vivo and in vitro evidence on the effects of nicotine on coronary microvasculature
Nicotinic acetylcholine receptors (nAChRs) are shown to express in coronary endothelial cells, and induce several pro-survival pathways upon normal physiologic stimulation [73]. However, their overstimulation in cells by chronic nicotine exposure causes aberrant microvascular dilatory functions via oxidative stress induction [74]. Induction of oxidative stress by overstimulation of nicotinic AChRs is due to their intrinsic cationic channel function [75]. Chronic nicotine exposure induces opening of these channels and an excessive influx of Na+ ions in to the cells. The following electrolyte imbalance results in overproduction of ROS and consequently cellular damage [76]. The effect of nicotine tested in vivo (rat model) demonstrated an impaired endothelial-dependent vascular function, concurrent with plasma increased level of inflammatory parameter CRP, as well as higher CD36, TNFα and IL1β in macrophages [77]. The pro-inflammatory effect of smoking and nicotine are on the other hand contradicted by findings of other in vivo and in vitro studies reporting that nicotine affects vascular endothelial cells and macrophages by reducing their production of inflammatory cytokines (e.g. TNF- α), and consequently their capacity for leukocyte recruitment and adhesion [78, 79]. The reason for the contradictory results regarding the pro- or anti-inflammatory impact of nicotine is not understood yet, and warrants further assessment in the future.
Impact of smoking on post-ischemic and PCI-induced coronary microvascular injury
Clinical studies on the impact of smoking on coronary microvasculature injury by reperfused myocardial infarction
Microvascular dysfunction as either microvascular intraluminal obstruction (MVO) or extravascular compression is one of the main non-reversible consequences of coronary ischemic reperfusion injury (IRI) caused by cardiac therapeutic interventions [80, 81]. Ischemia causes mitochondrial damage, plasma membrane and cytoskeleton disintegrity, and impaired enzymatic activities in the cells by lowering the pH and ATP levels, inducing dysfunction of the ion exchange factors and electrolyte imbalance. Upon reperfusion and reversal of oxygen supply, the impaired ion exchangers, enzymes, cellular membrane and mitochondria result in reciprocal enhancement of oxidative stress and inflammation that may result in severe cell damage or cell death [82]. Therefore, knowing the risk factors that exacerbate this problem or on the contrary preconditioning or therapeutic mechanisms, which protect against the excessive oxidative damage, inflammation and cell death in post-MI conditions or by IRI are currently under special attention of researchers and clinicians. Although cigarette smoking is an independent risk factor for cardiovascular disease, studies have reported controversial results on the effects of smoking on the mortality rate and prognosis of patients who underwent reperfusion with a percutaneous coronary intervention (PCI) or the extent of post-MI injury (smoker’s paradox phenomenon) which are previously reviewed elsewhere [55, 83–85]. Here we review studies which assessed the status of coronary microvasculature in these group of patients. Based on magnetic resonance (CMR) analysis in reperfused ST-segment elevation myocardial infarction (STEMI) patients, smoking displayed no significant association with microvascular obstruction (MVO) [86, 87] and index of microvascular resistance (IMR) assessed by intra-coronary sensor angiography [88].
However, smoking was associated with intramyocardial haemorrhage (IMH) in post-PCI STEMI patients [86, 88] and in the presence of IMH, the protective effect of smoking on post-PCI cardiovascular health was abolished suggesting higher microvascular injury by post-ischemic reperfusion in smokers [86]. IMH is a marker of severe and irreversible injury to the coronary microvasculature by ischemia–reperfusion which results to extravasation of erythrocytes [89].
This result is challenged by another study using CMR is reperfused STEMI patients in another population, which did not find a significant association between smoking and the frequency of IMH [90]. The effect of smoking on endothelial progenitor cells (EPC) as necessary factors for repair and regeneration of microvasculature post-ischemia [91] was investigated by several studies. Smoking is shown to reduce the number of EPC, their adhesive capacity, and colony forming abilities [56, 91–93]. Therefore, if the damage to the coronary microcirculation is not irreversible (IMH), smoking may have beneficial impact on coronary microvascular regeneration post ischemia and reperfusion. Further research is warranted to assess this phenomenon in the future.
Ex vivo and in vitro effects of smoking on post ischemic and PCI-induced microvascular injury
Animal studies indicate that nicotine has a pathologic angiogenic effect on coronary arteries and microvessels, and intimal hyperplasia post ischemia and in PCI [94, 95]. Nicotine angiogenic effects are mediated by nicotinic acetylcholine receptors in endothelial cells [95–97]. Neointimal formation induced by nicotine effect on VSMC post injury has been linked to ERK –Egr-1 signaling cascade, and the blockade of this pathway can revert the adverse effect of nicotine in coronary vascular remodeling [98].
Impact of alcohol on coronary microvasculature
Impact of alcohol on coronary microvasculature and stable CMD
Clinical impact of alcohol on the coronary microvascular function
Detrimental health effects of alcohol include cardiometabolic complications which account for 33% of death caused by alcohol [99]. Multiple studies demonstrated regular and irregular heavy drinking to markedly increase the risk of ischemic heart disease and hypertension [100–102]. However, the general effect of alcohol consumption on cardiovascular disease is considered to be complicated due to other reports which support a protective role for the low and moderate alcohol drinking in regard to the ischemic heart disease and MI [103, 104]. In this section we will review the current understanding of the impact of alcohol on coronary micro-vessels. Similar acute effect was observed for two moderate doses of red wine (not vodka or white wine) to improve CFR in healthy young individuals, indicating a vasodilatory and cardioprotective function. These doses of red wine correspond the amount of 0.5 and 1.0 g/kg ethanol. The level of CFR increase was correlated with the level of antioxidant capacity of plasma induced by alcohol containing red wine. Improtantly, de-alcoholized red wine had no such effects. The CFR was measured by transthoracic Doppler echocardiography and right after bevarage drinking (acute effect of ethanol) [105]. This was contradicted by another study that measured CFR (by myocardial contrast echocardiography) in response to 1–2 weeks consumption of moderate dose of ethanol (red wine) and observed no change in CFR. The difference could be due to the fact that in the later study measurements were made at least 12 h after alcohol consumption; therefore, it cannot represent the acute effects of alcohol consumption, compared to the abovementioned studies which assessed CFR right after drinking [106].
Mechanistic studies on the effects of alcohol on coronary microvasculature in human subjects
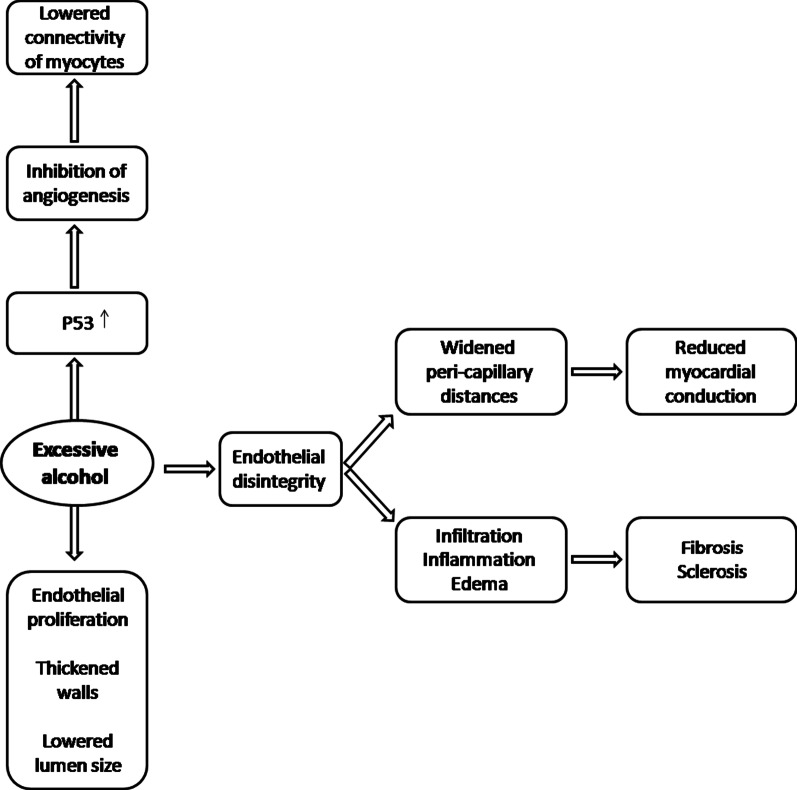
Heavy alcohol has been shown to result in deleterious remodeling and ultra-structural alterations of the cardiac microcirculation, depicted by case–control studies that used histochemical staining, and microscopy on cardiac biopsies obtained from alcoholic patients (angina patients with no CAD) after their death. Briefly in regard to the underlying pathophysiological mechanisms of alcohol-induced damage, the results showed disorganization of the layers of the micro-vessel walls, edema, perivascular fibrosis, sclerosis, interstitial inflammation, the degeneration of endothelial cells and higher density of capillary network [107, 108] (Fig. 2).
Fig. 2.
Pathophysiology of heavy alcohol-mediated coronary microvascular damage. Summary of the underlying mechanisms of coronary microvascular damage caused by heavy alcohol drinking
Mechanistic ex vivo and in vitro evidence on the effects of alcohol on coronary microvasculature
Animal studies indicate increased coronary microvascular wall thickness and an enrichment of the ATP-hydrolyzing small-caliber micorvessels [109]. Morphometric analysis by electron microscopy confirmed structural changes in the endothelial cells of the capillaries but not the muscle cells. Numerical density of endothelial cells was enhanced, whereas the volume density did not show a significant alteration, indicative of the proliferation of endothelial cells [110].
