Abstract
Objective:
To compare Phase 1 treatment, using the Frankel 2 (FR2) or the modified Twin Block (MTB), for Class II division 1 malocclusion in children and adolescents with respect to: treatment duration, number of appliance breakages, occlusal outcome, and patient and parent perspectives.
Materials and Methods:
Sixty participants with a Class II division 1 malocclusion were randomly assigned to either the FR2 or MTB appliance in a two-armed parallel randomized clinical trial with an allocation ratio of 1 to 1. Time to achieve a Class I incisor relationship was the primary outcome. The number of appliance breakages was recorded. The Peer Assessment Rating (PAR) index was used to evaluate pre- and post-treatment occlusal outcome on study models. Participants completed the child OHRQoL (oral health-related quality of life), Piers-Harris, Standard Continuum of Aesthetic Need (SCAN), and Oral Aesthetic Subjective Impact Score (OASIS) questionnaires pre- and post-treatment; parents completed a SCAN questionnaire.
Results:
Forty-two participants completed treatment (FR2: 20; MTB: 22). Multiple imputation was used to impute missing data for noncompleters. Mean treatment duration was similar for the two appliances (FR2: 376 days [SD 101]; MTB: 340 days [SD 102]; P = .41). There were no significant differences in mean number of appliance breakages (FR2: 0.3 SD 0.7; MTB: 0.4 SD 0.8; P = .67 or mean PAR score P = .48). Patient and parent perspectives did not differ between appliances (P > .05).
Conclusions:
Phase 1 treatment duration, number of appliance breakages, occlusal outcome, and patient and parent perspectives were similar in 11–14 year olds with Class II division 1 malocclusion treated using the FR2 or MTB appliance.
Keywords: Frankel appliance, Twin Block appliance, Class II division 1 malocclusion, Phase 1 treatment duration
INTRODUCTION
Class II division 1 malocclusion is common in Caucasian children1 and may lead to an increased risk of incisor trauma, teasing, and bullying.2,3 It may also have a negative impact on oral health-related quality of life (OHRQoL).4
A functional appliance may be used for initial correction (Phase 1) of Class II division 1 malocclusion in children and adolescents.5 Commonly, following this, a second phase with fixed appliances is required to complete treatment.6 The choice of functional appliance in management of Class II division 1 malocclusion may depend on operator and cost factors.7 The Frankel Regulator 2 (FR2) appliance8 is the only soft tissue-borne functional appliance, whereas the Twin block appliance (TBA)9 or a modification thereof (MTB),10 is a commonly used tooth-borne variant. The time to achieve a Class I incisor relationship (Phase 1), with either of these appliances, is an important consideration for the operator, patient and parent. The number of breakages and the cost of each appliance are further factors to consider, particularly if it were identified that each appliance produced a similar occlusal outcome. In addition, psychosocial benefits may accrue to both patient11 and parent12 from this form of treatment.
Whereas Phase 1 treatment has been assessed with each appliance separately,10,13 treatment duration, the number of appliance breakages, occlusal outcomes, as well as patient and parental perspectives have not been compared between the soft tissue-borne (FR2) appliance versus the tooth-borne appliance (MTB) in a randomized clinical trial. The primary aim of this study was to determine, in children and adolescents with a Class II division 1 malocclusion, whether there was a difference between the FR2 or MTB appliance in Phase 1 treatment duration. The secondary aims were to assess whether post-treatment differences existed between appliances with regard to:
the number of appliance breakages
occlusal outcome
child OHRQoL and self-concept
child and parental perception of esthetic impact of the malocclusion.
The following null hypotheses were tested:
there is no difference in Phase 1 treatment duration with either the FR2 or MTB appliance in children and adolescents with Class II division 1 malocclusion.
-
there is no difference following Phase 1 treatment with either the FR2 or MTB appliance in children and adolescents with Class II division 1 malocclusion with regard to:
-
○
the number of appliance breakages
-
○
occlusal outcome
-
○
child OHRQoL and self-concept
-
○
child and parental perception of esthetic impact of the malocclusion.
-
○
MATERIALS AND METHODS
Ethical approval was granted from the local Clinical Research Ethics Committee.
Trial Design
This was a single center randomized clinical trial with two parallel groups, FR2 or MTB.
