Abstract
Introduction
Catheter management strategies for suspected catheter-related bloodstream infection (CRBSI) remain a major challenge in intensive care units (ICUs). The objective of this study was to determine the incidence, risk factors, and mortality attributable to CRBSIs in those patients.
Methods
A population-based surveillance on suspected CRBSI was conducted from 2009 to 2018 in a tertiary care hospital in China. We used the results of catheter tip culture to identify patients with suspected CRBSIs. Demographics, systemic inflammatory response syndrome (SIRS) criteria, interventions, and microorganism culture results were analysed and compared between patients with and without confirmed CRBSIs. Univariate and multivariate analyses identified the risk factors for CRBSIs, and attributable mortality was evaluated with a time-varying Cox proportional hazard model.
Results
In total, 686 patients with 795 episodes of suspected CRBSIs were included; 19.2% (153/795) episodes were confirmed as CRBSIs, and 17.4% (119/686) patients died within 30 days. The multifactor model shows that CRBSIs were associated with fever, hypotension, acute respiratory distress syndrome, hyperglycaemia and the use of continuous renal replacement therapy. The AUC was 77.0% (95% CI 73.3%–80.7%). The population attributable mortality fraction of CRBSI in patients was 18.2%, and mortality rate did not differ significantly between patients with and without CRBSIs (95% CI 0.464–1.279, P = 0.312).
Conclusions
This initial model based on the SIRS criteria is relatively better at identifying patients with CRBSI but only in domains of the sensitivity. There were no significant differences in attributable mortality due to CRBSI and other causes in patients with suspected CRBSI, which prompt catheter removal and re-insertion of new catheter may not benefit patients with suspected CRBSIs.
Trial Registration
China Clinical Trials Registration number; ChiCTR1900022175.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-021-00429-3.
Keywords: Catheter-related bloodstream infections, Central venous catheter, Intensive care units, Mortality, Risk factor, Systemic inflammatory response syndrome
Key Summary Points
| Why carry out this study? |
| Catheter management strategies for suspected catheter-related bloodstream infection (CRBSI) remain a major challenge. |
| There is an urgent need to develop strong practical evidence to identify CRBSI for prevent unnecessary catheter removal and subsequent harm to patients. |
| To knowledge the attributable mortality of CRBSI is important that a key factor to the decisions made regarding the suspected CRBSIs management strategy in patients, but it is remains uncertain. |
| Why carry out this study? |
| This initial model based on the SIRS criteria is relatively better at identifying patients with CRBSI but only in domains of the sensitivity. |
| There were no significant differences in attributable mortality due to CRBSI and other causes in patients with suspected CRBSI, which prompt catheter removal and re-insertion of new catheter may not benefit patients with suspected CRBSIs. |
Digital Feautres
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14125796.
Introduction
Catheter-related bloodstream infections (CRBSIs) are common intensive care unit (ICU)-acquired infections [1,2], which have a significant effect on morbidity, mortality and associated health care costs [3–5]. CRBSIs are an important type of infection in ICU patients with sepsis [2], which is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [6], which compromises host homeostasis by the induction of the iron-sequestering ferritin H chain in response to polymicrobial infections and causes systemic inflammatory response syndrome (SIRS) [7,8]. However, to date, there is a lack of specific and reliable data to show that the indicators that are sensitive for the diagnosis of suspected sepsis can be used to identify CRBSI due to suspected but unconfirmed CRBSI. In addition, the attributable mortality to CRBSI remains uncertain and ranges from − 12.24% to 25.96% [9]; the mortality is an important factor affecting the decisions made regarding the central venous catheters (CVCs) management strategy in patients with suspected CRBSIs [2,9–11]. A recent systematic review reported that there was no robust evidence to guide the selection of the management strategy for patients with suspected CRBSIs [1], and knowledge of the attributable mortality of CRBSI is important.
The primary objective of this study was to determine the incidence of, risk factors for, and mortality attributable to CRBSIs in ICU patients with suspected but unconfirmed CRBSIs. Additionally, we also assessed the incidence of and mortality attributable to other infections during the same episodes.
