Abstract
Background
Lung adenocarcinoma (LUAD) is the most common pathological subtype of lung cancer. Ferroptosis, an oxidative, iron-dependent form of necrotic cell death, is highly associated with tumorigenesis and cancer progression. However, the prognostic value of ferroptosis progress in LUAD was still rarely be investigated.
Methods
Herein, we collected three mRNA expression profiles and 85 ferroptosis-related genes from public databases. The “limma” package was used to identify ferroptosis-related differentially expressed genes (DEGs). Univariate Cox regression analysis and LASSO regression analysis were applied to screen and develop a ferroptosis-related gene signature (FRGS) and a formula to calculate the risk score. Multivariate Cox regression analysis was implemented to determine independent prognostic predictors of overall survival (OS). The area under the receiver operating characteristic curve (AUC) and calibration plot were used to evaluate the predictive accuracy of the FRGS and nomogram.
Results
We developed a FRGS with five genes (CYBB, CISD1, FADD, SAT2, VDAC2). The AUC of the FRGS in TCGA cohort was 0.777 at 1-year, 0.721 at 3-year and 0.725 at 5-year, significantly superior to the AUC of TNM stage (1-year: 0.701, 3-year: 0.691, 5-year: 0.686). A similar phenomenon was observed in GEO cohort 1 and 2. Multivariate Cox regression analysis indicted TNM stage and risk score were independent prognostic predictors. Finally, we built a nomogram with TNM stage and FRGS, the AUCs of which markedly higher than that of FRGS or TNM stage alone.
Conclusion
We constructed a prognostic FRGS with five ferroptosis-related genes and a nomogram for predicting the 1-, 3- and 5-year survival rate of LUAD patients, which may provide a new understanding of the prognostic value of ferroptosis progress in LUAD and will benefit prognosis assessment of LUAD patients.
Keywords: Lung adenocarcinoma, Ferroptosis, Signature, Immune
Introduction
Lung cancer (LC), the highest incidence rate of malignancy globally, is estimated that nearly 234,000 new cases are diagnosed per year, and accounts for 13% and 14% new cancer cases in women and men, respectively (Bray et al., 2018; De Groot et al., 2018). Non-small cell lung cancer (NSCLC) is the most common subtype of LC (85%), and lung adenocarcinoma (LUAD) is the leading histology of NSCLC and accounts for over 65% of NSCLC cases (De Groot et al., 2018; Yu, Zhang & Zhang, 2020). With the advance of treatment strategies such as targeted therapy and immunotherapy, the long term survival rate of LUAD patients is continuously increasing. However, the five-year survival of LC patients at stage I remains poor at 65%, and for advanced patients is still less than 20% (Goldstraw et al., 2007; Herbst, Morgensztern & Boshoff, 2018). Thus, there is an urgent need to identify prognostic biomarkers and develop an effective prognostic model for predicting the prognosis of LUAD, which may be conducive to risk stratification and clinical treatment of LUAD.
Based on morphotype, cell death is classified into three types: apoptosis, autophagy and necrosis. In recent years, some novel discovered cell death processes have altered our traditional understanding of cell death (Liang et al., 2019; Proneth & Conrad, 2019). Among them, ferroptosis has gained considerable attention from researchers. Unlike autophagy and apoptosis, ferroptosis is defined as an oxidative, iron-dependent form of necrotic cell death, characterized by the generation of lethal amounts of lipid peroxidation products and reactive oxygen species (ROS) (Proneth & Conrad, 2019). Emerging evidence showed that some oncogenic pathways were related to ferroptosis and eradicate the carcinogenic cells by adjusting ferroptosis (Mou et al., 2019; Hassannia, Vandenabeele & Vanden Berghe, 2019). For example, P53, a well-studied tumor suppressor gene, could repress the expression of cystine/glutamate antiporter at the transcriptional level to regulate the ferroptosis pathway (Xie et al., 2017). In addition, clues demonstrated that extra-mitochondrial lipid peroxidation arising from an iron-dependent ROS accretion could trigger ferroptosis to inhibit tumorigenesis (Liang et al., 2019). Nevertheless, as reported in previous studies, ferroptosis process in lung tissues is generally inhibited due to the up-regulation of cystine/glutamate antiporter and iron reduction, which contribute to the tumorigenesis and development of LC (Lai et al., 2019). Whether ferroptosis process and relevant ferroptosis-related genes are correlated with the clinical outcomes of LUAD remains to be further elucidated.
In the present study, we collected ferroptosis-related genes and mRNA expression profiles to construct a novel ferroptosis-related gene signature (FRGS) and nomogram for predicting overall survival (OS) of LUAD patients, which may be help to understand the potential prognostic value of ferroptosis-related genes in LUAD, and provide a convenient tool for risk assessment and prognosis assessment in LUAD.
Materials and Methods
Data collection
Three independent cohorts were collected in this study, including TCGA cohort, GEO cohort 1 and GEO cohort 2. The level 3 RNA sequencing of TCGA cohort was retrieved from TCGA database (TCGA-LUAD, The Cancer Genome Altas, https://www.cancer.gov). The raw data of mRNA expression matrix of GSE68465 (GEO cohort 1) and GSE41271 (GEO cohort 1) were gathered from GEO database (Gene Expression Omnibus, https://www.ncbi.nlm.nih.gov). The platform of GSE68465 was GPL96 (Affymetrix Human Genome U133A Array), and for GSE41271, was GPL6884 (Illumina HumanWG-6 v3.0 expression beadchip), GSE68465 was normalized with the MAS5.0 method using the “affy” package, and GSE41271 was normalized with the Bioconductor package “lumi”. In addition, corresponding clinical information of all patients were also collected, such as age, gender, smoking, TNM stage, overall survival time (OS), and survival status. Cases lacking survival time and survival status would be removed. Ferroptosis-related genes were obtained from KEGG Pathway Database (https://www.genome.jp/dbget-bin/www_bget?pathway:map04216), and previous literature (Mou et al., 2019; Stockwell et al., 2017; Doll et al., 2019).
Construction and evaluation of FRGS
In TCGA cohort, the “limma” package in R was used to identify ferroptosis-related differentially expressed genes (DEGs) between normal and LUAD tissues. |log2FC| > 1 and false discovery rate (FDR) < 0.01 were set as the criteria. Then, DEGs were subjected to univariate Cox regression analysis to screen out OS-related genes using P-value < 0.05 as the cut-off criterion. Finally, LASSO regression analysis was applied to further filtrate prognostic candidate ferroptosis-related DEGs, and construct a FRGS and ferroptosis-related risk score formula with the “glmnet” R package.
