Abstract
Background
The effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in preventing pneumococcal pneumonia has been controversial.
Methods
To evaluate the effectiveness of the PPSV23 in elderly outpatients with chronic respiratory diseases, we carried out a case–control study, including 4128 outpatients aged ≥ 65 years, in the respiratory department.
Results
There were 320 vaccinated patients, of which 164 were diagnosed with pneumococcal pneumonia. The adjusted odds ratio was 0.39 (95% confidence interval (CI), 0.17 to 0.89). In the subsets consisting of age groups ≥ 70 and ≥ 75 years, the adjusted odds ratio (95% CI) was respectively 0.16 (0.04 to 0.67) and 0.15 (0.02 to 1.12).
Conclusion
This real-world study suggests that PPSV23 can be useful in preventing pneumococcal pneumonia in the elderly with chronic respiratory diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-021-01491-w.
Keywords: Chronic respiratory disease, Elderly, Pneumococcal polysaccharide vaccine, Pneumococcus, Pneumonia, Vaccine
Background
Streptococcus pneumoniae can cause pneumonia and invasive pneumococcal diseases (IPDs), which result in considerable morbidity and mortality worldwide [1, 2]. The Advisory Committee on Immunization Practices recommends the use of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) or the 13-valent pneumococcal conjugate vaccine (PCV13) for all the elderly (age ≥ 65 years) and for immunocompromised adults [3]. In Japan, the PPSV23 coverage of people aged ≥ 65 years was 32.4% in 2019 [4], and that of the PCV13, which is not covered by Japanese universal health insurance, has not been reported because of the extremely small number of people vaccinated. The effectiveness of the PPSV23 in preventing IPD has been reported, its effectiveness in preventing pneumococcal pneumoniae has been inconsistent [5–7]. Some researchers have targeted both healthy individuals and patients with various diseases at nursing home residences [6, 8].
We hypothesized that the PPSV23 would be useful in preventing pneumococcal pneumonia in elderly outpatients with chronic respiratory diseases. This study aimed to assess the effectiveness of the PPSV23 among elderly outpatients in clinical practice.
Methods
Study design and population
This study was a retrospective case–control design. The target population was defined as outpatients aged ≥ 65 years, with chronic respiratory diseases, treated between 2015 and 2017 in the respiratory department of Shizuoka General Hospital. From this sample, the case and control groups consisted of patients with and without pneumococcal pneumonia, respectively. Patients who had been vaccinated with PCV13 were excluded.
Diagnosis of pneumococcal pneumonia
Respiratory physicians diagnosed pneumonia based on clinical findings such as fever, hypothermia, chills, cough, sputum production, pleuritic chest pain, fatigue, tachypnea, white blood cell count > 9300, or < 4000 cells/mm3, and new pulmonary infiltrates on chest radiography [2]. In this study, all patients with pneumonia met these criteria. Pneumococcal pneumonia was diagnosed based on the positive results of urine pneumococcal antigen and sputum culture, but a negative blood culture for pneumococcus.
Definitions
The chronic respiratory diseases in this study included lung cancer, asthma, chronic obstructive pulmonary disease (COPD), interstitial pneumonia, pulmonary non-tuberculous mycobacteriosis (NTM), pulmonary tuberculosis, and others. The history of PPSV23 vaccination was obtained from medical records and declarations by patients or their families. Patients were considered vaccinated when they had received the PPSV23 within five years prior to the diagnosis of pneumonia. Patients without medical records or whose families had no knowledge of their vaccination statuses were considered unvaccinated.
Statistical analysis
The chi-squared tests for categorical variables and t-tests for continuous variables were used in comparing both groups. To evaluate the effectiveness of the PPSV23, we performed a logistic regression analysis, and then the odds ratio (OR), 95% confidence interval (CI), and p value (based on Wald test) were calculated. The adjusted OR was estimated by the quantile stratification method of propensity scores. The propensity score was estimated using multivariate logistic regression models with potential confounders as covariates, which included all variables of Table 1. We also made two subsets: those ≥ 70 years, and ≥ 75 years, and compared their adjusted ORs with that in all patients. As a sensitivity analysis, we estimated double-robust adjusted OR in case–control studies under causal inference [9], and we confirmed whether the point estimation of OR, as mentioned above, was overestimating the effect.
Table 1.
