Abstract
Objective:
To evaluate the cytotoxicity of stainless-steel orthodontic bands and their influence on the expression of the antioxidant genes in human gingival fibroblasts.
Materials and Methods:
Ten bands of each brand (Dentsply-Sirona, Dentaurum, TP Orthodontics, and Morelli) were conditioned in 0.2 g/mL culture medium at 37°C for 14 days, and the corresponding conditioned media were applied over the fibroblasts. Cell viability was assessed after 24, 48, and 72 hours of exposure to the conditioned media by trypan blue exclusion assay. Expression of the antioxidant defense genes peroxiredoxin 1 (PRDX1), superoxide dismutase 1 (SOD1), and glutathione peroxidase 1 (GPX1) were evaluated by quantitative polymerase chain reaction after 24 hours of exposure. These parameters were compared to those of the cells not exposed to the conditioned media of the bands (control).
Results:
All bands promoted a reduction in the number of viable cells in the periods of 48 and 72 hours (P < .01). Analysis of gene expression showed a significant increase in the levels of PRDX1 transcripts caused by the conditioned media of the Dentsply-Sirona, TP Orthodontics, and Morelli bands (P < .01) as well as induction of SOD1 by the conditioned media of the Dentaurum and Morelli (P < .01). Expression of GPX1 was not influenced by the conditioned media.
Conclusions:
The orthodontic bands showed toxicity to fibroblasts and increased the expression of PRDX1 and SOD1 antioxidant genes, indicating induction of oxidative stress in the cells.
Keywords: Orthodontic bands, Biocompatibility, Orthodontics
INTRODUCTION
Orthodontic bands are devices that have been used in orthodontic treatment since the beginning of the specialty in the late 19th century. They are usually fitted on molars and remain cemented to the teeth until the end of treatment. The alloy most commonly used in orthodontics is stainless steel (AISI 302, 304, 316l), which may contain carbon, silicon, manganese, chromium, nickel, phosphorus, selenium, iron, titanium, zinc, cobalt, molybdenum, and copper.1 This alloy forms a protective layer to the oxy-reduction process in the oral medium, but this barrier is not flawless and is generally dissolved by chemical agents and exposure to oxygen, as well as by contact with the oral environment.2,3
Corrosion of metallic devices in the oral environment is of concern to clinicians since the absorption of released metal ions can lead to local and systemic diseases of unknown magnitude. This situation occurs continuously in the oral environment as a result of abrasion by solid foods, acid diet, use of oral dentifrices and mouthwashes, fluoride, and friction generated during orthodontic mechanics.4 This metal release, especially that of iron and copper, is responsible for the toxicity of orthodontic devices, since they stimulate the production of the most reactive of oxygen-derived species, the hydroxyl radical.5–7 Different cellular enzymatic systems produce superoxide anion radicals by the monoelectronic reduction of molecular oxygen. Once formed, these radicals can be converted to hydrogen peroxide, which, in the presence of transition metals (Fe2+ or Cu1+), gives rise to hydroxyl radicals. Collectively, superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals are known as reactive oxygen species (ROS). They may cause DNA damage, lipid peroxidation, and depletion of sulfhydryls and may lead to cellular death, being associated with degenerative diseases such as cataracts, emphysema, Parkinson's disease, arthritis, diabetes, and cancer.8 To counterbalance these species, the cells are equipped with a complex antioxidant system, which comprises the enzymes superoxide dismutase (SOD), peroxiredoxins (Prxs), and glutathione peroxidase (GPx). SOD accelerates conversion of superoxide anion radicals to hydrogen peroxide, while Prxs and GPx convert hydrogen peroxide to water. Sometimes an overload of the antioxidant mechanism occurs, creating a situation known as “oxidative stress,” and from this stems the generation of diverse damage to biological systems.8 The steps that follow oxidative stress may include adaptation by activating the antioxidant response, tissue damage due to aggression, and cell death by necrosis or apoptosis.8
The role of metal components of orthodontic appliances as mediators of ROS production and cell damage has been studied mainly for brackets and orthodontic archwires, but the bands are the devices that remain longer in the oral cavity during orthodontic treatment. Thus, this study aimed to evaluate the cytotoxicity of stainless-steel orthodontic bands of different commercial brands, as well as their influence on the expression of antioxidant genes in human gingival fibroblasts.
