Abstract
Microbial production of antibiotics is common but our understanding of their roles in the environment is limited. Here, we explore long-standing observations that microbes increase production of redox-active antibiotics under phosphorus limitation. The availability of phosphorus, a nutrient required by all life on Earth and essential for agriculture, can be controlled by adsorption to and release from iron minerals via redox cycling. Using phenazine antibiotic production by pseudomonads as a case study, we show that phenazines are regulated by phosphorus and solubilize phosphate via reductive dissolution of iron oxides in the lab and field, concomitantly increasing microbial growth. Phenazines are just one of many examples of phosphorus-regulated antibiotics. Our work suggests a widespread but previously unappreciated role for redox-active antibiotics in phosphorus acquisition and cycling.
One Sentence Summary:
Antibiotics enhance P bioavailability.
The production of secondary metabolites by microbes is widespread, and there is growing recognition of the importance of these molecules for survival in the wild (1–3). However, with the exception of metal complexation by siderophores, the controlled use of secondary metabolites in nutrient acquisition has not been shown. In response to limitation for the essential nutrient phosphorus (P), several bacterial species increase production of secondary metabolites (4, 5). Many of these metabolites are considered to be antibiotics, and their toxicity is conferred by a variety of mechanisms, including the ability to engage in redox reactions. P-regulation of secondary metabolite production has been studied extensively in Actinobacteria but also occurs in Proteobacteria and Firmicutes (Fig. 1). The reason for this regulation is not totally understood but, changes in cell metabolism under slow growth and nutrient stress as well as a broad phosphate-starvation induced virulence response have been suggested (5, 6). An alternate, unexplored explanation is that under P limitation, rather than having toxic roles, the redox activity of some of these antibiotics might directly facilitate P acquisition.
Fig. 1. P regulates production of antibiotics in diverse bacteria.

Tree depicts species with experimental evidence for P-limited antibiotic production (pink text) and experimentally confirmed (filled green circle) or likely (open green circle) regulation by phoB/P and phoR. Data are largely from (5) and (6), see Table S1. Metabolite production in B. thailandensis and Serratia ATCC39006 was tested using chemostats (Fig. S1). Example structures for different antibiotics are shown with broad antibiotic type listed above each structure. The common metabolite name and producer are also listed. Producers of each example metabolite are also indicated with numbers around the tree. Tree was built from 241 small subunit rRNA sequences using RaxML (29) and is rooted for display.
Phosphorus availability affects global primary productivity in natural and agricultural systems (7–10) and can be influenced by microbial redox reactions involving iron (Fe) oxides over geologic as well as anthropologic time scales (7, 8, 11, 12). Phosphate and organic P are often immobilized via adsorption to positively charged surface sites on Fe(III)-(oxy)hydroxide minerals and subsequently solubilized by microbial reduction of Fe(III) to Fe(II) under anoxic conditions (7, 11–13). The controls on microbial P solubilization from Fe oxides and their contribution to P burial remain an under-constrained facet of the P cycle. Fe reduction by metabolites such as flavins (which are not known to be regulated by P) has been well studied (14). However, the primary purpose of these metabolites is thought to be respiration of Fe minerals, and any accompanying benefit from P solubilization has either been neglected or considered perfunctory.
Based on the precedent for P release via reductive dissolution of Fe oxides, we wondered if some antibiotics with redox activity might have a previously unappreciated role in this process, thus reconciling their stimulation by P limitation. We set out to quantify the stimulation of redox-active antibiotic production under P limitation and examine the resulting enhancement in P availability and microbial growth. Specifically, we sought to test the following hypotheses: i) redox-active antibiotic biosynthesis is regulated by P availability in many organisms and occurs via a conserved molecular pathway, ii) reductive dissolution of Fe minerals by these metabolites results in solubilization of adsorbed P and iii) the net result is increased P availability in lab cultures and natural microbial communities under P limitation.
We focused on phenazines (Fig. 1), a class of secondary metabolites with well understood redox properties (15) that are made by several pseudomonads as well as many other types of bacteria and are known to confer a variety of physiological benefits to the producing organism (3, 16). Phosphorus limitation was linked to phenazine production in Pseudomonas aeruginosa as early as 1947 (17), but it has been unclear whether this is widespread in pseudomonads. Additionally, when studied in batch culture, secondary metabolites are typically produced at the end of growth – due to a complex combination of nutrient exhaustion as well as the accumulation of quorum sensing (QS) molecules, which regulate biosynthesis of many secondary metabolites, including phenazines (18).
Chemostats provide a more controlled experimental setup than batch culture, allowing for precise tuning of both growth rate and cell density, thereby removing these complications. We designed a growth medium that maintains low cell densities (OD500 circa 0.1) based on limitation for either nitrogen or phosphorus and quantified phenazine production under these conditions in P. aeruginosa, P. aureofaciens, P. chlororaphis and P. fluorescens. We found that in all four Pseudomonas species tested, chemostats limited for phosphorus had much higher phenazine concentrations than those limited for nitrogen, ranging between 1 and 3 orders of magnitude. Slow growth rates also correlated with greater phenazine production (Fig. 2A, Fig S1), an observation that has been made by others (4, 19) but is not completely understood. These results are consistent with previous chemostat experiments in P. aeruginosa (19) as well as batch culture findings (4, 17, 18, 20) and show widespread enhancement of phenazine production under P limitation in pseudomonads.
Fig. 2. Phenazine production in different pseudomonads is regulated by phosphate via phoB.
