Abstract
Research question
Is there vertical transmission (from mother to baby antenatally or intrapartum) after SARS-CoV-2 (COVID-19) infected pregnancy?
Study design
A systematic search related to SARS-CoV-2 (COVID-19), pregnancy, neonatal complications, viral and vertical transmission. The duration was from December 2019 to May 2020.
Results
A total of 84 studies with 862 COVID positive women were included. Two studies had ongoing pregnancies while 82 studies included 705 babies, 1 miscarriage and 1 medical termination of pregnancy (MTOP). Most publications (50/84, 59.5%), reported small numbers (<5) of positive babies. From 75 studies, 18 babies were COVID-19 positive. The first reverse transcription polymerase chain reaction (RT-PCR) diagnostic test was done in 449 babies and 2 losses, 2nd RT-PCR was done in 82 babies, IgM tests were done in 28 babies, and IgG tests were done in 28 babies. On the first RT-PCR, 47 studies reported time of testing while 28 studies did not. Positive results in the first RT-PCR were seen in 14 babies. Earliest tested at birth and the average time of the result was 22 hours. Three babies with negative first RT-PCR became positive on the second RT-PCR at day 6, day 7 and at 24 hours which continued to be positive at 1 week.
Four studies with a total of 4 placental swabs were positive demonstrating SARS-CoV-2 localised in the placenta. In 2 studies, 10 tests for amniotic fluid were positive for SARS-CoV-2. These 2 babies were found to be positive on RT-PCR on serial testing.
Conclusion
Diagnostic testing combined with incubation period and placental pathology indicate a strong likelihood that intrapartum vertical transmission of SARS-CoV-2 (COVID-19) from mother to baby is possible.
Keywords: COVID-19, SARS-CoV-2, pregnancy, risks, vertical transmission
Introduction
In the current SARS-CoV-2 (COVID-19) pandemic, testing has become a cornerstone for public health advice, policies and political agenda. The unprecedented and exponential growth of non-peer- reviewed publications, systematic reviews lacking rigorous thoroughness and the repetitive inclusion of case reports is problematic (Bauchner, 2020). We critically appraised the limitations and manner of diagnostic testing in obstetric medicine (Bahadur et al., 2020). While the genetic sequence for SARS- CoV-2 became available (Wang et al., 2020), it was only after mid-April 2020 that profound concerns were aired about the very high false-negative rates for antibodies. This false-negative rate for antibodies has come down from almost 1 in 2 to 1 in 3 tests, possibly because the reverse transcription polymerase chain reaction (RT-PCR) tests were based on the previous SARS family virus (Bahadur et al., 2020).
The second major limitation is the fact that most researchers had not taken account of the incubation period, which for SARS-CoV-2 is 5-6 days (Li Q et al., 2020) and most publications have relied on a single test rather than serial checks, thereby producing scientifically unreliable conclusions. There is also the possibility that latent infections exist even before the onset of symptoms (Anderson et al., 2020) and the earliest trigger for transmission occurs before the onset of symptoms which could be one to two days if with viraemia (Zou et al., 2020). The safety margins to assess the likelihood of infection before the onset of infection should be revised towards 7-8 days, which is different from the World Health Organisation (WHO) RT-PCR testing recommendation for the asymptomatic or mildly symptomatic (WHO, 2020). Asymptomatic carriers acquiring and transmitting SARS-CoV-2 remains an underexplored area (Bai et al., 2020) against those cases with confirmed SARS- CoV-2 clinical symptoms (Huang et al., 2020).
Serological testing is a measure of a by-gone event (WHO, 2020) with probable clues on the long-term immunity or immunological memory and route to immunisation (Dijkstra et al., 2020). IgM provides the initial defence to viral infections, before generating the adaptive IgG response. From 173 SARS-CoV-2 infected patients and 535 plasma samples, the median seroconversion time for total antibody (Ab), IgM and then IgG was day 11, day 12 and day 14, respectively. The antibodies were present in <40% among patients within 1-week of onset, and rapidly increased to 100 % (Ab), 94.3% (IgM) and 79.8% (IgG) 15 days after onset (Jacofsky et al., 2020; Li Z et al., 2020). Furthermore, cross-reactivity against multiple coronavirus strains with similar antigen sites can become problematic (Jacofsky et al., 2020; Xiao et al., 2020).
This retrospective observational study on RT-PCR analyses on babies born to SARS-CoV-2 positive mothers sets out to analyse how SARS-CoV-2 diagnostic analyses and placental analyses were performed, their scientific limitations in interpreting data and whether intrapartum vertical transmission from mother to baby was possible.
Methods
Study design, size, duration
A systematic review searching terms related to SARS-CoV-2, COVID-19, pregnancy, neonatal complications, viral and vertical transmission was conducted. No language restriction was placed on published articles. The duration was from 1st December 2019 to 15th May 2020 for extracting the literature and screening the articles of potential interest.
Participants/materials, setting, methods
A systematic review following the PRISMA format was performed in PUBMED, EMBASE, CENTRAL, WEB of SCIENCE, Web of Knowledge, the WHO, RCOG, ESHRE, ASRM, NEJM, BMJ, Lancet, Welcome and Cochrane Central Register of Studies, UKOSS, Office of National Statistics (ONS-UK), Department of Health (UK), Google Scholar and any references of relevant articles. Data relevant to SARS-CoV-2 diagnostic interpretation was also collected. Case reports or case series of pregnant women with confirmed COVID-19, where neonatal outcomes were reported were assembled on an Excel spreadsheet.