Methods
Study Design and Participants
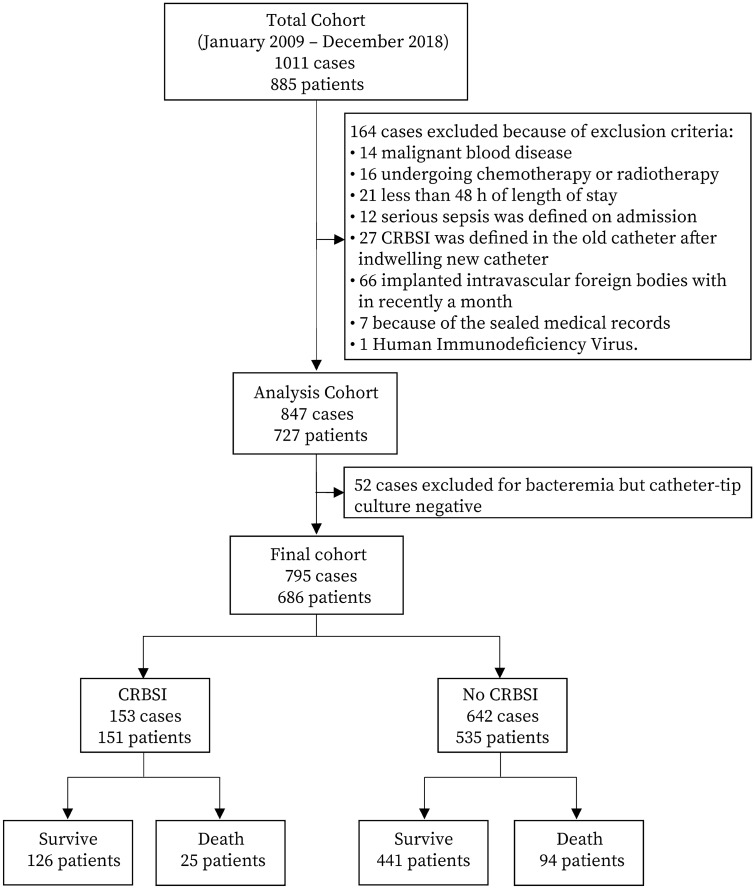
This was a retrospective cohort study conducted at the Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China. The ethics committee of institution approved this study and waived the need for informed consent on March 13, 2019 (PJ2018-066). This study was registered with the China Clinical Trials Registry on March 28, 2019 (ChiCTR1900022175). From January 2009 to December 2018. Antimicrobial catheters were not used in this ICU. According to the clinical practice guidelines of the Infectious Diseases Society of America (IDSA), catheter cultures were performed when a catheter was removed due to a suspected CRBSI. [12] We analyzed all data from patients aged ≥ 18 years who had suspected CRBSIs who had cultures performed of their catheter tips. The following exclusion criteria were used: malignant blood disease, chemotherapy or radiotherapy in the same period, length of stay less than 48 h, serious sepsis diagnosed on admission, CRBSI diagnosed of the old catheter after the reinsertion of new catheter, implantation of intravascular foreign bodies within the past month, and human immunodeficiency virus (Fig. 1).
Fig. 1.
Flow diagram and control patients for analysis
Variables and Data Sources
We used the medical charts and laboratory electronic database to collect demographic data; diagnoses; comorbidities; disease status on ICU admission; CVC information, treatment interventions; clinical symptoms; and laboratory values at the onset of the suspected CRBSI; the results of microbial cultures within 24 h before catheter removal; and 30-day mortality. Two classification systems were used to assess patients with times between ICU admission and the development of a suspected CRBSI: The Acute Physiology and Chronic Health Evaluation (APACHE) II score and Sequential Organ Failure Assessment (SOFA) score [2,6].
To assess the possible risk factors, we used the transformed data that were stratified according to the SIRS criteria of International Sepsis Definitions Conference (ISDC), including five aspects variable: general variable, inflammatory variables, hemodynamic variables, organ dysfunction variables, tissue perfusion variables. [13] However, for plasma glucose levels, we used the highest mean level three days before CVC removal, and the transformed data were stratified according to the values recommended in the international guidelines, with a plasma glucose target ≤ 10 mmol/L [14].
The outcome of this study was 30-day mortality in patients with suspected CRBSIs, and the mortality after 30 days was considered to be less likely to be related to CRBSIs [11,12].
Definitions
Catheter tip colonization was defined as a catheter tip with 15 or more colony-forming units [11]. The CRBSI definition adhered to the IDSA guidelines [12], which required the catheter tip to be colonized by a microorganism that was phenotypically the same as a microorganism isolated from a peripheral blood culture. A suspected CRBSI was identified when a patient developed a new episode of fever or sepsis [11], with at least 1 additional parameter described in the 2001 ISDC guidelines noted in the progress notes in the medical charts [13]. Fever was defined as a temperature > 38.3 °C[13]. Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, as in the Third International Consensus Definitions [6].
Study Sample Size
The study sample included patients aged ≥ 18 year who were suspected CRBSI from January 2009 to December 2018. All available patients were included: 642 in the non-CRBSI group and 153 in the CRBSI group. Assuming a mortality rate of 25.96% with CRBSI [15] and 10.9% with ICU-acquired infections [2], we calculated that group sample sizes of 209 in non-CRBSI group and 105 in CRBSI group achieve 80% power to detect a ratio in the group proportions of 0.5 by the PASS 11 software (NCSS, LLC. Kaysville, Utah, USA. www.ncss.com.).
Statistical Analysis
Categorical variables are shown as numbers (%), and continuous parameters are shown as the means (SDs) or medians (IQRs). Comparisons were made with Fisher’s exact test for categorical data and the Wilcoxon test for continuous data. In the final cohort, 52 cases of bacteraemia were excluded based on the absence of catheter colonization; these cases were considered possible deviations from the diagnosis of bacteraemia based on the analysis of the clinical symptoms. For the survival analysis, all patients were analyzed in aggregate and stratified by survival status at 30 days, and the last data were used as covariates when one patient had two or more episodes of suspected CRBSIs.
The hypothesis of this study was that the attributable mortality of CRBSI might be higher than other infections. The statistical analysis was performed in three steps. First, we used univariate and multivariate analyses to determine the risk factors for CRBSI. The quartiles of APACHE II scores and of SOFA scores per specific patient population were used as covariables [2,6]. A multivariable generalized linear model was used to evaluate the risk factors for CRBSI. A nonparametric receiver operating characteristic curve was constructed, and the area under the curve (AUC) was calculated. In the second step, we evaluated the mortality associated with CRBSI in the entire cohort using a time-varying Cox proportional hazard model [16], and the fraction of attributable mortality was used after the final model estimation command, the parameters of which were interpreted as log rate ratios [17]. Third, we assessed the incidence of and mortality attributable to other infections in patients with suspected CRBSIs during the same period. All statistical tests were two-tailed, and significance was set at α = 0.05.
The statistical analyses were performed with Stata/SE 15.1 (Stata Corp LLC 4905 Lakeway Drive College Station, TX 77,845 USA).