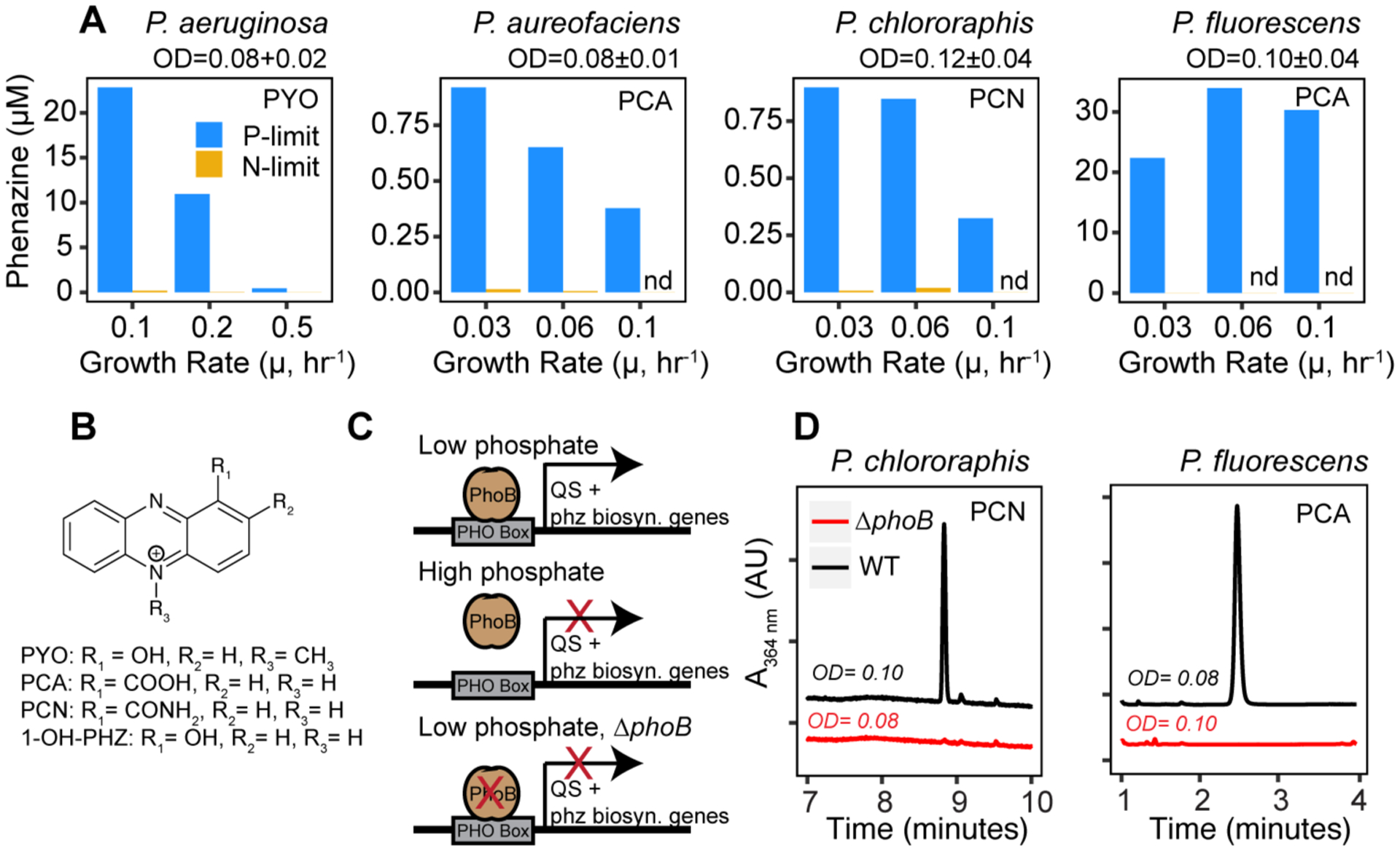
(A) Phenazine production in phosphorus- (blue) or nitrogen- (yellow) limited Pseudomonas chemostats. Phenazines produced are PCA: phenazine-1-carboxylic-acid; PCN: phenazine-1-carboxamide; PYO: pyocyanin. ODs were maintained at ~0.1, the range of ODs across chemostats for each experiment ± standard deviation is listed. (B) Phenazine structures. (C) PhoB is thought to increase phenazine production by binding predicted PHO boxes upstream of phenazine biosynthetic genes and QS genes (19, 21). (D) Phenazine production in wild type Pseudomonas and phoB mutants at μ=0.06. Reported growth rate (μ) is the dilution rate, the two are equivalent at steady state. Nd: not detected, OD: optical density at 500 nm.
In many bacteria, genes related to phosphorus acquisition are part of the PHO regulon, which is controlled by a two-component regulatory system comprising an inner membrane histidine kinase (PhoR) and a cytoplasmic response regulator (PhoB) that controls transcription by binding to conserved regulatory ‘PHO boxes’ (Fig. 2C, (6)). To test whether PhoB regulation might explain the trends seen in our Pseudomonas chemostat experiments, we constructed unmarked deletions of the phoB gene in P. fluorescens and P. chlororaphis. In both cases, the deletion of phoB abolished phenazine production in phosphorus-limited chemostats (Fig. 2D). These results are supported by previous findings that a P. aeruginosa phoB mutant produces less of the phenazine pyocyanin under P limitation and as well as in silico predictions of putative PHO boxes upstream of phenazine biosynthetic genes and QS genes (21). When considered with prior studies (Fig. 1, Table S1,(22)), our findings suggest that PhoB regulation of secondary metabolite production is conserved not only in pseudomonads but also across diverse species.
Phenazines are known to reduce Fe minerals (15) but, the potential effects of this process on P solubilization have not been investigated. To test whether phenazines could solubilize phosphate via reductive dissolution, phenazines were added to synthetic phosphated hydrous ferric oxides (herein HFO-P), and the release of phosphate (as elemental P), total Fe, and Fe(II) was tracked. Reduced 1-hydroxy-phenazine (1-OH-PHZ), phenazine-1-carboxamide (PCN) and phenazine-1-carboxylic acid (PCA) increased soluble phosphorus concentrations relative to controls, whereas oxidized forms of these phenazines did not (Fig. 3A). In addition, Fe(II) was only produced in incubations with reduced phenazines (Fig. 3A), consistent with HFO-P reductive dissolution, a mechanism that has been well established as an important control of environmental P availability (12).