Characteristics of patients with and without pneumococcal pneumonia
| Variable and category (reference) | Case group (n = 164) |
Control group (n = 4,054) |
p value |
|---|---|---|---|
| Age, yearsa | 76.2 ± 7.3 | 75.1 ± 6.7 | 0.127 |
| 65–69 | 32 (19.5) | 984 (24.3) | 0.153 |
| 70–74 | 44 (26.8) | 1,098 (27.1) | |
| 75–79 | 36 (22.0) | 950 (23.4) | |
| 80 + | 56 (34.1) | 1,063 (26.2) | |
| Male (vs. female) | 113 (68.9) | 2,525 (62.3) | 0.100 |
| Smoking | 0.001 | ||
| Non-smokers | 37 (22.6) | 1,198 (29.6) | |
| Current smokers | 108 (65.9) | 2,068 (51.0) | |
| Ex-smokers | 19 (11.6) | 788 (19.4) | |
| Chronic respiratory diseases (vs. absent) | |||
| Asthma | 30 (18.3) | 685 (16.9) | 0.671 |
| COPD | 42 (25.6) | 959 (23.6) | 0.574 |
| Lung cancer | 45 (27.4) | 1,594 (39.3) | 0.002 |
| Interstitial pneumonia | 29 (17.7) | 703 (17.3) | 0.916 |
| NTM | 10 (6.1) | 449 (11.1) | 0.054 |
| Othersb | 48 (29.3) | 746 (18.4) | 0.001 |
| Diabetes (vs. absent) | 65 (39.6) | 1,209 (29.8) | 0.023 |
| Chronic heart disease (vs. absent) | 95 (57.9) | 1,891 (46.6) | 0.005 |
| Chronic kidney disease (vs. absent) | 13 (7.9) | 299 (7.4) | 0.761 |
| Systemic corticosteroid user (vs. absent) | 65 (39.6) | 1,189 (29.3) | 0.007 |
Values are expressed as numbers and proportions in parentheses
aMean ± SD
bOther chronic respiratory diseases included chronic pulmonary aspergillosis, old pulmonary tuberculosis, sarcoidosis, and chronic cough
COPD, chronic obstructive pulmonary disease; NTM, non-tuberculous mycobacteriosis
To confirm the efficacy of the vaccine for each age group (65 to < 70 years, 70 to < 75 years, and ≥ 75 years), crude ORs, ORs adjusted for risk factors, and their 95% confidence intervals were calculated. Risk factors for pneumococcal pneumonia were identified as follows. Variables for which the p value of the comparison test between the case and control groups was less than 0.05 were considered as candidate risk factors, and these variables were entered into a multivariate logistic regression model. In this multivariate model, variables with p values less than 0.05 were identified as risk factors. Furthermore, to extract variables containing different categories of pneumococcal pneumonia proportions in different age groups, an interaction term test using a logistic regression model was performed.
A p value of < 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients background
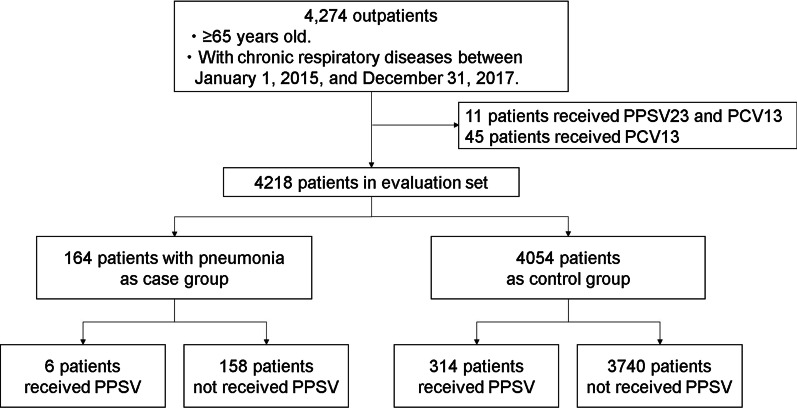
Between January 1, 2015, and December 31, 2017, 4274 outpatients aged ≥ 65 years with chronic respiratory diseases visited our department. Of 4274, 45 had been vaccinated with PCV13 and 11 with PPSV23 and PCV13. These were excluded from the study, which included 4218 patients. The patient flow is shown in Fig. 1. A total of 320 patients received the PPSV23, while 3898 did not. Of the 320 vaccinated patients, 6 developed pneumococcal pneumonia, compared to 158 of the 3 898 unvaccinated patients.
Fig. 1.
Patient flow. PPSV23: 23-valent pneumococcal polysaccharide vaccine, PCV13: 13-valent pneumococcal conjugate vaccine
The baseline characteristics of the case and control group on January 1, 2015, are shown in Table 1. Patients in the case group were more current-smokers, as well as had other chronic respiratory diseases, diabetes, chronic heart disease, and had higher use of systemic corticosteroids, than the control group. The number of patients in the case group with lung cancer was less than that of the control group.