Results
A total of 84 studies with 862 COVID-19 positives were included. Two studies had ongoing pregnancies while the remaining 82 studies included 705 babies, one miscarriage and one medical termination of pregnancy (MTOP).
Of the 84 studies, 1st RT-PCR was undertaken in 449 babies and two losses in 75 studies. Seven studies did not report testing, while two had ongoing pregnancies. Forty-one studies had only one RT- PCR testing done. 34 studies had a 2nd RT-PCR done. Overall excluding two losses, there were 431 negative, 14 positive and four unclear results (total=449) in the 1st RT-PCR. 356/431(82.5%) babies had only one negative RT-PCR (no serial testing). The time at which 1st RT-PCR was done was recorded in 47 studies (165/449 babies) but not in 28 studies (284/449 babies). Considering the 47 studies with 165 babies, the mean times of negative and positive results were 28 hours (day 1 after birth, day 1) and 22 hours (day of birth, day 0) respectively. 2nd RT-PCR was reported in 34 studies and 82 babies. 74 were negative and eight were positive. The mean time of negative result was 92 hours / day 4 (Figure 1).
Figure 1.
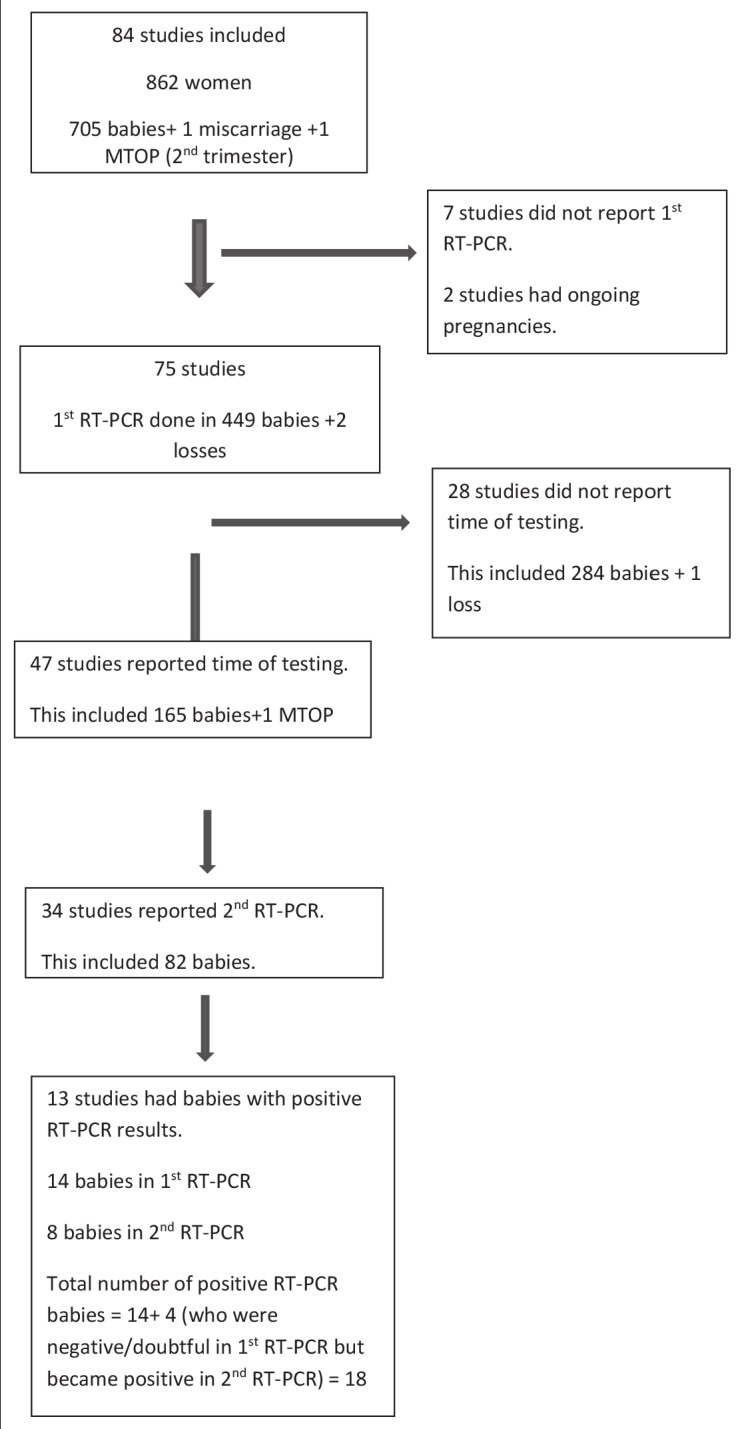
— 1st and 2nd RT PCR testing in babies born to COVID 19 positive mothers.
Positive results in 1st RT-PCR were documented in 14 babies. The earliest were tested at birth and the average time of the result was 22 hours/ day 0. Three babies with negative 1st RT-PCR became positive in 2nd RT-PCR at D6, D7 and at 24 hours which continued to test positive at 1 week. One baby with an equivocal result with 1st RT-PCR became positive in subsequent testing on day 3 (Figure 1, Table 1).
Table I.