Participants, Eligibility, and Setting
Children and adolescents aged 11–14 years with an overjet of at least 8mm who had good dental health and were awaiting orthodontic treatment, were recruited from a state-funded orthodontic service. Exclusion criteria were those of non-Caucasian origin, history of previous orthodontic treatment, or with a craniofacial syndrome.
Interventions
Positive assent/informed consent was obtained from each participant and/or their parents. Specialist orthodontists carried out all treatment. Prior to active treatment, clinical photographs, radiographs, and study models were taken for each participant. The total treatment time in days and the number of appliance breakages over Phase 1 treatment were recorded. In addition, the following data were collected at the start and end of Phase 1 treatment:
UK Weighted Peer Assessment Rating (PAR) scores14
Child OHRQoL15
Piers-Harris Children's Self-Concept Questionnaire16
Standard Continuum of Aesthetic Need (SCAN)17
Oral Aesthetic Subjective Impact Score (OASIS).18
A calibrated examiner undertook PAR scoring. The accompanying parent of each participant completed the SCAN questionnaire. All questionnaires were completed in a quiet room adjacent to the clinical area with no time limitation for the participant or parent.
The construction bite for the FR2 and the MTB was taken using a wax rim with the patient postured to an edge-to-edge occlusion. For the FR2, the anterior teeth were apart 3–4 mm to allow space for the lingual shield crossover wires.8 For the MTB, separation of the buccal segment teeth was around 7 mm.5 There was no subsequent advancement for either appliance.
Both functional appliances were constructed to standardized designs (Figure 1).8,10 The MTB had no labial bow10 and incorporated a Southend clasp (0.7 mm stainless steel) in the mandibular incisor region.
Figure 1.
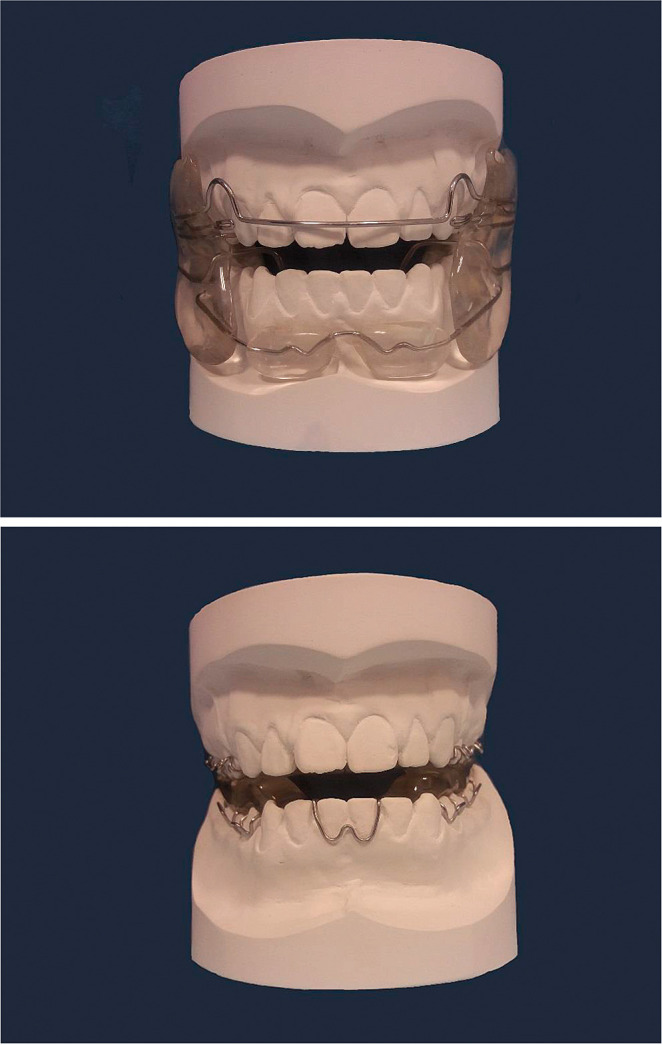
Appliance types used in this study: (a) FR2 and (b) MTB.