Others reported widened peri-capillary distances resulting in enforced remodeling changes in the size and connectivity of cardiomyocytes, and subsequently impaired myocardial conduction in animal models [111]. Evidence suggests that alcohol-induced hypoxia-mimetic and metabolically demanding condition [112] in the endothelial cells, lead to the endothelial remodeling and degeneration. Consequently, the damage of the micro-vessel endothelial cells precipitates in an increased infiltration of fluids and metabolites to the vessel walls and the perivascular space, which in turn results in edema and inflammation [111, 113]. This mechanism consequently induces the reported deposit of higher levels of collagen, perivascular fibrosis and sclerosis, and declined conductivity [111].
Impact of alcohol on post-ischemic and PCI-induced coronary microvascular injury
Clinical studies on the impact of alcohol on coronary microvasculature injury by reperfused myocardial infarction
Studies have shown better prognosis and lower mortality rate post MI [114–117] upon prior moderate but not heavy chronic alcohol consumption. In regard to PCI however, administration of a moderate dose of ethanol displayed an adverse effect on myocardial ischemic damage post PCI in STEMI patients [118]. Specific impact of moderate and heavy alcohol consumption on coronary microvascular function remains unfeatured as yet, warranting studies that assess the corresponding indices (e.g., CFR, MVO and IMR) in relation to beverage type, pattern and duration of alcohol consumption in MI patients.
Ex vivo and in vitro effects of alcohol on post ischemic and PCI-induced microvascular injury
The angiogenesis occurs after myocardial injuries such as ischemia to provide oxygen and supplies for the regeneration process of the myocardium. In vivo study in rat model preconditioned with either a high, or a modest dose of alcohol before the induction of MI, showed that the angiogenesis is significantly increased upon moderate alcohol preconditioning; whereas it was reduced in excessive dose of alcohol consumption prior to MI [119]. The level of VEGF expression did not change upon intake of high ethanol doses, while endostatin was upregulated. Conversely, upon moderate alcohol intake, VEGF level was shown to be up-regulated, whereas endostatin expression significantly declined [119].
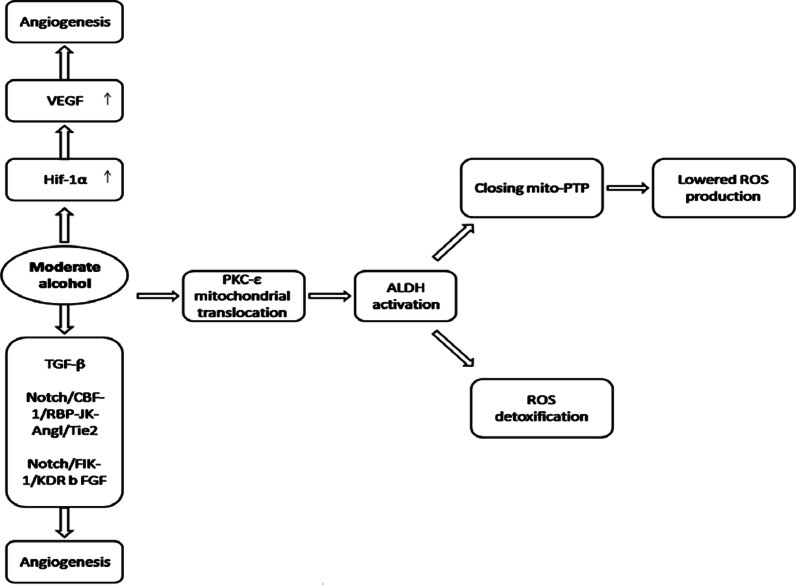
Angiogenic effects of moderate ethanol were concomitant with other beneficial cardio-protective effects including improved microvascular reactivity, endothelial function and myocardial perfusion in the ischemic regions of the myocardium [120, 121]. Interestingly, in the non-ischemic regions of myocardium distant from the ischemic territory, the blood flow and the endothelial microvascular reactivity showed no significant difference between the alcohol naïve and moderate EtOH group [120]. Moderate levels of ethanol is shown to positively regulate HIF-1α mRNA expression as the transcription factor upstream of VEGF [122]; and the HIF-1α protein is mainly stabilized upon hypoxic conditions while rapidly degraded at normal oxygen levels [123]. This could explain why the beneficial effects of alcohol only occur at ischemic regions, since HIF-1α is expressed, but degraded (Fig. 3). The other suggested mechanisms for the angiogenic effects of moderate ethanol are the induction of pro-angiogenic factors including basic fibroblast growth factor (bFGF), transforming growth factor-β1 (TGF-β1) [124], and Notch/CBF-1/RBP-JK -Ang1/Tie2 or Notch/ Flk-1/KDR pathways in endothelial cells as indicated by in vitro studies [125, 126]. On the other hand, inhibition of angiogenesis by high alcohol supplement may be attributed to the p53 up-regulation following excessive alcohol consumption [127]. P53 is known to have anti-angiogenic features [128]. The other potential mechanisms could be inhibited VEGF signaling as shown to occur in the endothelial cells upon intoxication by high doses of ethanol [129]. It was shown that ethanol inhibits the VEGF signaling in vitro regardless of the level of VEGF expression, via suppressing the phosphorylation and the expression level of VEGF receptors [129].
Fig. 3.
Beneficial effects of moderate alcohol consumption on coronary microcirculation. Positive impact of moderate alcohol consumption or ethanol preconditioning on coronary microvascular integrity and function, protecting against post-MI and PCI microvascular injuries
Moderate chronic ethanol preconditioning was indicated to protect against IRI [130–132], whereas consumption of acute alcohol before IRI does not confer cardioprotection [133, 134].
Proposed mechanisms of alcohol-mediated IRI protection include PKC-ε-ALDH activation, and mitoPTP (mitochondrial permeability transition pore) closing, which reduce the production and release of reactive oxygen species (ROS) [130, 131], as well as the VEGF-induced neovascularization which compensates for the IRI-induced cell death [132]. PKC‐ε activation and its cardiac mitochondrial translocation are triggered by moderate ethanol exposure. Inside mitochondria, it interacts with, and activates ALDH, which plays a critical role in reactive aldehydes detoxification and protection against mitochondrial-originated oxidative stress [130, 131, 135]. In addition, ALDH2 mitochondrial translocation inhibits opening of mitochondrial Permeability Transition Pore (mitoPTP), and thus leads to cardioprotective outcomes [136–138] MPTP is a mitochondrial membrane complex, which opens at highly stressed conditions of the cell (e.g. IRI, endotoxin, and anticancer agents), permitting the flow of the mitochondrial metabolites and ions, which leads to the induction of cell death [138]. Li et al., 2010 found that low concentration ethanol post-conditioning confers cardioprotection against IRI via inhibition of mitoPTP opening, associated with improved hemodynamics and smaller infarct size [136].
Further studies are essential to demonstrate the beneficial or harmful effects of alcohol-induced microvascular alterations, specifically in the setting of different cardiac diseases. Based on the studies available to this date, alcohol-induced microvascular remodeling can be directed toward a beneficial or disadvantageous path depending on the dose and the pattern of alcohol drinking.
Opioids and coronary microvascular function
Clinical effect of opioids on coronary microvasculature and CMD
Impact of opioids on coronary microvasculature and stable CMD
Many studies have associated opioid abuse with higher risk of CAD [139–152]. Nonetheless, the effect of opium on the coronary microvascular dysfunction (CMD) is under-studied. A cross-sectional study undertaken in a city of Iran with almost 30% rate of opioid addiction in the rural areas [153–155], analyzed stable angina patients, with normal angiography, diagnosed with CMD. The results implicated that opium addiction acts as an independent risk factor in CMD development [155]. In addition to opioid addiction, the effect of opioid-based anaesthetic substances on coronary microcirculation integrity showed microvascular perfusion impairment (long flow recovery times, and slower rate of oxygen re-saturation) [156].
Mechanistic ex vivo and in vitro evidence on the effects of opioid on coronary microvasculature
Moreover, experiments in animal models indicated that morphine aggravates the destructive effect of hypertension on coronary microvessels, via inhibition of angiogenesis and lowering the capillary density, as well as by deteriorating endothelial cell function in NO (nitrite oxide) production [157].
Impact of opioid on post-ischemic and PCI-induced coronary microvascular injury
Clinical studies on the impact of opioid on coronary microvasculature injury by reperfused myocardial infarction
Controversial reports on the impact of morphine and opioid agonists on mortality rate and myocardial damage post MI and PCI have been published suggesting a cardioprotective [158–160], adverse effect [161–163] or no significant change [164, 165]. In reperfused STEMI patients, CMR analysis suggested contradictory results by different studies as no impact of morphine on microvascular obstruction (MVO) [165] versus an adverse effect exacerbating the myocardial and microvascular damage (MVO) post PCI [162]. Future clinical trials are warranted to assess the effect of opioids on coronary microvascular function in post MI and Post-PCI patients to determine the safety of using opioid analgesics for pain treatment of ischemic cardiovascular patients.