RT-PCR results in babies tested positive.
| Study | No. of babies tested/total no. of babies in the study | 1st RT-PCR | Time at which 1st RT-PCR done | 2nd RT-PCR | Time at which 2nd RT-PCR done | 3rd and subsequent RT-PCR | Time at which test done |
|---|---|---|---|---|---|---|---|
| Yu N et al | 3/7 | 1 positive | 36 hours | 1 negative | within 2 weeks | 1 negative | Within 2 weeks |
| Zeng L et al | 33/33 | 3 positive | Day 2 & 4 | 3 negative | 2 on Day 6 & 1 on day 7 | ||
| Wang S et al | 1/1 | 1 positive | 36 hours | 1 negative | Day 15 | ||
| Ferrazzi E et al | 42/42 | 2 positive 1 doubtful |
Day 1 | 2 positive 1 positive |
Day 3 | ||
| Alzamora MC et al | 1/1 | 1 positive | 16 hrs | 1 positive | 48 hrs | ||
| Khan S et al | 17/17 | 2 positive | 24 hrs | Not tested | |||
| Nie R et al | 26/28 | 1 positive | 36 hrs | 1 negative | Day 4 | 1 negative | Day 8 & 15 |
| Carosso A et al | 1/1 | 1 positive | 0 hr | 1 negative | 37 hrs | ||
| Hu X et al | 7/7 | 1 positive | 36 hr | Not tested | |||
| Diaz SA et al | 1/1 | 1 negative | Day 6 | 1 positive | Day 8 | 1 positive | Day 13 |
| Zamaniyan M et al | 1/1 | 1 negative | 0 hr | 1 positive | 24 hrs | 1 positive | 1 week |
| Hantoushzadeh S et al | 5/9 | 5 negative | Day 1 | 1 positive | Day 7 | ||
| Kirtsman M et al | 1/1 | 1 positive | At birth | 1 positive | Day 2 | 1 positive | Day 3 |
This table includes all babies who were positive on RT-PCR at some point. Out of the 18 babies who were positive at some point, only 3 have positive result after 5 days of life. These 3 babies had initial negative result. All the rest had positive result within first 5 days and either became negative or were not tested further or continued to have positive result but these were within first 5 days.
Antibody testing (IgM/IgG) was done in 28 babies from six studies. Twenty-one babies had negative results, while 7 (25%) were positive (3 had positive IgM and all 7 had positive IgG). All three babies with positive IgM had negative RT-PCR. IgG could be acquired by transfer from mum through the placenta. All except one baby with positive IgG had a positive 1st RT-PCR. When this same swab was repeated after 37 hours, RT-PCR was negative. In the two studies where babies were positive for IgM, no information was available on placental swabs, cord blood or amniotic fluid.
One baby with negative antibody testing had positive 1st RT-PCR at 16 hrs after birth and the mother became antibody positive on postpartum day 4.
In order to confirm vertical transmission, amniotic fluid, and placenta and cord blood were assessed for evidence of infection with SARS-CoV-2 by RT-PCR results. In 15 studies (46 women) had sent placental swabs. Four studies with a total of four placental swabs were positive. Positive placental swabs on histology demonstrated SARS-CoV-2 localised predominantly to the syncytiotrophoblast cells of the placenta.
In 17 studies, amniotic fluid from 67 women was tested for COVID-19. In two studies, 10 tests were positive. In these, two babies were found to be positive on RT-PCR on serial testing.
Cord blood samples were tested in 77 cases (in 15 studies) and all except one was negative. This particular study included one woman who had a MTOP for maternal compromise with severe pre- eclampsia and COVID-19 infection with possible placental abruption. Fetal heart and lung tissues tested negative for SARS-CoV-2, whilst placenta and cord blood were found to be positive.
Out of the 705 babies and 2 non-viable fetuses, 18 babies were found to be positive on at least one RT-PCR testing. Timing and serial testing were done in 82 babies (>= 2 tests).
Discussion
Pregnant women are considered to be a high-risk group for SARS-CoV-2 infection, with potentially adverse maternal and perinatal outcomes, with an increased risk of admission to an intensive care unit (ICU) (Allotey et al., 2020). There was an increased likelihood of admission to the ICU (odds ratio (OR) 1.62, 95% CI 1.33 - 1.96) and requiring invasive ventilation (OR 1.88, 95% CI 1.36 -2.60) among pregnant women compared to non-pregnant women with COVID-19. Confirmed SARS-CoV-2 positive patients had a higher risk of delivering preterm and infants born to these mothers were more likely to be admitted to the neonatal intensive care unit. A quarter of all neonates from positive mothers were admitted to ICU. There appeared to be a 7% positivity rate among women universally screened for COVID-19 during pregnancy (Allotey et al., 2020). The rates of spontaneous and overall preterm birth were 6% and 17% respectively, while of the 11,000 pregnant women with SARS-CoV-2, 73 died (Allotey et al., 2020). Of 598 hospitalised pregnant women with SARS-Cov-2, about 55% were asymptomatic (Delahoy et al., 2020). Vertical transmission and congenital abnormalities experienced with Zika virus raise concerns about SARS-Cov-V-2 (Krauer et al., 2017; Hintley et al., 2020).
Table II.