Fig. 3. Phenazines solubilize P and promote P. aeruginosa growth on HFO-P.
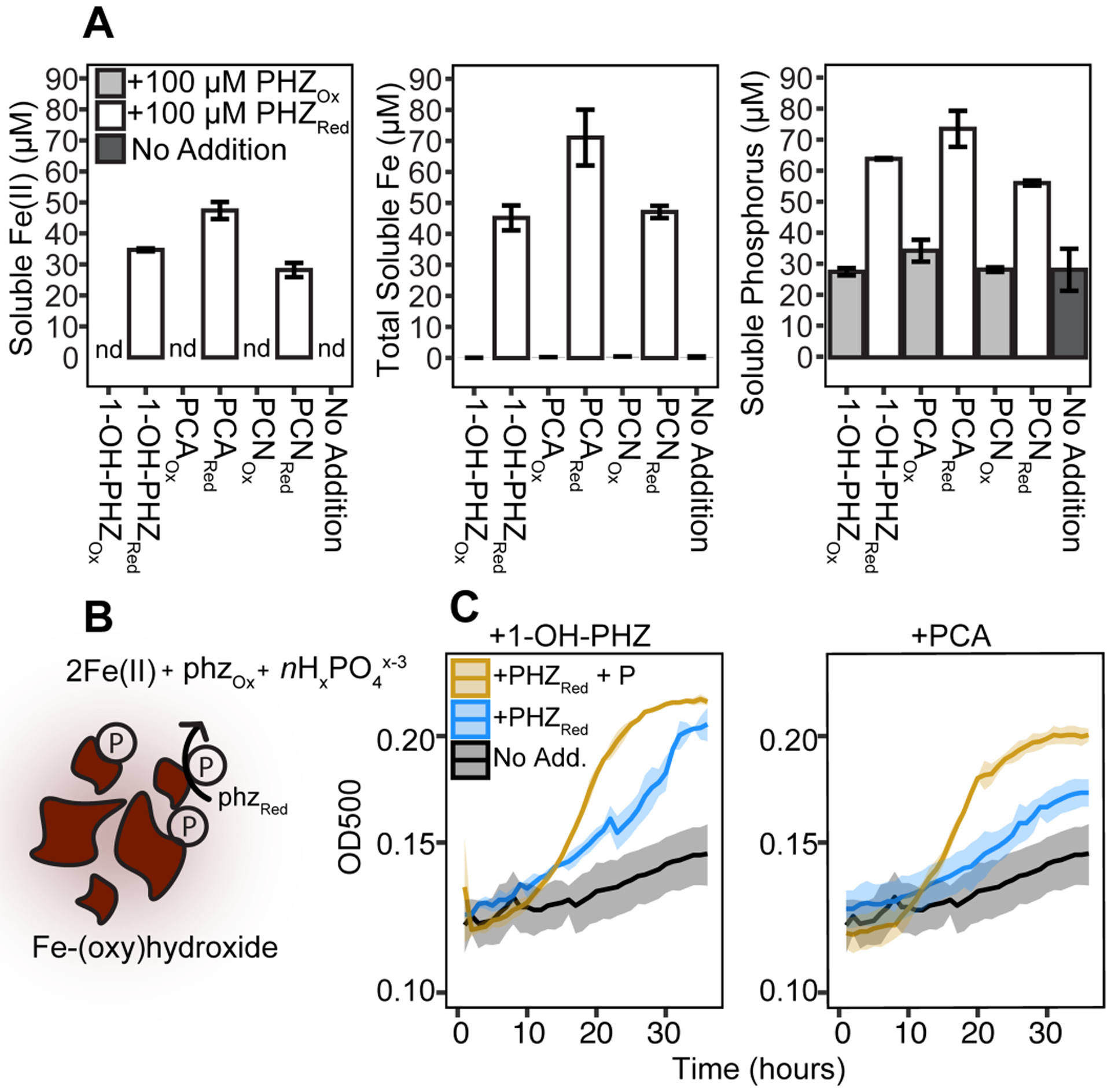
(A) Results of reactions between HFO-P and phenazines after 5 hours of anoxic incubation: Fe(II) (ferrozine assay), total soluble iron (ICP-MS), and total soluble phosphorus (ICP-MS). Nd: not detected. Error bars: standard deviations for duplicates. See Fig. S2 for details on P adsorption. (B) Model of reductive dissolution. The reaction of phenazine with Fe(III) is a two electron transfer yielding 2Fe(II) (15). Solubilized P is variable (indicate by n) depending on the extent of P surface coordination as dictated by P concentration, mineral composition, and pH (26). (C) Growth (as a denitrifier) of a P. aeruginosa mutant unable to make phenazines on HFO-P. Additions: 100 μM (reduced phenazine), 7mM (phosphate). Shaded area represents the standard deviation for biological duplicates.
To examine whether phenazines would stimulate microbial growth of P-limited cultures on HFO-P, we focused on 1-OH-PHZ and PCA as these phenazines are most reactive with Fe oxides and are made by soil and sediment dwelling pseudomonads (15, 23, 24). A P. aeruginosa mutant that cannot make phenazines was cultured under Fe-replete conditions with HFO-P as the sole P source. While this organism is best known for its role as an opportunistic pathogen, phenazine-producing P. aeruginosa strains have also been isolated from coastal marine sediments (24). To avoid oxidation of phenazines by oxygen and to provide an alternative electron acceptor, cells were grown anaerobically with nitrate. The addition of either reduced PCA or reduced 1-OH-PHZ increased growth (Fig. 3C). A further increase in growth was observed upon addition of P in the presence of phenazines (Fig. 3C), validating P limitation under these conditions. These results establish that reduced phenazines stimulate P-limited cultures in the presence of HFO-P, demonstrating their ability to increase the bioavailability of P (and possibly Fe) from the particulate phase.