Ex vivo and in vitro effects of opioid on post ischemic and PCI-induced microvascular injury
In vitro treatment of cultured endothelial cells and cardiac myocytes with morphine results in a marked reduction of VEGF expression. Subsequently the reduction of VEGF can lead to the inhibition of the neovascularization and the suppressed re-growth of the capillary network to restore the myocardial perfusion necessary to recover from ischemic injuries [166]. On the other hand, multiple studies suggest a cardioprotective role for opioids, indicating that the administration of opioid agonists such as morphine [167–171], fentanyl [172, 173] and methadone [174] attenuate the ischemic-triggered apoptosis and inflammation following myocardial ischemia, when used as a pre- or post-conditioning agent, or as pain treatment [170, 175–179]. Correspondingly, in vitro and ex vivo studies suggested that the enhanced opioid signaling diminished the cell death induced by ischemia-associated hypoxic injury [175, 180–183]. Summary of the human, animal and in vitro studies on the clinicopathology effects of substance use on the coronary microcirculation are presented in Table 1.
Table 1.
Summary of the human, animal and in vitro studies on the clinicopathology effects of substance use on the coronary microcirculation
| Patient or animal model | Study population | Substance | Administration duration/dose | Clinical test/experiment | Observed effect on coronary microcirculation | References |
|---|---|---|---|---|---|---|
| Young healthy smokers | 30 | Cigarette Smoking | Short-term chronic | Positron emission tomography measuring myocardial perfusion during rest, cold stress and dipyridamole-induced hyperemia | Impaired myocardial microcirculation function and regulation at cold stress (endothelial-dependent) | [184] |
| Male healthy twins | 360 | Cigarette Smoking | Chronic smokers | Positron emission tomography measuring myocardial perfusion at rest and adenosine vasodilation | Lowered CFR in smokers, even after adjusting for oxidative stress and inflammatory markers | [185] |
| Inflammation markers: IL-6, CRP | ||||||
| Oxidative stress markers: hydroperoxides, GSH,/GSSG ratio | ||||||
| Healthy young male smokers | 30 | Cigarette Smoking | Short-term chronic | Positron emission tomography measuring Myocardial blood flow (MBF) at rest, adenosine and cold stress | Reduced ratio of cold MBF to rest MBF (endothelium-dependent) | [186] |
| Healthy smokers | 19 | Cigarette Smoking | Chronic smokers | Positron emission tomography measuring coronary flow reserve (CFR), before and after vitamin C administration | Vitamin C restored CFR and the responsiveness of coronary microvessels | [187] |
| Angina patients, female, no CAD | 3568 | Cigarette Smoking | Chronic smokers | Doppler echocardiography measuring coronary flow velocity reserve (CFVR) at rest and high dose dipyridamole | Current smoking was identified as a predictor of impaired CFVR | [188] |
| Vasospastic angina pectoris (VSA) patients | 22 | Cigarette Smoking | Chronic smokers | Doppler echocardiography measuring coronary flow reserve (CFR), at rest and adenosine administration | Lowers CFR in smokers | [189] |
| CAD patients | 97 | Cigarette Smoking | Chronic smokers | Coronary angiography measuring CFR, index of microcirculatory resistance (IMR), and fractional flow reserve (FFR); at rest and adenosine-induced hyperemia | Higher IMR in current smokers, no difference in CFR or FFR | [190] |
| Healthy young volunteers | 20 | Cigarette smoking | Chronic and acute effect (chronic smokers with 4 h abstinence, smoking 2 cigarettes) | Doppler echocardiography measuring coronary flow velocity (CFV), and coronary vascular resistance index (CVRI) | No difference in CFR and CVRI at baseline, lower CFR and higher CVRI after smoking 2 cigarettes | [191] |
| Healthy young volunteers | 20 | Cigarette smoking | Acute (2 cigarette) | Doppler echocardiography measuring coronary flow reserve (CFR) | Similar reduction in CFR after light and regular cigarette smoking | [192] |
| Healthy young smokers | 62 | Cigarette smoking | Chronic and acute effect of light cigarette smoking vs. regular cigarette smoking | Doppler echocardiography measuring coronary flow velocity reserve (CFVR) | Both chronic and acute effects of regular and light cigarettes were similar, reducing the CFVR | [193] |
| Healthy smokers | 21 | Cigarette smoking | Acute, cigarettes with either > 1 mg, or < 1 mg nicotine content | Doppler echocardiography measuring coronary flow reserve (CFR) | Reduced CFR only in group smoking > 1 mg content cigarettes | [194] |
| Healthy smokers | 51 | Cigarette smoking | Chronic | Measuring plasma and urine biomarkers of inflammation ( IL-6, IL-8, ILβ1 and TNFα), endothelial injury (Intracellular adhesion molecule 1, metalloproteinase-9) and oxidative stress (myeloperoxidase, 8-isoprostane) | Biomarkers of inflammation, oxidative stress, immunity and tissue injury were increased in smokers | [195] |
| Human coronary arterioles (HCAs) | - | Cigarette Smoking | Chronic smokers | Dissected human coronary arterioles obtained from cardiac surgery; reactivity and responsiveness of microcirculation was tested by video microscopy | Smoking impaired Na+/K+ ATPase mediated vasodilation | [196] |
| PCI patients | 2765 | Cigarette Smoking | Chronic current or past smokers | Health related quality of life(HRQOL) and disease specific health status analyzed by questionnaires | Better cardiac health related outcomes in non-smokers and past smokers, compared to current smokers | [119] |
| Alcoholic patients expired for advanced liver disease, with no CAD symptoms | 18 | EtOH | Chronic (alcoholic) | Histology of endomyocardial biopsies | Endothelial cell degeneration, small lumen size, thickened micro-vessel walls with edema, perivascular fibrosis, vascular, subendothelial humps, and vascular wall inflammation | [129] |
| Rat model | 21 animals in each test group | EtOH | Chronic, 36% ethanol containing diet (4 weeks) | Histology | Thickened walls of micro-vessels and smaller lumen size, increased endothelial proliferation | [121] |
| Rabbit model | 10 animals per group | EtOH | Chronic ( diet containing 20% ethanol) 3 weeks | Histology and ultra-structural analysis of the myocardium and cardiac capillaries | Increased numerical density of the micro-vessels | [120] |
| Alcoholic patients | 40 | EtOH | Chronic (alcoholic patients) | Histopathology analysis on cardiac biopsies obtained | Increased capillary density with enhanced endothelial proliferation | [122] |
| C57BL/6 J mice | 7 animals per group | EtOH | Chronic (diet containing 36% ethanol) 12 weeks | Histology | Remodeling of the microcirculation, capillaries with widened peri-capillary distances | [125] |
| Rats preconditioned before myocardial infarction induction | 8 animals per group | EtOH | Chronic (preconditioned with low-dose ethanol (0.5 g/kg/day), high-dose ethanol (5 g/kg/day) of alcohol 4 weeks before MI induction | Immunohistochemistry | High dose: endostatin increased, no change in VEGF | [126] |
| Low dose: increased VEGF, lowered endostatin | ||||||
| Cultured small-vessel murine endothelial cells 4–10 (SVEC4-10) | - | EtOH | Acute, 100 mg/dl | In vitro angiogenesis assay, Endothelial cell tube formation assay | Impaired angiogenesis and reduction in VEGF receptors | [127] |
| Yorkshire swine | 14 | EtOH | Chronic 7 weeks after MI induction, 90 ml ethanol daily, | Dissected micro-vessels /vasodilator response and histopathology analysis | Increased angiogenesis, improved microvascular reactivity, endothelial function and myocardial perfusion in the ischemic regions of the myocardium | [155] |
| Yorkshire swine | 16 | EtOH | Chronic, 90 ml ethanol daily, 7 weeks | Dissected micro-vessels /vasodilator response and histopathology analysis | Increased capillary density, increased VEGF, no change in microvessel reactivity and myocardial perfusion | [156] |
| Coronary artery vascular smooth muscle cells | EtOH | Acute, 10–20 mM | protein and mRNA analysis | Increased VEGF | [166] | |
| Human umbilical vein endothelial cells (HUVECs) | EtOH | Acute, 24 h, 1–100 mM | Matrigel network formation assay, proliferation and migration assay, protein and mRNA analysis | Activation of CBF-1/RBP-Jk mediated angiogenesis | [23] | |
| Human coronary artery endothelial cells (HCAECs) | - | EtOH | Acute, 24 h, 1–50 mM | Matrigel network formation assay, protein and mRNA analysis | Activation of Flk-1/Notch mediated angiogenesis | [24] |
| Rat model | 26 | EtOH | Chronic, 3–24 month age, 12% in drinking water | mRNA extraction from left ventricles of the heart, qRT-PCR | Higher expression of p53 | [23] |
| Patients with chest pain, positive ETT, normal angiography | 250 | Opium | Chronic (addicted patients) | Coronary angiography | Opium addicted patients are more likely to develop CMD | [24] |
| Patients with CAD, scheduled to undergo coronary artery bypass grafting surgery | 35 | Opioid-based anesthetic (fentanyl) | Acute anesthetic dose of fentanyl up to 5 mg kg-1, 30 min) | Vascular occlusion testing (VOT) and near-infrared spectroscopy | Impaired coronary microvascular reactivity | [156] |
| Cultured cells: mouse endothelial cells and cardiac myocytes | - | Morphine | 1–100 ng/ml | Biomolecular tests for analysis of VEGF expression (qPCR, ELISA) | Reduced VEGF expression by cardiomyocytes and endothelial cells | [166] |
Conclusion
Overall, the presented evidence points to the importance of considering smoking, excessive alcohol use, and opioid addiction as independent risk factors in the development of the coronary microvascular disease. Moreover, evidence supports a cardioprotective role for moderate ethanol and opioids particularly against post-ischemic and intervention-mediated injuries to the coronary microcirculation. Almost 80% of the coronary vascular resistance is due to the microcirculation [23, 24]. Therefore the dysfunction of coronary microcirculatory network (CMD disease) can mainly impair the coronary blood perfusion, which results in cardiac damage [23, 24]. CMD has become a subject of attention among cardiologists and researchers over the last thirty years. Thus far, the studies have led to the identification of some of the mechanisms underlying the structural and functional impairment in the coronary microvasculature. Nevertheless, this is still a growing field and requires much further investigations. As of today, the diagnosis of CMD is still facing complications and misdiagnosis due to the technical limitations in imaging the coronary microcirculation, and the high cost and the invasiveness of the available clinical technologies. Therefore, more attention needs to be directed toward discovery of peripheral diagnostic markers for CMD, which are lacking.