Maternal characteristics.
| Study | Age | Parity | Gestation | Contact history | Co-morbidities | Outcome |
|---|---|---|---|---|---|---|
| Yu N et al | 38 | G3P2 | 39+6 | No | Hypothyroidism | recovered |
| Zeng L et al | 40 | NR x | NR | recovered | ||
| 40+4 | NR | NR | recovered | |||
| 31+2 | NR | NR | recovered | |||
| Wang S et al | 34 | 40 | Yes | Hypothyroidism | recovered | |
| Ferrazzi E et al | Mean-34.6 | NR | NR | recovered | ||
| Alzamora MC et al | 41 | G3P2 | 33 | Yes | Prev 2 CS Diabetes |
recovered |
| Khan S et al | 29(24-34) | 38(35+5 -41) | Yes | NR | recovered | |
| Nie R et al | NR | NR | recovered | |||
| Carosso A et al | 28 | G2P1 | 37 | NR | GDM | recovered |
| Hu X et al | 34 | 40 | Yes | None | recovered | |
| Diaz SA et al | 41 | 38+4 | Yes | Hypothyroidism, IVF pregnancy, severe PET | ventilated | |
| Zamaniyan M et al | 32+2 | Yes | hypothyroidism | Died | ||
| Hantoushzadeh S et al | 40-44 | G2P1 | 30+5 | No | Subclinical hypothyroidism, advanced maternal age | Died |
| Kirtsman M et al | 40 | G2P1 | 35+5 | NR | familial neutropenia, GDM, recurrent bacterial infection | recovered |
Hypothyroidism and advanced maternal age were a common maternal morbidity. Elderly mums. For babies with positive results after D5 – 2 mums were critically ill and died after delivery. xNR- Not recorded.
If more than half of SARS-CoV-2 infected pregnant women were asymptomatic there are healthcare implications in managing all patients and staff within a clinical setting (Delahoy et al., 2020). Equally the meaningfulness of the SARS-CoV-2 diagnostic analyses needs to be addressed against the backdrop of high levels of false negatives results relying on a single test which would thereby not inform us a true value of SARS-CoV-2 vertical transmission (Woloshin et al., 2020). Our data and interpretation suggest the need to improve our understanding when testing has to be performed and when to repeat to reach a meaningful clinical decision process and especially where validation of test had not occurred, the sensitivity not reported and where gold standard and controls remained obscure. What is now known is that there can be 100% false negatives on the first day of showing symptoms from an infected individual and dropping to 67% on day 4. Thus, the validity of the RT-PCR post symptoms would only be valid from days 5 to 12 after which RT-PCR becomes negative (Kucirka et al., 2020). In four cohorts, 100% of patients initially tested negative but turned positive after 2nd and 3rd re-tests for SARS-CoV-2 RNA (Kelly et al., 2020).
False-negative testing for SARS-CoV-2 is a serious clinically relevant problem and this should be overcome by serial testing in the 5-7 days incubation period post-infection (Kelly et al., 2020). In our study cohort there is no evidence that any of the researchers have been aware of the relevance of timing of testing or the need for serial testing to ensure the repeatability of an initial negative result thereby leading to inappropriate reassurances and conclusions about the health and status of SARS- CoV-2 infected mothers and babies with all its implications for healthcare workers in attendance.
The most striking feature from our data analyses of 84 studies was the over-reliance on a single RT- PCR negative test in 41 papers and the absence of documentation of time that the sample were taken in 28 papers (Figure 1). Given the incubation period, a single result cannot be used meaningfully, and conclusions remain unsupported by the available evidence. In 82.5% babies, only 1 negative RT- PCR done on an average on day 1 was relied upon. Even in babies having had 2nd negative RT-PCR, the test was done within 96 hours/ day 4. Based upon the understanding that the incubation period is > 5 days, the validity of this testing is questionable. In the few sequential tests performed these appeared more random rather than scientific reasoning in relation to the incubation period for SARS-CoV-2 viral infection.
Antibody testing has very limited value in unravelling the status of vertical transmission. IgG molecules can travel across the placental membranes; hence its’ presence does not support or refute vertical transmission. In two studies involving babies having positive IgM, there was no other supporting information supporting vertical transmission. In addition, RT-PCR in these babies was negative. From our analyses there remains sparse and meaningless information of the time for the production of IgM and IgG along with limited publication and possibly antibodies are more significant for long term immunity.
The strength of our study was the analysis of a substantial data set of publications and the manner in which the diagnostic tests were performed, revealing poor interpretation and meaningful follow up. Given the diagnostic tests were developed on the SARS Middle Eastern strain and in the period of study, the validation of these tests had not been established or undertaken and this adds to the weaknesses over which we do not have control.
Little information existed in the validation of diagnostic tests being utilised. Numerous tests have come onto the market since these studies were reported with differing sensitivities and specificities (Corman et al., 2020; Bahadur et al., 2020; Laureano and Riboldi, 2020) and its use to derive reassurances on major healthcare implication for mothers, babies and healthcare workers need caution. Single occasion screening is likely to miss potentially infected people (Viswanathan et al., 2020) and clinicians should not rely on unexpected negative results and performing serial tests could overcome an individual test’s limited sensitivity. Recent studies though not covered in our analyses are unlikely to alter the findings of this study as concerns about the lack of serial diagnostic analyses remain. As far as we can ascertain this risk is minimal in our cohort analyses and our study remains robust and contributes to the growing body of information on maternal-fetal effect during the SARS-CoV-2 pandemic.
Vertical transmission from mother to baby is rare and when this occurs there are serious health implications for the baby. The SARS-CoV-2 studies included in our cohort do cover placental pathology reports (Hosier et al., 2020, Kirstman et al., 2020, Schoenmakers et al., 2020, Shanes et al., 2020). The histological analyses in these reports point to a strong indication that intrapartum vertical transmission from mother to fetus had occurred (Table VIII). Our data also highlight the need to look at vertical transmission alongside placental pathologies where SARS-CoV-2 invasion of the placenta shows the potential for severe morbidity in pregnant women (Hosier et al., 2020). Another likely case of vertical transmission indicates neonates should have SARS-CoV-2 testing of the nasopharynx, placenta and cord blood as soon as possible after birth, while sample timing and collection methods should be documented (Kirtsman et al., 2020). There is limited information on how long the women were positive for SARS Cov2 virus and how soon transplacental transmission occurs. Assuming babies acquired infection in utero nearer delivery, it would be expected that the babies would be positive after the incubation period, most likely in the five days following delivery. In our review, there were only two babies who were negative soon after delivery but became positive five days later and one who became positive at 24 hours and continued to be positive at one week. All the other babies had positive RT-PCR within five days of birth. Of these three babies, two had positive amniotic fluid raising the possibility of vertical transmission (Table VI). Had it been contamination from amniotic fluid, RT- PCR would be expected to be positive in the initial testing soon after birth (Table VII).