Based on this formula, we calculated the risk score of each patient, and divided patients into low- and high- risk score groups according to the optimal cut-off value of risk score determined with R package “Survminer”. The principal component analysis (PCA) was performed to investigate the expression difference between low-and high-risk groups. The receiver operating characteristic curve (ROC) was depicted and the area under the curve (AUC) was calculated to evaluate the predictive power of the FRGS. Additionally, with a boot-strapping set of 1,000 resamples, the calibration plot was carried out to assess the FRGS. In the same way, the risk score of each patient was calculated, and the ROC and calibration plot were also performed in GEO cohort 1 and 2 to validate the predictive effectiveness of the FRGS.
Gene set enrichment analysis (GSEA)
To determine the potential altered signaling pathways between low- and high- risk patients, GSEA was performed with FDR < 0.05 as the threshold.
Single-sample gene set enrichment analysis (ssGSEA)
The ssGSEA was performed to assess the level of immune cells and the activity of immune-related pathways with BiocManager package: GSVA. The method for ssGSEA was based on a rank value of each gene, which defined a score representing the degree of absolute enrichment of a particular gene set in each sample. 29 gene sets specific (16 immune cell gene sets and 13 immune-related pathway sets) were acquired from other studies (Charoentong et al., 2017; Shi et al., 2020; Zuo et al., 2020).
Statistical analysis
All statistical analyses were performed with R 3.6.3 (https://www.r-project.org). For categorical data, group comparison was conducted with chi-square test, and for measurement data, was with t-test or one-way ANOVA. The Spearman correlation test analyzes the correlation among candidate ferroptosis-related candidate DEGs. Kaplan–Meier plot was carried out to investigate the relation of OS to the ferroptosis-related risk score as well as the expression of ferroptosis-related candidate DEGs. Univariate Cox regression analysis and multivariate Cox regression analysis were implemented to determine independent prognostic predictors of OS.
Results
Patient cohort
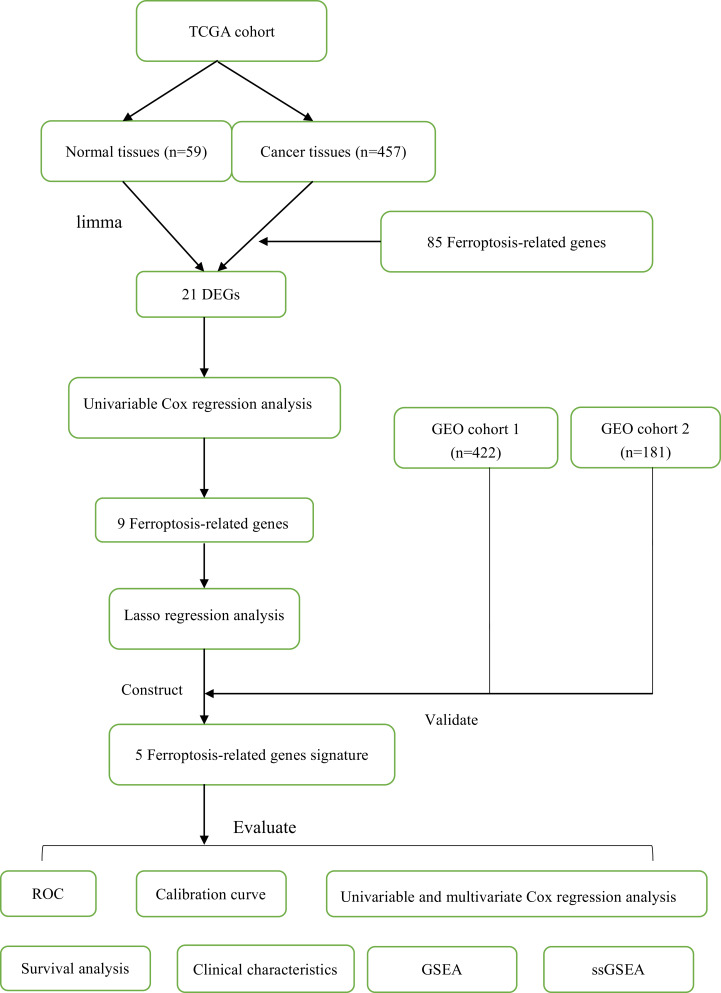
In this study, a total of 1060 LUAD patients were enrolled from three independent cohorts (TCGA cohort: 457; GEO cohort 1: 422; GEO cohort 2: 181). The detailed demographic and baseline characteristics of all samples were presented in Table 1. Besides, we obtained 85 ferroptosis-related genes. The flow diagram of this study was shown in Fig. 1.
Table 1. The baseline characteristics of lung adenocarcinoma patients in this study.
| Parameter | TCGA cohort | GEO cohort 1 | GEO cohort 2 |
|---|---|---|---|
| Database | TCGA | GSE68465 | GSE41271 |
| Gender | |||
| Female | 251 (54.92%) | 211 (50.00%) | 90 (49.72%) |
| Male | 206 (45.08%) | 211 (50.00%) | 91 (50.28%%) |
| Age | |||
| ≤65 | 233 (50.98%) | 219 (51.90%) | 102 (56.35%) |
| >65 | 224 (49.02%) | 203 (48.10%) | 79 (43.65%%) |
| Smoking | |||
| Never | 61 (13.35%) | 48 (11.37%) | 26 (14.36%) |
| Ever | 379 (82.93%) | 288 (68.25%) | 155 (85.64%%) |
| NA | 12 (2.62%) | 86 (20.38%) | 0 |
| TNM stage | |||
| I | 262 (57.33%) | 267 (63.27%) | 100 (55.25%) |
| II | 102 (22.32%) | 95 (22.51%) | 28 (15.47%) |
| III | 70 (15.32%) | 60 (14.22%) | 49 (27.07%) |
| IV | 23 (5.03%) | 0 | 4 (2.21%) |
| Tumor size | |||
| T1 | 149 (32.60%) | 146 (34.60%) | NA |
| T2 | 241 (52.74%) | 238 (56.40%) | NA |
| T3 | 49 (10.72%) | 27 (6.40%) | NA |
| T4 | 18 (3.93%) | 11 (2.60%) | NA |
| NA | 0 | 0 | 181 (100%) |
| Lymph node | |||
| N0 | 311 (68.05%) | 289 (68.48%) | NA |
| N1-3 | 146 (31.95%) | 133 (31.52%) | NA |
| NA | 0 | 0 | 181 (100%) |
| Metastasis | |||
| M0 | 434 (94.97%) | 422 (100%) | NA |
| M1 | 23 (5.03%) | 0 | NA |
| NA | 0 | 0 | 181 (100%) |
| Survival status | |||
| Alive | 306 (66.96%) | 193 (45.73%) | 112 (61.88%%) |
| Dead | 151 (33.04%) | 229 (54.27%%) | 69 (38.12%) |
| Risk score | |||
| Low | 272 (59.52%) | 89 (21.09%) | 122 (67.40%) |
| High | 185 (40.48%) | 333 (78.91%) | 59 (32.60%) |
| Total | 457 (100%) | 422 (100%) | 181 (100%) |
Note:
TCGA, The Cancer Genome Altas; GEO, Gene Expression Omnibus; NA, represents information not available.
