Abstract
Background
Immune checkpoint inhibitors (ICIs) are the new standard of care in microsatellite instability-high (MSI-H)/deficient mismatch repair (dMMR) metastatic colorectal cancer (mCRC). Since tumor response dynamic parameters already shown a strong association with survival outcomes in patients with mCRC treated with first-line therapy, we investigated the association of early tumor shrinkage (ETS) and depth of response (DoR) in patients with MSI-H/dMMR mCRC treated with ICIs.
Methods
This is a retrospective, multicenter, cohort study in patients with dMMR and/or MSI-high mCRC treated with ICIs (anti-PD-1/PD-L1 with or without anti-CTLA-4 agents) with measurable disease and at least one post-baseline radiological disease reassessment. The Kaplan-Meier method and Cox proportional-hazards regression models were used for survival analyses. A maximally selected statistics method in a Cox regression model for progression-free survival (PFS) was used to determine the optimal cut-offs for ETS and DoR.
Results
We included a total of 169 patients: 116 (68.6%) were treated with anti-PD-1 monotherapy, whereas 53 (31.4%) with anti-PD-1 plus anti-CTLA-4 agents. Patients with primary progressive disease (N=37, 21.9%), experienced an extremely poor overall survival (OS) and were evaluated separately. In patients with clinical benefit, we observed a significant association between ETS and DoR with both OS and PFS, and we identified a relative reduction of at least 1% as the optimal cut-off for ETS and a relative reduction of at least 50% as the optimal cut-off for DoR.
Conclusions
ETS and DoR are important prognostic factors in patients with MSI-high mCRC treated with ICIs that might be useful to design treatment intensification/deintensification strategies. A prospective validation of both is warranted.
Keywords: gastrointestinal neoplasms, immunotherapy, tumor biomarkers
Introduction
The efficacy of immune checkpoint inhibitors (ICIs) in microsatellite instability-high (MSI-H)/deficient mismatch repair (dMMR) metastatic colorectal cancer (mCRC) is unprecedented.1–3 Programmed cell death protein 1 (PD-1) blockade became a guideline-recommended, first-line treatment option for MSI-H/dMMR mCRC patients following the results of the landmark KEYNOTE-177 phase III trial.3 4 However, a non-negligible proportion of patients experiences primary resistance or short-term clinical benefit,1–3 and there are no validated clinical or biological predictive factors for the stratification of patients with MSI-H/dMMR mCRC candidate to ICI-treatment. Thus, the identification of biomarkers that could guide immunotherapy in this population represents an unmet clinical need.
Early tumor shrinkage (ETS) and depth of response (DoR) are novel, easy-evaluable on-treatment radiological parameters useful to assess the dynamic of tumor response, and both ETS and DoR showed a strong association with survival outcomes in mCRC patients receiving first-line treatment.5–9
Since the role of tumor response dynamic in predicting the outcomes of MSI-H/dMMR mCRC patients treated with ICIs is unknown, here we investigated the association of ETS and DoR with the outcomes and baseline characteristics of patients with MSI-H/dMMR mCRC treated with ICIs.
Methods
Patients’ population
This was a retrospective cohort study in patients with MSI-H/dMMR mCRC treated with ICIs (anti-PD-1/PD-L1 agents with or without cytotoxic T-lymphocyte-associated protein 4 [CTLA-4] blockade) at 6 Italian academic hospitals. We included patients with measurable disease and at least one post-baseline radiological disease reassessment. MSI/MMR status was assessed by means of immunohistochemistry (IHC) and/or polymerase chain reaction (PCR) as per international guidelines.10 Computed tomography (CT) scans were performed at baseline and every 8–9 weeks until disease progression.
Assessment of radiological parameters and tumor response dynamics
Tumor response dynamics were assessed according to RECIST V.1.1 criteria.11 For the assessment of ETS and DoR, the longest diameters of the RECIST-defined target lesions were measured and summed for each assessment. Changes in the sum of the longest diameters of the RECIST-defined target lesions were expressed as a relative change from baseline. For ETS, the relative change from baseline at the first tumor assessment (week 8/9) was considered, whereas for DoR the relative change from baseline at the nadir was considered. Non-target lesions were not considered in the measurement of change in tumor size, as previously reported,12 but the worsening of non-target lesions and/or newly occurring lesions identified per se a RECIST-defined disease progression independently from a favorable dynamics of target lesions. All the images were centrally reviewed at the Fondazione IRCCS Istituto Nazionale dei Tumori by a dedicated radiologist blinded to the clinical outcome of the patients.