Overall, more ultra-structural, molecular and histopathology assessments of the differential effects of various risk factors of CMD including smoking, alcohol, and drug addiction are essential. Combined with application of the most advanced imaging techniques for myocardial capillary network, future studies could lead to the development of novel specific diagnostic markers and therapeutic strategies for CMD, as well as preparation of accurate administration protocols for substances such as ethanol and opioid in preconditioning for ischemic cardiac disease in clinic.
Acknowledgements
Not Applicable
Abbreviations
- CVD
Cardiovascular diseases
- AMI
Acute myocardial infarction
- CAD
Coronary artery disease
- CMD
Coronary microvascular dysfunction
- CFR
Coronary flow reserve
- CVRI
Coronary vascular resistance index
- PCI
Percutaneous coronary intervention
- VEGF
Vascular endothelial growth factor
- IRI
Ischemic reperfusion injury
- MVO
Microvascular intraluminal obstruction
- mitoPTP
Mitochondrial permeability transition pore
Authors' contributions
EA carried out the design and conception of the article. The Execution and feasibility analysis of research was performed by EA; JZ, NS and KM collected and sorted out the data. JZ and EA analyzed and described the results. JZ wrote the thesis; EA and NS reviewed and amended the draft of the article. EA and JZ are supervisor, manager and responsible for the whole article. All authors read and approved of the final manuscript.
Funding
This project was supported by Rafsanjan University of Medical Sciences.
Availability of data and materials
All data used for this review study are available within the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
This contribution received no funding and the authors declare no conflict of interest with respect to the present manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shaw LJ, Shaw RE, Merz CNB, Brindis RG, Klein LW, Nallamothu B, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117(14):1787. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 2.Pacheco Claudio C, Quesada O, Pepine CJ, Noel Bairey Merz C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease) Clin Cardiol. 2018;41(2):185–93. doi: 10.1002/clc.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66(17):1918–33. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries: the ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59(7):655–62. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. J Am Coll Cardiol. 2010;362(10):886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiha J, Mitchell P, Gopinath B, Plant AJ, Kovoor P, Thiagalingam AJIH, et al. Gender differences in the severity and extent of coronary artery disease. IJC Heart Vascul. 2015;8:161–6. doi: 10.1016/j.ijcha.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 8.Members TF, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 9.Zimarino M, Cappelletti L, Venarucci V, Gallina S, Scarpignato M, Acciai N, et al. Age-dependence of risk factors for carotid stenosis: an observational study among candidates for coronary arteriography. Atherosclerosis. 2001;159(1):165–73. doi: 10.1016/S0021-9150(01)00477-4. [DOI] [PubMed] [Google Scholar]
- 10.Kutty S, Bisselou Moukagna KS, Craft M, Shostrom V, Xie F, Porter TR. Clinical outcome of patients with inducible capillary blood flow abnormalities during demand stress in the presence or absence of angiographic coronary disease. Circ Cardiovasc Imaging. 2018;11(10):e007483. doi: 10.1161/CIRCIMAGING.117.007483. [DOI] [PubMed] [Google Scholar]
- 11.Camici PG, Crea F. Microvascular angina: a women’s affair? Am Heart Assoc. [DOI] [PubMed]
- 12.Crea F, Lanza GA, Camici PG. Mechanisms of coronary microvascular dysfunction. Coronary microvascular dysfunction: Springer; 2014. p. 31-47.
- 13.Pries AR, Reglin BJ. Coronary microcirculatory pathophysiology: Can we afford it to remain a black box? Eur Heart J. 2017;38(7):478–88. doi: 10.1093/eurheartj/ehv760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12(1):48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 15.Sechtem U, Brown D, Godo S, Lanza GA, Shimokawa H, Sidik N. Coronary microvascular dysfunction in stable ischaemic heart disease (non-obstructive coronary artery disease and obstructive coronary artery disease) Cardiovasc Res. 2020;116(4):771–86. doi: 10.1093/cvr/cvaa005. [DOI] [PubMed] [Google Scholar]
- 16.Konijnenberg LS, Damman P, Duncker DJ, Kloner RA, Nijveldt R, van Geuns R-JM, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. CardiovascRes. 2020;116(4):787–805. doi: 10.1093/cvr/cvz301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101–11. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Wei J, AlBadri A, Zarrini P, Merz CNB. Coronary microvascular dysfunction-epidemiology, pathogenesis, prognosis, diagnosis, risk factors and therapy. Circ J. 2016;16:1002. doi: 10.1253/circj.CJ-16-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britten MB, Zeiher AM, Schächinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coronary Artery Dis. 2004;15(5):259–64. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 20.Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN. Ischemia and no obstructive coronary artery disease (INOCA): What is the risk? J Am Heart Assoc. 2018;7(17):e008868. doi: 10.1161/JAHA.118.008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM, et al. Ischemia and no obstructive coronary artery disease (INOCA) developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–92. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC. 2015;8(11):1445–53. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Libby P. Braunwald’s heart disease: a textbook of cardiovascular medicine. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 24.De Lemos J, Omland T. Chronic coronary artery disease: a companion to Braunwald's heart disease E-book: Elsevier Health Sciences; 2017.
- 25.Feher A, Sinusas AJ. Quantitative assessment of coronary microvascular function: dynamic single-photon emission computed tomography, positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circulation. 2017;10(8):e006427. doi: 10.1161/CIRCIMAGING.117.006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew RC, Bourque JM, Salerno M, Kramer CM. Cardiovascular imaging techniques to assess microvascular dysfunction. Cardiovasc Imaging. 2020;13(7):1577–90. doi: 10.1016/j.jcmg.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha A, Rahman H, Perera D. Ischaemia without obstructive coronary artery disease: the pathophysiology of microvascular dysfunction. Curr Opin Cardiol. 2020;35(6):720–5. doi: 10.1097/HCO.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 28.Camici PG, Crea F. Coronary microvascular dysfunction. New Eng J Med. 2007;356(8):830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 29.Madsen MM, Bøttcher M, Nielsen TT, Czernin J. Altered regulation of the myocardial microcirculation in young smokers. Cardiology. 2000;94(2):91–8. doi: 10.1159/000047298. [DOI] [PubMed] [Google Scholar]
- 30.Rooks C, Faber T, Votaw J, Veledar E, Goldberg J, Raggi P, et al. Effects of smoking on coronary microcirculatory function: a twin study. Atherosclerosis. 2011;215(2):500–6. doi: 10.1016/j.atherosclerosis.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwado Y, Yoshinaga K, Furuyama H, Ito Y, Noriyasu K, Katoh C, et al. Decreased endothelium-dependent coronary vasomotion in healthy young smokers. Eur J Nucl Med Mol Imaging. 2002;29(8):984–90. doi: 10.1007/s00259-002-0818-1. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann PA, Gnecchi-Ruscone T, Di Terlizzi M, Schäfers KP, Lüscher TF, Camici PG. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102(11):1233–8. doi: 10.1161/01.CIR.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 33.Akasaka T, Hokimoto S, Sueta D, Tabata N, Sakamoto K, Yamamoto E, et al. Sex differences in the impact of CYP2C19 polymorphisms and low-grade inflammation on coronary microvascular disorder. Am J Physiol Heart Circ Physiol. 2016;310(11):H1494–H500. doi: 10.1152/ajpheart.00911.2015. [DOI] [PubMed] [Google Scholar]
- 34.Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Am Heart Assoc. 2016;5(3):e003064. doi: 10.1161/JAHA.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashikaga T, Nishizaki M, Fujii H, Niki S, Maeda S, Yamawake N, et al. Examination of the microcirculation damage in smokers versus nonsmokers with vasospastic angina pectoris. Am J Cardiol. 2007;100(6):962–4. doi: 10.1016/j.amjcard.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki T, Ashikaga T, Ohigashi H, Komura M, Kobayashi K, Isobe M. Impact of smoking on coronary microcirculatory resistance in patients with coronary artery disease. Int Heart J. 2015;56(1):29–36. doi: 10.1536/ihj.14-189. [DOI] [PubMed] [Google Scholar]