Figure 1. The flow diagram of this study.
Construction, validation and evaluation of the prognostic FRGS
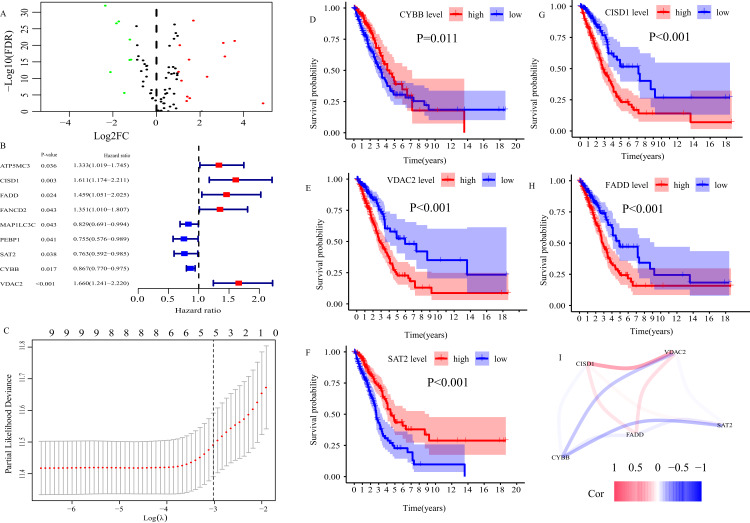
In TCGA cohort, 21 ferroptosis-related DEGs between normal and LUAD tissues were determined (Fig. 2A). Then, 21 DEGs were subjected to univariate Cox regression analysis, and 9 genes highly related to OS of LUAD patients were screened out (P < 0.05, Fig. 2B). Finally, we applied LASSO regression analysis to analyze those 9 genes, and constructed a prognostic FRGS with 5 candidate ferroptosis-related genes (CYBB, CISD1, FADD, SAT2, VDAC2, Fig. 2C). Survival analysis showed that high-level CYBB (P = 0.011, Fig. 2D) and SAT2 (P < 0.001, Fig. 2F) along with low-level CISD1 (P < 0.001, Fig. 2G), FADD (P < 0.001, Fig. 2H) and VDAC2 (P < 0.001, Fig. 2E) predicted better favorable outcomes in LUAD patients. The correlation among 5 candidate ferroptosis-related genes is presented in Fig. 2I. The risk score formula was presented as follows.
Figure 2. Construction of ferroptosis-related gene signature (FRGS) in TCGA cohort.
(A) Volcano plot of ferroptosis-related DEGs between normal and LUAD tissues with |log2FC| > 1 and FDR < 0.01. Red dots represent 13 up-regulated genes and green dots represent eight down-regulated genes. (B) Univariate Cox regression analysis identified nine ferroptosis-related genes were related to overall survival (P < 0.05). (C) “Leave- one-out-cross-validation” for parameter selection in LASSO regression to filter out five candidate genes. (D) Survival analysis of CYBB, (E) CISD1, (F) VDAC2, (G) FADD, (H) SAT2. (I) The correlation network of five candidate genes. DEG, differentially expressed genes; FDR, false discovery rate.
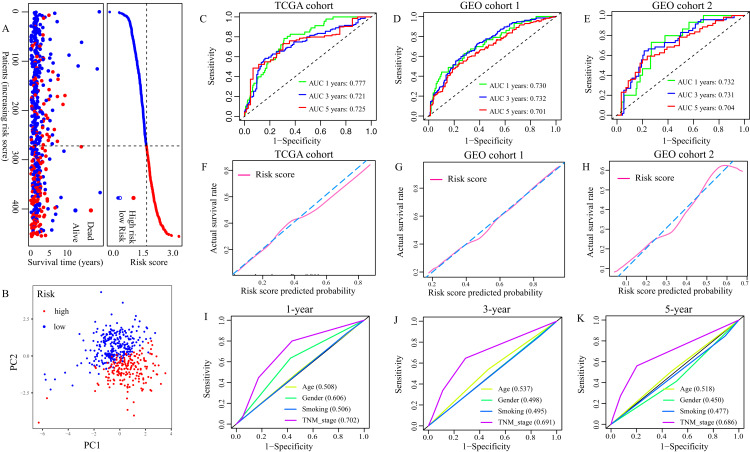
According to the formula, the risk score of each patient in TCGA cohort was calculated, and patients were divided into low- and high-risk groups based on the optimal cut-off risk score:1.515 (Fig. S1A). So did patients in GEO cohort 1 and 2. The distributions of risk score and survival status in TCGA cohort were shown in Fig. 3A. PCA of TCGA cohort demonstrated that the patients in different risk groups were gathered in two areas (Fig. 3B), which was confirmed in GEO cohort 1 and 2 (Figs. S1B and S1C).
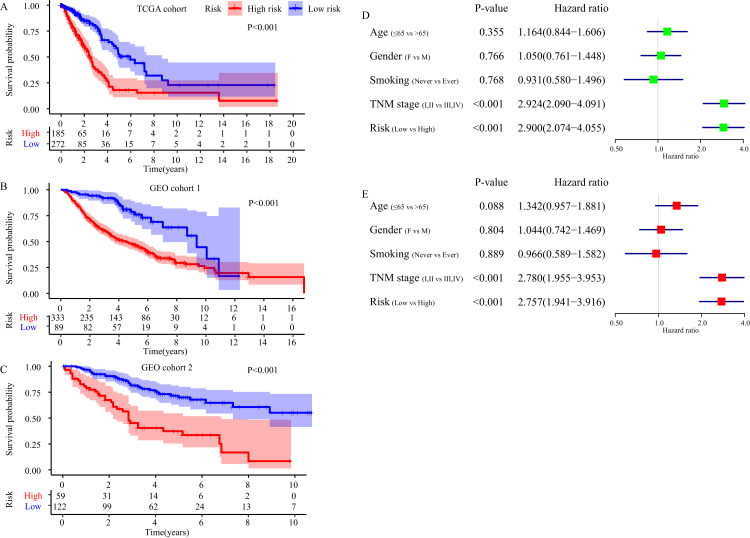
Figure 3. Evaluation of the FRGS.
