Abstract
Objective:
Ginger has protective effects on the kidney, however the molecular mechanism of this effect has not yet been fully elucidated. Therefore, this work studied molecular mechanisms of ginger effects on ethanol-induced kidney injury.
Materials and Methods:
Twenty-four male Sprague-Dawley rats were randomly divided into four groups: control, ginger (1 g/kg/day ginger extract by oral gavage), ethanol (4 g/kg/day ethanol by oral gavage) and ginger-ethanol group and treated daily for 28 days. Kidney function, expression of nuclear factor erythroid 2-related factor 2 (NRF2) and tumor necrosis factor (TNF)-α genes and oxidative stress parameters in kidney tissue, were evaluated. Total phenolic content (TPC) and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity of ginger extract were also evaluated.
Results:
Hydroethanolic extract of ginger showed a good level of DPPH scavenging activity and TPC. In the ethanol group, serum level of urea, creatinine and uric acid and the expression of NRF2 and TNF-α significantly increased compared to control group, while co-treatment with ginger in ginger+ethanol group significantly ameliorated them compared to the ethanol group. Ethanol exposure significantly reduced the activity of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) compared to the control values ,while the level of malondialdehyde (MDA) significantly increased. Ginger significantly ameliorated the level of MDA and activity of SOD, GPx and CAT in the ginger-ethanol group compared to the ethanol group.
Conclusion:
The results showed that ginger's protective effects against ethanol renotoxicity were mediated via enhancing the NRF2 and TNF-α expression.
Key Words: Kidney, Ethanol, Ginger, Oxidative stress, NRF2, TNF-α
Introduction
Nowadays people's lifestyle is closely linked with exposure to radio waves, chemicals such as toxins and insecticides, and or smoking and alcohol (Akbari et al., 2016 ▶; Akbari et al., 2017 ▶). Alcohol is a common and popular drink and its excessive intake is known as a risk factor of chronic diseases such as liver disease, cancer, diabetes mellitus, reproductive disorders and renal failure (Shield et al., 2013 ▶). Excessive intake of alcohol after smoking and high blood pressure can lead to death in middle and high-income countries. Ethanol is mainly metabolized in the liver and to some extent in kidneys by several enzymes such as cytochrome P450 2E1 (CYP2E1) monooxygenase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX2). This metabolism causes the production of acetaldehyde, superoxide (O2•−) and other reactive oxygen species (ROS). Over production of ROS can cause oxidative stress and damage the cells (Altamirano et al., 2012 ▶). Kidney is a crucial bean-shaped organ involved in formation of urine, hormone secretion, blood pressure regulation, acid-base balance, regulation of osmolality and metabolism. Kidneys are responsible for blood clearance and are constantly exposed to toxic chemicals such as drugs and their metabolites; in addition central parts of the kidneys receive limited blood suply, therefore, they are prone to oxidative damage (George et al., 2017 ▶). Nephrotoxicity or kidney injury is usually detected through evaluating the plasma level of blood urea nitrogen (BUN), creatinine, urea and acid uric (Kim and Moon, 2012 ▶). Evaluation of these parameters is clinically useful to check patients with kidney diseases. Oxidative stress, imbalance between ROS production and the ability of neutralization by antioxidants, along with inflammation, are the most important players in the pathogenesis of chronic renal failure associated with long-term alcohol consumption. Evidence indicated that ROS overproduction, lipid peroxidation, and depletion of antioxidant systems in epithelial tubular cells, are the main pathomechanisms associated with nephrotoxicity induced by ethanol (Latchoumycandane et al., 2014 ▶). NRF2/Keap-1/HO-1 pathway which regulates the cellular redox and phase II detoxification responses, has attracted many researchers' attention in recent years. The NRF2/Keap-1/HO-1 pathway is inactive under physiological conditions but it is activated by ROS overproduction (Chen and Maltagliati, 2018 ▶; Fathi et al., 2020 ▶). Therefore, strengthening the endogenous antioxidant through the use of herbal supplements containing phenolic and flavonoid compounds such as ginger, can prevent the development of many diseases (Nimrouzi et al., 2020a ▶; Jelodar et al., 2020 ▶; Ostovar et al., 2020 ▶).
Ginger (Zingiber officinale Roscoe) belongs to the Zingiberaceae family. Rhizome or ginger root is widely used as a spice and a folk medicine worldwide (Ross, 2005 ▶). Ginger contains phytochemical compounds such as flavonoids, phenols and proteins which are used to prevent and treat many disorders including cardiovascular (Nicoll and Henein, 2009 ▶), eye (Akbari et al., 2019a ▶), renal (Rafieian-kopaei, 2013 ▶), reproductive (Akbari et al., 2017 ▶) and hepatic (Akbari et al., 2019b ▶) disorders. Zingerone, gingerdiol, zingiberene, gingerols and shogoals are the main compounds of ginger with antioxidant (Ghasemzadeh et al., 2010 ▶), anti-inflammatory (Lantz et al., 2007 ▶), anti-diabetic and hypo-lipidemic properties (Al-Amin et al., 2006 ▶). Shanmugam et al. (2010) ▶ showed that ginger in a dose-dependent manner (100 and 200 mg/kg) could reverse ethanol-induced oxidative damage in rat kidney (Shanmugam et al., 2010 ▶). Although the therapeutic potential of ginger in these studies (Hamed et al., 2012 ▶, Shanmugam et al., 2010 ▶) was well documented, the molecular mechanisms of such effects have not yet been elucidated. Therefore, the purpose of this study was to investigate the protective effects of ginger extract on ethanol-induced nephrotoxicity and oxidative stress in rats.
Materials and Methods
Materials
Ethanol 96% was purchased from Razi Chemical Company (Tehran, Iran) and other chemicals, reagents and standard solutions used in the study were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO, USA).