Results
Baseline Characteristics
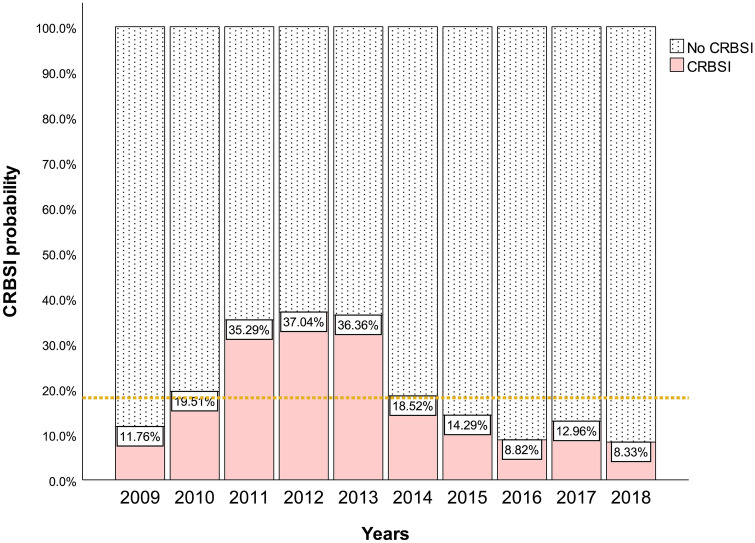
A total of 7653 critically ill patient with 12,245 CVCs were admitted to the ICU, among which CVC removal was undergone in 5623 (45.9%) cases for various reasons. Among 1011 (18.0%) cases whose CVCs were removed due to suspected CRBSI, 164 cases were excluded because they met the exclusion criteria. We studied 686 patients with 795 ICU episodes in the final analysis (Fig. 1); 93 patients had at least 2 or more episodes of suspected CRBSIs. Of all episodes of suspected CRBSIs, 153 (19.2%) were defined as CRBSIs based on microorganism culture results from among 627 (78.9%) documented infections. Figure 2 shows that the range of suspected CRBSIs has expanded, but the rate of confirmed CRBSIs has trended downward, especially in 2018, in which only 8.3% of suspected CRBSIs were confirmed. In addition, regarding the pathogenic microorganisms causing CRBSIs, we found 40 (25.5%) gram-positive cocci, 79 (50.3%) gram-negative bacilli and 38 (24.2%) fungi; 4 of the 153 cases of CRBSIs had multiple microbes colonizing the same catheter tip (Supplemental e-Table 1).
Fig. 2.
The incidence of CRBSI in patients with suspected CRBSI from 2009 to 2018. The percentage expression of the documented CRBSI cases. The horizontal dashed line shows the average documented rate of CRBSI in ten years. CRBSI: Catheter-Related Bloodstream Infection
Risk Factors Analysis
A total of 795 ICU episodes were included in the analysis of the risk factors for CRBSI in patients with suspected CRBSIs. We did not find significant differences between the CRBSI and non-CRBSI groups in the baseline cohort with regard to age, sex, or admission diagnosis (Supplemental e-Table 2). We used a multifactor generalized linear model to assess the risk factors for CRBSI based on the results of univariate analysis (Table 1). The model showed an elevated risk of CRBSI in patients with fever (> 38.3 °C), arterial hypotension (mean arterial pressure [MAP] < 70 mmHg), hyperglycemia (plasma glucose > 10 mmol/L), acute respiratory distress syndrome (ARDS), and the use of continuous renal replacement therapy (CRRT). However, a lower risk of CRBSI was observed in patients with diabetes mellitus (95% CI 0.194–0.644, P = 0.001) and creatinine ≥ 133 mmol/L (95% CI 0.173–0.538, P < 0.001). The AUC of the model was 77.0% (95% CI 73.3%-80.7%). In the expanded baseline cohort, patients in whom CVCs were reinserted (within 24 h) had a higher relative risk of CRBSIs than those in whom CVCs were not reinserted (30.0% vs 19.2%, 95% CI 1.145–2.121, P = 0.007) (Supplemental e-Table 2). Hence, it may be necessary to perform a more comprehensive assessment of the clinical significance of the relative risk in patients in whom CVCs are reinserted.
Table 2.