(A) The distribution of risk score and survival status in TCGA cohort. (B) PCA showed that the patients in different risk groups were gathered in two areas. (C–E) The ROC of the FRGS for predicting 1-, 3- and 5-year OS in TCGA cohort, GEO cohort 1, and GEO cohort 2. (F–H) The calibration plots of TCGA cohort, GEO cohort 1 and GEO cohort 2. (I–K) The ROC of clinical indexes for predicting 1-, 3- and 5-year OS in TCGA cohort. PCA, principal component analysis; ROC, the receiver operating characteristic curve.
Then, we depicted the ROC of the FRGS, and estimated the value of AUC at 1-, 3- and 5- year. The AUC of the FRGS in TCGA cohort was 0.777 at 1-year, 0.721 at 3-year and 0.725 at 5-year (Fig. 3C), higher than that of clinical indexes (Figs. 3I–3K). To confirm the accuracy of the survival probabilities of the FRGS, we validate the finding in GEO cohort 1 with AUCs at 1-, 3- and 5-year OS reaching 0.730, 0.732 and 0.701, respectively (Fig. 3D), and in GEO cohort 2 with AUCs at 1-, 3- and 5-year OS reaching 0.732, 0.731 and 0.704, respectively (Fig. 3E). Similarly, the AUCs in GEO cohort 1 and 2 were more excellent than that of clinical indexes (Fig. S2). The calibration plots of three cohorts were presented in Figs. 3F and 3H.
Correlation between FRGS and clinical characteristics
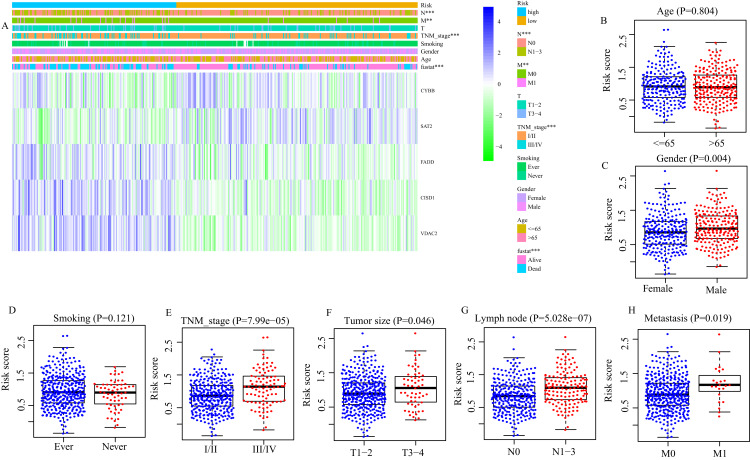
Next, we evaluated the correlation between FRGS and clinical characteristics (age, gender, smoking, TNM stage, tumor size, lymph node metastasis and distant metastasis). Between low- and high-risk groups, the distributions of patients at different TNM stage (P < 0.001), with or without lymph node metastasis (P < 0.001) and distant metastasis (P = 0.013) was significantly different (Fig. 4A). Moreover, the risk score in male patients was significantly increased (P = 0.004, Fig. 4C). Similar results were observed in patients staged III/IV (P < 0.001, Fig. 4E), Tumor size at T3/4 (P = 0.045, Fig. 4F), with lymph node metastasis (P < 0.001, Fig. 4G), and with distant metastasis (P = 0.019, Fig. 5H). However, we did not found the effect of age (P = 0.804, Fig. 4B) and smoking (P = 0.121, Fig. 4D) on the risk score.
Figure 4. Correlation between risk score and clinical characteristics.
(A) The distribution of clinicopathological features between high- and low- risk group. (B) The difference of risk score between Age ≤ 65 and Age > 65, (C) between different gender, (D) smoking and non-smoking, (E) TNM stage I/II and III/IV, (F) T1/2 and T3/4, (G) between with and without lymph node metastasis, (H) between with and without distance metastasis. **P < 0.01, ***P < 0.001.
Figure 5. The FRGS is an independent prognostic factor for the prognosis of LUAD patients.
(A–C) Survival difference between high- and low-risk group in TCGA cohort, GEO cohort 1 and GEO cohort 2. (D) Result of the univariate Cox regression analysis in TCGA cohort. (E) Results of the multivariate Cox regression analysis in TCGA cohort.
Independent prognostic value of the FRGS
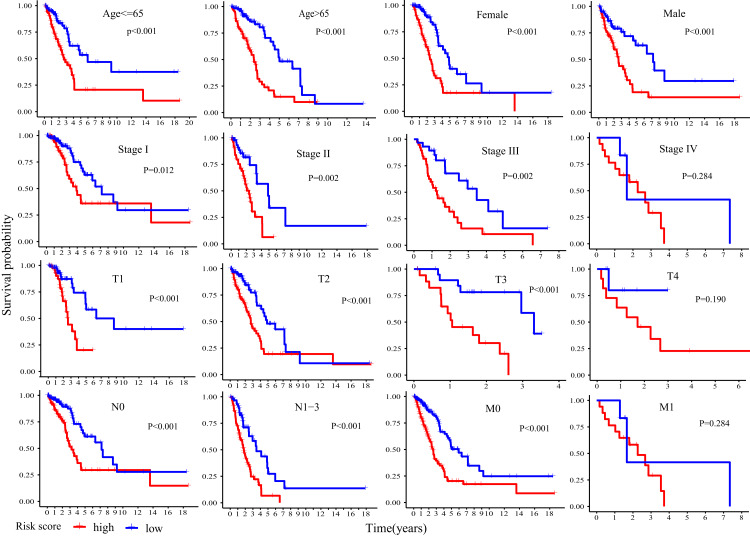
Kaplan-Meier plot was executed to explore overall survival difference between two risk groups, and the result in TCGA cohort demonstrated that the prognosis of high-risk patients was worse than that of low-risk patients (P < 0.001, Fig. 5A), in line with the results in GEO cohort 1 (P < 0.001, Fig. 5B) and GEO cohort 2 (P < 0.001, Fig. 5C). Furthermore, stratification analyses indicated high-risk patients exhibited poorer clinical outcomes in each subgroup except in subgroup Stage IV, T1 and M1 (Fig. 6).