Effectiveness of the vaccine against pneumococcal pneumonia
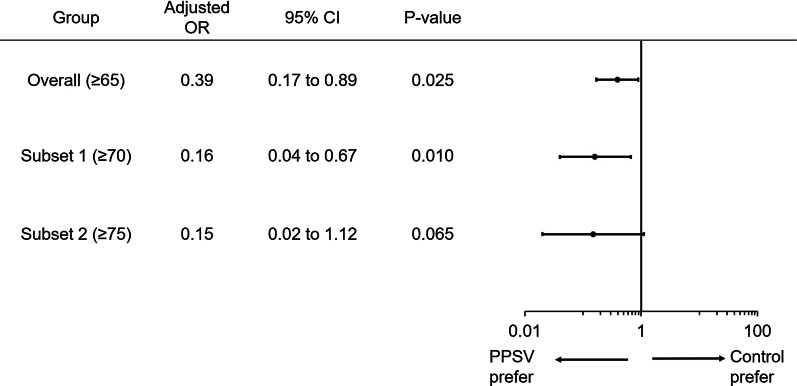
Pneumococcal pneumonia prevention crude OR (95% CI) was 0.45 (0.20–1.03, p = 0.059), and the adjusted OR was 0.39 (0.17–0.89) (Fig. 2). There was a trend toward lower adjusted ORs in the subset restricted to only the more elderly: OR (95% CI): 0.39 (0.17–0.89) for those aged ≥ 65 years, 0.16 (0.04–0.67) for subset 1 (≥ 70 years), and 0.15 (0.02–1.12) for subset 2 (≥ 75 years). In the sensitivity analysis, the double-robust adjusted ORs in patients of ≥ 65, ≥ 70, and ≥ 75 years old were respectively 0.35, 0.14 and 0.13, thus, the above adjusted ORs were conservative and not overestimating.
Fig. 2.
Effectiveness of vaccines in pneumococcal pneumonia in age-related subsets. OR, odds ratio; CI, confidence interval; PPSV23, 23-valent pneumococcal polysaccharide vaccine. The lower adjusted ORs in subsets of older patients were estimated, suggesting that the PPSV23 can be more effective for older patients. The adjusted OR was adjusted using the quantile category of propensity scores
Age-specific effectiveness and patients’ characteristics
Age-specific patients’ characteristics were presented in Table 2. The crude ORs (95% CI) by age for vaccine efficacy against pneumococcal pneumonia in those aged 65 to < 70 years, 70 to < 75 years, and ≥ 75 years were 1.44 (0.49–4.22), 0.24 (0.03–1.74), and 0.17 (0.02–1.24), respectively (Table 2). The results of feeding the variables that were significant in Table 1 into a multivariate logistic regression model are shown in Additional file 1: Table 1. In this multivariate model, smoking, lung cancer, other chronic respiratory diseases, and systemic corticosteroid user were considered as risk factors. The ORs (95% CI) adjusted for these risk factors for each age group were 1.34 (0.45–4.02), 0.22 (0.03–1.59), and 0.17 (0.02–1.20) (Table 2).
Table 2.