On fitting each appliance, participants received the written and verbal instructions used in the clinic. The importance of appliance wear was emphasized. Participants in the FR2 group were directed to wear the appliance full time except for eating, contact sports, swimming, oral hygiene, and appliance cleaning. Instructions were similar for the MTB group except that participants were directed to wear the appliance for eating. When required, those in the MTB group turned the upper midline expansion screw for arch coordination. Appliance wear was assessed at each review appointment by checking speech with the appliance in and then by examining it for signs of use.10 The overjet and the molar relationships were also noted.
Outcomes Primary Outcome Measure
The primary outcome measure was Phase 1 treatment duration, defined as the time, in days, from fitting the functional appliance until a Class I incisor relationship19 was achieved.
Secondary Outcome Measures
The secondary outcome measures were:
the number of appliance breakages
-
post-treatment:
-
○
mean PAR score
-
○
child OHRQoL and self-concept
-
○
child and parental perception of the aesthetic impact of the malocclusion.
-
○
Sample Size
The sample size calculation was based on the primary outcome measure. Mean treatment duration data for the TBA (7.02 months [95% CI: 6.34, 7.70]) was used.20 On the basis that 84 days (12 weeks) would be a clinically meaningful difference in mean treatment duration between the two appliances, for a power of 80% and P < .05, 15 participants were required for each group. Thirty participants were recruited to each group to allow for dropouts.
Randomization
Randomization was carried out using a computer generated random number sequence in blocks of 10 with an allocation ratio of 1:1. An administrative assistant held opaque sealed envelopes in a secure location remote from the clinic and allocated each participant.
Blinding
Due to the nature of the study, it was not possible to blind the operator or the participant to the intervention. Coding of all data, however, was undertaken prior to analyses to ensure blinding of the statistician.
Statistical Analyses
All primary and secondary outcome measures were compared between treatment groups using linear models. Treatment group and patient sex were included as factors. Pre-treatment age, overjet, and PAR were included as covariates. Natural logarithmic transformations were applied when required to normalize the residuals. An intention to treat analysis was performed.
All data that were missing either due to incomplete questionnaires, dropout or lost to follow-up, were imputed using multiple imputation based on 100 imputations. SAS (Version 9.4, Cary, North Carolina, USA) was used for all statistical analyses and multiple imputations.
RESULTS
Sixty participants (27 males and 33 females) were enrolled in the trial (FR2: 14 males and 16 females; MTB: 13 males and 17 females). Forty-two participants completed treatment (FR2: 20, MTB: 22) with seven males and 13 females in the FR2 group, and 10 males and 12 females in the MTB group (Figure 2). At baseline, there were no significant differences between groups with regard to sex (P = .80), mean age, mean overjet, or mean PAR score (Table 1; all P > .05). At the end of Phase 1 treatment, there was no significant difference between groups with regard to drop out rates (P = .78) or final overjet (mean difference: 0.6 mm (95% CI: −0.7, 2.0; P = .35). No harms were reported with either appliance.
Figure 2.
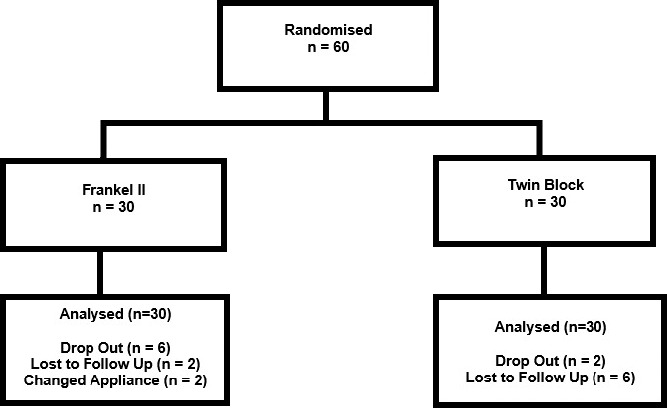
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
Table 1.
Baseline Demographics and Clinical Characteristics for FR2 and MTB Groupsa
| FR2, Mean (SD) |
MTB, Mean (SD) |
Difference (95% CI) |
|
| Age, years | 12.6 (1.4) | 13.1 (1.0) | −0.5 (−1.1, 0.1) |
| Overjet, mm | 10.8 (1.3) | 10.3 (1.3) | 0.6 (−0.1, 1.2) |
| PAR score | 41.8 (10.4) | 42.6 (9.2) | −0.8 (−5.8, 4.3) |
CI indicates confidence interval; SD, standard deviation.