Figure 6. Stratification analyses of overall survival difference between high- and low- risk patients in different subgroups.
Then, we took advantage of univariate and multivariate Cox regression model to compare the risk score with clinical parameters (age, gender, smoking, TNM stage) in TCGA cohort to determine whether the risk score was an independent prognostic predictor for OS of LUAD patients. Univariate Cox regression analysis showed that the risk score was an important OS-related influence factor (HR = 2.900, 95% CI [2.074–4.055], P < 0.001, Fig. 5D). We verified the result in GEO cohort 1 (HR = 2.820, 95% CI [1.831, 4.342], P < 0.001, Fig. S3A) and GEO cohort 2 (HR = 3.511, 95% CI [2.170–5.681], P < 0.001, Fig. S3C), and obtained the same results. In addition, multivariate Cox regression model in TCGA cohort indicated the risk score was an independent influence factor for OS of LUAD patients (HR = 2.757, 95% CI [1.941–3.916], P < 0.001, Fig. 5E). It was validated in GEO cohort 1 (HR = 2.418, 95% CI [1.543–3.789], P < 0.001, Fig. S3B) and GEO cohort 2 (HR = 3.390, 95% CI [2.062–5.574], P < 0.001, Fig. S3D).
GSEA
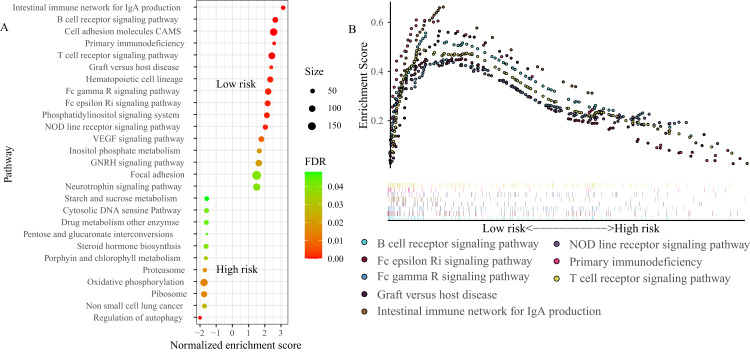
To explore the basic biological mechanisms of the FRGS, we performed GSEA analysis. A total of 27 pathways were identified, including 16 pathways in low-risk group and 11 pathways in high-risk group (Fig. 7A). Of note, several immune-related pathways were enriched in low-risk group, such as B cell receptor signaling pathway (Normalized enrichment score (NES): 2.72, FDR < 0.001), T cell receptor signaling pathway (NES: 2.52, FDR < 0.001), Intestinal immune network for IgA production (NES: 3.19, FDR < 0.001), NOD line receptor signaling pathway (NES: 2.1, FDR = 0.001), Fc epsilon Ri signaling pathway (NES: 2.25, FDR = 0.001), Fc gamma R signaling pathway (NES: 2.29, FDR = 0.001), and Graft versus host disease (NES: 2.44, FDR = 0.001, Fig. 7B).
Figure 7 . Gene set enrichment analysis (GSEA) between high- and low-risk groups.
(A) A total of 27 pathways were identified, including 16 pathways in low-risk group and 11 pathways in high-risk group. (B) Eight immune-related pathways enriched in low-risk group. NES, normalized enrichment score; ES, enrichment score.
ssGSEA
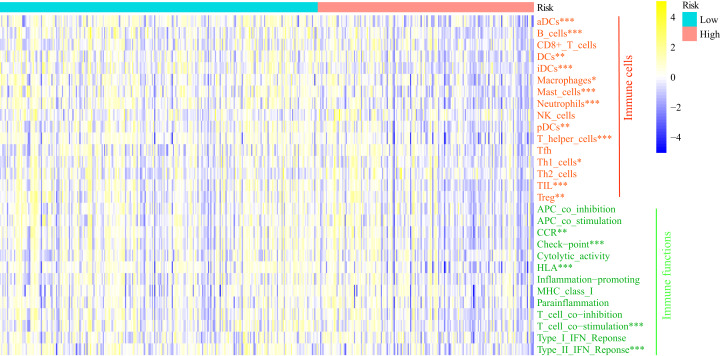
GSEA analysis showed the FRGS was highly associated with immune status. Thus, we carried out ssGSEA to investigate the relationship of FRGS to the enrichment score of 16 immune cells and 13 immune-related pathways (Fig. 8). Interestingly, the enrichment score of aDCs, DCs, iDCs, pDCs, B cells, Macrophages, Mast cells, Neutrophils, T helper cells, Th1 cells, TIL and Treg was significantly increased in low-risk group. Meanwhile, low-risk group had a higher score of CCR, the activity of checkpoint molecules, HLA, T cell co−stimulation and IFN Reponse Type II.
Figure 8. Comparison of the enrichment score of ssGSEA between different risk groups.
aDCs, activated dendritic cells; DCs, dendritic cells; iDCs, immature dendritic cells; pDCs, plasmacytoid dendritic cells; Tfh, T follicular helper cells; Th1_cells, type 1 helper cells; Th2_cells, type 2 helper cells; TIL, tumor infiltrating lymphocytes; Treg, regulatory T cells; HLA, human leukocyte antigen; CCR: C–C chemokine receptor; APC, antigen presenting cells; MHC, major histocompatibility complex. *P < 0.05, **P < 0.01, ***P < 0.001.
Construction, validation and evaluation of of nomogram
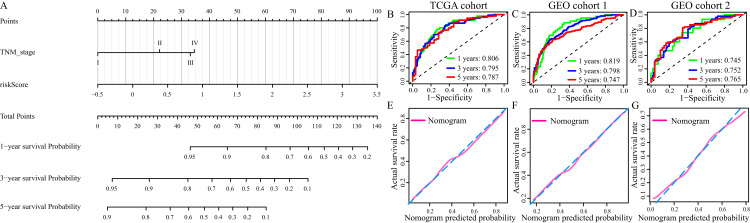
Multivariate Cox regression analysis identified TNM stage and risk score were independent OS-related predictors. Therefore, we used TNM stage and risk score to develop a prognostic nomogram in TCGA cohort (Fig. 9A). The AUCs of the nomogram for predicting 1-, 3- and 5- year OS in TCGA cohort were 0.806, 795 and 0.787, respectively (Fig. 8B). Then, we validated the predictive power of the nomogram in GEO cohort 1 and 2. The AUCs in GEO cohort 1 reached 0.819 at 1-year, 0.798 at 3-year and 0.747 at 5-year, and in GEO cohort 2 reached 0.745 at 1-year, 0.752 at 3-year and 0.765 at 5-year (Figs. 8C and 8D). The calibration plots of the nomogram in three cohorts were presented in Figs. 8E and 8F.