Age-specific effectiveness and patients’ characteristics
| Variable and category (reference) | 65 to < 70 years | 70 to < 75 years | ≥ 75 years | p value of interaction term with age category | |||
|---|---|---|---|---|---|---|---|
| Case (n = 30) |
Control (n = 974) |
Case (n = 44) |
Control (n = 1,085) |
Case (n = 90) |
Control (n = 1,995) |
||
| PPSV23 (vs. absent) | 4 (13.3) | 94 (9.7) | 1 (2.3) | 97 (8.9) | 1 (1.1) | 123 (6.2) | 0.090 |
| Crude odds ratio (95% CI) | 1.44 (0.49–4.22) | 0.24 (0.03–1.74) | 0.17 (0.02–1.24) | ||||
| Adjusted odds ratioa (95% CI) | 1.34 (0.45–4.02) | 0.22 (0.03–1.59) | 0.17 (0.02–1.20) | 0.102 | |||
| Male (vs. female) | 19 (63.3) | 585 (60.1) | 33 (75.0) | 696 (64.2) | 61 (67.8) | 1244 (62.4) | 0.736 |
| Smoking (vs. non-smokers) | 0.727 | ||||||
| Current smokers | 21 (70.0) | 522 (53.6) | 33 (75.0) | 578 (53.3) | 54 (60.0) | 968 (48.5) | |
| Ex-smokers | 5 (16.7) | 215 (22.1) | 4 (9.1) | 222 (20.5) | 10 (11.1) | 351 (17.6) | |
| Chronic respiratory diseases | |||||||
| Asthma (vs. absent) | 5 (16.7) | 180 (18.5) | 11 (25.0) | 189 (17.4) | 14 (15.6) | 316 (15.8) | 0.506 |
| COPD (vs. absent) | 6 (20.0) | 210 (21.6) | 16 (36.4) | 282 (26.0) | 20 (22.2) | 467 (23.4) | 0.360 |
| Lung cancer (vs. absent) | 9 (30.0) | 415 (42.6) | 19 (43.2) | 409 (37.7) | 17 (18.9) | 770 (38.6) | 0.013 |
| Interstitial pneumonia (vs. absent) | 7 (23.3) | 167 (17.2) | 8 (18.2) | 195 (18.0) | 14 (15.6) | 340 (17.0) | 0.646 |
| NTM (vs. absent) | 11 (36.7) | 167 (17.2) | 10 (22.7) | 191 (17.6) | 27 (30.0) | 388 (19.5) | 0.405 |
|
Others chronic respiratory diseasesb (vs. absent) |
5 (16.7) | 87 (8.9) | 4 (9.1) | 118 (10.9) | 28 (31.1) | 332 (16.6) | 0.218 |
| Diabetes (vs. absent) | 9 (30.0) | 253 (26.0) | 26 (59.1) | 350 (32.3) | 30 (33.3) | 606 (30.4) | 0.036 |
| Chronic heart disease (vs. absent) | 12 (40.0) | 350 (35.9) | 25 (56.8) | 504 (46.5) | 58 (64.4) | 1036 (51.9) | 0.736 |
| Chronic kidney disease (vs. absent) | 0 | 28 (2.9) | 3 (6.8) | 25 (2.3) | 0 | 64 (3.2) | 0.998 |
| Systemic corticosteroid user (vs. absent) | 15 (50.0) | 311 (31.9) | 26 (59.1) | 363 (33.5) | 24 (26.7) | 515 (25.8) | 0.029 |
Bold value indicates statistical significance
aThe odds ratios for PPSV23 were calculated for adjusting smoking, lung cancer, other chronic respiratory diseases, and systemic corticosteroid user
bOther chronic respiratory diseases included chronic pulmonary aspergillosis, old pulmonary tuberculosis, sarcoidosis, and chronic cough
CI, confidence interval; COPD, chronic obstructive pulmonary disease; NE, not evaluated; NTM, non-tuberculous mycobacteriosis; PPSV23, 23-valent pneumococcal polysaccharide vaccine
The variables containing categories with different rates of pneumococcal pneumonia according to age according to the interaction term test were lung cancer (p = 0.013), diabetes mellitus (p = 0.036), and presence of systemic corticosteroid use (p = 0.029) (Table 2).
Discussion
This study found that the PPSV23 prevented pneumococcal pneumonia in older patients (age ≥ 65 years) with chronic respiratory diseases, and could be more effective for the elderly (patients aged ≥ 70 years). To the best of our knowledge, this is the first real-world study that assesses the effectiveness of the PPSV23 in older patients with chronic respiratory disease in a single center.
In previous studies, the effectiveness of the vaccine against pneumococcal pneumonia was controversial. In the results of a meta-analysis of 18 randomized trials [5], the PPSV23 reduced the risk of IPDs such as bacteremia, meningitis, and bacteremic pneumonia (OR [95% CI]: 0.26 [0.15–0.46]) and pneumococcal pneumonia (0.46 [0.25–0.84]). However, some studies reported that the PPSV23 did not reduce pneumococcal pneumonia [6, 7, 10]. The EVAN-65 study in community-dwelling patients [11] showed that the hazard ratios (HR) for the risk of pneumococcal pneumonia in vaccinated patients compared with non-vaccinated patients were not different (HR [95% CI]: 0.61 [0.35–1.06]). Similarly, another study in elderly patients with chronic respiratory diseases showed no difference (0.76 [0.30–1.90]) [12]. These conflicting results can be due to the difficulties of accurately diagnosing pneumococcal pneumonia and the use of non-validated diagnostic tests [13]. In this study, we included non-invasive cases according to specific diagnostic criteria of pneumonia, urine pneumococcal antigen, and sputum culture, and we are convinced that these results are close to correct.