To extend these findings to the environment, we conducted anaerobic incubations of phenazines with soils (Site 1: 33° 26.1008’ N, 118° 30.1868’ W, August 2019) and sediments (Site 2: 33° 25.7952’ N, 118° 30.2765’ W, August and October 2019) collected from Catalina Harbor on Catalina Island, CA, a location where pseudomonads have been previously isolated (25). Our experiments showed a variety of P solubilization responses to phenazine additions (Fig. 4), ranging from no change to significant increases; and these differences were correlated with variations in Fe:P ratios of soil/sediments. Experiments from Site 1, which had a very high Fe:P ratio of ~88 (Fig. 4A), showed little response to phenazine additions (Fig. 4B). Oxidation of a large fraction of added PCA by Fe at P-free mineral surface sites and re-adsorption of solubilized P could explain these muted effects. In contrast, at Site 2, where the Fe:P ratio was substantially lower (~26, Fig. 4A) reduced PCA significantly increased total soluble phosphorus in two separate experiments, but with very different temporal dynamics (Figs. 4C,D, S3, S4). In the first set of experiments conducted in August 2019, reduced PCA led to an immediate and sustained elevation in phosphorus concentration. In experiments from October 2019, reduced PCA led to dramatic but delayed increases in soluble phosphorus starting at 72 hours. Experiments with 1-OH-PHZ at Site 2 in August showed little effect (Fig. S3, S4), likely owing to greater reactivity of 1-OH-PHZ (15) and subsequent formation of secondary mineral phases.
Fig. 4. Phenazines solubilize P in marine sediments.
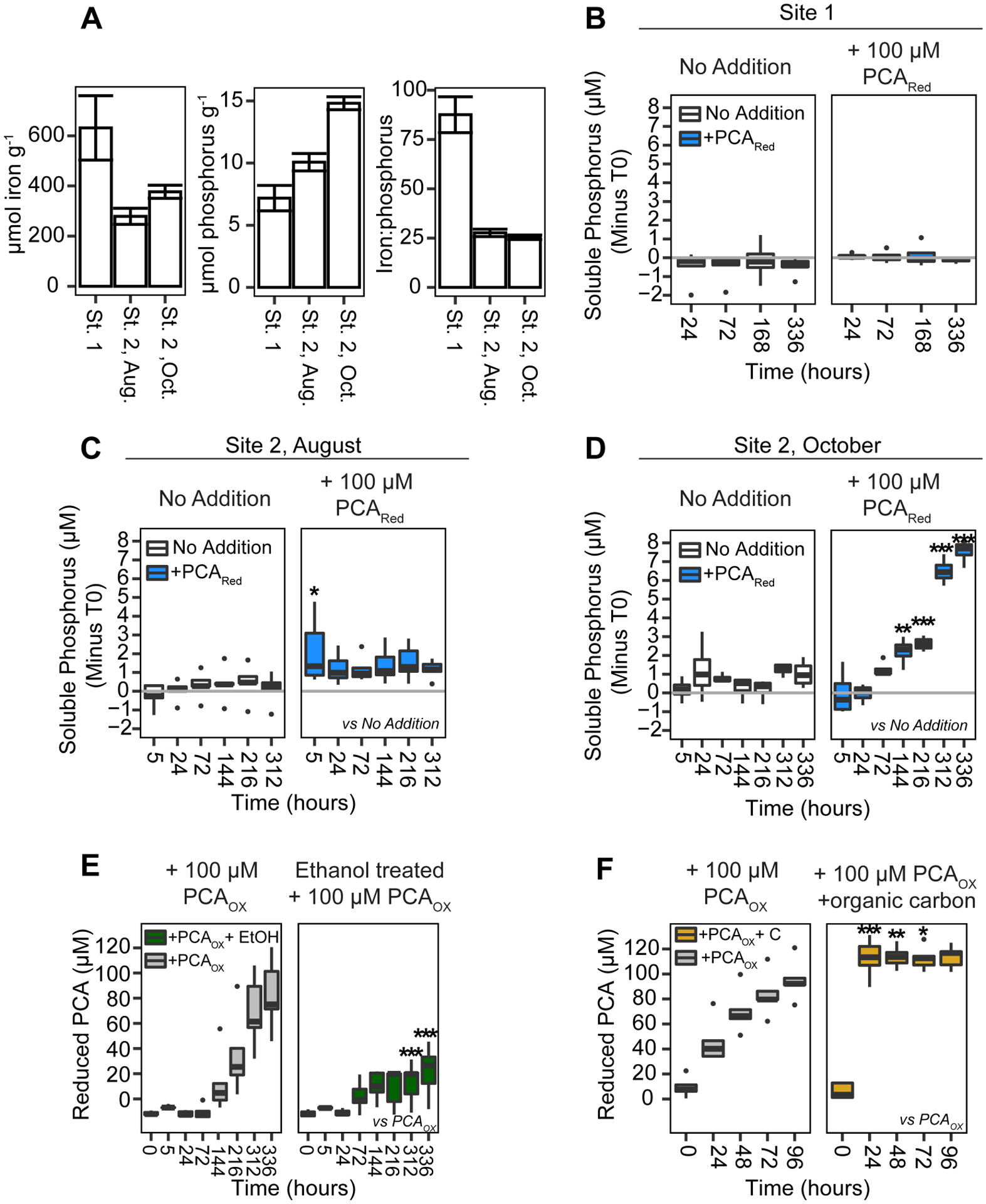
(A) Nitric acid extractable iron and phosphorus from sampling sites. Error bars: standard deviations from triplicate digestions. (B-D) Phosphorus solubilization in Catalina Island sediments from Site 1 collected in August (B), and Site 2 collected in August (C) or October (D). Y-axes in (B-D) reflect difference in total soluble phosphorus (ICP-MS) from the initial time point, horizontal lines depict no change. See also Figs. S3, S4. (E) PCA reduction is suppressed in sediments treated with ethanol. (F) PCA reduction is stimulated by organic carbon (10 mM glucose + 10 mM lactate). Sediments in (F) were starved before PCA additions and cannot be compared directly to (E). For (B-F) Plots reflect data from 4 or 5 replicates, outliers (single black dots) are >1.5x interquartile range, p values reflect comparison to the control treatment (as indicated on the figure) at a specific time point. * p<0.05, ** p<0.005, ***, p<0.0001.