Baseline cohort of patients who survived at 30 days after CVC remval due to suspected CRBSI
| Baseline characteristics | All patients (n = 686) | Survive (n = 567) | Death (n = 119) | RR (95% CI) | P-value |
|---|---|---|---|---|---|
| Male | 503(73.3) | 414(73.0) | 89(74.8) | 1.079(0.740–1.574) | 0.6908 |
| Age 1st quartile (18–51) | 173(25.2) | 154(27.2) | 19(16.0) | 0.563(0.356–0.892) | 0.0106 |
| Age 2nd quartile (52–66) | 174(25.4) | 153(27.0) | 21(17.6) | 0.631(0.407–0.978) | 0.0333 |
| Age 3rd quartile (67–77) | 186(26.9) | 154(27.2) | 32(26.9) | 0.989(0.684–1.429) | 0.9520 |
| Age 4th quartile (78–97) | 153(22.3) | 106(18.7) | 47(39.5) | 2.274(1.650–3.133) | 0.0000 |
| Medical | 400(58.3) | 307(54.1) | 93(78.2) | 2.558(1.702–3.844) | 0.0000 |
| Surgical | 187(27.3) | 172(30.3) | 15(12.6) | 0.385(0.230–0.644) | 0.0001 |
| Traumatology | 99(14.4) | 88(15.5) | 11(9.2) | 0.604(0.337–1.081) | 0.0765 |
| APACHE 1st quartile (2–15) | 195(28.4) | 177(31.2) | 18(15.1) | 0.449(0.280–0.720) | 0.0004 |
| APACHE 2nd quartile (16–19) | 149(21.7) | 126(22.2) | 23(19.3) | 0.863(0.569–1.311) | 0.4863 |
| APACHE 3rd quartile (20–25) | 208(30.3) | 174(30.7) | 34(28.6) | 0.919(0.640–1.321) | 0.6479 |
| APACHE 4th quartile (26–46) | 134(19.5) | 90(15.9) | 44(37.0) | 2.417(1.754–3.331) | 0.0000 |
| Chronic Obstructive Pulmonary Disease | 95(13.8) | 74(13.1) | 21(17.6) | 1.333(0.877–2.026) | 0.1870 |
| Acute Respiratory Distress Syndrome | 76(11.1) | 67(11.8) | 9(7.6) | 0.657(0.348–1.241) | 0.1789 |
| Diabetes mellitus | 121(17.6) | 92(16.2) | 29(24.4) | 1.505(1.040–2.177) | 0.0341 |
| Malignancy | 73(10.6) | 62(10.9) | 11(9.2) | 0.855(0.483–1.514) | 0.5865 |
| Chronic renal failure | 23(3.4) | 14(2.5) | 9(7.6) | 2.358(1.378–4.037) | 0.0050 |
| Corticosteroids | 141(20.6) | 107(18.9) | 34(28.6) | 1.546(1.087–2.198) | 0.0173 |
| Anticoagulant | 203(29.6) | 159(28.0) | 44(37.0) | 1.396(0.999–1.950) | 0.0523 |
| Antibiotics (Combined use [> 2 types]) | 479(69.8) | 382(67.4) | 97(81.5) | 1.905(1.235–2.938) | 0.0022 |
| Tracheostomy | 292(42.6) | 246(43.4) | 46(38.7) | 0.850(0.607–1.190) | 0.3427 |
| Artificial respiration | 437(63.7) | 346(61.0) | 91(76.5) | 1.852(1.249–2.746) | 0.0014 |
| Positive end expiratory pressure | 373(54.4) | 288(50.8) | 85(71.4) | 2.098(1.452–3.032) | 0.0000 |
| CVC removal with new catheter | 484(70.8) | 381(67.2) | 103(86.6) | 2.687(1.629–4.432) | 0.0000 |
| SOFA score 1st quartile (1–8) | 192(28.0) | 174(30.7) | 18(15.1) | 0.459(0.286–0.736) | 0.0006 |
| SOFA score 2nd quartile (9–10) | 186(27.1) | 161(28.4) | 25(21.0) | 0.715(0.476–1.075) | 0.0994 |
| SOFA score 3 rd quartile (11–13) | 177(25.8) | 142(25.0) | 35(29.4) | 1.198(0.840–1.709) | 0.3222 |
| SOFA score 4 th quartile (14–24) | 131(19.1) | 90(15.9) | 41(34.5) | 2.227(1.606–3.087) | 0.0000 |
| CRBSI | 151(22.0) | 126(22.2) | 25(21.0) | 0.942(0.063–1.409) | 0.7714 |
| Pneumonia | 412(60.1) | 337(59.4) | 75(63.0) | 1.134(0.807–1.592) | 0.4673 |
| Abdominal Infection | 19(2.8) | 14(2.5) | 5(4.2) | 1.540(0.712–3.328) | 0.2951 |
| Gastrointestinal infection | 10(1.5) | 8(1.4) | 2(1.7) | 1.156(0.331–4.035) | 0.8234 |
| Soft tissue infections | 13(1.9) | 11(1.9) | 2(1.7) | 0.885(0.245–3.200) | 0.8504 |
| Urinary tract infections | 41(6.0) | 29(5.1) | 12(10.1) | 1.764(1.063–2.927) | 0.0376 |
| Central nervous system infections | 11(1.6) | 11(1.9) | 0 | – | 0.1256 |
| Chest infection | 1(0.1) | 1(0.2) | 0 | – | 0.6466 |
| Biliary tract infection | 2(0.3) | 1(0.2) | 1(0.8) | 2.900(0.718–11.701) | 0.2220 |
| Multiple infections | 126(18.4) | 102(18.0) | 24(20.2) | 1.123(0.750–1.682) | 0.5768 |
| Unknown | 146(21.3) | 124(21.9) | 22(18.5) | 0.839(0.548–1.283) | 0.4125 |
Survive group and death group according to 30 days follow up
APACHE acute physiology and chronic health evaluation, CI confidence interval, CRBSI catheter-related bloodstream infection, CVC central venous catheter, IQR interquartile ranges, RR relative risk, SD standard deviation, SOFA sequential organ failure assessment, PaO2 oxygen partial pressure
Table 1.