Table VIII.
Placental histology.
| Study | No. of women | No. of babies | Placental histology |
|---|---|---|---|
| Schoenmakers S et al | 1 | 1 negative | Placenta showed the presence of SARS-CoV-2 particles with generalized inflammation characterized by histiocytic intervillositis with diffuse perivillous fibrin depositions with damage to the syncytiotrophoblasts.The maternal side of the placenta had a viral load of 4.42 log copies /mL, while the fetal side had 7.15 log copies /mL. |
| Hosier H et al | 1 | MTOP-negative | The placenta was remarkable for the presence of diffuse perivillous fibrin and an inflammatory infiltrate composed of macrophages as well as T lymphocytes, consistent with histiocytic intervillositis. SARS–CoV-2 localized predominantly to the syncytiotrophoblast cells of the placenta. |
| Shanes ED | 16 | 15 babies negative 1 miscarriage- not tested |
Placentas show increased prevalence of decidual arteriopathy and other features of maternal vascular malperfusion, a pattern of placental injury reflecting abnormalities in oxygenation within the intervillous space associated with adverse perinatal outcomes. |
| Kirtsman M et al | 1 | 1 positive | Placenta showed multiple areas of infiltration by inflammatory cells and extensive early infarction, largely confined to the intervillous space, consistent with chronic histiocytic intervillositis. There was extensive early necrosis of the syncytiotrophoblast layer. |
*MTOP- Medical termination of pregnancy
Table VI.
Placenta, amniotic fluid, cord blood, breast milk, vaginal swab results.
| Study | Placenta | Amniotic fluid | Cord blood | Vagina | Breast milk |
|---|---|---|---|---|---|
| Yu N et al | Negative | Negative | |||
| Zeng L et al | Negative | Negative | Negative | ||
| Negative | Negative | Negative | |||
| Negative | Negative | Negative | |||
| Wang S et al | Negative | Negative | Negative | ||
| Ferrazzi E et al | |||||
| Alzamora MC et al | |||||
| Khan S et al | |||||
| Nie R et al | Negative | negative | |||
| Carosso A et al | fetal and maternal side negative | negative | |||
| Hu X et al | negative | ||||
| Diaz SA et al | |||||
| Zamaniyan M et al | Positive | Negative | Negative | ||
| Hantoushzadeh S et al | Positive | ||||
| Kirtsman M et al | Positive | Not tested | Positive | Positive |
Table VII.
Evidence for and against vertical transmission - Inconsistencies in the timing and repetition of RT-PCR makes it difficult to come to an inference
| Study | 1st RT-PCR | Time done | 2nd RT-PCR | Time done | For vertical transmission | Against vertical transmission |
|---|---|---|---|---|---|---|
| Yu N et al | 1 pos | 36 hours | 1 neg | Within 2 week | CS, and baby was isolated for 14 days | Placenta and cord blood neg. Contamination- baby showed mild SOB Don’t know exactly when baby became negative |
| Zeng L et al | 1pos | Day 2&4 | 1 neg | Day 6 | Cs, isolation | Cord blood and amniotic fluid neg. Positive in <5 days |
| 1pos | Day 2&4 | 1 neg | Day 6 | Cs isolation | Cord blood and amniotic fluid neg. Positive in <5 days |
|
| 1pos | Day 2&4 | 1 neg | Day 7 | If vag fluid contamination- why amniotic fluid neg? | Cord blood and amniotic fluid neg. Prematurity complication. Vaginal fluid contamination(PPROM) Ex utero infec? |
|
| Wang S et al | 1 pos | 36 hrs | 1 neg | Day 15 | Long gap between 1st and 2nd RT-PCR- difficult to know if baby was positive within a week (5-7 days) | Placenta and cord blood neg Baby asymptomatic |
| Ferrazzi E et al | 2 pos | Day 1 | 2 pos | Day 3 | Vaginal fluid contamination Likely contamination. RT-PCR not repeated after 5 days. Babies were relatively asymptomatic. |
|
| 1 doubtful | Few hours after birth | 1 pos | Day 3 | -do- | ||
| Alzamora MC et al | 1 pos | 16 hrs | 1 pos | 48 hrs | CS Diabetes |
Baby positive <5 days. Had resp difficulty and needed ventilated but was premature at 33 weeks? Cause is prematurity. |
| Khan S et al | 2 pos | 24 hrs | Not tested | CS neonatal pneumonia |
Insufficient information- pos at 24 hours and not repeated. | |
| Nie R et al | 1 pos | 36 hrs | 1 neg | Day 4 | Neg at D4 Placenta cord blood neg Isolated for 16 days ?contamination |
|
| Carosso A et al | 1 pos | 0 hr | 1 neg | 37 hrs (on same swab) | Vaginal contamination highly likely as same swab neg after 37 hours. Possibly low titres... | |
| Hu X et al | 1 pos | 36 hr | Not tested | Positive >day 7 Isolation CS-?vertical |