Outcome Measures
All comparisons of outcome measures between the FR2 and MTB groups were adjusted for age, sex, pre-treatment overjet, and pre-treatment PAR.
Primary Outcome Measure
The mean treatment duration in the FR2 group was 376 days (SD: 101) and 340 days (SD: 102) in the MTB group; mean difference: 36 days (95% CI: [−44, 95]). The difference was not statistically significant (P = .41).
Secondary Outcome Measures
Breakages were reported with four (mean 0.3 [SD: 0.7]) FR2 and five (mean 0.4 [SD: 0.8]) MTB appliances (P = .67). Of these, three FR2 and four MTB appliances required replacement and one of each appliance type was repaired.
The mean post-treatment PAR score in the FR2 group was 26.8 (SD: 19.5) and 25.5 (SD: 17.0) in the MTB group; mean difference: 4.1 (95% CI: [−7.3, 15.5]). This difference was not statistically significant (P = .48), although both appliances demonstrated a decrease in mean PAR score from pre-treatment (Table 1) to post-treatment (FR2: 15.0; MTB: 17.1).
There was no significant difference in child OHRQoL or Piers-Harris scores between participants in the two appliance groups when analyses included pre-treatment scores for each questionnaire (all P > .05; Table 2 and Table 3).
Table 2.
Post-treatment Child OHRQoL Domain Scores for FR2 and MTB Groupsa
| Domain |
FR2, Mean (SD) |
MTB, Mean (SD) |
Difference (95% CI) |
P |
| Child OHRQoL | 46.0 (12.8) | 45.4 (11.9) | 1.0 (−6.6, 8.6) | .78 |
| About you | 8.1 (2.9) | 8.3 (3.0) | 0.0 (−2.0, 2.0) | .89 |
| Oral problems | 18.1 (5.1) | 18.4 (4.9) | −0.4 (−3.5, 2.7) | .76 |
| Feelings | 11.0 (4.8) | 10.8 (4.8) | 0.7 (−2.3, 3.7) | .62 |
| Spare-time and activities | 8.8 (3.4) | 7.9 (3.3) | 0.8 (−1.4, 2.9) | .39 |
CI indicates confidence interval; SD, standard deviation.
Table 3.
Post-Treatment Piers-Harris T-scores and SCAN (Most Common Image Chosen and Score) for FR2 and MTB Groupsa
| FR2, Mean (SD) |
MTB, Mean (SD) |
Difference (95% CI) |
P |
|
| Piers-Harris T-scores | ||||
| Total score | 54.3 (12.4) | 54.8 (11.9) | −0.5 (−8.8, 7.8) | .91 |
| Behavioral adjustment | 53.4 (12.0) | 55.8 (10.4) | −1.3 (−8.9, 6.3) | .74 |
| Intellectual and school status | 50.6 (13.0) | 51.8 (12.1) | −0.8 (−9.4, 7.9) | .86 |
| Physical appearance and attributes | 52.5 (12.5) | 50.0 (11.4) | 2.4 (−5.6, 10.4) | .56 |
| Freedom from anxiety | 53.5 (12.3) | 53.9 (12.1) | −1.0 (−8.8, 6.9) | .81 |
| Popularity | 54.7 (13.2) | 53.3 (12.2) | 0.9 (−8.4, 10.2) | .84 |
| Happiness and satisfaction | 52.7 (11.2) | 52.8 (11.4) | 0.2 (−6.9, 7.3) | .95 |
| SCAN most common image chosen | ||||
| Parent | 3 | 3 | ||
| Child | 3 | 3 | ||
| SCAN score | ||||
| Parent | 6.3 (3.1) | 6.2 (2.6) | −0.3 (−2.0, 1.5) | .76 |
| Child | 5.4 (3.2) | 5.9 (2.8) | −0.6 (−2.5, 1.2) | .50 |
CI indicates confidence interval; SD, standard deviation.
No statistically significant differences were found between the two appliance groups for either child or parental perception adjusted for all aforementioned variables including pre-treatment SCAN scores (Table 3). The most commonly chosen photograph in the SCAN assessment pre-treatment by participants and parents was nine and post-treatment was three.