MATERIALS AND METHODS
Orthodontic Bands
Ten molar bands were used from each of the following manufacturers:
Dentsply-Sirona, York, Pa (Ideal);
Dentaurum GmbH & Co, Inspringen, Germany (Dentaform);
TP orthodontics Inc, LaPorte, Ind (GripTite); and
Morelli, Sorocaba/SP, Brazil (universal orthodontic band).
All bands were composed of stainless steel and were sterilized in an autoclave prior to the start of the laboratory tests.
Cell Lines
Human fibroblast cell lines were obtained from the Cell Bank of the São Leopoldo Mandic Institute and Research Center. These cells were previously isolated through primary culture from gums removed from three patients who attended the dental clinic of the institute using the explant technique. Permission to conduct the study was approved by the São Leopoldo Mandic research ethics committee (No.1.303.768). The cells were used for all experiments described in biological triplicate.
Cell Culture
Human fibroblasts were cultured in Dulbecco's minimum modified essential medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic solution (penicillin-streptomycin). The cells were incubated under standard cell culture conditions (37°C, 100% humidity, 95% air, and 5% CO2).
Preparation of Conditioned Medium and Exposure of Cells
The band samples were conditioned in DMEM medium at a ratio of 0.2 g/mL, according to the International Organization for Standardization, at 37°C for 14 days. After the conditioning period, cells at the density of 110 cells/mm2 were cultured with conditioned medium for 24, 48, and 72 hours at 37°C. As a control, the cells were cultured in DMEM medium without prior exposure to the materials to be tested.
Cell Proliferation Assay
For the evaluation of cell proliferation, the vital trypan blue exclusion method was used. After reaching subconfluency, the cells were enzymatically removed from the plates, and the cell pellet resulting from the centrifugation was suspended in 1 mL of medium. Ten microliters of the cell suspension was added to 10 μL of trypan blue, and 1 μL of this solution was placed in a hemocytometer (Neubauer-Fisher Scientific, Pittsburgh, Pa) and examined under a phase microscope (Nikon, Eclipse TS100, Tokyo, Japan) for counting and observation of cells.
Evaluation of the Expression of Antioxidant Genes
RNA extraction.
Total RNA was extracted from 1 × 106 cells using the Trizol reagent (ThermoFisher Scientific, MA, USA) according to the manufacturer's instructions. Briefly, the cells were collected and homogenized with 1 mL of Trizol, and separation of the aqueous and organic phases was performed with the addition of chloroform (0.2 mL) followed by centrifugation (12,000 g, 15 minutes, 4°C). RNA was precipitated from the aqueous phase with 0.5 mL of isopropanol (12,000 g, 15 minutes, 4°C), washed with 75% ethanol, and suspended in water.
Reverse transcription.
One-microgram RNA samples were treated with 1U DNAse I. Complementary DNA (cDNA) synthesis by reverse transcription was performed using the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, MA, USA) reagent, according to the manufacturer's instructions. Briefly, reactions occurred from 1 μg of RNA, 0.5 μg of oligo (dT) 18, 1 mM of the dNTP mix, 200 U RevertAid H Minus M-MuLV Transcriptase, and 20 U of RiboLockRNAse Inhibitor at 42°C for 60 minutes. The reactions were then terminated by heating at 70°C for 5 minutes.
Real-time or quantitative polymerase chain reaction.