Amniotic fluid pos ?ex-utero inf |
|
| Diaz SA et al | 1 neg | Day 67 | 1 pos | Day 8 | High likelihood of ex-utero infection from Mum- Mum BF till D6 when baby showed symptoms and was tested. | |
| Zamaniyan M et al | 1 neg | 0 hr | 1 pos | 24 hrs | Amniotic fluid positive. If it was amniotic fluid contamination-should have been positive at birth as well. Cord blood was negative so unlikely vertical transmission? infection exutero? |
|
| Yu N et al | 1 pos | 36 hours | 1 neg | Within 2 week | CS, and baby was isolated for 14 days | Placenta and cord blood neg. Contamination- baby showed mild SOB Don’t know exactly when baby became negative |
| Zeng L et al | 1pos | Day 2&4 | 1 neg | Day 6 | Cs, isolation | Cord blood and amniotic fluid neg. Positive in <5 days |
| 1pos | Day 2&4 | 1 neg | Day 6 | Cs isolation | Cord blood and amniotic fluid neg. Positive in <5 days |
|
| 1pos | Day 2&4 | 1 neg | Day 7 | If vag fluid contamination- why amniotic fluid neg? | Cord blood and amniotic fluid neg. Prematurity complication. Vaginal fluid contamination(PPROM) Ex utero infec? |
*CS-Caesarean section; **PPROM-Prelabour premature rupture of membranes
Given that the majority of pregnant women appear asymptomatic or have nonspecific symptoms (Table III), it was remarkable to have confirmation of SARS-CoV-2 placental damage, detection of SARS-CoV-2 RNA and the presence of whole viral particles on both maternal and fetal aspects of the placenta (Schoenmakers et al., 2020). Placental histological changes (Kirtsman et al., 2020; Shanes et al., 2020) provide important supporting tools to SARS-CoV-2 diagnostic testing when deciphering vertical transmission, especially considering that the majority of pregnant women appear asymptomatic although other infection routes such as through being born vaginally, breastfed or remaining with the infected mother (Walker et al., 2020). Several infected babies within 12 hours suggest an incubation period pre-existed the delivery to suggest vertical transmission (Table V). PCR positive SARS-CoV-2 tests in the amniotic fluid and infant provide important support for vertical transmission in SARS-CoV-2 infected pregnant women (Zamaniyan et al., 2020). A recent report outside our study cohort provides compelling data on vertical transmission and where the RT-PCR viral load was much higher in placental tissue than in amniotic fluid and maternal or neonatal blood (Vivanti et al., 2020) (Table VIII). Recent studies suggest SARS-CoV-2 vertical transmission, the associated chronic placental insufficiency, as well as miscarriage and fetal growth restriction (Gengler et al., 2020), while a systematic analysis shows a 3.3% intrapartum vertical transmission (Raschetti et al., 2020). Strong evidence of transplacental transmission in early pregnancy associated with hydrops fetalis and fetal demise due to SARS-CoV-2 is also reported (Shende et al., 2020).
Table III.
Maternal symptoms and investigation.
| Study | Symptoms | Lymphopenia | Thrombocytopenia | Transaminitis | Others | RT-PCR (all positive) |
|---|---|---|---|---|---|---|
| Yu N et al | Fever | Yes | yes | ↑ CRP CT-pneumonia |
Throat swab positive, Sputum & nasopharyngeal postpartum neg | |
| Zeng L et al | Fever, pneumonia | Nasopharyngeal | ||||
| Cough, pneumonia | Nasopharyngeal | |||||
| Pneumonia | Nasopharyngeal | |||||
| Wang S et al | Fever, Pneumonia | yes | ↑ CRP Neutrophilia |
Pharyngeal | ||
| Ferrazzi E et al | Pneumonia postpartum | Throat postpartum | ||||
| Alzamora MC et al | malaise, fatigue, fever, SOB | Metabolic acidosis, pancytopenia, raised CRP | Nasopharyngeal | |||
| Khan S et al | Fever, SOB, cough | yes | yes | Pharyngeal | ||
| Nie R et al | Fever | Throat | ||||
| Carosso A et al | mild fever, dry persistent cough | Nasopharyngeal swab positive rectal and stool swab postdelivery positive, vaginal swab postdelivery negative |
||||
| Hu X et al | Fever | yes | Yes | Throat | ||
| Diaz SA et al | Postpartum-fever, pneumonia | Test not specified | ||||
| Zamaniyan M et al | Myalgia, SOB, anorexia, nausea, non-productive cough, fever | yes | ↑ CRP CT-pneumonia |
Nasal and throat | ||
| Hantoushzadeh S et al | Fever, cough | yes | ↑ CRP CT-pneumonia |
Nasopharyngeal | ||
| Kirtsman M et al | myalgia, decreased appetite, fatigue, dry cough, fever | yes | Raised aPTT | Nasopharyngeal |
*CRP- C-reactive protein; **CT-Computed tomography; ***SOB- Shortness of breath
Table V.