One previous case–control study suggested that 85–90% of adults aged 55 and younger achieved the prevention of invasive pneumococcal infections, but this effect was reduced with increasing age, and no protection was shown in the population aged ≥ 80 years [14]. Another study suggested that the prevention of community-onset pneumococcal pneumonia was effective in people aged 65-75 years but not effective in those aged ≥ 75 years in Japan [15]. The population-based retrospective cohort study in Germany reported that the prevention of pneumococcal pneumonia was not effective at all in people aged ≥ 80 years [16]. These studies suggest that poorer effectiveness might be due to immunosenescence, which refers to the decline of the immune system associated with aging [17]. However, this study found that the OR was much decreased in older people, which could imply that older people can acquire antibodies with the PPSV23 vaccination, and we proposed that older people be re-vaccinated because of an anticipated decline in the effectiveness of the vaccine over time.
Almost all patients with chronic respiratory diseases in this study had risk factors for pneumonia [18]. Previously, most studies on the PPSV23 targeted nursing home residences or healthy adults, and controversial results have been reported on non-invasive pneumonia [8]. Our findings suggest the importance of vaccinating chronic respiratory patients in clinical practice.
There were several limitations. First, this study was a retrospective single-center study, and a complete confounding adjustment was not done. Second, we did not assess the severity of the underlying diseases. The study population was biased, consisting mainly of moderate to severe disease cases. Third, we did not regularly identify the serotype of each pneumococcal pneumonia. Fourth, the vaccination status of unvaccinated persons was defined based on the lack of medical records of vaccination, and some vaccinated patients may have been misclassified as unvaccinated. Fifth, in this study, we were not able to investigate the patients' history of seasonal influenza vaccination. This may affect the risk of pneumococcal infections.
In summary, the PPSV23 can be useful in preventing pneumococcal pneumonia among the elderly with chronic respiratory diseases.
Supplementary Information
Additional file 1: Table 1. The result of multivariable logistic regression analysis to identify risk factors for pneumococcal pneumonia.
Acknowledgements
Not applicable.
Authors' contributions
Study concept and design: TM, EN, TS; acquisition of data: TM, TS, TA, KT, ST, YT, HW, YE, TS, MS, AY, SM, KA; data analysis: TM, EN, YS; interpretation of data: TM, EN, YS, TS; first draft of the manuscript: TM. All authors read and approved the final manuscript.
Funding
There was no funding support.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study conforms to the Ethical Principles for Medical Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology in Japan. Following these guidelines, the Shizuoka General Hospital Research Ethical Committee determined that individual patient informed consent was not required because this study was an analysis study of existing information and patients were given the right to refuse participation by disclosure. After obtaining the approval of this committee (SGHIRB#2017062) and publishing the disclosure document on Shizuoka General Hospital's website, the information of each individual was anonymized, and the analysis was conducted.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Said MA, Johnson HL, Nonyane BAS, Deloria-Knoll M, O’brien KL, for the AAPBST Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8:e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 4.Number of people immunized against Streptococcus pneumoniae in the elderly. Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/topics/bcg/other/5.html. Accessed 25 Mar 2021.
- 5.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;23:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 7.Musher DM, Rueda-Jaimes AM, Graviss EA, Rodriguez-Barradas MC. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin Infect Dis. 2006;43:1004–1008. doi: 10.1086/507699. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004. doi: 10.1136/bmj.c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchetgen Tchetgen EJ, Rotnitzky A. Double-robust estimation of an exposure-outcome odds ratio adjusting for confounding in cohort and case-control studies. Stat Med. 2011;30:335–347. doi: 10.1002/sim.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis. 2006;43:860–868. doi: 10.1086/507340. [DOI] [PubMed] [Google Scholar]
- 12.Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine. 2008;26:1955–1962. doi: 10.1016/j.vaccine.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27:229–241. doi: 10.1016/j.idc.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17:313–321. doi: 10.1016/S1473-3099(17)30049-X. [DOI] [PubMed] [Google Scholar]
- 16.Kolditz M, Schmitt J, Pletz MW, Tesch F. Impact of pneumococcal polysaccharide vaccine on incidence and mortality after pneumonia in adults aged >/=60 years-a population-based retrospective cohort study. Clin Microbiol Infect. 2018;24:500–504. doi: 10.1016/j.cmi.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Pera A, Campos C, Lopez N, Hassouneh F, Alonso C, Tarazona R, et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Ishiguro T, Takayanagi N, Yamaguchi S, Yamakawa H, Nakamoto K, Takaku Y, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med. 2013;52:317–324. doi: 10.2169/internalmedicine.52.8830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. The result of multivariable logistic regression analysis to identify risk factors for pneumococcal pneumonia.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.