- 37.Park SM, Shim WJ, Song WH, Lim DS, Kim YH, Ro Y. Effects of smoking on coronary blood flow velocity and coronary flow reserve assessed by transthoracic Doppler echocardiography. Echocardiography. 2006;23(6):465–70. doi: 10.1111/j.1540-8175.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 38.Ciftci O, Caliskan M, Gullu H, Erdogan D, Topcu S, Guler O, et al. Acute effects of smoking light cigarettes on coronary microvascular functions. Clin Cardiol. 2009;32(4):210–4. doi: 10.1002/clc.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gullu H, Caliskan M, Ciftci O, Erdogan D, Topcu S, Yildirim E, et al. Light cigarette smoking impairs coronary microvascular functions as severely as smoking regular cigarettes. Heart. 2007;93(10):1274–7. doi: 10.1136/hrt.2006.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka T, Oka Y, Tawara I, Sada T, Kira Y. Acute effects of nicotine content in cigarettes on coronary flow velocity and coronary flow reserve in men. Am J Cardiol. 1998;82(10):1275–8. doi: 10.1016/S0002-9149(98)00614-6. [DOI] [PubMed] [Google Scholar]
- 41.Health UDo, Services H. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease; 2006. [PubMed]
- 42.Oberg M, Jaakkola M, Prüss-Üstün A, Peruga A, Woodward A, Organization WH. Global estimate of the burden of disease from second-hand smoke. 2010. [DOI] [PubMed]
- 43.He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease—a meta-analysis of epidemiologic studies. New Engl J Med. 1999;340(12):920–6. doi: 10.1056/NEJM199903253401204. [DOI] [PubMed] [Google Scholar]
- 44.Law MR, Morris J, Wald N. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ. 1997;315(7114):973–80. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111(20):2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 46.Steenland K. Risk assessment for heart disease and workplace ETS exposure among nonsmokers. Environ Health Perspect. 1999;107(suppl 6):859–63. doi: 10.1289/ehp.99107s6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law MR, Wald NJ. Environmental tobacco smoke and ischemic heart disease. Progress in cardiovascular diseases. Prog Cardiovasc Dis. 2003;46(1):31–8. doi: 10.1016/S0033-0620(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 48.Raupach T, Schäfer K, Konstantinides S, Andreas S. Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J. 2006;27(4):386–92. doi: 10.1093/eurheartj/ehi601. [DOI] [PubMed] [Google Scholar]
- 49.Aronow WS. Effect of passive smoking on angina pectoris. New Engl J Med. 1978;299(1):21–4. doi: 10.1056/NEJM197807062990105. [DOI] [PubMed] [Google Scholar]
- 50.Argacha J-F, Adamopoulos D, Gujic M, Fontaine D, Amyai N, Berkenboom G, et al. Acute effects of passive smoking on peripheral vascular function. Hypertension. 2008;51(6):1506–11. doi: 10.1161/HYPERTENSIONAHA.107.104059. [DOI] [PubMed] [Google Scholar]
- 51.Leone A, Giannini D, Bellotto C, Balbarini A. Passive smoking and coronary heart disease. Curr Vasc Pharmacol. 2004;2(2):175–82. doi: 10.2174/1570161043476366. [DOI] [PubMed] [Google Scholar]
- 52.Prugger C, Wellmann J, Heidrich J, De Bacquer D, Perier M-C, Empana J-P, et al. Passive smoking and smoking cessation among patients with coronary heart disease across Europe: results from the EUROASPIRE III survey. Eur Heart J. 2014;35(9):590–8. doi: 10.1093/eurheartj/eht538. [DOI] [PubMed] [Google Scholar]
- 53.Prugger C, Wellmann J, Heidrich J, De Bacquer D, De Backer G, Périer M-C, et al. Readiness for smoking cessation in coronary heart disease patients across Europe: results from the EUROASPIRE III survey. Eur J Prev Cardiol. 2015;22(9):1212–9. doi: 10.1177/2047487314564728. [DOI] [PubMed] [Google Scholar]
- 54.Critchley JA, Capewell SS. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane database of systematic reviews. 2000(2).
- 55.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Arch Intern Med. 2000;160(7):939–44. doi: 10.1001/archinte.160.7.939. [DOI] [PubMed] [Google Scholar]
- 56.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscleros Thromb Vas Biol. 2004;24(8):1442–7. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 57.Samim A, Nugent L, Mehta PK, Shufelt C, Merz CNB. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med. 2010;12(4):355–64. doi: 10.1007/s11936-010-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan NA, Lawyer G, McDonough S, Wang Q, Kassem NO, Kas-Petrus F, et al. Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers. Tobacco Control. 2020;29(Suppl 2):s102–s9. doi: 10.1136/tobaccocontrol-2019-054958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puri BK, Treasaden IH, Cocchi M, Tsaluchidu S, Tonello L, Ross BM. A comparison of oxidative stress in smokers and non-smokers: an in vivo human quantitative study of n-3 lipid peroxidation. BMC Psychiatry. 2008;8(S1):S4. doi: 10.1186/1471-244X-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dandekar A, Mendez R, Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Stress responses: Springer; 2015. p. 205–14 [DOI] [PubMed]
- 61.Tona F, Serra R, Di Ascenzo L, Osto E, Scarda A, Fabris R, et al. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis. 2014;24(4):447–53. doi: 10.1016/j.numecd.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Caliskan Z, Gokturk HS, Caliskan M, Gullu H, Ciftci O, Ozgur GT, et al. Impaired coronary microvascular and left ventricular diastolic function in patients with inflammatory bowel disease. Microvasc Res. 2015;97:25–30. doi: 10.1016/j.mvr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30(15):1837–43. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 64.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC. 2013;6(6):660–7. doi: 10.1016/j.jcmg.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki M, Shimizu H, Miyoshi A, Takagi Y, Sato S, Nakamura Y. Association of coronary inflammation and angiotensin II with impaired microvascular reperfusion in patients with ST-segment elevation myocardial infarction. Int J Cardiol. 2011;146(2):254–6. doi: 10.1016/j.ijcard.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 66.Schroder J, Mygind ND, Frestad D, Michelsen M, Suhrs HE, Bove KB, et al. Pro-inflammatory biomarkers in women with non-obstructive angina pectoris and coronary microvascular dysfunction. IJC Heart Vasc. 2019;24:100370. doi: 10.1016/j.ijcha.2019.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ugur MG, Kutlu R, Kilinc I. The effects of smoking on vascular endothelial growth factor and inflammation markers: a case–control study. Clin Respir J. 2018;12(5):1912–8. doi: 10.1111/crj.12755. [DOI] [PubMed] [Google Scholar]
- 68.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91(2):142–9. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–66. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 70.Luetragoon T, Rutqvist LE, Tangvarasittichai O, Andersson BÅ, Löfgren S, Usuwanthim K, et al. Interaction among smoking status, single nucleotide polymorphisms and markers of systemic inflammation in healthy individuals. Immunology. 2018;154(1):98–103. doi: 10.1111/imm.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi W-J, Lee J-W, Cho AR, Lee Y-J. Dose-dependent toxic effect of cotinine-verified tobacco smoking on systemic inflammation in apparently healthy men and women: a nationwide population-based study. Int J Environ Res Public Health. 2019;16(3):503. doi: 10.3390/ijerph16030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miura H, Toyama K, Pratt PF, Gutterman DD. Cigarette smoking impairs Na+-K+-ATPase activity in the human coronary microcirculation. Am J Physiol Heart Circ Physiol. 2011;300(1):H109–H17. doi: 10.1152/ajpheart.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smedlund K, Tano JY, Margiotta J, Vazquez G. Evidence for operation of nicotinic and muscarinic acetylcholine receptor-dependent survival pathways in human coronary artery endothelial cells. J Cell Biochem. 2011;112(8):1978–84. doi: 10.1002/jcb.23169. [DOI] [PubMed] [Google Scholar]
- 74.Mayhan WG, Sharpe GM. Chronic exposure to nicotine alters endothelium-dependent arteriolar dilatation: effect of superoxide dismutase. J Appl Physiol. 1999;86(4):1126–34. doi: 10.1152/jappl.1999.86.4.1126. [DOI] [PubMed] [Google Scholar]
- 75.Changeux J-P. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J Biol Chem. 2012;287(48):40207–15. doi: 10.1074/jbc.R112.407668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krautwurst D, Seifert R, Hescheler J, Schultz G. Formyl peptides and ATP stimulate Ca2+ and Na+ inward currents through non-selective cation channels via G-proteins in dibutyryl cyclic AMP-differentiated HL-60 cells. Involvement of Ca2+ and Na+ in the activation of β-glucuronidase release and superoxide production. Biochem J. 1992;288(3):1025–35. doi: 10.1042/bj2881025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu C, Zhou M-S, Li Y, Wang A, Chadipiralla K, Tian R, et al. Oral nicotine aggravates endothelial dysfunction and vascular inflammation in diet-induced obese rats: role of macrophage TNFα. PLoS ONE. 2017;12(12):e0188439. doi: 10.1371/journal.pone.0188439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201(7):1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Báez-Pagán CA, Delgado-Vélez M, Lasalde-Dominicci JA. Activation of the macrophage α7 nicotinic acetylcholine receptor and control of inflammation. J Neuroimmune Pharmacol. 2015;10(3):468–76. doi: 10.1007/s11481-015-9601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Investig. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sezer M, van Royen N, Umman B, Bugra Z, Bulluck H, Hausenloy DJ, et al. Coronary microvascular injury in reperfused acute myocardial infarction: a view from an integrative perspective. J Am Heart Assoc. 2018;7(21):e009949. doi: 10.1161/JAHA.118.009949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediat Inflamm. 2017;2017. [DOI] [PMC free article] [PubMed]