Preparation of ginger ( Zingiber officinale Roscoe) extract
Rhizome of ginger was purchased from a herbal shop (Attari) in Shiraz, Iran. Then, it was verified by a botanist in Shiraz University of Medical Sciences and assigned with the voucher number: PM-948. Afterwards, 250 g of dried ginger powder was mixed with 500 ml of 70% ethanol and water in an Erlenmeyer. It was then filtered using a filter paper after 48 hr and evaporated at 40°C. The residual was the extract of ginger.
Evaluation of total phenolic content (TPC) and total antioxidant capacity
The evaluation of the extract total phenol content was done using modified Folin-Ciocalteu spectrophotometric method as described by Waterhouse (2002) ▶ (Waterhouse, 2002 ▶).
Total antioxidant capacity of ginger extract was evaluated by Diphenylpicyl hydrazine (DPPH) method as previously described (Leong and Shui, 2002 ▶).
Animals
All stages of this research were conducted in accordance with the "Guidelines for the Care and Use of Research Animals" approved by Shiraz University. Twenty-four adult male Sprague-Dawley rats (220±15 g) were housed in an animal room under controlled conditions: lighting (12 hr light: 12 hr darkness) and temperature (20±2ºC); animals had free access to pelleted food and tap water.
Experimental design
The protective effects of ginger extract against toxicity induced by ethanol in rat kidney were studied by dividing animals into four groups, each group included six rats.
Group I: Vehicle or control group which received normal saline (1 ml/day)(Lapin, 1995 ▶, Receno et al., 2018 ▶)
Group II: Ethanol group which received ethanol (4 g/kg of Body Weight (B.W)/day) for 28 consecutive days (Alirezaei et al., 2012 ▶)
Group III: Ginger group was assigned to receive ginger rhizome extract (1 g/kg of B.W/day) (Al-Qudah et al., 2016 ▶)
Group IV: Ginger-ethanol group which received ethanol (4 g/kg of B.W/day) after administration of ginger (1 g/kg of B.W/day) for 28 consecutive days.
The vehicle and the extract of ginger was administered by gavage daily for 28 days.
Sampling and assessment of kidney function and oxidative status
Animals were killed by anesthetizing with ether at the end of the study period after a fasting night. Blood samples were collected through heart puncture. After blood clotting, the sera were used to evaluate creatinine and uric acid and urea. Creatinine and uric acid were determined by Jaffe reaction and enzymatic method (uricase), respectively. Urea was measured by diacetyl monoxime method. The right kidney was immediately dissected and rinsed in ice saline. It was then manually homogenized using phosphate buffered saline (0.1 M, pH 7.4), and centrifuged at 3000 g for 10 min. The upper clear supernatants were used for evaluating biochemical and molecular parameters. Total antioxidant capacity of the kidney tissue was evaluated by Ferric Reducing Ability of Plasma (FRAP) method as previously described by (Benzie and Strain, 1999 ▶). The activities of SOD and GPx were measured by detection kit (Ransod and Ransel kits, respectively; RANDOX Company, UK). The level of MDA was evaluated by a modified method as described previously (Alirezaei et al., 2012 ▶). Catalase activity and total protein were determined according to methods described by Aebi (Aebi, 1984 ▶) and Lowry et al. (1951) ▶(Lowry et al., 1951 ▶), respectively.
RNA extraction and cDNA synthesis
The characteristics of primer sequences are presented in Table 1. RNA samples were isolated from rat’s kiddney tissue using the extraction kit (Qiagen RNeasy Mini Kit, Germany) according to manufacturer's instruction. Quantification and qualification of total RNA concentration was estimated using Nanodrop ND-1000 spectrophotometer (Thermo Scientific). Reverse Transcriptase (M-MLV RT) (Yekta Tajhiz Azma, Iran) and Oligo-dT primer for the synthesis of cDNA were used. From each sample, 2 μg of total RNA was used to synthesize cDNA. In this study, TNFα and GAPDH primers were designed using Primer Premeir 5 software and NRF2 primers were used from the study by Liang et al. (2017).
Table 1.
The characterizations of primer sequences used in this study
| Tissue | Gene | Primer sequences | Product length (bp) |
|---|---|---|---|
| Kiddney | NRF2 | Forward 5´-GCTGCCATTAGTCAGTCGCTCTC-3´ Reverse 5´-ACCGTGCCTTCAGTGTGCTTC-3´ |
104 |
| TNFα | Forward 5'- CTT CAG GGA TAT GTG ATG GAC TC-3' Reverse 5'- GGA GAC CTC TGG GGA GAT GT -3' |
186 | |
| GAPDH | Forward 5´-GGCAAGTTCAACGGCACAG-3´ Reverse 5´-GACGCCAGTAGACTCCACGAC-3´ |
144 |
Determination of relative quantity in Real Time PCR was done by measuring the increase of fluorescence light, as the result of attaching SYBR Green color to DNA. During this step, the Polymerase chain Reaction was performed on cDNA samples to amplify NRF2 and TNFα genes and GAPDH as the reference gene, using RealQ Plus 2x Master Mix Green (Ampliqon, Denmark) in Rotor Gene 6000 (Corbett Research, Australia). Real Time PCR reactions were performed in a final volume of 20 μl and each reaction was duplicated. The reaction mixture contained 3 μl of cDNA (50 ng/μl), 8 μl of RealQ Plus 2x Master Mix Green, 0.4 μl of each of primers (10 pmol) and 8.2 μl of ribonuclease-free water. The temperature program was as follows: enzyme activation 95°C for 13 min, followed by 40 cycles of denaturation at 95°C for 30 sec, primer annealing at 58°C (GAPDH) and 60°C (NRF2 and TNFα) for 35 sec and extension at 72°C for 30 sec, melting curve at 60°C for 5 sec, and the final step at 95°C as continuous; 2-∆∆CT method was used to calculate the fold change in genes expression (Rao et al., 2013 ▶).