Figure 9. Construction and evaluation of the prognostic nomogram.
(A) The prognostic nomogram for the prediction of 1-, 3- and 5-year overall survival in LUAD. (B–D) The ROC of the nomogram for predicting 1-, 3- and 5-year OS in TCGA cohort, GEO cohort 1, and GEO cohort 2. (E–G) The calibration plots of TCGA cohort, GEO cohort 1 and GEO cohort 2.
Discussion
In recent years, due to air pollution, smoking and other factors, the incidence rate of LC is continuously rising. And, LUAD has become the most common histological subtype of LC (Bray et al., 2018; De Groot et al., 2018). Although, increasingly advanced diagnosis and treatment strategy greatly improved the long term survival rate of LUAD patients. The five-year survival rate remains less than 20% (Herbst, Morgensztern & Boshoff, 2018). TNM stage (AJCC) is still the most commonly used parameter for clinical decision and prognosis evaluation in LUAD. However, emerging clinical evidences show that patients with the same TNM stage and treatment strategy have different prognoses, indicating that prognosis assessment based on TNM stage alone may be not adequate in LC.
Ferroptosis, as an oxidative, iron-dependent form of necrotic cell death, has attracted a lot of attention. Researches realized that several oncogenic pathways render tumor cells extremely susceptible to ferroptosis through regulating key checkpoints, which could promote cancer cells death and repress tumor progression (Mou et al., 2019; Hassannia, Vandenabeele & Vanden Berghe, 2019) Previous studies showed modulation of ferroptosis progress could alter proliferation, colony formation, and cell death of LC cells and improve the therapeutic effect on xenograft of LC (Alvarez et al., 2017; Wang et al., 2019a; Gai et al., 2020). However, the role of ferroptosis on LUAD patients’ OS remains largely unknown.
In the current study, we collected 85 ferroptosis-related genes and three mRNA expression matrixes of LUAD patients (TCGA-LUAD, GSE68465 and GSE41271). In TCGA cohort, 21 ferroptosis-related DEGs were identified between normal and LUAD tissues. After screened by univariate Cox regression analysis and LASSO regression analysis, 5 ferroptosis-related genes (CYBB, CISD1, FADD, SAT2, VDAC2) were applied to construct a novel ferroptosis-related gene signature (FRGS). Survival analysis illustrated that all of those 5 genes were highly related to the OS of LUAD. FADD (Fas Associated Via Death Domain) is an adaptor molecule that, through recruiting caspase-8 or caspase-10, could activate Fas (CD95) or TNFR-1 receptors to mediate cell death signals. In addition, it is also involved in cell cycle progression, T-cell proliferation, and interferon-mediated immune response (Marín-Rubio et al., 2019). Previous researches have proven that overexpression of FADD predicted unfavorable clinical outcomes in NSCLC (Bhojani et al., 2005; Chen et al., 2005; Chen et al., 2020), which was in line with the finding of this study. Experimental studies showed that NSCLC cells could release FADD which was positively related to the progression and aggressiveness of LC and phosphorylated FADD can induce cell-cycle progression and cell proliferation in LC (Chen et al., 2005; Chen et al., 2020). VDAC2 (Voltage Dependent Anion Channel 2) encodes a protein that participates in metabolite diffusion across the mitochondrial outer membrane and mitochondrial apoptotic pathway (Chin et al., 2018). A basic study about melanoma demonstrated VDAC2 could suppress ferroptosis (Yang et al., 2020). Additionally, Wang et al. found increased VDAC2 protected pancreatic cancer cells from chemotherapy via restraining apoptosis (Chin et al., 2018). The protein encoded by CISD1 (CDGSH Iron Sulfur Domain 1) localizes to the outer membrane of mitochondria, and binds to a redox-active [2Fe-2S] cluster (Taminelli et al., 2008; Yuan et al., 2016). It plays an important role in the regulation of oxidation and iron metabolism. And, CISD1 could limit mitochondrial iron uptake and therefore inhibit ferroptosis (Taminelli et al., 2008). CYBB (Cytochrome B-245 Beta Chain) is a primary component of the oxidase system of phagocytes, and is involved in the killing effect of multiple immune cells (Monteiro et al., 2013). SAT2 (Spermidine/Spermine N1-Acetyltransferase Family Member 2) could catalyze the N-acetylation of the amino acid thialysine, and enhance NF-kappaB-dependent transcription (Vogel, Boeke & Ashburner, 2006). Although, many previous studies have reported the biological functions of those 5 ferroptosis-related genes. Up to now, little is known regarding the role of those genes on LUAD especially CYBB, CISD1, SAT2 and VDAC2. Hence, it is needed more basic and clinical researches to further clarify the biological functions of those 5 ferroptosis-related genes in LUAD.
Herein, we developed and validated a FRGS for predicting OS of LUAD patients. Survival analysis demonstrated that high-risk patients had poorer clinical outcomes. And stratification analyses showed the same results in each subgroup except in subgroup Stage IV, T1 and M1, which may be because of insufficient sample size. The AUCs of the FRGS in TCGA cohort were 0.777 at 1-year, 0.721 at 3-year and 0.725 at 5-year, significantly superior to the AUCs of TNM stage (1-year: 0.701, 3-year: 0.691, 5-year: 0.686). A similar phenomenon was observed in GEO cohort 1 and 2. Additionally, the calibration plots demonstrated a good fit between the predicted probability and the actual survival rate. Multivariate Cox regression analysis identified TNM stage and risk score were independent factors for OS. Therefore, we applied those two parameters to construct a novel prognostic nomogram for estimating 1-, 3- and 5-year survival rate of LUAD patients. The AUCs of the nomogram in three cohorts were similar, and markedly higher than that of FRGS or TNM stage alone, indicating the nomogram is stable and reliable for predicting 1-, 3- and 5-year survival rate of LUAD. Moreover, the calibration plots showed better consistency than that of FRGS along. In recent years, several prognostic signatures based on RNA-seq or microarray expression have been established for exploring prognosis-related biomarkers and predicting OS of LUAD. For example, a previous study developed an autophagy-related gene prognostic signature with the AUC = 0.615 at 5-year (Zhu, Wang & Hu, 2020). And, an immune signature for 1- and 3-year survival rate of LUAD with AUCs reaching 0.70 and 0.68 (Guo et al., 2020). Both the AUCs of them were inferior to that of the nomogram. All data suggested that the established prognostic nomogram is suitable for predicting the 1-, 3- and 5-year survival probability of LUAD patients. Previously, Gao et al. established a prognostic signature with 12 ferroptosis genes (Gao et al., 2021). Those 12 ferroptosis genes were totally different with the ferroptosis-related genes in the study, which may be caused by different way used in developing signature. Gao et al. only applied LASSO regression analysis to screen prognostic candidate ferroptosis-related genes, and construct a signature. Herein, we firstly used univariate Cox regression analysis to filter out OS-related genes. Then, we applied LASSO regression analysis to further filtrate prognostic ferroptosis-related genes, and develop a FRGS. In addition, we collected more ferroptosis-related genes, and patient cohorts.