- 83.Ma W-Q, Wang Y, Sun X-J, Han X-Q, Zhu Y, Yang R, et al. Impact of smoking on all-cause mortality and cardiovascular events in patients after coronary revascularization with a percutaneous coronary intervention or coronary artery bypass graft: a systematic review and meta-analysis. Coronary Artery Dis. 2019;30(5):367–76. doi: 10.1097/MCA.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 84.Mulcahy R. Influence of cigarette smoking on morbidity and mortality after myocardial infarction. Brit Heart J. 1983;49(5):410. doi: 10.1136/hrt.49.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aune E, Røislien J, Mathisen M, Thelle DS, Otterstad JE. The "smoker's paradox" in patients with acute coronary syndrome: a systematic review. BMC Med. 2011;9(1):97. doi: 10.1186/1741-7015-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Symons R, Masci PG, Francone M, Claus P, Barison A, Carbone I, et al. Impact of active smoking on myocardial infarction severity in reperfused ST-segment elevation myocardial infarction patients: the smoker's paradox revisited. Eur Heart J. 2016;37(36):2756–64. doi: 10.1093/eurheartj/ehv738. [DOI] [PubMed] [Google Scholar]
- 87.Redfors B, Furer A, Selker HP, Thiele H, Patel MR, Chen S, et al. Effect of Smoking on Outcomes of Primary PCI in Patients With STEMI. J Am Coll Cardiol. 2020;75(15):1743–54. doi: 10.1016/j.jacc.2020.02.045. [DOI] [PubMed] [Google Scholar]
- 88.Haig C, Carrick D, Carberry J, Mangion K, Maznyczka A, Wetherall K, et al. Current smoking and prognosis after acute ST-segment elevation myocardial infarction: new pathophysiological insights. JACC. 2019;12(6):993–1003. doi: 10.1016/j.jcmg.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Basso C, Corbetti F, Silva C, Abudureheman A, Lacognata C, Cacciavillani L, et al. Morphologic validation of reperfused hemorrhagic myocardial infarction by cardiovascular magnetic resonance. Am J Cardiol. 2007;100(8):1322–7. doi: 10.1016/j.amjcard.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 90.Reinstadler SJ, Eitel C, Fuernau G, de Waha S, Desch S, Mende M, et al. Association of smoking with myocardial injury and clinical outcome in patients undergoing mechanical reperfusion for ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2017;18(1):39–45. doi: 10.1093/ehjci/jew030. [DOI] [PubMed] [Google Scholar]
- 91.Leal V, Ribeiro CF, Oliveiros B, António N, Silva S. Intrinsic vascular repair by endothelial progenitor cells in acute coronary syndromes: an update overview. Stem Cell Rev Rep. 2019;15(1):35–47. doi: 10.1007/s12015-018-9857-2. [DOI] [PubMed] [Google Scholar]
- 92.Kaur S, Jayakumar K, Kartha CC. The potential of circulating endothelial progenitor cells to form colonies is inversely proportional to total vascular risk score in patients with coronary artery disease. Indian Heart J. 2007;59(6):475–81. [PubMed] [Google Scholar]
- 93.Ivanova V, Kostin S, Popovich I, Chebanu N, Kobets V, Popovich M. Qualitative and quantitative changes of circulating in blood endotheliocyte precursor cells in patients with hypercholesterolemia. Kardiologiia. 2010;50(12):27–31. [PubMed] [Google Scholar]
- 94.Ruixing Y, Qi B, Tangwei L, Jiaquan L. Effects of nicotine on angiogenesis and restenosis in a rabbit model. Cardiology. 2007;107(2):122–31. doi: 10.1159/000094658. [DOI] [PubMed] [Google Scholar]
- 95.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7(7):833–9. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 96.Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol. 1998;84(6):2089–98. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- 97.Heeschen C, Weis M, Cooke JP. Nicotine promotes arteriogenesis. J Am Coll Cardiol. 2003;41(3):489–96. doi: 10.1016/S0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- 98.Vazquez-Padron RI, Mateu D, Rodriguez-Menocal L, Wei Y, Webster KA, Pham SM. Novel role of Egr-1 in nicotine-related neointimal formation. Cardiovasc Res. 2010;88(2):296–303. doi: 10.1093/cvr/cvq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Organization WH. Global status report on alcohol and health 2018: World Health Organization; 2019.
- 100.Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12(1):182. doi: 10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roerecke M, Rehm J. Chronic heavy drinking and ischaemic heart disease: a systematic review and meta-analysis. Open Heart. 2014;1(1):e000135. doi: 10.1136/openhrt-2014-000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roerecke M, Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am J Epidemiol. 2010;171(6):633–44. doi: 10.1093/aje/kwp451. [DOI] [PubMed] [Google Scholar]
- 103.Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta-analysis. Addiction. 2012;107(7):1246–60. doi: 10.1111/j.1360-0443.2012.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leong DP, Smyth A, Teo KK, McKee M, Rangarajan S, Pais P, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case–control study. Circulation. 2014;113:7627. doi: 10.1161/CIRCULATIONAHA.113.007627. [DOI] [PubMed] [Google Scholar]
- 105.Shimada K, Watanabe H, Hosoda K, Takeuchi K, Yoshikawa J. Effect of red wine on coronary flow-velocity reserve. The Lancet. 1999;354(9183):1002. doi: 10.1016/S0140-6736(99)03478-9. [DOI] [PubMed] [Google Scholar]
- 106.Kaul S, Belcik T, Kalvaitis S, Jayaweera AR, Choi S-W, Wei K. Effect of modest alcohol consumption over 1–2 weeks on the coronary microcirculation of normal subjects. Eur J Echocardiogr. 2010;11(8):683–9. doi: 10.1093/ejechocard/jeq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Factor SM. Intramyocardial small-vessel disease in chronic alcoholism. Am Heart J. 1976;92(5):561–75. doi: 10.1016/S0002-8703(76)80075-0. [DOI] [PubMed] [Google Scholar]
- 108.Vaideeswar P, Chaudhari C, Rane S, Gondhalekar J, Dandekar S. Cardiac pathology in chronic alcoholics: a preliminary study. J Postgrad Med. 2014;60(4):372. doi: 10.4103/0022-3859.143958. [DOI] [PubMed] [Google Scholar]
- 109.Herrmann H, Morvai V, Ungváry G, Norden C, Mühlig P. Long-term effects of ethanol on coronary microvessels of rats. MicrocircEndoth Lymph. 1984;1(5):589–610. [PubMed] [Google Scholar]
- 110.Mall G, Mattfeldt T, Rieger P, Volk B, Frolov V. Morphometric analysis of the rabbit myocardium after chronic ethanol feeding—early capillary changes. Basic Res Cardiol. 1982;77(1):57–67. doi: 10.1007/BF01908131. [DOI] [PubMed] [Google Scholar]
- 111.Lai Y-J, Hung C-L, Hong R-C, Tseng Y-M, Lin C-I, Ko Y-S, et al. Slow conduction and gap junction remodeling in murine ventricle after chronic alcohol ingestion. J Biomed Sci. 2011;18(1):72. doi: 10.1186/1423-0127-18-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steiner JL, Lang CH. Etiology of alcoholic cardiomyopathy: mitochondria, oxidative stress and apoptosis. Int J Biochem Cell Biol. 2017;89:125–35. doi: 10.1016/j.biocel.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y, Davis-Gorman G, Watson RR, McDonagh PF. Ethanol modulates coronary permeability to macromolecules in murine AIDS. Alcohol Alcohol. 2002;37(6):555–60. doi: 10.1093/alcalc/37.6.555. [DOI] [PubMed] [Google Scholar]
- 114.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285(15):1965–70. doi: 10.1001/jama.285.15.1965. [DOI] [PubMed] [Google Scholar]
- 115.Pai JK, Mukamal KJ, Rimm EB. Long-term alcohol consumption in relation to all-cause and cardiovascular mortality among survivors of myocardial infarction: the health professionals follow-up study. Eur Heart J. 2012;33(13):1598–605. doi: 10.1093/eurheartj/ehs047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosenbloom JI, Mukamal KJ, Frost LE, Mittleman MA. Alcohol consumption patterns, beverage type, and long-term mortality among women survivors of acute myocardial infarction. Am J Cardiol. 2012;109(2):147–52. doi: 10.1016/j.amjcard.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Churchill EN, Disatnik M-H, Budas GR, Mochly-Rosen D. Ethanol for cardiac ischemia: the role of protein kinase c. Therap Adv Cardiovasc Dis. 2008;2(6):469–83. doi: 10.1177/1753944708094735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niccoli G, Altamura L, Fabretti A, Lanza GA, Biasucci LM, Rebuzzi AG, et al. Ethanol abolishes ischemic preconditioning in humans. J Am Coll Cardiol. 2008;51(3):271–5. doi: 10.1016/j.jacc.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Yuan H, Sun Y, Wang Y, Wang A. The effects of ethanol on angiogenesis after myocardial infarction, and preservation of angiogenesis with rosuvastatin after heavy drinking. Alcohol. 2016;54:27–32. doi: 10.1016/j.alcohol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 120.Elmadhun NY, Sabe AA, Lassaletta AD, Sellke FW. Ethanol promotes new vessel growth in remote nonischemic myocardium. JSurgRes. 2015;193(2):536–42. doi: 10.1016/j.jss.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lassaletta AD, Elmadhun NY, Liu Y, Feng J, Burgess TA, Karlson NW, et al. Ethanol promotes arteriogenesis and restores perfusion to chronically ischemic myocardium. Circulation. 2013;128(11 suppl 1):S136–S43. doi: 10.1161/CIRCULATIONAHA.112.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu J-W, Elam J, Sartin A, Li W, Roach R, Adair TH. Moderate levels of ethanol induce expression of vascular endothelial growth factor and stimulate angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R365–R72. doi: 10.1152/ajpregu.2001.281.1.R365. [DOI] [PubMed] [Google Scholar]