Histopathological examination
Kidney was removed and fixed in a 10% formalin solution for at least three weeks and was then embedded in paraffin. After that, sections were cut at 5 μm and stained with hematoxylin and eosin. These sections were then examined under a light microscope (×40, BX-51, Olympus Corporation, Tokyo, Japan) for evaluating the degenerative changes in tubules and congestion in glomerular capillaries and medullary vessels in rat kidney.
Statistical analysis
The results were analyzed using one-way analysis of variance (ANOVA) followed by post hoc multiple comparisons Tukey test to compare the results of different treatment groups. All data were recorded using Statistical Package for Social Sciences (SPSS 17.0). The descriptive results are expressed as means±standard error of mean (Mean±SEM). The statistical significance was set at p<0.05.
Results
The results of TAC of extract of ginger are presented in Figure 1. The results showed that the ginger extract exhibited a good DPPH scavenging activity (IC50 = 354.782 mµ/mL). In addition, the TPC in ginger extract was calculated from equation of calibration curve and was expressed as milligrams of gallic acid equivalents per gram of dry extract (mg of GAE/g of dE). It was 89.84mg GAE/g dE.
Figure 1.
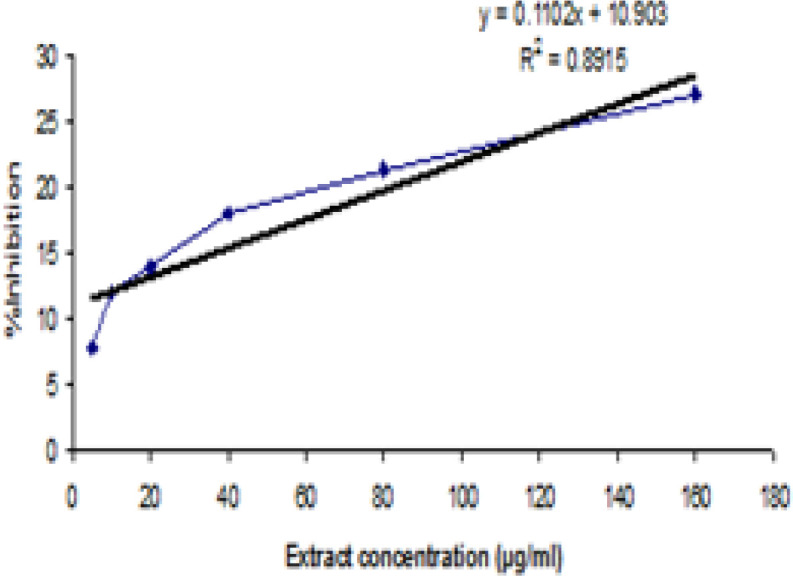
DPPH radical scavenging activity of extract of ginger
The serum level of urea, creatinine and uric acid was measured to evaluate kidney function (Table 2). In the ethanol group, the serum level of urea, cratinine and uric acid significantly increased compared to other groups (p<0.01, Table 2); while, the level of these parameters in the ginger-ethanol group was significantly lower compared to the ethanol group (p<0.05, Table 2).
Table 2.
The mean value (±SEM) for urea, cratinine and uric acid in studied groups
| Group | Control | Ginger | Ethanol | Ethanol-Ginger |
|---|---|---|---|---|
| Urea (mg/dl) | 14.15±1.36 | 15.78±1.45 | 28.8±1.67* | 16.5±1.14 |
| Creatinine (mg/dl) | 0.46±0.12 | 0.48±0.07 | 1.73±0.18# | 1.06±0.15* |
| Uric acid (mg/dl) | 6.15±0.47 | 6.94±0.39 | 11.5±0.44* | 6.96±0.49 |
* p<0.05 vs. other groups, # p<0.01 vs. control and ginger groups.
The renal expression of NRF2 and TNFα genes is presented in Figure 2. Our results showed that renal expression of TNFα gene was significantly higher in the ethanol group compared to other groups, while co-treatment with ginger significantly decreased expression of this gene in the ginger-ethanol group compared to the ethanol group (p<0.001, Figure 2B). The results also showed that there is no significant difference between ginger and control group regarding the experssion of this gene (Figure 2B).
Figure 2.
Comparison of the expression of NRF2 (A) and TNFα (B) among control and treated groups (n=6). Data are expressed as mean±SEM
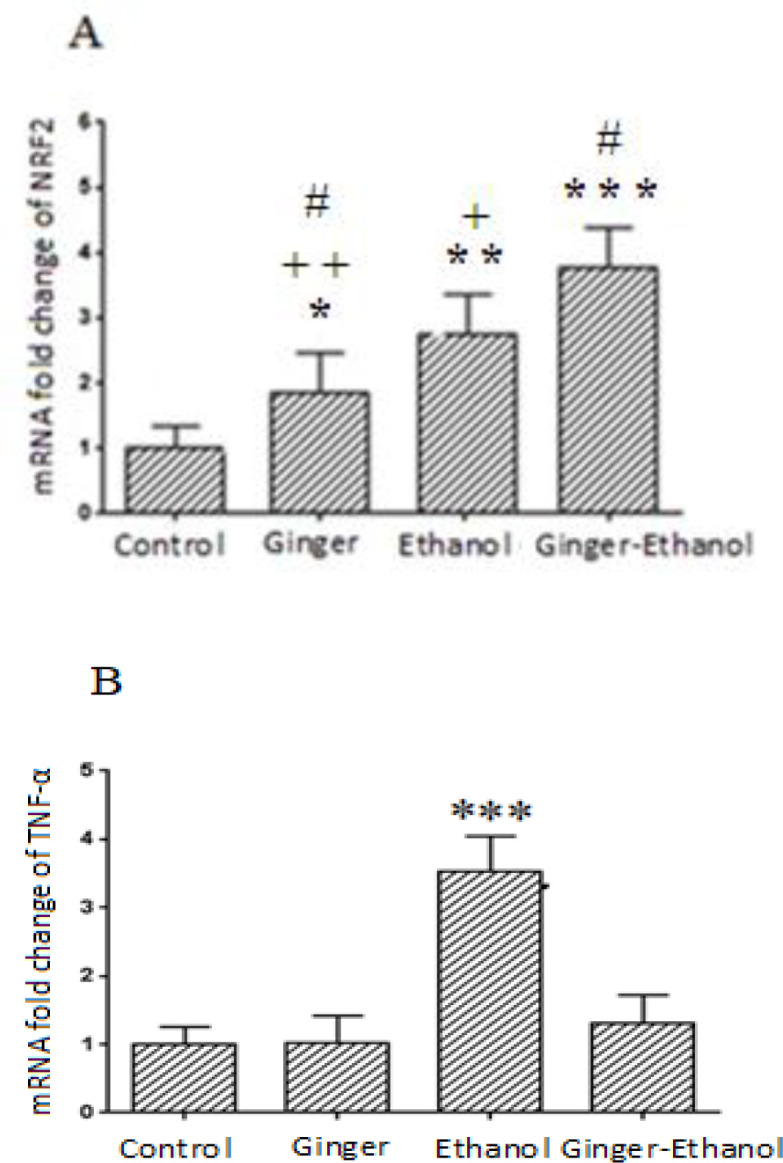
A. *p<0.05, **p<0.01 and ***p<0.001 significant difference compared to the control group. +p<0.05, ++p<0.01: significant difference compared to the Ginger-Ethanol group. #p<0.05: significant difference compared to the ethanol group.