Recently, it has been realized that ferroptosis is involved in tumor immunization and cancer immunotherapy. In the present study, we performed GSEA and ssGSEA to investigate the basic biological mechanisms of the FRGS, and the relation of the FRGS to immune system. GSEA analysis demonstrated several immune-related pathways were enriched, especially in low-risk group. Meanwhile, ssGSEA results showed the enrichment score of multiple immune cells and immune-related pathways in low-risk group were increased, hinting that the patients with low-risk score may have better immune status and immune function. Wang et al. reported that CD8+ T cells could drive ferroptosis in tumor cells, and the immune system can regulate the sensitization of tumor cells to ferroptosis to suppress tumorigenesis (Wang et al., 2019b). In addition, immunotherapy enhanced tumor lipid oxidation and ferroptosis by repressing SLC7A11 to improve tumor control (Lang et al., 2019). All of these evidences highlighted the promising role of ferroptosis in cancer therapy, and targeting tumor ferroptosis pathway may be a new therapeutic way in combination with immunotherapy. Yet, undeniably, more work is warranted to explore the immunomodulatory role of ferroptosis in anti-tumor immunity.
Although the prognostic FRGS and nomogram presented a well predictive accuracy and effectiveness for OS of LUAD patients in this study, there were still some limitations that needed to be addressed. Firstly, it was a retrospective study, and all cases were retrospective samples. Hence, validation of prospective samples was still needed. Secondly, owing to all samples were collected from public databases, the potential selection bias could not be excluded. Thirdly, the signature was constructed based on microarray expression and RNA-seq data, which is costly and time-consuming. And, it lacked validation using PCR or IHC. Hence, further investigation is demanded to examine the discovery of this research both in vitro and in vivo.
Together, in this study, we constructed a prognostic FRGS with 5 ferroptosis-related genes (CYBB, CISD1, FADD, SAT2, VDAC2), and a nomogram for predicting the 1-, 3- and 5-year survival rate of LUAD patients. Evidences indicated that the nomogram was stable and reliable for the prediction of LUAD prognosis. Our study provides a new understanding of the prognostic value of ferroptosis progress in LUAD and will benefit the prognosis assessment of LUAD patients. However, the underlying mechanisms of ferroptosis-related genes on LUAD, and its relation to tumor immune status remain relatively enigmatic and warrant further investigation.
Supplemental Information
(A) Determination of the optimal cut-off risk score:1.515. (B) PCA showed that the patients in different risk groups were gathered in two areas in GEO cohort 1. (C) PCA showed that the patients in different risk groups were gathered in two areas in GEO cohort 2.
(A) Result of the univariate Cox regression analysis in GEO cohort 1. (B) Results of the multivariate Cox regression analysis in GEO cohort 1. (C) Result of the univariate Cox regression analysis in GEO cohort 2. (D) Results of the multivariate Cox regression analysis in GEO cohort 2.
Abbreviations
- LC
Lung cancer
- NSCLC
Non-small cell lung cancer
- LU
AD lung adenocarcinoma
- DEGs
differentially expressed genes
- FRG
S ferroptosis-related gene signature
- TC
GA The Cancer Genome Altas
- GE
O Gene Expression Omnibus
- RO
C the receiver operating characteristic curve
- AU
C the area under curve of ROC
- O
S overall survival
- G
SEA Gene set enrichment analysis
- N
ES Normalized enrichment score
- s
sGSEA Single-sample gene set enrichment analysis
- F
DR false discovery rate
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Sheng Wang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Chunlei Wu performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Dehua Ma conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Quanteng Hu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
All data came from the TCGA dataset (TCGA-LUAD, The Cancer Genome Altas, https://www.cancer.gov) and the GEO database (GSE68465, GSE41271).
References
- Alvarez et al. (2017).Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, Possemato R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551(7682):639–643. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojani et al. (2005).Bhojani MS, Chen G, Ross BD, Beer DG, Rehemtulla A. Nuclear localized phosphorylated FADD induces cell proliferation and is associated with aggressive lung cancer. Cell Cycle. 2005;4(11):1478–1481. doi: 10.4161/cc.4.11.2188. [DOI] [PubMed] [Google Scholar]
- Bray et al. (2018).Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Charoentong et al. (2017).Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, Hackl H, Trajanoski Z. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Reports. 2017;18(1):248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2005).Chen G, Bhojani MS, Heaford AC, Chang DC, Laxman B, Thomas DG, Griffin LB, Yu J, Coppola JM, Giordano TJ, Lin L, Adams D, Orringer MB, Ross BD, Beer DG, Rehemtulla A. Phosphorylated FADD induces NF-kappaB, perturbs cell cycle, and is associated with poor outcome in lung adenocarcinomas. Proceedings of the National Academy of Sciences USA. 2005;102(35):12507–12512. doi: 10.1073/pnas.0500397102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2020).Chen L, Xie G, Feng J, Wen Q, Zang H, Lu J, Zhan Y, Fan S. Overexpression of FADD and Bcl-XS proteins as novel prognostic biomarkers for surgically resected non-small cell lung cancer. Cancer Biomark. 2020;30(2):145–154. doi: 10.3233/CBM-190018. [DOI] [PubMed] [Google Scholar]
- Chin et al. (2018).Chin HS, Li MX, Tan IKL, Ninnis RL, Reljic B, Scicluna K, Dagley LF, Sandow JJ, Kelly GL, Samson AL, Chappaz S, Khaw SL, Chang C, Morokoff A, Brinkmann K, Webb A, Hockings C, Hall CM, Kueh AJ, Ryan MT, Kluck RM, Bouillet P, Herold MJ, Gray DHD, Huang DCS, Van Delft MF, Dewson G. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nature Communications. 2018;9(1):4976. doi: 10.1038/s41467-018-07309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot et al. (2018).De Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Translational Lung Cancer Research. 2018;7(3):220–233. doi: 10.21037/tlcr.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll et al. (2019).Doll S, Freitas FP, Shah R, Aldrovandi M, Da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O’Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- Gai et al. (2020).Gai C, Yu M, Li Z, Wang Y, Ding D, Zheng J, Lv S, Zhang W, Li W. Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. Journal of Cellular Physiology. 2020;235(4):3329–3339. doi: 10.1002/jcp.29221. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2021).Gao X, Tang M, Tian S, Li J, Liu W. A ferroptosis-related gene signature predicts overall survival in patients with lung adenocarcinoma. Future Oncology. 2021;17(12):1533–1544. doi: 10.2217/fon-2020-1113. [DOI] [PubMed] [Google Scholar]