Generalized linear models for risk factors for CRBSI in patients due to suspected CRBSI
| Robust Std. Err | z-Value | RR (95% Conf. Interval) | P-value | |
|---|---|---|---|---|
| Admission | ||||
| APACHE1st quartile (2–14) (reference) | 1 | 1 | 1 | 1 |
| APACHE2nd quartile (15–18) | 0.301 | − 0.07 | 0.977(0.534–1.788) | 0.940 |
| APACHE3rd quartile (19–24) | 0.402 | 0.8 | 1.285(0.696–2.371) | 0.423 |
| APACHE4th quartile (25–46) | 0.541 | 1.71 | 1.714(0.924–3.182) | 0.088 |
| Comorbidities | ||||
| Acute Respiratory Distress Syndrome | 0.684 | 2.52 | 2.194(1.191–4.042) | 0.012 |
| Diabetes mellitus | 0.108 | − 3.4 | 0.354(0.194–0.644 | 0.001 |
| Kidney injury | 0.209 | − 1.74 | 0.421(0.159–1.114) | 0.082 |
| Severity of disease during suspected CRBSI | ||||
| SOFA1st quartile (1–7) (reference) | 1 | 1 | 1 | 1 |
| SOFA2nd quartile (8–9) | 0.196 | − 1.56 | 0.600(0.316–1.139) | 0.118 |
| SOFA3rd quartile (10–12) | 0.189 | − 1.66 | 0.584(0.310–1.100 | 0.096 |
| SOFA4th quartile (13–24) | 0.226 | − 1.29 | 0.628(0.310–1.273) | 0.197 |
| Central venous catheter | ||||
| Jugular (reference) | 1 | 1 | 1 | 1 |
| Subclavian | 0.053 | -5.65 | 0.167(0.090–0.311) | 0.000 |
| Femoral | 0.189 | − 1.23 | 0.725(0.434–1.209) | 0.218 |
| Catheter days (> 7) | 0.322 | 1.85 | 1.491(0.977–2.275) | 0.064 |
| General variables | ||||
| Temperature (> 38.3 °C) | 0.375 | 2.19 | 1.646(1.053–2.573) | 0.029 |
| Hemodynamic variables | ||||
| Arterial hypotension (MAP < 70 mmHg) | 0.549 | 2.57 | 2.016(1.182–3.437) | 0.010 |
| Vasoconstrictor Agents | 0.176 | − 1.87 | 0.553(0.297–1.030) | 0.062 |
| Inflammatory variables | ||||
| Leukocytes (WBC count > 12.000 μL-1) | 0.204 | − 0.33 | 0.930(0.605–1.429) | 0.740 |
| Leukopenia (WBC count < 4000 μL-1) | 3.681 | 0.88 | 2.977(0.264–33.596) | 0.378 |
| Plasma Procalcitonin (> 1 ng/ml) | 0.235 | 0.22 | 1.050(0.676–1.629) | 0.829 |
| Organ dysfunction variables | ||||
| Creatinine increase (> 133 μmol/L) | 0.088 | − 4.11 | 0.305(0.173–0.538) | 0.000 |
| Continuous renal replacement therapy | 1.098 | 4.54 | 3.761(2.122–6.666) | 0.000 |
| Hyperglycemia (plasma glucose > 10 mmol/L) | 0.390 | 2.46 | 1.737(1.119–2.697) | 0.014 |
| Glasgow Coma Scale (< 12) | 0.480 | 1.76 | 1.664(0.945–2.929) | 0.078 |
| Activated Partial Thromboplastin Time (> 60 secs) | 0.384 | 0.17 | 1.064(0.525–2.158) | 0.863 |
| International Normalized Ratio (> 1.5) | 0.237 | − 0.78 | 0.792(0.440–1.425) | 0.436 |
| Thrombocytopenia (platelet count < 100,000 μL−1) | 0.238 | − 0.49 | 0.875(0.513–1.492) | 0.623 |
| Tissue perfusion variables | ||||
| Hyperlactatemia (Lactic acid > 1 mmol/L) | 0.175 | − 1.37 | 0.713(0.440–1.155) | 0.169 |
Nonparametric ROC estimation to models: AUC 77.0% (95% CI 73.3%–80.7%)
APACHE Acute Physiology and Chronic Health Evaluation, CI confidence interval, CRBSI Catheter-related bloodstream infections, MAP mean arterial pressure, RR relative risk, SOFA Sequential Organ Failure Assessment, WBC white blood cell
Mortality Risk Analysis
We included all 686 patients in the analysis of survival. The mean age was 62.9 years (SD 17.1), 503 (73.3%) were male, and 183 (26.7%) were female. A total of 119 (17.4%) patients with suspected CRBSIs died within 30 days. Table 2 showed univariate relative risk factors that there were significant differences between death and survivors.
A multivariate Cox proportional hazards model was used to identify the association of CRBSI with mortality. The models were adjusted for the variables with significant results in univariate analysis. A crude univariate model showed that CRBSI did not increase the hazard ratio for 30-day mortality (95% CI 0.599–1.433, P = 0.731). Four Cox models were constructed, all of which indicated that CRBSI was not associated with an increase in 30-day mortality (Supplemental e-Table 3). The final model (Table 3) showed that the following four factors were associated with an increased risk of mortality: age (> 77 years) (95% CI 1.087–3.437, P = 0.025), APACHE II score (> 25) (95% CI 1.165–3.938, P = 0.014), SOFA score (> 13) (95% CI 1.232–3.988, P = 0.008), and the reinsertion of new CVC (95% CI 1.334–4.066, P = 0.003). In contrast, the surgical admission of critically ill patients was associated with lower hazard ratios for 30-day mortality than was the medical admission of critically ill patients (95% CI 0.227–0.698, P = 0.001).
Table 3.