The amplification reactions occurred from 40 ng of cDNA and 0.3 μM of pairs of primers for antioxidant defense genes peroxiredoxin 1 (PRDX1), superoxide dismutase 1 (SOD1), and glutathione peroxidase 1 (GPX1) (Table 1), added to the Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific, MA, USA). Reaction conditions were as follows: 10 minutes at 95°C, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute, using the 7500 Fast Real Time PCR System (Thermo Fisher Scientific, MA, USA). The levels of expression were quantified using the SDS System program (Thermo Fisher Scientific, MA, USA), and the relative expression among the samples was calculated according to the method of comparison of Ct (threshold cycle), based on the formula 2−ΔΔCt. For the normalization of expression levels, the GAPDH gene was used.
Table 1.
Analyzed Genes and Sequences of the Primers Used
| Gene Name |
Gene Symbol |
Gene Bank (NM) |
Sequence of Primers |
| Peroxiredoxin I | PRDX1 | 181696.1a 181697.1a 002574.2a | F 5′′-GGATTCTCACTTCTGTCATCTAGCA-3′ R 5′-TGTTCATGGGTCCCAGTCCT-3′ |
| Glutathione Peroxidase I | GPX1 | 000581.2a 201397.1a | F 5′-CCGACCCCAAGCTCATCA-3′ R 5′-GAAGCGGCGGCTGTACCT-3′ |
| Superoxide dismutase 1 | SOD1 | 000454.4 | F 5′-AGGTCCTCACTTTAATCCTCTATCCA-3′ R 5′-ACCATCTTTGTCAGCAGTCACATT -3′ |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 002046.3 | F 5′-ACCCACTCCTCCACCTTTGA-3′ R 5′-TGTTGCTGTAGCCAAATTCGTT-3′ |
Variants of transcripts.
Statistical Analysis
Results were expressed as mean and standard deviation and were evaluated by analysis of variance (one criteria) followed by the Bonferroni test for multiple comparisons, with a significance level of 5%.
RESULTS
Cell Viability
To assess the toxicity of the orthodontic bands, fibroblasts were exposed to the respective conditioned media and the number of viable cells was determined. The results demonstrated that none of the extracts from the bands significantly influenced cell viability in the 24-hour exposure period (Figure 1). However, all the conditioned media promoted a reduction in the number of viable cells, compared to the control group, in the periods of 48 and 72 hours (P < .05) (Figure 1). This reduction was observed to a greater degree for the conditioned media from the Dentsply-Sirona and Morelli bands (Figure 1).
Figure 1.
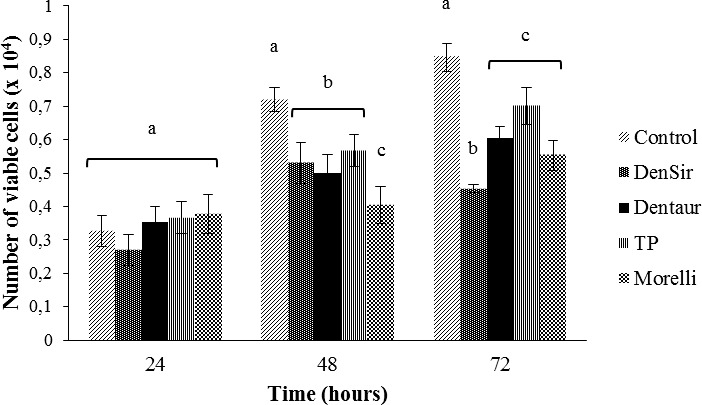
Influence of the conditioned medium of orthodontic bands on the viability of human gingival fibroblasts. Gingival fibroblasts were exposed to the conditioned media of Dentsply-Sirona (DenSir), Dentaurum (Dentaur), TP Orthodontics (TP), and Morelli bands or to the culture medium (control) for 24, 48, and 72 hours, and the number of viable cells was obtained by the vital trypan blue exclusion test. The data represent means and standard deviations. Different letters indicate statistically significant differences (P < .05) among the control and conditioned media from the bands within each time interval.