B. ***p<0.001 vs.other groups.
Our results also indicated that renal expression of NRF2 significantly increased in ginger, ethanol and ginger-ethanol groups compared to the control (p<0.05 to p<0.001, Figure 2A). The renal expression of this gene significantly increased in the ethanol group compared to the control and ginger groups. Moreover, in the ginger group, the expression of NRF2 was higher than the control but lower than the ethanol and ginger-ethanol groups. The results also showed that the NRF2 gene expression has the highest level in the ginger-ethanol group compared to other groups (p<0.05, Figure 2A).
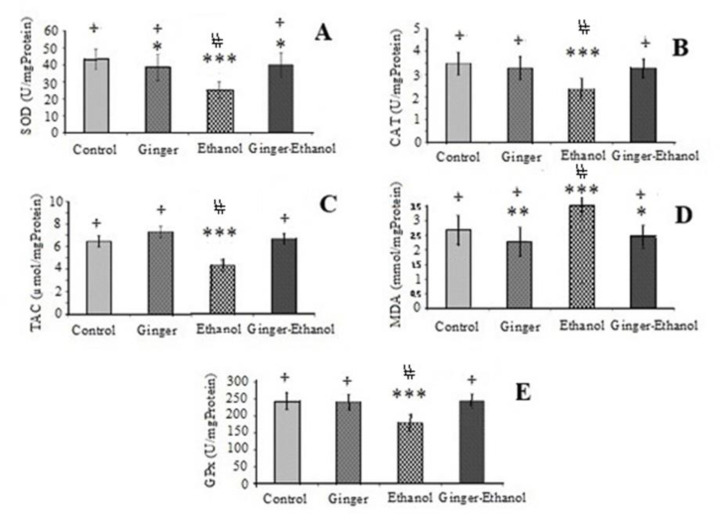
The protective effect of ginger on kidney toxicity induced by ethanol was evaluated by monitoring the renal level of SOD, GPx and CAT activity and the level of TAC and MDA. The mean value (±SEM) of these parameters is presented in Figure 3. Based on our results, the activity of SOD, GPx and CAT and the level of TAC in the ethanol group significantly decreased compared to control group (p<0.001, Figure 3). While, the administration of ginger could increase these parameters in the ginger-ethanol group compared to the ethanol group (p<0.05, Figure 3). Additionally, the increased level of MDA by alcohol, was reduced through co-treatment with ginger (p<0.05, Figure 3).
Figure 3.
Comparison of SOD (superoxide dismutase. A), CAT (catalase, B), TAC (total antioxidant capacity, C), MDA (Malondialdehyde, D) and GPx (glutathione peroxidase, E) among different groups (n=6). Data are expressed as mean±SEM
*p<0.05, **p<0.01 and ***p<0.001 significant difference compared to the control group. +p<0.001 significant difference compared to the ethanol group. #p<0.001 significant difference compared to the ginger-ethanol group.
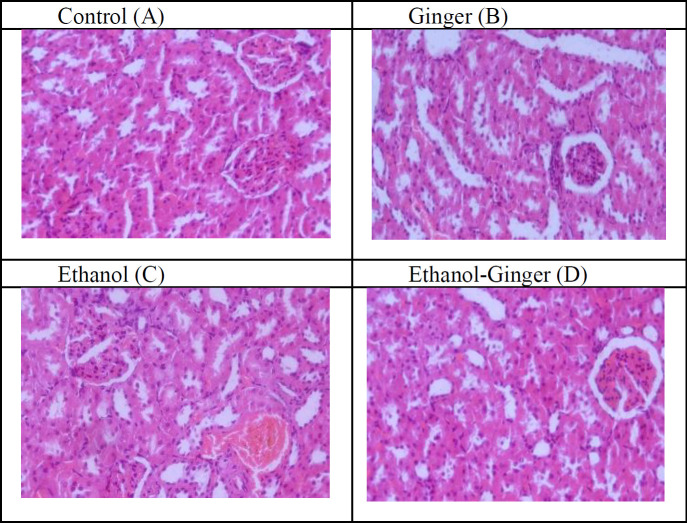
The histological finding of the rat kidney sections in the studied groups, is presented in Figure 4. Light microscopic examination of kidney sections from control and ginger groups showed that there were no congestion in glomerular capillaries and medullary vessels (Figure 4A and 4B). The evaluation of kidney sections from the ethanol group showed that ethanol induced congestion in glomerular capillaries and medullary vessels (Figure 4C), while co-treatment with ginger in animals receiving ethanol, significantly improved these abnormalities in the cortex and medulla of kidney (Figure 4D).
Figure 4.