For the OASIS outcome measure, there was no statistically significant difference between the mean total scores for the FR2 (15.3 [SD 7.8]) or MTB (13.6 [SD 6.6]) groups adjusted to include pre-treatment OASIS scores (P = .32) (Table 4).
Table 4.
Post-Treatment OASIS Scores and Treatment Need (n [%]) for FR2 and MTB Groupsa
| FR2, Mean (SD) |
MTB, Mean (SD) |
Difference |
P |
|
| Total score | 15.3 (7.8) | 13.6 (6.6) | 2.3 (−2.2, 6.7) | .32 |
| Treatment need, n (%) | ||||
| No or little need (5-10) | 10 (34) | 13 (44) | ||
| Borderline (11-25) | 16 (54) | 16 (52) | ||
| Great need (26-35) | 4 (12) | 1 (5) |
SD indicates standard deviation.
DISCUSSION
This clinical trial was conducted by specialist orthodontists in a state-funded orthodontic service with participants of one ethnic group. These factors should be considered in conjunction with the findings. Care must be taken when comparing results of the present study with those of others as their end point may have differed and reported functional alone21 or functional followed by fixed appliance phases of treatment.10 Both the FR2 and MTB appliances had comparable Phase 1 treatment duration of Class II division 1 malocclusion in children and adolescents, which was consistent with the findings of studies with similar appliances.10,21,22 The initial and final sample sizes compared favorably with other TBA studies where the intention was to analyze the duration of Phase 1 treatment.10,21 Previous TBA studies recorded dropout rates from 6% to almost 50%.21–23 The percentage of participants in the MTB group lost to follow-up was similar to that recorded formerly20 and the greater discontinuation rate in the FR2 group mirrored that previously reported.24 Despite losses in both appliance groups, the study remained adequately powered for the primary outcome measure.
The design of the MTB differed from that used in other clinical trials.6,10,21 No labial bow was incorporated as it did not influence skeletal or dental outcomes.10 The mandible was advanced in one step for each appliance, which was shown to be as effective as incremental advancement with the TBA.20
The mean treatment duration for the MTB was comparable to the 11.2 months reported with a TBA of different design6 but longer mean treatment duration (14.7–36.5 months) was identified with Frankel appliance treatment.25 Although the monthly rate of overjet reduction was unlikely to be constant, interestingly, with the MTB this was 0.34 mm, which is analogous to 0.23 mm.6
The number of breakages for each treatment group was similar. A high breakage rate, although unspecified, was reported with the Herbst appliance in a previous trial that made comparison to a TBA.6
The mean change in PAR scores for the FR2 and MTB groups indicated an overall “improved” occlusion for each. The mean pre-treatment PAR scores of both groups exceeded those in previous trials,5,6,26 which reflected a greater severity of malocclusion. The mean post-treatment PAR score for the FR2 group (26.8 [SD: 19.5]) was similar to that recorded with this appliance after 12 months (27.27 [SD: 10.29])26 but a greater mean change in PAR score from pre- to post-treatment was recorded in the present trial (15.0 compared with 6.4).
Kadkhoda et al27 found no difference in child OHRQoL for participants wearing the TBA or headgear. The design of the TBA was unspecified, however, in that study and a different version of the questionnaire was used and their sample was of a different ethnic background.
Comparison with previously reported changes in Piers Harris scores is not valid as the post-treatment total scores included functional followed by fixed appliance phases of treatment and it appeared that an earlier version of the questionnaire was used.6 In the current study, the changes in the individual domain scores were similar for both treatment groups.
Before treatment, assessment of the malocclusion by the participant using OASIS indicated a borderline need for treatment (FR2: 63%; MTB: 53%); objective assessment, however, indicated that all but one had a great need for treatment based on overjet.28 The percentage of participants indicating a borderline treatment need had reduced scores post-treatment (FR2: 54%; MTB: 52%) but the difference was not statistically significant between groups. There was no study to which comparisons could be made. The photograph most commonly chosen17 by both parents and children separately (pre-treatment: 9; post-treatment: 3) reflected similarity in patient and parent perception of treatment need on esthetic grounds, the post-treatment score also in agreement with the change in PAR scores.14
This study identified no difference in mean treatment duration between the FR2 and the MTB appliances for Phase 1 treatment of Class II division 1 malocclusion in 11–14 year olds. Where this study was conducted, the FR2 cost double that of the MTB, providing an economic reason to choose one appliance over the other.