Gene Expression
To evaluate the ability of orthodontic bands to induce oxidative stress and antioxidant adaptive cellular response, expression analysis of PRDX1, SOD1, and GPX1 antioxidant genes was performed in fibroblasts exposed to the conditioned media of the bands, and the levels were compared to those of the cells not exposed (control). This analysis revealed a significant increase in the levels of PRDX1 transcripts caused by conditioned media of the Dentsply-Sirona, TP Orthodontics, and Morelli bands (P < .01), as well as induction of SOD1 by conditioned media of the Dentaurum and Morelli bands (P < .01) (Figure 2). The highest levels of expression of these genes were observed with exposure to the Morelli bands conditioned medium (Figure 2). Expression of GPX1 was not significantly influenced by any of the media (Figure 2).
Figure 2.
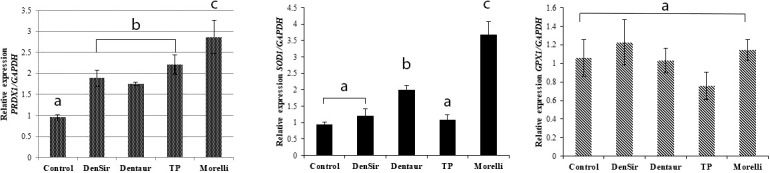
Influence of the conditioned medium of orthodontic bands on the expression of antioxidant genes in human gingival fibroblasts. Gingival fibroblasts were exposed to the conditioned media of the Dentsply-Sirona (DenSir), Dentaurum (Dentaur), TP Orthodontics (TP), and Morelli bands or to the culture medium (control) for 24 hours, and the expression of PRDX1, SOD1, and GPX1 was assessed by quantitative polymerase chain reaction. The data represent means and standard deviations. Different letters indicate statistically significant differences (P < 5%) among the control and conditioned media from the bands.
DISCUSSION
The orthodontic literature presents few reports about the toxicity and oxidative stress generated by orthodontic bands in cells of the oral cavity, which prompted development of this in vitro study. Human gingival fibroblasts were chosen for this study because this type of cell is one of the most abundant in the oral cavity, clinically exposed to potential toxic effects of orthodontic appliances during treatment. This was shown by other studies1,9–13 that evaluated the cytotoxicity of orthodontic materials composed of stainless steel using human or rat fibroblast cell lines. The results showed that all the conditioned media from the orthodontic bands promoted a reduction in the number of viable cells in the periods of 48 and 72 hours compared to the control group. The literature14,15 suggests that the reduction in cell viability occurred as a result of metallic ion release when orthodontic bands are present in the buccal environment. This is mainly due to the presence of iron, copper, zinc, and nickel ions, which are capable of altering cell metabolism, with the ion iron, followed by copper, being the most frequently found, according to previous studies.10,14,16,17
The analysis of the activation of oxidative stress response evidenced a significant increase in the levels of PRDX1 transcripts caused by the conditioned media of the Dentsply Sirona, TP Orthodontics, and Morelli bands, as well as induction of SOD1 by the conditioned media of the Dentaurum and Morelli bands. The induction of these antioxidant genes suggested an attempt by the cells to counteract ROS production, most likely favored by the release of metals such as iron, copper, and chromium from the orthodontic bands. However, the suggested activation of the oxidative stress response seemed to be not entirely efficient, so a reduction of the number of viable cells was observed. Thus, these results advocate that the band cytotoxicity was related, at least in part, to the formation of ROS.
ROS-mediated cytotoxicity of orthodontic bands was also suggested by Gonçalves et al.,14 who compared the cytotoxicity, genotoxicity, and detachment of metal ions from stainless-steel orthodontic bands, with or without silver soldered joints, in the HepG2 and HOK cell lines. The quantification of the metals was performed by atomic absorption spectroscopy. The MTT assay was used to evaluate cytotoxicity, and DNA damage was evaluated by the Comet test. Their results14 showed that the higher levels of nickel and iron ion detachment from the bands with silver solder were correlated with the higher genotoxicity and cytotoxicity effects observed for these bands, when compared to the bands without silver solder.