Measurements at the end of experiments (see Methods) showed the presence of sulfide in incubations from October but not August. Increased activity of sulfate reducing bacteria (SRB) explains the sharp increase in P solubilization seen later in the year as sulfide promotes reductive dissolution of Fe(III) oxides and release of sorbed P (26). Fe(III) reduction in the October incubations was directly observed as a peak in dissolved Fe(II) at 72 hours (Fig. S5), followed by a decrease as expected from the subsequent precipitation of Fe(II)-S phases with increasing S(-II) concentration. Interestingly, sulfide was found only in reduced PCA treatments, suggesting reduced PCA indirectly increases P solubilization by stimulating sulfate reduction, possibly by assuaging SRB limitation for Fe and/or P (27). Overall, our Catalina Island experiments show that phenazines can solubilize P from natural sediments through both direct and indirect processes.
A potentially advantageous feature of redox-active metabolites is their capacity to be re-reduced and cycled by the microbial community (16). There is some evidence for this occurrence in our experiments at Catalina Island: when oxidized phenazines were added (Figs. S3, S4), treatments showed modest increases in soluble phosphorus over time, consistent with P solubilization via phenazine that was reduced in situ. To test this possibility, we tracked the reduction of PCA in samples of sediments (using a fluorescence assay, see Methods) from Site 2 and either suppressed biological activity with ethanol or stimulated it with organic carbon. Ethanol dramatically suppressed PCA reduction (Fig. 4E) while organic carbon stimulated it (Fig. 4F), as expected for a biologically-driven process. The capacity of native microbial communities to reduce phenazines is notable as it suggests a small concentration of such metabolites could have a large effect on P solubilization.
In conclusion, we demonstrate an ecophysiological role for redox-active antibiotics in phosphorus solubilization and acquisition. This phenomenon is potentially widespread since, like phenazines, phosphate-regulated metabolites including tetracyclines (which can reduce ferrihydrite (28)) and those with putatively redox-active moieties (quinone, phenoxazine, Fig. 1, Table S1) may also solubilize P via reductive dissolution. Given that global reserves of mineable phosphorus rock, the source of P fertilizers, are predicted to be exhausted shortly (9), and that phenazine producers are found in crop soils (23) our findings in marine sediments may also have applied relevance for the P cycle in agricultural contexts. Overall, this work expands our knowledge of beneficial physiological roles for antibiotics, indicating that they also contribute to the acquisition of macronutrients and biogeochemical cycling.
Supplementary Material
Acknowledgments:
We thank N. Dalleska (Caltech) for help with ICP-MS and LC-MS analysis, K. Nealson (USC) as well as L. Sadler and K. Spafford (USC Wrigley Marine Science Center) for assistance with Catalina Island sampling and S. Lim (Caltech) for help with sulfide measurements. We are grateful to Newman lab members M. Bergkessel and M. Spero for guidance on mutant construction, S. Wilbert for field assistance, and L. Tsypin for help with translation of papers. We also thank F.M.M. Morel for support and advice.
Funding:
This work was supported by grants from the ARO (W911NF-17-1-0024) and NIH (1R01AI127850- 01A1) to D.K.N. D.L.M. was supported by a division postdoctoral fellowship from Biology and Biological Engineering at Caltech as well as the Simons Foundation postdoctoral fellowship in Marine Microbial Ecology.
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: All data is available in the main text or the supplementary materials.