Limitations of the Study
Compliance with appliance wear was assessed using standard clinical protocol. Although microsensors have been incorporated in functional appliances as an objective measure of compliance, further development of microsensor software is required.29 A no-treatment control group was not incorporated in this study as the primary aim was to compare Phase 1 treatment duration between two appliances, ie, a head-to-head randomized clinical trial. In addition, it would not have been ethical to include a matched control group with a high treatment need (mean overjet in both groups over 10 mm).
CONCLUSIONS
Phase 1 treatment duration, number of appliance breakages, occlusal outcome, and patient and parent perspectives were similar in 11- to 14-year-olds with Class II division 1 malocclusion treated using the FR2 or MTB appliance.
REFERENCES
- 1.Chestnutt IG, Burden DJ, Steele JG, Pitts NB, Nuttall N, Morris AJ. The orthodontic condition of children in the United Kingdom in 2003. Br Dent J. 2006;200:609–612. doi: 10.1038/sj.bdj.4813640. [DOI] [PubMed] [Google Scholar]
- 2.Petti S. Over two hundred million injuries to anterior teeth attributable to large overjet: a meta-analysis. Dent Traumatol. 2015;31(1):1–8. doi: 10.1111/edt.12126. [DOI] [PubMed] [Google Scholar]
- 3.DiBiase AT, Sandler PJ. Malocclusion, orthodontics and bullying. Dent Update. 2. 28:464–466. doi: 10.12968/denu.2001.28.9.464. 001; [DOI] [PubMed] [Google Scholar]
- 4.Johal A, Cheung MYH, Marcenes W. The impact of two different malocclusion traits on quality of life. Br Dent J. 2007. 27:202(2):E2. [DOI] [PubMed]
- 5.O'Brien K, Wright J, Conboy F, et al. Effectiveness of early orthodontic treatment with the Twin-block appliance: a multicenter, randomized, controlled trial. Part 1 Dental and Skeletal effects. Am J Orthod Dentofacial Orthop. 2003;124(3):234–243. doi: 10.1016/S0889540603003524. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien K, Wright J, Conboy F, et al. Effectiveness of treatment for Class II malocclusion with the Herbst or Twin-block appliances: A randomized, controlled trial. Am J Orthod Dentofacial Orthop. 2003;124(2):128–137. doi: 10.1016/s0889-5406(03)00345-7. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick SM, Banks P, Wright JL. The use of myofunctional appliances in the UK: a survey of British orthodontists. Dent Update. 1998;25(7):302–308. [PubMed] [Google Scholar]
- 8.Frankel R, Frankel C. Orofacial Orthopaedics with the Function Regulator. Basel: Karger; 1989. [Google Scholar]
- 9.Design Clark W. and management of Twin Blocks: reflections after 30 years of clinical use. J Orthod. 2010;37(3):209–216. doi: 10.1179/14653121043110. [DOI] [PubMed] [Google Scholar]
- 10.Yaqoob O, DiBiase AT, Fleming PS, Cobourne MT. Use of the Clark Twin Block functional appliance with and without an upper labial bow: a randomized controlled trial. Angle Orthod. 2012;82(2):363–369. doi: 10.2319/041411-268.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien K, Wright J, Conboy F, et al. Effectiveness of early orthodontic treatment with the Twin-block appliance: a multicenter, randomized, controlled trial. Part 2: Psychosocial effects. Am J Orthod Dentofacial Orthop. 2003;124:488–494. doi: 10.1016/S0889540603006425. [DOI] [PubMed] [Google Scholar]
- 12.Sinha I, Jones L, Smyth RL, Williamson PR. A systematic review of studies that aim to determine which outcomes to measure in clinical trials in children. PLoS Med. 2008;5(4):e96. doi: 10.1371/journal.pmed.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestrini-Biavati A, Alberti G, Silvestrini-Biavati F, Signori A, Castaldo A, Migliorati M. Early functional treatment in class II division 1 subjects with mandibular retrognathia using Frankel II appliance. A prospective controlled study Eur J Paediatr Dent. 2012;13(4):301–306. [PubMed] [Google Scholar]