- 123.Lee J-W, Bae S-H, Jeong J-W, Kim S-H, Kim K-W. Hypoxia-inducible factor (HIF-1) α: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 124.Gavin TP, Wagner PD. Acute ethanol increases angiogenic growth factor gene expression in rat skeletal muscle. J Appl Physiol. 2002;92(3):1176–82. doi: 10.1152/japplphysiol.00929.2001. [DOI] [PubMed] [Google Scholar]
- 125.Morrow D, Cullen JP, Cahill PA, Redmond EM. Ethanol stimulates endothelial cell angiogenic activity via a Notch-and angiopoietin-1-dependent pathway. Cardiovasc Res. 2008;79(2):313–21. doi: 10.1093/cvr/cvn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morrow D, Hatch E, Hamm K, Cahill PA, Redmond EM. Flk-1/KDR mediates ethanol-stimulated endothelial cell Notch signaling and angiogenic activity. J Vasc Res. 2014;51(4):315–24. doi: 10.1159/000367807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jänkälä H, Eriksson PC, Eklund K, Sarviharju M, Härkönen M, Mäki T. Effect of chronic ethanol ingestion and gender on heart left ventricular p53 gene expression. AlcoholClinExpRes. 2005;29(8):1368–73. doi: 10.1097/01.alc.0000175043.67463.e5. [DOI] [PubMed] [Google Scholar]
- 128.Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med. 2007;85(11):1175–86. doi: 10.1007/s00109-007-0221-2. [DOI] [PubMed] [Google Scholar]
- 129.Radek KA, Kovacs EJ, Gallo RL, DiPietro LA. Acute ethanol exposure disrupts VEGF receptor cell signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2008;295(1):H174. doi: 10.1152/ajpheart.00699.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Churchill EN, Disatnik M-H, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of ɛPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46(2):278–84. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Budas GR, Disatnik M-H, Chen C-H, Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCɛ) knockout mice. J Mol Cell Cardiol. 2010;48(4):757–64. doi: 10.1016/j.yjmcc.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Louboutin J-P, Marusich E, Gao E, Agrawal L, Koch WJ, Strayer DS. Ethanol protects from injury due to ischemia and reperfusion by increasing vascularity via vascular endothelial growth factor. Alcohol. 2012;46(5):441–54. doi: 10.1016/j.alcohol.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 133.Hale S, Kloner R. Ethanol does not exert myocardial preconditioning in an intact rabbit model of ischemia/reperfusion. Heart Dis. 2001;3(5):293–6. doi: 10.1097/00132580-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 134.Itoya M, Morrison JD, Downey HF. Effect of ethanol on myocardial infarct size in a canine model of coronary artery occlusion–reperfusion. Myocardial Ischemia and Reperfusion: Springer; 1998. p. 35–41. [PubMed]
- 135.Budas GR, Disatnik M-H, Mochly-Rosen D. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target? Trends Cardiovasc Med. 2009;19(5):158–64. doi: 10.1016/j.tcm.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Z, Jiang C, Xia M, Ye H, Guan S, Gao Q. Activation of mitochondrial aldehyde dehydrogenase 2 and inhibition of mitochondrial permeability transition pore involved in cardioprotection of ethanol postconditioning. Zhejiang da xue xue bao Yi xue ban JZhejiang UnivMedSci. 2010;39(6):566–71. doi: 10.3785/j.issn.1008-9292.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75(3):530–5. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morciano G, Bonora M, Campo G, Aquila G, Rizzo P, Giorgi C, et al. Mechanistic role of mPTP in ischemia-reperfusion injury. Mitochondrial Dynamics in Cardiovascular Medicine: Springer; 2017. p. 169-89. [DOI] [PubMed]
- 139.Masoudkabir F, Sarrafzadegan N, Eisenberg MJ. Effects of opium consumption on cardiometabolic diseases. Nat Rev Cardiol. 2013;10(12):733–40. doi: 10.1038/nrcardio.2013.159. [DOI] [PubMed] [Google Scholar]
- 140.Marmor M, Penn A, Widmer K, Levin RI, Maslansky R. Coronary artery disease and opioid use. Am J Cardiol. 2004;93(10):1295–7. doi: 10.1016/j.amjcard.2004.01.072. [DOI] [PubMed] [Google Scholar]
- 141.Sadeghian S, Darvish S, Davoodi G, Salarifar M, Mahmoodian M, Fallah N, et al. The association of opium with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2007;14(5):715–7. doi: 10.1097/HJR.0b013e328045c4e9. [DOI] [PubMed] [Google Scholar]
- 142.Sadeghian S, Graili P, Salarifar M, Karimi AA, Darvish S, Abbasi SH. Opium consumption in men and diabetes mellitus in women are the most important risk factors of premature coronary artery disease in Iran. Int J Cardiol. 2010;141(1):116–8. doi: 10.1016/j.ijcard.2008.11.063. [DOI] [PubMed] [Google Scholar]
- 143.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/S0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 144.Masoomi M, Ramezani MA, Karimzadeh H. The relationship of opium addiction with coronary artery disease. Int J Prev Med. 2010;1(3):182. [PMC free article] [PubMed] [Google Scholar]
- 145.Masoumi M, Shahesmaeili A, Mirzazadeh A, Tavakoli M, Ali AZ. Opium addiction and severity of coronary artery disease: a case–control study. J Res Med Sci. 2010;15(1):27. [PMC free article] [PubMed] [Google Scholar]
- 146.Hosseini SA, Salehi A. The relationship between coronary risk factors and coronary artery involvement based on angiogrpahy findings. Koomesh. 2012;14(1):7–12. [Google Scholar]
- 147.Rezvani MR, Ghandehari K. Is opium addiction a risk factor for ischemic heart disease and ischemic stroke? J Res Med Sci. 2012;17(10):958. [PMC free article] [PubMed] [Google Scholar]
- 148.AZIMZADE SB, Gholamreza Y, Narooey S. A case–control study of effect of opium addiction on myocardial infarction. 2005.
- 149.Niaki MRK, Hamid M, Farshidi F, Mohammadpour M, Omran MTS. Evaluation of the role of opium addiction in acute myocardial infarction as a risk factor. Caspian J Intern Med. 2013;4(1):585. [PMC free article] [PubMed] [Google Scholar]
- 150.Safaei N. Outcomes of coronary artery bypass grafting in patients with a history of opiate use. Pak J Biol Sci. 2008;11(22):2594. doi: 10.3923/pjbs.2008.2594.2598. [DOI] [PubMed] [Google Scholar]
- 151.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of myocardial infarction amongst adults. J Intern Med. 2013;273(5):511–26. doi: 10.1111/joim.12035. [DOI] [PubMed] [Google Scholar]
- 152.Khademi H, Malekzadeh R, Pourshams A, Jafari E, Salahi R, Semnani S, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50 000 adults in Iran. BMJ. 2012;344:e2502. doi: 10.1136/bmj.e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ahmad Jamalizadeh ZK, Nadimi AE, Nejadghaderi M, Saeidi A, Porkarami A. Prevalence of smoking and high blood pressure, two major risk factors for non-communicable diseases: the SuRF NCD (surveillance of risk factors of non-communicable disease) report 2012. J Cardiovasc Thorac Res. 2016;8(4):183. doi: 10.15171/jcvtr.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, et al. prospective epidemiological research studies in IrAN (The PERSIAN Cohort): Rationale, objectives and design. American journal of epidemiology. 2017. [DOI] [PMC free article] [PubMed]
- 155.Nadimi AE, Amiri FP, Fathollahi MS, Hassanshahi G, Ahmadi Z, Sayadi AR. Opium addiction as an independent risk factor for coronary microvascular dysfunction: a case–control study of 250 consecutive patients with slow–flow angina. Int J Cardiol. 2016;219:301–7. doi: 10.1016/j.ijcard.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 156.Vandenbulcke L, Lapage KG, Vanderstraeten KV, De Somer FM, De Hert SG, Moerman AT. Microvascular reactivity monitored with near-infrared spectroscopy is impaired after induction of anaesthesia in cardiac surgery patients: an observational study. Eur J Anaesthesiol. 2017;34(10):688–94. doi: 10.1097/EJA.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 157.Zeinivand M, Pourshanazari A, Hassanshahi G. Coronary angiogenesis during morphine and nicotine withdrawal in two-kidney one clip hypertensive (2K1C) rats. Bratislavske lekarske listy. 2015;116(9):554–9. [PubMed] [Google Scholar]
- 158.Murphy GS, Szokol JW, Marymont JH, Avram MJ, Vender JS. Opioids and cardioprotection: the impact of morphine and fentanyl on recovery of ventricular function after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2006;20(4):493–502. doi: 10.1053/j.jvca.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 159.Tanaka K, Kersten J, Riess ML. Opioid-induced cardioprotection. Current Pharm Des. 2014;20(36):5696–705. doi: 10.2174/1381612820666140204120311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC. 2010;3(1):49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 161.Meine T, Roe M, Chen A, Whitling D. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE quality improvement initiative. AmHeart J. 2006;30(1):123. doi: 10.1016/j.ahj.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 162.de Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Urban D, et al. Intravenous morphine administration and reperfusion success in ST-elevation myocardial infarction: insights from cardiac magnetic resonance imaging. Clin Res Cardiol. 2015;104(9):727–34. doi: 10.1007/s00392-015-0835-2. [DOI] [PubMed] [Google Scholar]