The final model for time-varying analysis for the effect of CRBSI on mortality
| Sensitivity Variables | Robust Std. Err | z-Value | Hazard ratio (95% Confidence Interval) | P-Value |
|---|---|---|---|---|
| Catheter-Related Bloodstream Infection | 0.199 | − 1.01 | 0.770 (0.464–1.279) | 0.312 |
| Urinary Tract Infection | 0.395 | 0.47 | 1.170 (0.604–2.268) | 0.642 |
| Age 1st quartile (18–51) (reference) | 1 | 1 | 1 | 1 |
| Age 2nd quartile (52–66) | 0.318 | − 0.05 | 0.982(0.521–1.851) | 0.956 |
| Age 3rd quartile (67–77) | 0.355 | 0.41 | 1.136(0.616–2.096) | 0.683 |
| Age 4th quartile (78–97) | 0.568 | 2.24 | 1.933(1.087–3.437) | 0.025 |
| Medical (reference) | 1 | 1 | 1 | 1 |
| Surgical | 0.114 | − 3.21 | 0.398 (0.227–0.698) | 0.001 |
| Traumatology | 0.189 | − 1.68 | 0.579 (0.306–1.097) | 0.094 |
| APACHE 1st quartile (2–15) (reference) | 1 | 1 | 1 | 1 |
| APACHE 2nd quartile (16–19) | 0.431 | 1.01 | 1.372(0.741–2.539) | 0.314 |
| APACHE 3rd quartile (20–25) | 0.375 | 0.67 | 1.227(0.674–2.232) | 0.504 |
| APACHE 4th quartile (26–46) | 0.666 | 2.45 | 2.142(1.165–3.938) | 0.014 |
| Diabetes mellitus | 0.257 | 1.08 | 1.248(0.833–1.869) | 0.282 |
| Chronic renal failure | 0.646 | 1.34 | 1.676 (0.788–3.566) | 0.180 |
| Corticosteroids | 0.271 | 1.34 | 1.317 (0.880–1.972) | 0.181 |
| Anticoagulant | 0.169 | − 1.04 | 0.803(0.532–1.214) | 0.299 |
| Antibiotics | 0.352 | 1.33 | 1.399(0.854–2.291) | 0.182 |
| Artificial respiration | 0.509 | 0.21 | 1.100(0.444–2.722) | 0.837 |
| Positive end expiratory pressure | 0.761 | 1.19 | 1.702(0.708–4.088) | 0.234 |
| Immediately new central venous catheter | 0.662 | 2.97 | 2.329(1.334–4.066) | 0.003 |
| SOFA score 1st quartile (1–8) (reference) | 1 | 1 | 1 | 1 |
| SOFA score 2nd quartile (9–10) | 0.361 | 0.51 | 1.169 (0.638–2.143) | 0.613 |
| SOFA score 3rd quartile (11–13) | 0.441 | 1.3 | 1.474(0.820–2.651) | 0.195 |
| SOFA score 4th quartile (14–24) | 0.664 | 2.65 | 2.216 (1.232–3.988) | 0.008 |
APACHE Acute Physiology and Chronic Health Evaluation, CRBSI: Catheter Related Bloodstream Infection, SOFA Sequential Organ Failure Assessment
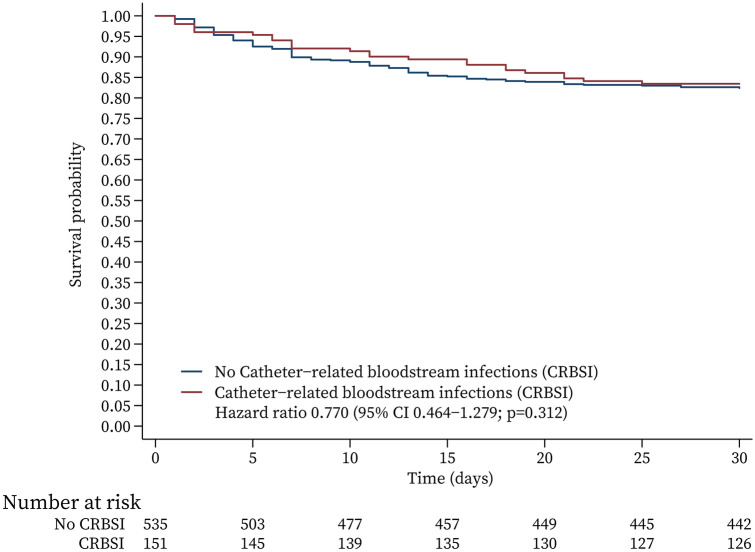
With regard to the final model, which contains variables that did and did not satisfy the assumption, in the Kaplan–Meier survival analysis (Fig. 3), we used a stratified Cox model (CRBSI and no CRBSI) [17]. We did not find significant differences between the CRBSI group and the no CRBSI group. The test of the proportional hazards assumption for the Cox model [18] showed that it was met (chi-square = 28.20, P = 0.1346) but did not meet proportional hazards assumptions in the subgroup of patients with CRBSI who survived (Spearman = 0.142, chi-square = 4.33, P = 0.0375).
Fig. 3.
Multivariate Cox survival analysis function by means of covariates. Multivariate variables including age, admission diagnosis, APACHE II score, diabetes mellitus, corticosteroids, anticoagulants, antibiotics, positive end-expiratory pressure, artificial respiration, reinsertion of new catheters, plasma procalcitonin, platelets, bilirubin, creatinine, and stratification according to mortality of CRBSI and no CRBSI
Population Attributable Mortality Fraction Analysis
The population attributable fraction analysis was based on the Cox proportional hazards model, and it demonstrated that the fraction of 30-day mortality attributable to CRBSIs was 18.2% (95% CI − 21.3% to 44.8%, P = 0.318), that attributable to pneumonia was 1.8% (95% CI − 14.5% to 15.8%, P = 0.816), and that attributable to multiple infections was 13.8% (95% CI − 27.4% to 41.7%, P = 0.457) (Supplemental e-Table 4).