Baby symptoms & treatment details..
| Study | Symptoms & signs | Treatment | NNU admission (days) | Isolation | Breast feeding |
|---|---|---|---|---|---|
| Yu N et al | mild SOB | not ventilated | 14 | NR | |
| Zeng L et al | lethargy,fever, pneumonia | NR | 2 | NR | |
| lethargy,fever, pneumonia vomiting | NR | 4 | NR | ||
| resp distress, pneumonia, sepsis, coagulopathy | non-invasive ventilation, anti-biotic, caffiene | 11 | NR | ||
| Wang S et al | Clinically well, Ct chest showed changes | Not ventilated, monitored | 13 | Yes | |
| Ferrazzi E et al | NR | NR | NR | No | Yes |
| GI symptoms. Had resp symptoms after 3 days | Ventilated 24 hrs | 1 | Yes | No | |
| Alzamora MC et al | mild resp difficulty, sporadic cough | Ventilated | NR | yes | No |
| Khan S et al | Neonatal pneumonia | NR | NR | NR | |
| Nie R et al | Pulmonary infection | No treatment required | 16 | Yes | |
| Carosso A et al | Asymptomatic | Not ventilated | NR | Yes | |
| Hu X et al | Asymptomatic | NR | NR | Yes | |
| Diaz SA et al | Respiratory distress | CPAP x2hours | 5 | Yes (isolated on day 6 when mum positive) | Yes |
| Zamaniyan M et al | Fever | Not ventilated | NR | Yes | |
| Hantoushzadeh S et al | pneumonia, lymphopenia, prematurity | Ventilated | NR | Yes | |
| Kirtsman M et al | hypoglycemia, feeding difficulty, hypothermia | not ventilated, antibiotics glucose | 1 | Yes | Yes |
*NR- Not recorded ; ** CPAP- Continuous Positive Airway Pressure; ***CT- Computed tomography
Table IV.
Baby characteristics.
| Study | No. of positive babies | Gestation | Mode of delivery | Apgar score @ 1,5,10 min | Birth weight (gm) |
|---|---|---|---|---|---|
| Yu N et al | 1 | 39+6 | CS | 8-9,9-10 | 3250 |
| Zeng L et al | 1 | 40 | CS | NR | 3250 |
| 1 | 40+4 | CS | NR | 3360 | |
| 1 | 31+2 | CS | 3,4,5 | 1580 | |
| Wang S et al | 1 | 40 | CS | 8,9 | 3205 |
| Ferrazzi E et al | 2 | SVD | >7 at 5 min | 3224 | |
| SVD | NR (Good condition) | NR | |||
| Alzamora MC et al | 1 | 33 | CS | 6,8 | 2970 |
| Khan S et al | 2 | 38(35+5 -41) | CS | 9,10 | 3104 (mean) |
| Nie R et al | 1 | CS | 8-10,9-10 | NR | |
| Carosso A et al | 1 | 37 | SVD in neg pressure room | 9,10 | 3120 |
| Hu X et al | 1 | 40 | CS | 8,9 | 3250 |
| Diaz SA et al | 1 | 38+4 | CS | 7,9 | 2500 |
| Zamaniyan M et al | 1 | 32+2 | CS | 8,9 | 2350 |
| Hantoushzadeh S et al | 1 | 30+5 | CS | 9,10 | 2100 |
| Kirtsman M et al | 1 | 35+5 | CS | 9,9 | 2930 |
*Caesarean section; **SVD-Spontaneous vaginal delivery; ***NR- Not recorded
It seems that the timing of diagnostic tests and interpretation of results are not standardised in an obstetric medicine setting. From our data, it appears there is inconsistency in the tests, timing and repeatability of RT-PCR, adding to the uncertainty to reach credible inferences leading to likely false and inconsistent healthcare reassurances in relation to SARS-CoV-2 infectivity. Importantly, the incubation period and the timing of testing which could account for false-negative results appear to have been overlooked or not factored-in most studies in our cohort. Where repeat testing was performed this was not based on any critical scientific rationale. Despite a large volume of publications being added every month, there remains a fundamental absence of serial testing and over-reliance on a single test that underestimates the true positive cases as highlighted with our study.
The most important question in reproductive and fetal medicine to be answered is whether there is a vertical transmission of SARS-CoV-2 virus from mother to baby and if there is an increased incidence of congenital malformations. This paper reveals that vertical transmission of SARS-CoV-2 based on diagnostic analyses is not accurately detected and it disregards the viral incubation period, placental pathologies and peripheral supporting data, thereby under-reporting mother to baby vertical transmission. In conclusion, the vast body of information generated during this SARS-CoV-2 pandemic may falsely reassure the public about the overall level of mother to baby viral transmission since serial testing after a negative test was not performed in over 80% of newborns. It is more important to amalgamate the diagnostic testing with good microscopic and histological analyses of the placenta for vertical transmission of SARS-CoV-2 to be assessed. The collective information of diagnostic analysis, incubation periods, placental and amniotic fluid data and increasingly positive cases suggest the argument for intrapartum vertical transmission is compelling and transmission from mother to baby cannot be dismissed.
Table IX.
List of studies with babies positive for COVID 19.