- 163.Bellandi B, Zocchi C, Xanthopoulou I, Scudiero F, Valenti R, Migliorini A, et al. Morphine use and myocardial reperfusion in patients with acute myocardial infarction treated with primary PCI. Int J Cardiol. 2020;221:567–71. doi: 10.1016/j.ijcard.2016.06.204. [DOI] [PubMed] [Google Scholar]
- 164.Bonin M, Mewton N, Roubille F, Morel O, Cayla G, Angoulvant D, et al. Effect and safety of morphine use in acute anterior ST-segment elevation myocardial infarction. J Am Heart Assoc. 2018;7(4):e006833. doi: 10.1161/JAHA.117.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eitel I, Wang J, Stiermaier T, Fuernau G, Feistritzer H-J, Joost A, et al. Impact of morphine treatment on infarct size and reperfusion injury in acute reperfused ST-elevation myocardial infarction. J Clin Med. 2020;9(3):735. doi: 10.3390/jcm9030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Balasubramanian S, Ramakrishnan S, Charboneau R, Wang J, Barke RA, Roy S. Morphine sulfate inhibits hypoxia-induced vascular endothelial growth factor expression in endothelial cells and cardiac myocytes. J Mol Cell Cardiol. 2001;33(12):2179–87. doi: 10.1006/jmcc.2001.1480. [DOI] [PubMed] [Google Scholar]
- 167.Schultz JEJ, Hsu AK, Gross GJ. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996;78(6):1100–4. doi: 10.1161/01.RES.78.6.1100. [DOI] [PubMed] [Google Scholar]
- 168.Wang T-L, Chang H, Hung C-R, Tseng Y-Z. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997;80(12):1532–5. doi: 10.1016/S0002-9149(97)00788-1. [DOI] [PubMed] [Google Scholar]
- 169.Wang T-L, Chang H, Hung C-R, Tseng Y-Z. Morphine preconditioning attenuates neutrophil activation in rat models of myocardial infarction. Cardiovasc Res. 1998;40(3):557–63. doi: 10.1016/S0008-6363(98)00192-8. [DOI] [PubMed] [Google Scholar]
- 170.Groban L, Vernon JC, Butterworth J. Intrathecal morphine reduces infarct size in a rat model of ischemia-reperfusion injury. Anesth Anal. 2004;98(4):903–9. doi: 10.1213/01.ANE.0000105878.96434.05. [DOI] [PubMed] [Google Scholar]
- 171.Lu Y, Dong C, Yu J, Li L. Role of central and peripheral opioid receptors in the cardioprotection of intravenous morphine preconditioning. Irish J Med Sci. 2011;180(4):881. doi: 10.1007/s11845-011-0734-0. [DOI] [PubMed] [Google Scholar]
- 172.Kato R, Ross S, Foex P. Fentanyl protects the heart against ischaemic injury via opioid receptors, adenosine A1 receptors and KATP channel linked mechanisms in rats. Brit J Anaesth. 2000;84(2):204–14. doi: 10.1093/oxfordjournals.bja.a013404. [DOI] [PubMed] [Google Scholar]
- 173.Kato R, Foex P. Fentanyl reduces infarction but not stunning via δ-opioid receptors and protein kinase C in rats. Brit J Anaesth. 2000;84(5):608–14. doi: 10.1093/bja/84.5.608. [DOI] [PubMed] [Google Scholar]
- 174.Gross ER, Hsu AK, Gross GJ. Acute methadone treatment reduces myocardial infarct size via the δ-opioid receptor in rats during reperfusion. Anesth Anal. 2009;109(5):1395. doi: 10.1213/ANE.0b013e3181b92201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Okubo S, Tanabe Y, Takeda K, Kitayama M, Kanemitsu S, Kukreja RC, et al. Ischemic preconditioning and morphine attenuate myocardial apoptosis and infarction after ischemia-reperfusion in rabbits: role of δ-opioid receptor. Am J PhysioL. 2004;287(4):H1786–H91. doi: 10.1152/ajpheart.01143.2003. [DOI] [PubMed] [Google Scholar]
- 176.Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Distinct components of morphine effects on cardiac myocytes are mediated by the κ and δ opioid receptors. J Mol Cell Cardiol. 1997;29(2):711–20. doi: 10.1006/jmcc.1996.0313. [DOI] [PubMed] [Google Scholar]
- 177.Ludwig LM, Patel HH, Gross GJ, Kersten JR, Pagel PS, Warltier DC. Morphine enhances pharmacological preconditioning by isofluranerole of mitochondrial KATPchannels and opioid receptors. Anesthesiol JAmSoc Anesthesiol. 2003;98(3):705–11. doi: 10.1097/00000542-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 178.Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth Anal. 2005;101(4):942–9. doi: 10.1213/01.ane.0000171931.08371.a2. [DOI] [PubMed] [Google Scholar]
- 179.Schultz JJ, Hsu AK, Gross GJ. Ischemic preconditioning and morphine-induced cardioprotection involve the delta (δ)-opioid receptor in the intact rat heart. J Mol Cell Cardiol. 1997;29(8):2187–95. doi: 10.1006/jmcc.1997.0454. [DOI] [PubMed] [Google Scholar]
- 180.Tanaka K. Kersten rJ, L Riess M 2014 Opioid-induced cardioprotection. Curr Pharma Des. 2014;20(36):5696–705. doi: 10.2174/1381612820666140204120311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Romano MA, Seymour EM, Berry JA, McNish RA, Bolling SF. Relative contribution of endogenous opioids to myocardial ischemic tolerance. J Surg Res. 2004;118(1):32–7. doi: 10.1016/j.jss.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 182.Takasaki Y, Wolff RA, Chien GL, van Winkle DM. Met5-enkephalin protects isolated adult rabbit cardiomyocytes via δ-opioid receptors. Am J Physiol Heart Circ Physiol. 1999;277(6):H2442–H50. doi: 10.1152/ajpheart.1999.277.6.H2442. [DOI] [PubMed] [Google Scholar]
- 183.Chien GL, Van Winkle DM. Naloxone blockade of myocardial ischemic preconditioning is stereoselective. J Mol Cell Cardiol. 1996;28(9):1895–900. doi: 10.1006/jmcc.1996.0182. [DOI] [PubMed] [Google Scholar]
- 184.Miyazaki T, Ashikaga T, Ohigashi H, Komura M, Kobayashi K, Isobe M. Impact of smoking on coronary microcirculatory resistance in patients with coronary artery disease. Int Heart J. 2015;56(1):29–36. doi: 10.1536/ihj.14-189. [DOI] [PubMed] [Google Scholar]
- 185.Park SM, Shim WJ, Song WH, Lim DS, Kim YH, Ro YM. Effects of smoking on coronary blood flow velocity and coronary flow reserve assessed by transthoracic Doppler echocardiography. Echocardiography. 2006;23(6):465–70. doi: 10.1111/j.1540-8175.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 186.Ciftci O, Caliskan M, Gullu H, Erdogan D, Topcu S, Guler O, et al. Acute effects of smoking light cigarettes on coronary microvascular functions. Clin Cardiol. 2009;32(4):210–4. doi: 10.1002/clc.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gullu H, Caliskan M, Ciftci O, Erdogan D, Topcu S, Yildirim E, et al. Light cigarette smoking impairs coronary microvascular functions as severely as smoking regular cigarettes. Heart. 2007;93(10):1274–7. doi: 10.1136/hrt.2006.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tanaka T, Oka Y, Tawara I, Sada T, Kira Y. Acute effects of nicotine content in cigarettes on coronary flow velocity and coronary flow reserve in men. Am J Cardiol. 1998;82(10):1275–8. doi: 10.1016/S0002-9149(98)00614-6. [DOI] [PubMed] [Google Scholar]
- 189.Chaumont M, De Becker B, Zaher W, Culié A, Deprez G, Mélot C, et al. Differential effects of e-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: a randomized crossover trial. Sci Rep. 2018;8(1):10378. doi: 10.1038/s41598-018-28723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Khan NA, Lawyer G, McDonough S, Wang Q, Kassem NO, Kas-Petrus F, et al. Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers. Tobacco Control. 2019:tobaccocontrol-2019-054958. [DOI] [PMC free article] [PubMed]
- 191.Miura H, Toyama K, Pratt PF, Gutterman DD. Cigarette smoking impairs Na+-K+-ATPase activity in the human coronary microcirculation. Am J Physiol Heart Circ Physiol. 2010;300(1):H109–H17. doi: 10.1152/ajpheart.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jang J-S, Buchanan DM, Gosch KL, Jones PG, Sharma PK, Shafiq A, et al. Association of smoking status with health-related outcomes after percutaneous coronary intervention. CircCardiovascInterven. 2015;8(5):e002226. doi: 10.1161/CIRCINTERVENTIONS.114.002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Factor SM. Intramyocardial small-vessel disease in chronic alcoholism. Am Heart J. 1976;92(5):561–75. doi: 10.1016/S0002-8703(76)80075-0. [DOI] [PubMed] [Google Scholar]
- 194.Herrmann H, Morvai V, Ungváry G, Norden C, Mühlig P. Long-term effects of ethanol on coronary microvessels of rats. Microcirc Endoth Lymph. 1984;1(5):589–610. [PubMed] [Google Scholar]
- 195.Vaideeswar P, Chaudhari C, Rane S, Gondhalekar J, Dandekar S. Cardiac pathology in chronic alcoholics: a preliminary study. J Postgrad Med. 2014;60(4):372. doi: 10.4103/0022-3859.143958. [DOI] [PubMed] [Google Scholar]
- 196.Lai Y-J, Hung C-L, Hong R-C, Tseng Y-M, Lin C-I, Ko Y-S, et al. Slow conduction and gap junction remodeling in murine ventricle after chronic alcohol ingestion. J Biomed Sci. 2011;18(1):72. doi: 10.1186/1423-0127-18-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for this review study are available within the manuscript.