Studies that evaluated the biological effects of stainless-steel orthodontic devices other than bands also supported the role of metal-mediated ROS production in cytotoxicity. Ortiz et al.1 evaluated the release of metal ions from brackets and orthodontic tubes made of stainless steel, titanium, and nickel-free alloys, as well as their toxicity and DNA damage to human fibroblasts. The authors detected the release of titanium, chromium, manganese, cobalt, nickel, molybdenum, iron, copper, and zinc ions, mainly from stainless-steel brackets, which led to a significant decrease in cell viability, reaching 4.60%. Additionally, greater DNA damage was observed for groups containing stainless-steel and nickel-free brackets.1 Orthodontic archwires made of stainless steel, with and without esthetic coating, were also demonstrated11 to be toxic to human gingival fibroblasts. Taken together, the current results and those of previous work11 indicated that regardless of the orthodontic device, the fact that they are made of stainless steel seems to determine that there will be a deleterious biological effect. This information may be valuable for clinicians as they are choosing which orthodontic appliances to use during treatment.
It has been suggested4,18 that in vivo studies are crucial because the human mouth provides a hostile environment that favors corrosion of fixed orthodontic appliances. As an electrolyte and medium for chemical reactions between metals, saliva can cause corrosion. Though it is constantly renewed, the metallic appliance remains in the oral cavity, so the potential to release metal ions is continuous. This release may even be stimulated by thermal and pH alterations in the mouth, in addition to exposure to foods and drinks, microbial activity, mechanical loads, and abrasion.4,18 Singh et al.19 showed a significant increase in salivary nickel and chromium concentrations at 1 week and 3 weeks after insertion of fixed orthodontic appliances. Additionally, orthodontic treatment itself may turn the oral environment into a pro-corrosion state, as a significant increase in total colony counts of Candida albicans, Streptococcus mutans, and Lactobacillus acidophilus and a significant saliva pH decrease were observed20 after 6, 12, and 18 weeks of fixed orthodontic treatment. Regarding the effect on buccal mucosa cells, results from a longitudinal in vivo study21 showed that compared to the cells collected before treatment, fixed orthodontic appliances decreased cellular viability, induced DNA damage, and increased the nickel and chromium contents of the buccal mucosa cells collected at 3, but not at 6, months after appliance placement. The authors suggested that the adaptive capacity of the cells, possibly through the induction of defense and repair molecules, could explain the reverse in effects from 3 to 6 months. Part of this defense was evaluated in the current study with regard to induction of three antioxidant enzymes. Even considering the limitations of each model (ie, lack of saliva turnover in the in vitro model and biological variations among patients and among cell types in the in vivo model), taken together, the results from studies using both models were in agreement regarding the toxicity of orthodontic appliances.
The present study evaluated four different brands of orthodontic bands, demonstrating cytotoxicity and induction of the oxidative stress response in human gingival fibroblasts. The literature on this topic is quite consistent with the current study results in reporting DNA damage and decreased cell viability using different cell lines in in vitro studies involving brackets and metal orthodontic arches. Although the benefit of using orthodontic appliances is undeniable, the evaluation of different brands is essential to guide clinicians in the choice of safer orthodontic appliances.
CONCLUSION
Orthodontic bands showed toxicity to fibroblasts and increased the expression of the antioxidant genes PRDX1 and SOD1, indicating induction of the oxidative stress response in human gingival fibroblasts.