| Study | Year | Title | Link |
|---|---|---|---|
| Yu N et al | 2020 | Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. | https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30176-6/fulltext |
| Zeng L et al | 2020 | Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China. | https://jamanetwork.com/journals/jamapediatrics/fullarticle/2763787 |
| Wang S et al | 2020 | A Case Report of Neonatal 2019 Coronavirus Disease in China. | https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa225/5803274 |
| Ferrazzi E et al | 2020 | Mode of Delivery and Clinical Findings in COVID-19 Infected Pregnant Women in Northern Italy. | https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3562464 |
| Alzamora MC et al | 2020 | Severe COVID-19 during Pregnancy and Possible Vertical Transmission. | https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0040-1710050.pdf |
| Khan S et al | 2020 | Association of COVID-19 infection with pregnancy outcomes in healthcare workers and general women. | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7141623/pdf/main.pdf |
| Nie R et al | 2020 | Clinical features and the maternal and neonatal outcomes of pregnant women with coronavirus disease 2019. | https://www.medrxiv.org/content/10.1101/2020.03.22.20041061v1.full.pdf |
| Carosso A et al | 2020 | Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? | https://www.ejog.org/article/S0301-2115(20)30202-5/pdf |
| Hu X et al | 2020 | Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vertical Transmission in Neonates Born to Mothers With Coronavirus Disease 2019 (COVID-19) Pneumonia. |
https://journals.lww.com/greenjournal/Citation/9000/Severe_Acute_Respiratory_Syndrome_Coronavirus_2.97384.aspx DOI: 10.1097/AOG.0000000000003926 |
| Diaz SA et al | 2020 | Neonatal first case of SARS-CoV-2 in Spain First case of neonatal infection due to SARS-CoV-2 in Spain. | https://www.sciencedirect.com/science/article/pii/S1695403320301302?via%3Dihub |
| Zamaniyan M et al | 2020 | Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. | https://obgyn.onlinelibrary.wiley.com/doi/epdf/10.1002/pd.5713 |
| Hantoushzadeh S et al | 2020 | Maternal Death Due to COVID-19 Disease. | https://www.ajog.org/article/S0002-9378(20)30516-0/pdf |
| Schoenmakers S et al | 2020 | SARS-CoV-2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. | https://doi.org/10.1101/2020.06.08.20110437 |
| Hosier H et al | 2020 | SARS–CoV-2 infection of the placenta | https://doi.org/10.1172/JCI139569 |
| Shanes ED | 2020 | Placental pathology in COVID. | https://doi.org/10.1093/ajcp/aqaa089 |
| Kirtsman M et al | 2020 | Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. | doi: 10.1503/cmaj.200821 |
Footnotes
Conflict of interests: The authors declare that they have no competing interests.
Funding: There is no funding for this project.
COVID-related papers and webinars are receiving special attention with the covid repository at the Centers for Disease Control and Prevention (CDC) Atlanta.
Hopkins Center for Humanitarian Health, Johns Hopkins Bloomberg School of Public Health Baltimore: http://hopkinshumanitarianhealth.org/empower/advocacy/covid-19/covid-19-children-and-nutrition/
MedPage is also especially reporting pregnancy covid related papers: https://www.medpagetoday.com/infectiousdisease/covid19
References
- 1.Allotey J, Stallings E, Bonet M, et al. For PregCOV-19 living systematic review consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RM, Heesterbeek H, Klinkenberg D, et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahadur G, Acharya S, Muneer A, et al. SARS-CoV-2: diagnostic and design conundrums in the context of male factor infertility. Reprod Biomed Online. 2020;41:365–369. doi: 10.1016/j.rbmo.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauchner H, Golub RM, Zylke J. Editorial concern-possible reporting of the same patients with COVID-19 in different reports. JAMA. 2020;323:1256. doi: 10.1001/jama.2020.3980. [DOI] [PubMed] [Google Scholar]
- 6.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eur Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delahoy MJ, Whitaker M, O’Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 States, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep. ePub: 16 September 2020. [DOI] [PMC free article] [PubMed]
- 8.Dijkstra J, Hashimoto K. Expected immune recognition of COVID-19 virus by memory from earlier infections with common coronaviruses in a large part of the world population. F1000RES. 2020;9:285. doi: 10.12688/f1000research.23458.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gengler C, Dubruc E, Favre G, et al. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect. 2020:S1198-743X(20)30603-0. doi: 10.1016/j.cmi.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosier H, Farhadian SF, Morotti R, et al. SARS–CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huntley BJF, Huntley ES, Di Mascio D, et al. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome Coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol. 2020;136:303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 13.Jacofsky D, Jacofsky EM, Jacofsky M. Understanding antibody testing for COVID-19. J Arthroplasty. 2020;35:74–81. doi: 10.1016/j.arth.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly JC, Dombrowksi M, O'Neil-Callahan M, et al. False-negative testing for severe acute respiratory syndrome coronavirus 2: consideration in obstetrical care. Am J Obstet Gynecol. 2020;2:100130. doi: 10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:647–650. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020:18;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauer F, Riesen M, Reveiz L, et al. WHO Zika causality working group. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré Syndrome: systematic review. PLoS Med. 2017;14:e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laureano AFS, Riboldi M. The different tests for the diagnosis of COVID-19: a review in Brazil so far. JBRA Assist Reprod. 2020;24:340–346. doi: 10.5935/1518-0557.20200046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raschetti R, Vivanti AJ, Vauloup-Fellous C, et al. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11:5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenmakers S, Snijder P, Verdijk RM, et al. SARS-CoV-2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 23.Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shende P, Gaikwad P, Gandhewar M, et al. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum Reprod. 2020 doi: 10.1093/humrep/deaa367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viswanathan M, Kahwati L, Jahn B, et al. Universal screening for SARS-CoV-2 infection: a rapid review. Cochrane Database Syst Rev. 2020;9 doi: 10.1002/14651858.CD013718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker KF, O'Donoghue K, Grace N, et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG. 2020;127:1324–1336. doi: 10.1111/1471-0528.16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. WHO - World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance. 2020.
- 30.Woloshin S, Patel N, Kesselheim AS. False-negative tests for SARS-CoV-2infection-challenges and implications. NEJM. 2020;381:38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 31.Xiao SY, Wu Y, Liu H. Evolving status of the 2019 novel coronavirus infection: Proposal of conventional serologic assays for disease diagnosis and infection monitoring. J Med Virol. 2020;92:464–467. doi: 10.1002/jmv.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamaniyan M, Ebadi A, Aghajanpoor S, et al. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat Diagn. 2020;40:1759–1761. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]