Histological finding of the rat kidney sections in the studied groups (×40). Light microscopic examination of kidney sections from control (A) and ginger (B) groups showed normal arrangement of glomerulus and no congestion in glomerular capillaries and medullary vessels. Evaluation of kidney sections from the ethanol group (C) showed that ethanol induced injury in the kidney which is characterized by abnormal glomerulus and congestion in glomerular capillaries, while co-treatment with ginger in animals treated with ethanol, improved these injuries (D)
Discussion
In the current study, the serum level of urea, creatinine and uric acid as the indices of kidney function, increased by ethanol which is in agreement with previous reports (Altamirano et al., 2012 ▶; Latchoumycandane et al., 2014 ▶; Shirpoor et al., 2016 ▶). The results also showed that the level of these parameters improved by pre-treatment with ginger. Shanmugam et al. (2010) ▶ showed that ethanol (2 g/kg body weight, once daily for 30 days) induces oxidative stress and changes kidney tissue, while treatment with ethanolic extract of ginger (100 and 200 mg/kg body weight, once daily for 30 days) return these parameters to normal levels (Shanmugam et al., 2010 ▶). Moreover, Shirpoor et al. (2016) ▶ showed that ethanol induces oxidative DNA damage, functional and structural changes in kidney of rats, meanwhile ginger alleviated functional and structural alterations in kidney of rats (Shirpoor et al., 2016 ▶). However, Latchoumycandane et al. (2014) ▶ stated that ethanol-induced kidney dysfunction correlates with leukocyte infiltration and activation, and not primarily from metabolism of ethanol by CYP2E1. CYP2E1 metabolism may be necessary to initiate inflammation in the liver (Altamirano et al., 2012 ▶), but kidneys contain significantly less of this monooxygenase than the liver, and the liver was only modestly affected by ethanol in this animal model. Conversely, other researchers discovered that neutrophils were abundant in kidneys after ethanol feeding. Neutrophil type 2 NADPH oxidase has a critical role in ethanol-induced liver damage (Kono et al., 2001 ▶; Kono et al., 2000 ▶), and may contribute to oxidative stress in kidneys as well (Latchoumycandane et al., 2014 ▶). Latchoumycandane et al., (2014) ▶ suggested that ethanol acts as an indirect nephrotoxin to induce Acute Kidney Injury. Chronic ethanol metabolism induces an unappreciated cycle of leukocyte infiltration and activation necessary to induce its nephrotoxic effects. However, we did not evaluate the status and activity of leukocytes in kidneys in our study, nor did they evaluate the activity of renal antioxidant and inflammatory system. In fact, the major contradiction between this study and our study is that we hypothesized that ethanol directly induced kidney damage via oxidative stress and inflammation. In addition, regular ethanol intake increases the blood pressure, which is a risk factor for kidney damage. It should be also noted that a very large volume of blood circulates in the kidneys daily, and the role of these cells (leukocyte and neutrophil) cannot be considered in inducing renal injury. However, strengthening anti-inflammatory and antioxidant systems of the kidneys is essential to improve their function. Accordingly, we studied the expression of NRF2 and TNFα genes along with oxidative status in kidneys. Our results showed that the renal expression of TNFα and NRF2 was increased by ethanol which is in agreement with previous reports (Dong et al., 2008 ▶; Perrien et al., 2003 ▶; Luedemann et al., 2005 ▶; Shirpoor et al., 2016 ▶). The results also showed that the expression of TNFα increased by ethanol, while co-treatment with ginger could decrease its expression in rat kidney. TNFα is a cell signaling protein which is involved in systemic inflammation. Moreover, it was reported that ethanol could up-regulate the level of type 1 TNF-receptor (TNF-R1) in different cells and may augment TNF-α-mediated cell injury in different tissues (Rodriguez et al., 2004 ▶). Co-treatment with ginger could decrease the expression of TNFα in kidneys. Studies showed that co-treatment and treatment with ginger could reduce the level of TNFα in patients with type 2 diabetes (Mahluji et al., 2013 ▶) and tuberculosis (Kulkarni and Deshpande, 2016 ▶). In addition, Luettig et al. (2016) ▶ indicated that ginger by inhibiting the PI3K/Akt and NF-κB signaling prevents inflammation (Luettig et al., 2016 ▶). Isa et al. (2008) ▶ stated that 6-shogaol and 6-gingerol as ginger ingredients could inhibit TNFα signaling in 3T3-L1 adipocytes (Isa et al., 2008 ▶). Grzanna et al. (2004) ▶ showed that ginger has an effect on several genes encoding cytokines, chemokines and the inducible enzyme cyclo-oxygenase-2 (COX-2) (Grzanna et al., 2004 ▶). To sum up, in addition to down-regulating TNFα expression, ginger is able to inhibit synthesis of pro-inflammatory cytokines such as IL-1 and IL-8 (Tjendraputra et al., 2001 ▶) and NF-κB signaling pathways (Saedisomeolia et al., 2019 ▶). NF-κB plays a key role in activating subsequent signaling pathways, especially the regulation of pro-inflammatory molecules (Mashhadi et al., 2013 ▶).
The results of this study showed that the level of SOD, TAC, CAT and GPx decreased and the level of MDA increased by ethanol, while co-treatment with ginger could improve these levels which is in agreement with previous reports (Albano, 2006 ▶; Akbari et al., 2017 ▶; Ilkhanizadeh et al., 2016 ▶; Shanmugam et al., 2010 ▶).