Supplementary Materials: Materials and Methods
References and Notes:
- 1.Davies J, Ryan KS, Introducing the parvome: bioactive compounds in the microbial world. ACS chemical biology 7, 252–259 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Demain AL, Fang A, in History of Modern Biotechnology, Fietcher A, Ed. (Springer-Verlag, Berlin, Heidelberg, 2000), pp. 1–39. [Google Scholar]
- 3.Price-Whelan A, Dietrich LEP, Newman DK, Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nature Chemical Biology 2, 71–78 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Whooley MA, McLoughlin AJ, The regulation of pyocyanin production in Pseudomonas aeruginosa. European Journal of Applied Biotechnology, 1–8 (1982). [Google Scholar]
- 5.Martín JF, in Advances in Biochemical Engineering, Ghose T, Fietcher A, Blakebrough N, Eds. (Springer, Berlin, Heidelberg, 1977), vol. 6, pp. 105–127. [Google Scholar]
- 6.Santos-Beneit F, The Pho regulon: a huge regulatory network in bacteria. Frontiers in microbiology 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froelich PN, Bender ML, Luedtke NA, Heath GR, DeVries T, The marine phosphorus cycle. American Journal of Sciences 282, 474–511 (1982). [Google Scholar]
- 8.Van Cappellen P, Ingall ED, Redox stabilization of the atmosphere and oceans by phosphorus-limited marine productivity. Science 271, 493–496 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Obersteiner M, Peñuelas J, Ciais P, van der Velde M, Janssens IA, The phosphorus trilemma. Nature Geoscience 6, 897–898 (2013). [Google Scholar]
- 10.Du E et al. , Global patterns of terrestrial nitrogen and phosphorus limitation. Nature Geoscience 13, 221–226 (2020). [Google Scholar]
- 11.Crosby SA, Millward GE, Butler EI, Turner DR, Whitfield M, Kinetics of phosphate adsorption by iron oxyhydroxides in aqueous systems. Estuarine, Coastal and Shelf Science 19, 257–270 (1984). [Google Scholar]
- 12.Borch T, Fendorf S, in Developments in Earth and Environmental Science, Barnett MO, Kent DB, Eds. (Elsevier, 2008), vol. 7. Adsorption of Metals by Geomedia II, chap. 12, pp. 321–348. [Google Scholar]
- 13.Peretyazhko T, Sposito G, Iron(III) reduction and phosphorous solubilization in humid tropical forest soils. Geochemica et Cosmochimica Acta 69, 3643–3652 (2005). [Google Scholar]
- 14.Brutinel ED, Gralnick JA, Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Applied Microbiology and Biotechnology 93, 41–48 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Newman DK, Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environmental Science & Technology 42, 2380–2386 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasser NR, Saunders SH, Newman DK, The colorful world of extracellular electron shuttles. Annual Review of Microbiology 71, 731–751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton MO, Campbell J, Eagles BA, The mineral requirements for pyocyanin production. Canadian Journal of Reseach 26, 15–22 (1947). [DOI] [PubMed] [Google Scholar]
- 18.Sakhtah H, Price-Whelan A, Dietrich LEP, Chincholkar S, Thomashow LS, Eds. (Springer, Berlin, Heidelberg, Berlin, Heidelberg, 2013), pp. 19–42. [Google Scholar]
- 19.Mellbye B, Schuster M, Physiological framework for the regulation of quorum sensing-dependent public goods in Pseudomonas aeruginosa. Journal of Bacteriology 196, 1155–1164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin X-J et al. , iTRAQ-based quantitative proteomic analysis reveals potential factors associated with the enhancement of phenazine-1-carboxamide production in Pseudomonas chlororaphis P3. Scientific reports 6, 27393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen V et al. , RhlR Expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. Journal of Bacteriology 188, 8601–8606 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sola-Landa A, Moura RS, Martín JF, The two-component PhoR-PhoP system controls both primary metabolism and secondary metabolite biosynthesis in Streptomyces lividans. Proceedings of the National Academy of Sciences 100, 6133–6138 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavrodi DV et al. , Accumulation of the antibiotic phenazine-1-carboxylic acid in the rhizosphere of dryland cereals. Applied and Environmental Microbiology 78, 804–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L et al. , Antagonistic activity and mode of action of phenazine-1-carboxylic acid, produced by marine bacterium Pseudomonas aeruginosa PA31x, against Vibrio anguillarum in vitro and in a zebrafish in vivo model. Frontiers in Microbiology 8, 229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowe AR, Chellamuthu P, Lam B, Okamoto A, Nealson KH, Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism. Frontiers in microbiology 5, 1269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumm W, Sulzberger B, The cycling of iron in natural environments: considerations based on laboratory studies of heterogenous redox processes. Geochimica et Cosmochimica Acta Supplement 56, 3233–3257 (1992). [Google Scholar]
- 27.Sundareshwar PV, Morris JT, Koepfler EK, Fornwalt B, Phosphorus limitation of coastal ecosystem processes. Science 299, 563–565 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Wu T et al. , Mechanistic insight into interactions between tetracycline and two iron oxide minerals with different crystal structures. Chemical Engineering Journal 366, 577–586 (2019). [Google Scholar]
- 29.Stamatakis A, RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics (Oxford, England) 22, 2688–2690 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Sunda WG, Price NM, Morel FMM, in Algal Culturing Techniques, Andersen RA, Ed. (Elsevier Academic Press, Burlington, MA, 2005), pp. 35–63. [Google Scholar]
- 31.Redfield AC, On the proportions of organic derivatives in sea water and their relation to the composition of plankton. Daniel RJ, Ed., (University Press of Liverpool, 1934), pp. 177–192. [Google Scholar]
- 32.Seyedsayamdost MR et al. , Quorum-sensing-regulated bactobolin production by Burkholderia thailandensis E264. Organic Letters 12, 716–619 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanks RMQ, Caiazza NC, Hinsa SM, Toutain CM, O’Toole GA, Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl. Environ. Microbiol 72, 5027–5036 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson DG et al. , Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods 6, 343–345 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Choi K-H, Schweizer HP, mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nature Protocols 1, 153–161 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Schwertmann U, Cornell RM, Iron oxides in the laboratory: preparation and characterization. (Wiley-VCH, Germany, 2000). [Google Scholar]