- Goldstraw et al. (2007).Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. Journal of Thoracic Oncology. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2020).Guo D, Wang M, Shen Z, Zhu J. A new immune signature for survival prediction and immune checkpoint molecules in lung adenocarcinoma. Journal of Translational Medicine. 2020;18(1):394. doi: 10.1186/s12967-020-02286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia, Vandenabeele & Vanden Berghe (2019).Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Herbst, Morgensztern & Boshoff (2018).Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- Lai et al. (2019).Lai Y, Zhang Z, Li J, Li W, Huang Z, Zhang C, Li X, Zhao J. STYK1/NOK correlates with ferroptosis in non- small cell lung carcinoma. Biochemical and Biophysical Research Communications. 2019;519(4):659–666. doi: 10.1016/j.bbrc.2019.09.032. [DOI] [PubMed] [Google Scholar]
- Lang et al. (2019).Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, Liao P, Zhou J, Zhang Q, Dow A, Saripalli AL, Kryczek I, Wei S, Szeliga W, Vatan L, Stone EM, Georgiou G, Cieslik M, Wahl DR, Morgan MA, Chinnaiyan AM, Lawrence TS, Zou W. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discovery. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang et al. (2019).Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Advanced Materials. 2019;31(51):e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- Marín-Rubio et al. (2019).Marín-Rubio JL, Vela-Martín L, Fernández-Piqueras J, Villa-Morales M. FADD in cancer: mechanisms of altered expression and function, and clinical implications. Cancers (Basel) 2019;11(10):1462. doi: 10.3390/cancers11101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro et al. (2013).Monteiro MB, Patente TA, Mohammedi K, Queiroz MS, Azevedo MJ, Canani LH, Parisi MC, Marre M, Velho G, Corrêa-Giannella ML. Sex-specific associations of variants in regulatory regions of NADPH oxidase-2 (CYBB) and glutathione peroxidase 4 (GPX4) genes with kidney disease in type 1 diabetes. Free Radical Research. 2013;47(10):804–810. doi: 10.3109/10715762.2013.828347. [DOI] [PubMed] [Google Scholar]
- Mou et al. (2019).Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. Journal of Hematology & Oncology. 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proneth & Conrad (2019).Proneth B, Conrad M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death and Differentiation. 2019;26(1):14–24. doi: 10.1038/s41418-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2020).Shi J, Jiang D, Yang S, Zhang X, Wang J, Liu Y, Sun Y, Lu Y, Yang K. LPAR1, correlated with immune infiltrates, is a potential prognostic biomarker in prostate cancer. Frontiers in Oncology. 2020;10:846. doi: 10.3389/fonc.2020.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell et al. (2017).Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taminelli et al. (2008).Taminelli GL, Sotomayor V, Valdivieso AG, Teiber ML, Marín MC, Santa-Coloma TA. CISD1 codifies a mitochondrial protein upregulated by the CFTR channel. Biochemical and Biophysical Research Communications. 2008;365(4):856–862. doi: 10.1016/j.bbrc.2007.11.076. [DOI] [PubMed] [Google Scholar]
- Vogel, Boeke & Ashburner (2006).Vogel NL, Boeke M, Ashburner BP. Spermidine/Spermine N1-Acetyltransferase 2 (SSAT2) functions as a coactivator for NF-kappaB and cooperates with CBP and P/CAF to enhance NF-kappaB-dependent transcription. Biochimica et Biophysica Acta. 2006;1759(10):470–477. doi: 10.1016/j.bbaexp.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019a).Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, Shi Y, Chen L, Xiao D, Yu F, Wang X, Zhou H, Cao Y, Liu S, Yan Q, Tao Y, Zhang B. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death and Differentiation. 2019a;26(11):2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019b).Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019b;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al. (2017).Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, Lotze MT, Zeh HJ, 3rd, Kang R, Kroemer G, Tang D. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Reports. 2017;20(7):1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2020).Yang Y, Luo M, Zhang K, Zhang J, Gao T, Connell DO, Yao F, Mu C, Cai B, Shang Y, Chen W. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nature Communications. 2020;11(1):433. doi: 10.1038/s41467-020-14324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2016).Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochemical and Biophysical Research Communications. 2016;478(2):838–844. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Yu, Zhang & Zhang (2020).Yu X, Zhang X, Zhang Y. Identification of a 5-gene metabolic signature for predicting prognosis based on an integrated analysis of tumor microenvironment in lung adenocarcinoma. Journal of Oncology. 2020;2020(4):1–12. doi: 10.1155/2020/5310793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo et al. (2020).Zuo S, Wei M, Wang S, Dong J, Wei J. Pan-cancer analysis of immune cell infiltration identifies a prognostic immune-cell characteristic score (ICCS) in lung adenocarcinoma. Frontiers in Immunology. 2020;11:1218. doi: 10.3389/fimmu.2020.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Wang & Hu (2020).Zhu J, Wang M, Hu D. Development of an autophagy-related gene prognostic signature in lung adenocarcinoma and lung squamous cell carcinoma. PeerJ. 2020;8(5):e8288. doi: 10.7717/peerj.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Determination of the optimal cut-off risk score:1.515. (B) PCA showed that the patients in different risk groups were gathered in two areas in GEO cohort 1. (C) PCA showed that the patients in different risk groups were gathered in two areas in GEO cohort 2.
(A) Result of the univariate Cox regression analysis in GEO cohort 1. (B) Results of the multivariate Cox regression analysis in GEO cohort 1. (C) Result of the univariate Cox regression analysis in GEO cohort 2. (D) Results of the multivariate Cox regression analysis in GEO cohort 2.
Data Availability Statement
The following information was supplied regarding data availability:
All data came from the TCGA dataset (TCGA-LUAD, The Cancer Genome Altas, https://www.cancer.gov) and the GEO database (GSE68465, GSE41271).