- 14.Richmond S, Shaw WC, O'Brien KD, et al. The development of the PAR Index (Peer Assessment Rating): reliability and validity. Eur J Orthod. 1992;14(2):125–139. doi: 10.1093/ejo/14.2.125. [DOI] [PubMed] [Google Scholar]
- 15.Locker D, Jokovic A, Tompson B. Health-related quality of life of children aged 11 to 14 years with orofacial conditions. Cleft Palate Craniofac J. 2005;42(3):260–266. doi: 10.1597/03-077.1. [DOI] [PubMed] [Google Scholar]
- 16.Piers EV, Harris DB, Herzberg DS. Piers-Harris Children's Self-Concept Scale, 2nd ed. Torrance, Calif: WPS; 2002. [Google Scholar]
- 17.Evans R, Shaw W. Preliminary evaluation of an illustrated scale for rating dental attractiveness. Eur J Orthod. 1987;9(4):314–318. doi: 10.1093/ejo/9.4.314. [DOI] [PubMed] [Google Scholar]
- 18.Mandall NA, McCord JF, Blinkhorn AS, Worthington HV, O'Brien KD. Perceived aesthetic impact of malocclusion and oral self-perceptions in 14-15-year-old Asian and Caucasian children in greater Manchester. Eur J Orthod. 2002;22(2):175–183. doi: 10.1093/ejo/22.2.175. [DOI] [PubMed] [Google Scholar]
- 19.British Standards Institution. British Standards Glossary of Dental Terms. 1983. BS-4492 London: BSI;
- 20.Banks P, Wright J, O'Brien K. Incremental versus maximum bite advancement during twin-block therapy: a randomized controlled clinical trial. Am J Orthod Dentofacial Orthop. 2004;126(5):583–588. doi: 10.1016/j.ajodo.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Trenouth MJ, Desmond S. A randomized clinical trial of two alternative designs of Twin-block appliance. J Orthod. 2012;39(1):17–24. doi: 10.1179/14653121226788. [DOI] [PubMed] [Google Scholar]
- 22.Thiruvenkatachari B, Sandler J, Murray A, Walsh T, O'Brien K. Comparison of Twin Block and Dynamax appliances for the treatment of Class II malocclusion in adolescents: a randomized controlled trial. Am J Orthod Dentofacial Orthop. 2010;138(2):144.e1–9. doi: 10.1016/j.ajodo.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell S, Cook P. Predicting the outcome of twin block functional appliance treatment: a prospective study. Eur J Orthod. 1999;21(5):533–539. doi: 10.1093/ejo/21.5.533. [DOI] [PubMed] [Google Scholar]
- 24.Ghafari J, Shofer FS, Jacobsson-Hunt U, Markowitz DL, Laster LL. Headgear versus function regulator in the early treatment of Class II, division 1 malocclusion: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 1998;113(1):51–61. doi: 10.1016/s0889-5406(98)70276-8. [DOI] [PubMed] [Google Scholar]
- 25.Perillo L, Cannavale R, Ferro R, et al. Meta-analysis of skeletal mandibular changes during Fränkel appliance treatment. Eur J Orthod. 2011;33(1):84–92. doi: 10.1093/ejo/cjq033. [DOI] [PubMed] [Google Scholar]
- 26.Wijayaratne D, Harkness M, Herbison P. Functional appliance treatment assessed using the PAR index. Aust Orthod J. 2000;16(3):118–126. [PubMed] [Google Scholar]
- 27.Kadkhoda S, Nedjat S, Shirazi M. Comparison of oral-health-related quality of life during treatment with headgear and functional appliances. Int J Paediatr Dent. 2011;21(5):369–373. doi: 10.1111/j.1365-263X.2011.01133.x. [DOI] [PubMed] [Google Scholar]
- 28.Brook PH, Shaw WC. The development of an index of orthodontic treatment priority. Eur J Orthod. 1989;11(3):309–320. doi: 10.1093/oxfordjournals.ejo.a035999. [DOI] [PubMed] [Google Scholar]
- 29.Brierley CA, Benson PE, Sandler J. How accurate are TheraMon® microsensors at measuring intraoral wear-time? Recorded vs. actual wear times in five volunteers. J Orthod. 2017;44(4):241–248. doi: 10.1080/14653125.2017.1365220. [DOI] [PubMed] [Google Scholar]