REFERENCES
- 1.Ortiz AJ, Fernández E, Vicente A, et al. Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: toxicity and DNA damage. Am J Orthod Dentofacial Orthop. 2011;140:e115–e122. doi: 10.1016/j.ajodo.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Kerosuo H, Hensten-Pettersen A. Salivary nickel and chromium in subjects with different types of fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 1997;111:595–598. doi: 10.1016/s0889-5406(97)70310-x. [DOI] [PubMed] [Google Scholar]
- 3.Oh KT, Kim KM. Iron release and cytotoxicity of stainless steel wires. Eur J Orthod. 2005;27:533–540. doi: 10.1093/ejo/cji047. [DOI] [PubMed] [Google Scholar]
- 4.Huang TH, Ding SJ, Min Y, et al. Metal ion release from new and recycled stainless steel brackets. Eur J Orthod. 2004;26:171–177. doi: 10.1093/ejo/26.2.171. [DOI] [PubMed] [Google Scholar]
- 5.David A, Lobner D. In vitro cytotoxicity of orthodontic archwires in cortical cell cultures. Eur J Orthod. 2004;26:421–426. doi: 10.1093/ejo/26.4.421. [DOI] [PubMed] [Google Scholar]
- 6.Spalj S, Zrinski MM, Spalj VT, et al. In-vitro assessment of oxidative stress generated by orthodontic archwires. Am J Orthod Dentofacial Orthop. 2012;141:583–589. doi: 10.1016/j.ajodo.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Free Radical in Biology and Medicine 4th ed. Oxford: Oxford University Press USA; 2006. [Google Scholar]
- 9.Retamoso LB, Luz TB, Marinowic DR, et al. Cytotoxicity of esthetic, metallic, and nickel-free orthodontic brackets: cellular behavior and viability. Am J Orthod Dentofacial Orthop. 2012;142:70–74. doi: 10.1016/j.ajodo.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Freitas MP, Oshima HM, Menezes LM, et al. Cytotoxicity of silver solder employed in orthodontics. Angle Orthod. 2009;79:939–944. doi: 10.2319/101108-530.1. [DOI] [PubMed] [Google Scholar]
- 11.Rongo R, Valletta R, Bucci R, et al. In vitro biocompatibility of nickel-titanium esthetic orthodontic archwires. Angle Orthod. 2016;86:789–795. doi: 10.2319/100415-663.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Issa Y, Brunton P, Waters C, et al. Cytotoxicity of metal ions to human oligodendroglial cells and human gingival fibroblasts assessed by mitochondrial dehydrogenase activity. Dent Mater. 2008;24:281–287. doi: 10.1016/j.dental.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Buljan ZI, Ribaric SP, Abram M, et al. In vitro oxidative stress induced by conventional and self-ligating brackets. Angle Orthod. 2012;82:340–345. doi: 10.2319/061811-395.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonçalves TS, de Menezes LM, Trindade C, et al. Cytotoxicity and genotoxicity of orthodontic bands with or without silver soldered joints. Mutat Res Genet Toxicol Environ Mutagen. 2014;762:1–8. doi: 10.1016/j.mrgentox.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Mockers O, Deroze D, Camps J. Cytotoxicity of orthodontic bands, brackets and archwires in vitro. Dent Mater. 2002;18:311–317. doi: 10.1016/s0109-5641(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 16.Wataha JC, Lockwood PE, Schedle A. Effect of silver, copper, mercury, and nickel ions on cellular proliferation during extended, low-dose exposures. J Biomed Mater Res. 2000;52:360–364. doi: 10.1002/1097-4636(200011)52:2<360::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Schmalz G, Garhammer P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002;18:396–406. doi: 10.1016/s0109-5641(01)00063-x. [DOI] [PubMed] [Google Scholar]
- 18.Eliades T, Bourauel C. Intraoral aging of orthodontic materials: the picture we miss and its clinical relevance. Am J Orthod Dentofacial Orthop. 2005;127:403–412. doi: 10.1016/j.ajodo.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Singh DP, Sehgal V, Pradhan KL, et al. Estimation of nickel and chromium in saliva of patients with fixed orthodontic appliances. World J Orthod. 2008;9:196–202. [PubMed] [Google Scholar]
- 20.Arab S, Nouhzadeh Malekshah S, Abouei Mehrizi E, et al. Effect of fixed orthodontic treatment on salivary flow, pH and microbial count. J Dent (Tehran) 2016;13:18–22. [PMC free article] [PubMed] [Google Scholar]
- 21.Hafez HS, Selim EMN, Eid FHK, et al. Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: a longitudinal in-vivo study. Am J Orthod Dentofacial Orthop. 2011;140:298–308. doi: 10.1016/j.ajodo.2010.05.025. [DOI] [PubMed] [Google Scholar]