- 37.Wang X et al. , Effect of ferrihydrite crystallite size on phosphate adsorption reactivity. ACS Publications 47, 10322–10331 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Mallet M, Barthélémy K, Ruby C, Renard A, Naille S, Investigation of phosphate adsorption onto ferrihydrite by X-ray photoelectron spectroscopy. Journal of Colloid and Interface Science 407, 95–101 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Stookey LL, Ferrozine--a new spectrophotometric reagent for iron. Analytical Chemistry 42, 779–781 (1970). [Google Scholar]
- 40.Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, Newman DK, The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Molecular Microbiology 61, 1308–1321 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Sullivan NL, Tzeranis DS, Wang Y, So PTC, Newman DK, Quantifying the dynamics of bacterial secondary metabolites by spectral multiphoton microscopy. ACS chemical biology 6, 893–899 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cline JD, Spectrophotometric determination of hydrogen sulfide in natural waters. Limnology and Oceanography 14, 454–458 (1969). [Google Scholar]
- 43.RCoreTeam. (R Foundation for Statistical Computing, Vienna, Austria, 2020). [Google Scholar]
- 44.Glöckner FO et al. , 25 years of serving the community with ribosomal RNA gene reference databases and tools. Journal of Biotechnology 261, 169–176 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Pruesse E, Peplies J, Glöckner F, SINA: accurate high through-put multiple sequence alignment of RNA genes. Bioinformatics (Oxford, England) 28, 1823–1829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Letunic I, Bork P, Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic acids research 47, W256–W259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Witney FR, Failla ML, Weinberg ED, Phosphate inhibition of secondary metabolism in Serratia marcescens. Appl. Environ. Microbiol 33, 1042–1046 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slater H, Crow M, Everson L, Salmond GPC, Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Molecular Microbiology 47, 303–320 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Im J, Lee J, Löffler FE, Interference of ferric ions with ferrous iron quantification using the ferrozine assay. Journal of microbiological methods 95, 366–367 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Nakano H, Tomita F, Yamaguchi K, Nagashima M, Suzuki T, Corynecin (chloramphenicol analogs) fermentation studies: selective production of corynecin I by Corynebacterium hydrocarboclastus grown on acetate. Biotechnology and Bioengineering 19, 1009–1018 (1977). [DOI] [PubMed] [Google Scholar]
- 51.Kirpekar A, Kirwan D, Stieber R, Effects of glutamate, glucose, phosphate, and alkali metal ions on cephamycin C production by Nocardia lactamdurans in defined medium. Biotechnology and Bioengineering 38, 1100–1109 (1991). [DOI] [PubMed] [Google Scholar]
- 52.Egorov NS, Torpova ES, Suchkova LA, Effect of phosphorus metabolism in Proactinomyces fructiferi Var Ristomycini on the biosynthesis of ristomycin. Mikrobiologiia 40, 475–480 (1971). [PubMed] [Google Scholar]
- 53.Asturias JA, Martin JF, Liras P, Biosynthesis and phosphate control of candicidin by Streptomyces acrimycini JI2236: effect of amplication of the pabAB gene. Journal of Industrial Microbiology, 183–189 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Kutty RM, Kannan LV, Rehacek Z, Effect of phosphate on biosynthesis of antimycin A and production and utilization of poly-B-hydroxybutyrate by Streptomyces antibioticus. Indian Journal of Biochemistry 6, 230–231 (1969). [PubMed] [Google Scholar]
- 55.Torbochkina LI, Dormidoshina TA, Saizeva LP, Carbohydrate metabolism in the oleandomycine producing Actinomyces antibioticus. Mikrobiologiia 33, 162–166 (1964). [PubMed] [Google Scholar]
- 56.Abou-Zeid A-ZA, Abou-el-Atta A.-e.-S. Y., The antifungal antibiotic (AYE) produced by Streptomyces aureofaciens. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheitin und Hygiene 126, 371–375 (1970). [PubMed] [Google Scholar]
- 57.Prokofieva-Belgovskaya A, Popova L, The influence of phosphorus on the development of Streptomyces aureofaciens and on its ability to produce chlortetracycline. Journal of general microbiology 20, 462–472 (1959). [DOI] [PubMed] [Google Scholar]
- 58.Yang R et al. , The PhoP transcription factor negatively regulates avermectin biosynthesis in Streptomyces avermitilis. Applied Microbiology and Biotechnology 99, 10547–10557 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Lilley G, Clark AE, Lawrence GC, Control of the production of cephamycin C and thienamycin by Streptomyces cattleya NRRL 8057. 31, 127–134 (1981). [Google Scholar]
- 60.Lebrihi A, Germain P, Lefebvre G, Phosphate repression of cephamycin and clavulanic acid production by Streptomyces clavuligerus. Applied Microbiology and Biotechnology 26, 130–135 (1987). [Google Scholar]
- 61.Hobbs G, Frazer CM, Gardner DCJ, Flett F, Oliver SG, Pigmented antibiotic production by Streptomyces coelicolor A3(2): kinetics and the influence of nutrients. Microbiology (Reading, England) 136, 2291–2296 (1990). [Google Scholar]
- 62.Doull JL, Vining L, Nutritional control on actinorhodin production by Streptomyces coelicolor A3(2): suppressive effects of nitrogen and phosphate. Applied Microbiology and Biotechnology 32, 449–454 (1990). [DOI] [PubMed] [Google Scholar]
- 63.Santos-Beneit F, Rodríguez-García A, Sola-Landa A, Martín JF, Cross-talk between two global regulators in Streptomyces: PhoP and AfsR interact in the control of afsS, pstS and phoRP transcription. Molecular Microbiology 72, 53–68 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Thomas L et al. , Metabolic switches and adaptations deduced from the proteomes of Streptomyces coelicolor wild type and phoP mutant grown in batch culture. Molecular & Cellular Proteomics 11, M111.013797 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donovick R, Brown WE, Vanek Z, Hostalek Z, Eds. (Publishing House of the Czechoslovak Acadmet of Sciences, Prague, Czech Republic, 1965), chap. 22, pp. 281–286.