Many studies demonstrated that ginger extract reduce lipid proxidation and oxidative damage induced by ethanol (Akbari et al., 2017 ▶; Shanmugam et al., 2010 ▶), streptozocin-induced diabetes (Ilkhanizadeh et al., 2016 ▶), CCL4 (Hamed et al., 2012 ▶), lead (Reddy et al., 2014 ▶) and iron (Gholampour et al., 2017 ▶) induced renal toxicity in male rats. The mechanisms involved in the induction of oxidative stress by ethanol are well-known (Luo et al., 2018 ▶; Lu and Cederbaum, 2008 ▶). Ethanol is a nephrotoxin that acts through a cycle of leukocyte infiltration and activation and ROS production (Latchoumycandane et al., 2014 ▶). Our results showed that the expression of NRF2 was up-regulated by ethanol and ginger. It was well reported that changes in ROS production are one of the most important stimuli for expression of this gene. The level of ROS was increased by ethanol, therefore it can up-regulate the expression of NRF2. However, the question is how the expression of this gene increased in healthy animals using ginger. It is likely that ginger constituents such as 6-shogaol and 6-gingerol were able to increase the expression of NRF2 gene through alterations in cellular signaling pathways (Bak et al., 2012 ▶; Schadich et al., 2016 ▶). Interestingly, the expression of this gene was significantly higher in ginger-ethanol group than the ethanol and ginger groups. In fact, this increase in expression is a potent response to the fight against ethanol damage. As previously mentioned, NRF2 is able to inhibit inflammation (Luo et al., 2018 ▶). Loboda et al. (2016) ▶ stated that NRF2, as a cytoprotective factor, not only regulates the expression of genes coding for anti-oxidant, anti-inflammatory and detoxifying proteins, but it is also a powerful modulator of species longevity (Loboda et al., 2016 ▶). Studies showed that NRF2/HO-1 pathway plays a protective role in oxidative and inflammatory responses induced by ethanol (Xu et al., 2018 ▶) and inhibits apoptosis in HEI193 Schwann cells (Jeong et al., 2019 ▶). Moreover, NRF2/Keap-1/HO-1 pathway has an crucial role in preventing the development of chronic diseases by inhibiting oxidative damage and infllamation (Nimrouzi et al., 2020b ▶; Tu et al., 2019 ▶). According to our results, co-treatment with ginger could improve oxidative damage and histological damages induced by ethanol in the kidney which is in agreement with previous reports (Shanmugam et al., 2010 ▶, Hamed et al., 2012 ▶, Gholampour et al., 2017 ▶). Gholampour et al. (2017) ▶ showed that pretreatment with ginger improves the congestion in glomerular capillaries and medullary vessels induced by iron in rat (Gholampour et al., 2017 ▶). Shanmugam et al. (2010) ▶ showed that ethanol induces degenerative changes in tubules, diffused cellular infiltration and congestion of blood vessels in rat kidney, while treatment with ginger (2 g/kg body weight, once daily for 30 days) improved the damage caused by ethanol (Shanmugam et al., 2010 ▶). In fact, the cause of these injuries can simply be considered oxidative damage and inflammation. It is well-known that high levels of ROS react with lipids, proteins and DNA, leading to cells and tissues injury (Akbari et al., 2014 ▶). Lipid peroxidation as a physiological process in membrane, and MDA as a marker for lipid peroxidation is increased by high levels of ROS. Excessive ethanol intake induces structural and functional changes in kidneys and plays a central role in the development of chronic renal failure (Epstein, 1997 ▶). Structural injuries might be associated with the absorption potential of renal tubules after ethanol exposure (Cigremis et al., 2006 ▶). Ginger treatment significantly ameliorates the injurious effects of ethnaol on renal morphology. Previous research showed that ginger had reno-protective effect against CCL4 and alcohol (Shanmugam et al., 2010 ▶; Hamed et al., 2012 ▶). Besides, the protective effects of ginger against damage induced by ethanol can be due to antioxidant and anti-inflammatory activity or improvement of trace elements such as zinc (Akbari et al., 2019a ▶; Akbari et al., 2017 ▶). Ginger contains high levels of gingerdiol, zingerone, shogoals, gingerols and zingiberene which inhibit oxidative damage (Semwal et al., 2015 ▶). Our study confirms that ginger precludes the toxic impact of ethanol against kidney damage at both histological and biochemical levels.
The findings of this study indicated that ethanol induced renal oxidative damage and ginger could improve all of these condition. It should be noted that ginger extract due to its role as an antioxidant and its interference with other processes such as homeostasis of essential elements and inflammation, could improve the damages caused by ethanol.
Acknowledgment
We are grateful to Shiraz University and University of Mazandaran for supporting this study.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Aebi H. [13] Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Akbari A, Jelodar G, Nazifi S. The prophylactic effect of vitamin C on oxidative stress indexes following exposure to radio frequency wave generated by a BTS antenna model in rat liver and kidney. Zahedan J Res Med Sci. 2014;16:19–23. [Google Scholar]
- Akbari A, Jelodar G, Nazifi S. The proposed mechanisms of radio frequency waves (RFWs) on nervous system functions impairment. Com Clin Path. 2016;25:1289–1301. [Google Scholar]
- Akbari A, Nasiri K, Heydari M. Ginger (Zingiber officinale Roscoe) extract can improve the levels of some trace elements and total homocysteine and prevent oxidative damage induced by ethanol in rat eye. Avicenna J Phytomed. 2019a;10:364–371. [PMC free article] [PubMed] [Google Scholar]
- Akbari A, Nasiri K, Heydari M, Mosavat SH, Iraji A. The protective effect of hydroalcoholic extract of Zingiber officinale Roscoe (Ginger) on ethanol-induced reproductive toxicity in male rats. Evid Based Complement Alternat Med. 2017;22:609–617. doi: 10.1177/2156587216687696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari A, Nasiri K, Heydari M, Nimrouzi M, Afsar T. Ameliorating Potential of Ginger (Zingiber officinale Roscoe) Extract on Liver Function and Oxidative Stress Induced by Ethanol in Male Rats. Zahedan J Res Med Sci. 2019b;21:e86464. [Google Scholar]
- Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R & Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96:660–666. doi: 10.1079/bjn20061849. [DOI] [PubMed] [Google Scholar]
- Al-Qudah MMA, Haddad MA & El-Qudah J MF. The effects of aqueous ginger extract on pancreas histology and on blood glucose in normal and alloxan monohydrate-induced diabetic rats. Biomed Res. 2016;27:350–356. [Google Scholar]
- Albano E. Alcohol, oxidative stress and free radical damage. P Nutr Soc. 2006;65:278–290. doi: 10.1079/pns2006496. [DOI] [PubMed] [Google Scholar]
- Alirezaei M, Jelodar G, Ghayemi Z. Antioxidant defense of betaine against oxidative stress induced by ethanol in the rat testes. Int J Pept Res Ther. 2012;18:239–247. [Google Scholar]
- Altamirano J, Fagundes C, Dominguez M, Garcia E, Michelena J, Cardenas A, Guevara M, Pereira G, Torres-Vigil K, Arroyo V, Caballeria J, Gines P, Bataller R. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Bak MJ, Ok S, Jun M, Jeong WS. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012;17:8037–8055. doi: 10.3390/molecules17078037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IF & Strain J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 1999 doi: 10.1016/s0076-6879(99)99005-5. Elsevier. [DOI] [PubMed] [Google Scholar]
- Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50:77–97. doi: 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigremis Y, Turkoz Y, Tuzcu M, Ozen H, Kart A, Gaffaroglu M, Erdogan K, Akgoz M, Ozugurlu F. The effects of chronic exposure to ethanol and cigarette smoke on the formation of peroxynitrite, level of nitric oxide, xanthine oxidase and myeloperoxidase activities in rat kidney. Mol Cell Biochem. 2006;291:127–138. doi: 10.1007/s11010-006-9205-8. [DOI] [PubMed] [Google Scholar]
- Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008;10:2023–2033. doi: 10.1089/ars.2007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. Alcohol’s impact on kidney function. Alcohol Health Res World. 1997;21 [PMC free article] [PubMed] [Google Scholar]
- Fathi R, Nasiri K, Akbari A, Ahmadi-KaniGolzar F, Farajtabar Z. Exercise protects against ethanol-induced damage in rat heart and liver through the inhibition of apoptosis and activation of Nrf2/Keap-1/HO-1 pathway. Life Sci. 2020;256:117958. doi: 10.1016/j.lfs.2020.117958. [DOI] [PubMed] [Google Scholar]
- George B, You D, Joy MS, Aleksunes LM. Xenobiotic transporters and kidney injury. Adv Drug Deliv Rev. 2017;116:73–91. doi: 10.1016/j.addr.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh A, Jaafar HZ & Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe) Molecules. 2010;15:4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholampour F, Ghiasabadi FB, Owji SM, Vatanparast J. The protective effect of hydroalcoholic extract of Ginger (Zingiber officinale Rosc ) against iron-induced functional and histological damages in rat liver and kidney. Avicenna J phytomed. 2017;7:542. [PMC free article] [PubMed] [Google Scholar]
- Grzanna R, Phan P, Polotsky A, Lindmark L, Frondoza CG. Ginger extract inhibits beta-amyloid peptide-induced cytokine and chemokine expression in cultured THP-1 monocytes. J Altern Complement Med. 2004;10:1009–1013. doi: 10.1089/acm.2004.10.1009. [DOI] [PubMed] [Google Scholar]
- Hamed MA, Ali SA, El-Rigal NS. Therapeutic potential of ginger against renal injury induced by carbon tetrachloride in rats. Scientific World J. 2012;2012:840421. doi: 10.1100/2012/840421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilkhanizadeh B, Shirpoor A, Nemati S, Rasmi Y. Protective effects of ginger (Zingiber officinale) extract against diabetes-induced heart abnormality in rats. Diabetes Metab. 2016;40:46–53. doi: 10.4093/dmj.2016.40.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa Y, Miyakawa Y, Yanagisawa M, Goto T, Kang MS, Kawada T, Morimitsu Y, Kubota K, Tsuda T. 6-Shogaol and 6-gingerol, the pungent of ginger, inhibit TNF-α mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2008;373:429–434. doi: 10.1016/j.bbrc.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Jelodar G, Mohammadi M, Akbari A & Nazifi S. Cyclohexane extract of walnut leaves improves indices of oxidative stress, total homocysteine and lipids profiles in streptozotocin‐induced diabetic rats. Physiol Rep. 2020;8:e14348. doi: 10.14814/phy2.14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JY, Cha HJ, Choi EO, Kim CH, Kim GY, Yoo YH, Hwang HJ, Park HT, Yoon HM, Choi YH. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int J Med Sci. 2019;16:145–155. doi: 10.7150/ijms.27005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY & Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomol Ther. 2012;20:268. doi: 10.4062/biomolther.2012.20.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–12. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RA, Deshpande AR. Anti-inflammatory and antioxidant effect of ginger in tuberculosis. J Complement Integr Med. 2016;13:201–206. doi: 10.1515/jcim-2015-0032. [DOI] [PubMed] [Google Scholar]
- Lantz RC, Chen G, Sarihan M, Solyom A, Jolad S, Timmermann B. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–128. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Only controls: effect of handling, sham injection, and intraperitoneal injection of saline on behavior of mice in an elevated plus-maze. J Pharmacol Toxicol Methods. 1995;34:73–77. doi: 10.1016/1056-8719(95)00025-d. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Nagy LE, Mcintyre TM. Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation. Free Radic Biol Med. 2014;69:403–416. doi: 10.1016/j.freeradbiomed.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong L, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. [Google Scholar]
- Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. 2016;Cell Mol Life Sci 73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedemann C, Bord E, Qin G, Zhu Y, Goukassian D Losordo DW, Kishore R. Ethanol modulation of tnf‐alpha biosynthesis and signaling in endothelial cells: synergistic augmentation of tnf‐alpha mediated endothelial cell dysfunctions by chronic ethanol. Alcohol Clin Exp Res. 2005;29:930–938. doi: 10.1097/01.alc.0000171037.90100.6b. [DOI] [PubMed] [Google Scholar]
- Luettig J, Rosenthal R, Lee IM, Krug SM, Schulzke JD. The ginger component 6-shogaol prevents TNF-alpha-induced barrier loss via inhibition of PI3K/Akt and NF-kappaB signaling. Mol Nutr Food Res. 2016;60:2576–2586. doi: 10.1002/mnfr.201600274. [DOI] [PubMed] [Google Scholar]
- Luo JF, Shen XY, Lio CK, Dai Y, Cheng CS, Liu JX, Yao YD, Yu Y, Xie Y, Luo P, Yao XS, Liu ZQ, Zhou H. Activation of Nrf2/HO-1 pathway by nardochinoid c inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front Pharmacol. 2018;9:911–911. doi: 10.3389/fphar.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahluji S, Ostadrahimi A, Mobasseri M, Ebrahimzade Attari V, Payahoo L. Anti-inflammatory effects of zingiber officinale in type 2 diabetic patients. Adv Pharm Bull. 2013;3:273–276. doi: 10.5681/apb.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhadi NS, Ghiasvand R, Askari G, Hariri M, Darvishi L, Mofid MR. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int J Prev Med. 2013;4:S36. [PMC free article] [PubMed] [Google Scholar]
- Nicoll R, Henein M. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int J Cardiol. 2009;131:408–409. doi: 10.1016/j.ijcard.2007.07.107. [DOI] [PubMed] [Google Scholar]
- Nimrouzi M, Ruyvaran M, Zamani A, Nasiri K, Akbari A. Oil and extract of safflower seed improve fructose induced metabolic syndrome through modulating the homeostasis of trace elements, TNF-α, and fatty acids metabolism. J Ethnopharmacol. 2020a:112721. doi: 10.1016/j.jep.2020.112721. [DOI] [PubMed] [Google Scholar]
- Nimrouzi M, Abolghasemi J, Sharifi MH, Nasiri K, Akbari A. Thyme oxymel by improving of inflammation, oxidative stress, dyslipidemia and homeostasis of some trace elements ameliorates obesity induced by high-fructose/fat diet in male rat. Biomed Pharmacother. 2020b;126:110079. doi: 10.1016/j.biopha.2020.110079. [DOI] [PubMed] [Google Scholar]
- Ostovar M, Akbari A, Anbardar MH, Iraji A, Salmanpour M, Ghoran SH, Heydari M, Shams M. Effects of Citrullus colocynthis in a rat model of diabetic neuropathy. J Integr Med. 2020;18:59–67. doi: 10.1016/j.joim.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Perrien DS, Liu Z, Wahl EC, Bunn RC, Skinner RA, Aronson J, Fowlkes J, Badger TM, Lumpkin JrC K. Chronic ethanol exposure is associated with a local increase in TNF-α and decreased proliferation in the rat distraction gap. Cytokine. 2003;23:179–189. doi: 10.1016/s1043-4666(03)00225-4. [DOI] [PubMed] [Google Scholar]
- Rafieian-Kopaei M. Medicinal plants for renal injury prevention. J Ren Inj Prev. 2013;2:63. doi: 10.12861/jrip.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
- Receno CN, Glausen TG, Deruisseau LR. Saline as a vehicle control does not alter ventilation in male CD-1 mice. Physiol Rep. 2018;6:e13702. doi: 10.14814/phy2.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy YA, Chalamaiah M, Ramesh B, Balaji G, Indira P. Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J Food Sciand Tech. 2014;51:908–914. doi: 10.1007/s13197-011-0568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez DA, Moncada C, Nunez MT, Lavandero S, Ponnappa BC, Israel Y. Ethanol increases tumor necrosis factor-alpha receptor-1 (TNF-R1) levels in hepatic, intestinal, and cardiac cells. Alcohol. 2004;33:9–15. doi: 10.1016/j.alcohol.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Ross IA. Zingiber Officinale. Springer; 2005. [Google Scholar]
- Saedisomeolia A, Arzati MM, Abdolahi M, Sedighiyan M, Rangel A, Muench G, Zarezadeh M, Jafarieh A, Honarvar NM. Mechanisms of action of ginger in nuclear factor-kappaB signaling pathways in diabetes. J Herb Med. 2019;16:100239. [Google Scholar]
- Schadich E, Hlaváč J, Volná T, Varanasi L, Hajdúch M, Džubák P. Effects of ginger phenylpropanoids and quercetin on nrf2-are pathway in human bj fibroblasts and hacat keratinocytes. Biomed Res Int. 2016;2016:3275–3275. doi: 10.1155/2016/2173275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semwal RB, Semwal DK, Combrinck S, Viljoen AM. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry. 2015;117:554–568. doi: 10.1016/j.phytochem.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Shanmugam KR, Ramakrishna CH, Mallikarjuna K, Reddy KS. Protective effect of ginger against alcohol-induced renal damage and antioxidant enzymes in male albino rats. Indian J Exp Biol. 2010;48:143–149. [PubMed] [Google Scholar]
- Shield KD, Parry C, Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2013;35:155–173. [PMC free article] [PubMed] [Google Scholar]
- Shirpoor A, Rezaei F, Fard AA, Afshari AT, Gharalari FH, Rasmi Y. Ginger extract protects rat’s kidneys against oxidative damage after chronic ethanol administration. Biomed Pharmacother. 2016;84:698–704. doi: 10.1016/j.biopha.2016.09.097. [DOI] [PubMed] [Google Scholar]
- Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke C C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- Tu W, Wang H, Li S, Liu Q & Sha H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019;10:637. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AL. Determination of total phenolics. Current protocols Food Analytical Chemistry. 2002;6:I1–1. [Google Scholar]
- Xu L, Yu Y, Sang R, Li J, Ge B, & Zhang X. Protective Effects of Taraxasterol against Ethanol-induced liver injury by regulating CYP2E1/Nrf2/HO-1 and NF-kappa B signaling pathways in mice. Oxid Med Cell Longev. 2018;2018:8284107. doi: 10.1155/2018/8284107. [DOI] [PMC free article] [PubMed] [Google Scholar]