Discussion
This large cohort study supports the use of the adjusted SIRS criteria to identify CRBSIs in patients with suspected CRBSIs. There were no significant differences in mortality between patients confirmed with and without CRBSIs who underwent CVC removal due to suspected CRBSIs, regardless of the variables used to adjust the Cox model. However, we determined that the reinsertion of new CVCs was associated with an increased hazard ratio of mortality.
There are five aspects of the SIRS criteria. First, the general variable fever was confirmed to be a specific and sensitive indicator of CRBSIs in this study. The guidelines report that fever is the most sensitive clinical finding that is indicative of CRBSI but that it has poor specificity [12]. In addition, our data did show a relatively higher risk of CRBSIs in patients using the cutoff value for the glucose level of > 10 mmol/L rather than 7.7 mmol/L [13], but the rate of CRBSIs was relatively lower in patients with diabetes mellitus. A recent cohort study reported that relative hypoglycemia is common in ICU patients with diabetes [19].
Second, with regard to inflammatory variables, we found no statistically significant difference in the value of the index regardless of whether they were included in the variables used for adjustment. These inflammatory variables reflect the host response to “danger” in the form of infection or other insults [6], but they have poor discriminant validity due to their presence in many hospitalized patients [20].
Third, with regard to the hemodynamic variables in patients with suspected CRBSIs, the relative risk of CRBSI was significantly higher in patients with arterial hypotension (MAP < 70 mmHg) than in those without hypotension, which is consistent with the SIRS criterion in patients with sepsis and can be explained by the presence of shock due to bloodstream infection, which causes circulatory abnormalities that are sufficiently profound to increase mortality [6].
Fourth, with regard to the organ dysfunction variables, the interesting finding in our study was that the rate of CRBSI was relatively lower in patients with a high creatinine level (> 133 μmol/L), which is in contrast to the common perception and current guidelines for the care of ICU patients [12]. However, at the same time, our data show that the relative risk of CRBSI was elevated in patients receiving CRRT; CRRT is widely used in ICU patients, and although it reduces creatinine levels in patients, it does not reduce mortality [21].
Fifth, tissue perfusion variables were not helpful with regard to identifying CRBSI. Septic shock involves both hypotension and hyperlactatemia because there is not only cellular dysfunction but also cardiovascular compromise [6].
This study found that ARDS is also an independent risk factor for CRBSI. To the best of our knowledge, to date, no study has reported a direct correlation between ARDS and CRBSI. Microbial pathogen-associated molecular patterns activate innate immunocytes through pattern recognition receptors, and the consequent cellular injury provides a key link between inflammation and SIRS [8]. Acute lung injury is caused by the systemic inflammatory response; dipeptidase-1 is the target and has been shown to be a physical adhesion receptor for neutrophil sequestration independent of a major adhesion receptor on the lung [22]. Patients with CRBSI normally have severe disease, which manifests as SIRS, leading to fever and pneumonia. Hence, ARDS may be a good predictor of CRBSIs in patients with suspected CRBSIs, but the clear exclusion of other causes of lung injury is needed.
In the recent decade, these clinical data also confirmed a lower rate of CRBSI in patients with a subclavian insertion site than in those with other insertion sites [23]. Therefore, our multifactor model for the identification of CRBSI may be beneficial with regard to selecting the correct management strategy for suspected CRBSIs, and it merits further validation in a cohort study. Our results provided rational explanations for the common perceptions and current guidelines, but we do not support the use of a single risk factor as a strategy for identifying CRBSI. The complexity of real-world clinical practice is greater than that reflected in a selected cohort of patients.
When analyzing the mortality of CRBSI, which was consistent in all Cox models and subgroup analyses, CRBSIs were not associated with increased ICU mortality. The results are consistent with those of a recent cohort study that did not find differences in mortality between patients with CRBSIs and those with other ICU-acquired infections [2], another cohort study that patients with CRBSIs had lower mortality rates than those with other infections [11]. Hence, in this study, these findings regarding the relatively low mortality and attributable mortality are informative for future studies. First, most patients with suspected CRBSIs should be treated conservatively, such as with watchful waiting rather than prompt CVC removal, as is commonly performed in patients with suspected CRBSIs while waiting for blood culture results [11,12]. A recent observational study reported a treatment success rate of 85% and no deaths within six weeks in hemodialysis patients with CRBSIs who did not undergo catheter removal [24]. Second, we determined that the reinsertion of a new CVC was a risk factor for mortality in our final model. Thus, a more comprehensive assessment is needed in future studies. Third, there is a lack of high-level evidence [10], and it should be possible to design a randomized controlled trial to compare mortality between patients with suspected CRBSIs managed with prompt CVC removal and those managed with watchful waiting.
The following four independent risk indicators for mortality in patients with CRBSIs were identified: age, APACHE II score, SOFA score, and reinsertion of new CVC. Other than the re-insertion of new CVCs, these risk factors for mortality have been established in previous studies [2,6]. As mentioned above, previous studies did not provide the evidence needed to assess the harm or benefit associated with this clinical practice [10], while a systematic review suggested that CVC removal and reinsertion may be associated with marked discomfort, severe risks and possible disruptions of or delays in treatment in critically ill patients [10]. Therefore, the potential reason underlying the increased risk of mortality in patients in whom CVCs are reinserted needs to be confirmed in future research.