- 66.Vu-Trong K, Bhuwapathanapun S, Gray PP, Metabolic regulation in tylosin-producing Streptomyces fradiae: phosphate control of tylosin biosynthesis. Antimicrobial Agents and Chemotherapy 19, 209–212 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boeck L, Clem GM, Wilson MM, Westhead JE, A9145, a new adenine-containing antifungal antibiotic: fermentation. Antimicrobial Agents and Chemotherapy 3, 49–56 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liras P, Villanueva JR, Martín JF, Sequential expression of macromolecule biosynthesis and candicidin formation in Streptomyces griseus. Microbiology 102, 269–277 (1977). [DOI] [PubMed] [Google Scholar]
- 69.Liras P, Asturias JA, Martín JF, Phosphate control sequences involved in transcriptional regulation of antibiotic biosynthesis. Trends in Biotechnology 8, 184–189 (1990). [DOI] [PubMed] [Google Scholar]
- 70.Rebollo A, Gil JA, Liras P, Asturias JA, Martín JF, Cloning and characterization of a phosphate-regulated promoter involved in phosphate control of candicidin biosynthesis. Gene 79, 47–58 (1989). [DOI] [PubMed] [Google Scholar]
- 71.Abou-Zeid A-ZA, Production of cyclohexamide by Streptomyces sp. . Acta Microbiologica Polonica 4, 83–88 (1972). [PubMed] [Google Scholar]
- 72.Ohnishi Y et al. , Structures of grixazone A and B, A-factor-dependent yellow pigments produced under phosphate depletion by Streptomyces griseus. The Journal of Antibiotics 57, 218–223 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Perlman D, Wagman GH, Studies on the utilization of lipids by Streptomyces griseus. Journal of Bacteriology 63, 253–262 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martín JF, Ramos A, Liras P, Regulation of geldanamycin biosynthesis by cluster-situated transcription factors and the master regulator PhoP. Antibiotics (Basel, Switzerland) 8, 87 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hall MJ, Hassall CH, Production of the monamycins, novel depsipeptide antibiotics. Applied Microbiology 19, 109–112 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basak K, Majumdar SK, Mineral nutrition of Streptomyces kanamyceticus for kanamycin formation. Antimicrobial Agents and Chemotherapy 8, 391–395 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zyuzina ML, Efimova TP, Effect of inorganic phosphate on levorin biosynthesis and composition of Streptomyces levoris mycelium. Antibiotiki 24, 656–659 (1979). [PubMed] [Google Scholar]
- 78.Mendes MV et al. , The two-component phoR-phoP system of Streptomyces natalensis: Inactivation or deletion of phoP reduces the negative phosphate regulation of pimaricin biosynthesis. Metabolic Engineering 9, 217–227 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Hoeksema H, Smith CG, Novobiocin. Progress in Industrial Microbiology 3, 91–139 (1960). [PubMed] [Google Scholar]
- 80.Müller PJ et al. , Effect of phosphate on the biosynthesis of nourseothricin by Streptomyces noursei JA 3890b. Zeitschrift für allengemeine Mikrobiologic 24, 555–564 (1984). [PubMed] [Google Scholar]
- 81.Mertz FP, Doolin L, The effect of inorganic phosphate on the biosynthesis of vancomycin. Canadian Journal of Microbiology 19, 263–270 (1973). [DOI] [PubMed] [Google Scholar]
- 82.Zygmunt WA, Influence of inorganic phosphorus on oxytetracycline formation by Streptomyces rimosus. Applied Microbiology 12, 195–196 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDowall KJ, Thamchaipenet A, Hunter IS, Phosphate control of oxytetracycline production by Streptomyces rimosus is at the level of transcription from promoters overlapped by tandem repeats similar to those of the DNA-binding sites of the OmpR family. Journal of Bacteriology 181, 3025–3032 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Masuma R, Tanaka Y, Tanaka H, Omura S, Production of nanaomycin and other antibiotics by phosphate-depressed fermentation using phosphate-trapping agents. The Journal of Antibiotics 39, 1557–1564 (1986). [DOI] [PubMed] [Google Scholar]
- 85.Hege-Treskatis D, King R, Wolf H, Gilles E-D, Nutritional control of nikkomycin and juglomycin production by Streptomyces in continuous culture. Applied Microbiology and Biotechnology 36, 440–445 (1992). [DOI] [PubMed] [Google Scholar]
- 86.Martínez-Castro M et al. , Taxonomy and chemically semi-defined media for the analysis of the tacrolimus producer ‘Streptomyces tsukubaensis’. Applied Microbiology and Biotechnology 97, 2139–2152 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Jakeman DL, Graham CL, Young W, Vining LC, Culture conditions improving the production of jadomycin B. Journal of Industrial Microbiology and Biotechnology 33, 767–772 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Kishimoto K, Park YS, Okabe M, Akiyama S-I, Effect of phosphate ion on mildiomycin production by Streptoverticillium rimofaciens. The Journal of Antibiotics 49, 775–780 (1996). [DOI] [PubMed] [Google Scholar]
- 89.Vandamme EJ, Demain AL, Nutrition of Bacillus brevis ATCC 9999, the producer of gramicidin s. Antimicrobial Agents and Chemotherapy 10, 265–273 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haavik HI, Studies on the formation of bacitracin by Bacillus licheniformis: effect of inorganic phosphate. Journal of General Microbiology 84, 226–230 (1974). [DOI] [PubMed] [Google Scholar]
- 91.Kuratsu Y, Sakurai M, Inuzuka K, Suzuki T, Effect of phosphate ion and ammonia-nitrogen on colistin production by Bacillus polymyxa. Journal of Fermentation Technology 61, 365–371 (1983). [Google Scholar]
- 92.van Rij ET, Wesselink M, Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ, Influence of environmental conditions on the production of phenazine-1-carboxamide by Pseudomonas chlororaphis PCL1391. Molecular Plant-Microbe Interactions 17, 557–566 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Romano S, Schulz-Vogt HN, González JM, Bondarev V, Phosphate limitation induces drastic physiological changes, virulence-related gene expression, and secondary metabolite production in Pseudovibrio sp. strain FO-BEG1. Applied and Environmental Microbiology 81, 3518–3528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gristwood T, Fineran PC, Everson L, Williamson NR, Salmond GP, The PhoBR two-component system regulates antibiotic biosynthesis in Serratia in response to phosphate. BMC Microbiology 9, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