The strengths of this study were that it covered the longest time span and included a list with an extensive number of known, calculable risk factors for in the analysis of attributable mortality in patients with CRBSI. However, our study has several limitations. First, this was a single-center, retrospective study that was subject to potential unmeasured confounding factors. Nevertheless, to date, it includes the largest cohort of patients with CVCs and suspected CRBSIs, and it includes more established prognostic factors than previous studies [11,25], as well as known sensitive indicators for the development of CRBSI [13]. Second, in this cohort, it was difficult to distinguish between delayed and prompt removal [11]. However, based on the general consensus and common practice of the medical team in the participating institution, prompt CVC removal was considered a coordinate strategy in patients with suspected CRBSIs. Third, it is possible that the CVCs were not the origin of the CRBSIs in patients with suspected CRBSIs [11]. Finally, data bias could have occurred as we excluded patients without catheter tip cultures.
Conclusion
This initial model based on the SIRS criteria is relatively better at identifying patients with CRBSI but only in terms of the sensitivity. There were no significant differences in attributable mortality due to CRBSI and other causes in patients with suspected CRBSI, which prompt catheter removal and re-insertion of new catheter may not benefit patients with suspected CRBSIs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the department of medical records of Affiliated Hospital of Guangdong Medical University for clinical data support.
Funding
This study was funded by the National Natural Science Foundation of China (81671957 and 81873951) and Guangdong Natural Science Foundation (2018B030311038). In addition, data collection and storage were supported by the Clinical Research Fund of Affiliated Hospital of Guangdong Medical University (LCYJ 2018C010). The journal's Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Yiyue Zhong and Limin Zhou contributed equally to this manuscript and Liangqing Zhang, Zhifeng Liu and Jing Tang contributed equally to this manuscript.
Disclosures
Yiyue Zhong, Limin Zhou, Xiaolei Liu, Liehua Deng, Ruona Wu, Zhengyuan Xia, Guixi Mo, Liangqing Zhang,Zhifeng Liu, Jing Tang have nothing to disclose.
Compliance with Ethics Guidelines
The ethics committee of the Affiliated Hospital of Guangdong Medical University approved this study and waived the need for informed consent on March 13, 2019 (PJ2018-066). This study was registered with the China Clinical Trials Registry on March 28, 2019 (ChiCTR1900022175). From January 2009 to December 2018.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Liehua Deng, Email: glinson@126.com.
Zhifeng Liu, Email: zhifengliu7797@163.com.
Jing Tang, Email: tanglitangjing@126.com.
References
- 1.Takashima M, Schults J, Mihala G, Corley A, Ullman A. Complication and failures of central vascular access device in adult critical care settings. Crit Care Med. 2018;46(12):1998–2009. doi: 10.1097/ccm.0000000000003370. [DOI] [PubMed] [Google Scholar]
- 2.van Vught LA, Klein Klouwenberg PM, Spitoni C, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315(14):1469–1479. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283–2289. doi: 10.1097/CCM.0b013e3181a02a67. [DOI] [PubMed] [Google Scholar]
- 5.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Internal Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weis S, Carlos AR, Moita MR, et al. Metabolic adaptation establishes disease tolerance to sepsis. Cell. 2017;169(7):1263–1275.e1214. doi: 10.1016/j.cell.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29–36. doi: 10.1007/s15010-014-0689-y. [DOI] [PubMed] [Google Scholar]
- 10.Janum S, Afshari A. Central venous catheter (CVC) removal for patients of all ages with candidaemia. Cochrane Database Syst Rev. 2016;7(7):Cd011195. doi:10.1002/14651858.CD011195.pub2 [DOI] [PMC free article] [PubMed]
- 11.Lorente L, Martin MM, Vidal P, Rebollo S, Ostabal MI, Sole-Violan J. Should central venous catheter be systematically removed in patients with suspected catheter related infection? Crit Care. 2014;18(5):564. doi: 10.1186/s13054-014-0564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol. 1999;20(6):396–401. doi: 10.1086/501639. [DOI] [PubMed] [Google Scholar]
- 16.Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 17.Xue X, Xie X, Gunter M, et al. Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol. 2013;13:88. doi: 10.1186/1471-2288-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tijmstra J. Why checking model assumptions using null hypothesis significance tests does not suffice: A plea for plausibility. Psychon Bull Rev. 2018;25(2):548–559. doi: 10.3758/s13423-018-1447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwan TN, Zwakman-Hessels L, Marhoon N, et al. Relative hypoglycemia in diabetic patients with critical illness. Crit Care Med. 2019;48(3):e233–e240. doi: 10.1097/ccm.0000000000004213. [DOI] [PubMed] [Google Scholar]
- 20.Churpek MM, Zadravecz FJ, Winslow C, Howell MD, Edelson DP. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015;192(8):958–964. doi: 10.1164/rccm.201502-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury SR, Babes L, Rahn JJ, et al. Dipeptidase-1 is an adhesion receptor for neutrophil recruitment in lungs and liver. Cell. 2019;178(5):1205–1221.e1217. doi: 10.1016/j.cell.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Parienti JJ, Mongardon N, Megarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220–1229. doi: 10.1056/NEJMoa1500964. [DOI] [PubMed] [Google Scholar]
- 24.Mandolfo S, Anesi A, Maggio M, Rognoni V, Galli F, Forneris G. High success rate in salvage of catheter-related bloodstream infections due to Staphylococcus aureus, on behalf of project group of Italian society of nephrology. J Vasc Access. 2020;21(3):336–341. doi: 10.1177/1129729819875323. [DOI] [PubMed] [Google Scholar]
- 25.Mishra SB, Misra R, Azim A, et al. Incidence, risk factors and associated mortality of central line-associated bloodstream infections at an intensive care unit in northern India. Int J Quality Health Care. 2017;29(1):63–67. doi: 10.1093/intqhc/mzw144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.