Statistical analysis
Progression-free survival (PFS) was defined as the interval from the initiation of treatment with ICIs to the evidence of progressive disease (PD) or death from any cause, whichever occurred first. Overall survival (OS) was defined as the interval from the initiation of treatment with ICIs to death from any cause.
Median and interquartile range (IQR) were used to report distribution of continuous variables and the non-parametric Kruskal-Wallis test was used to examine baseline differences between groups. Logistic regression was used to describe and explain the relationship between dependent binary variables and independent variables. Odds ratio (OR) together with 95% confidence interval (CI) were provided for logistic regression analyses. Independent variable statistically significant in the univariate analyses were used to build the multivariate models. The Kaplan-Meier method and Cox proportional hazards regression models were used for survival analyses. Hazard ratio (HR) together with 95% CI were provided for Cox proportional hazards regression analyses. A maximally selected statistics method in a Cox regression model for PFS was used to determine the optimal cut-offs for ETS and DoR. Statistical significance threshold was set to a two-tailed 0.05 value. Statistical analyses were performed using R software (V.3.5.0).
Results
We included a total of 169 patients: 116 (68.6%) were treated with anti-PD-1 monotherapy, whereas 53 (31.4%) with anti-PD-1 plus anti-CTLA-4 agents. Clinicopathological and treatment characteristics are illustrated in table 1. Online supplemental figure 1 shows the waterfall plots of ETS values and DoR according to the type of ICI regimen. Median follow-up time was 30.4 months (95% CI 28.2 to 32.3). OS and PFS in the entire study population are shown in online supplemental figure 2. No pseudoprogressions were observed.
Table 1.
Clinicopathological and treatment characteristics
| Characteristics | Study population (N=169) N (%) |
| Sex | |
| Female | 78 (46.2) |
| Male | 91 (53.8) |
| Age (years) | |
| <70 | 118 (69.8) |
| ≥70 | 51 (30.2) |
| ECOG PS | |
| 0 | 104 (61.5) |
| ≥1 | 65 (38.5) |
| Primary tumor resection | |
| No | 4 (2.4) |
| Yes | 165 (97.6) |
| Primary tumor sidedness | |
| Left | 49 (29.0) |
| Right | 120 (71.0) |
| RAS and BRAF mutational status | |
| All wild-type | 65 (38.5) |
| RAS mutated | 48 (28.4) |
| BRAF mutated | 56 (33.1) |
| Synchronous metastases | |
| No | 76 (45.0) |
| Yes | 93 (55.0) |
| Liver metastases | |
| No | 110 (65.1) |
| Yes | 59 (34.9) |
| Lung metastases | |
| No | 129 (76.3) |
| Yes | 40 (23.7) |
| Lymph nodal metastases | |
| No | 59 (34.9) |
| Yes | 110 (65.1) |
| Peritoneal metastases | |
| No | 100 (59.2) |
| Yes | 69 (40.8) |
| Bone metastases | |
| No | 159 (94.1) |
| yes | 10 (5.9) |
| No of metastatic sites | |
| 1 | 68 (40.2) |
| ≥2 | 101 (59.8) |
| Prior systemic treatment for metastatic disease | |
| No | 36 (21.3) |
| Yes | 133 (78.7) |
| Time from metastatic condition to ICI treatment start | |
| <18 months | 114 (67.5) |
| ≥18 months | 55 (32.5) |
| ICI regimen | |
| Anti-PD-1 | 116 (68.6) |
| Anti-CTLA-4+ anti-PD-1 | 53 (31.4) |
ICI, immune checkpoint inhibitor.
jitc-2021-002501supp001.pdf (78.8KB, pdf)
jitc-2021-002501supp002.pdf (96.5KB, pdf)
Primary progression to treatment
In order to allow a proper and clinically sound interpretation of the data about ETS and DoR, patients experiencing a PD as per RECIST criteria V.1.1 at the first tumor reassessment (ie, patients with primary progressive disease, N=37, 21.9%) were evaluated separately. Indeed, these patients experienced an extremely poor OS (1-year OS rate: 21%; HR: 17.29, 95% CI 9.33 to 32.06; p<0.001) (online supplemental figure 3). Table 2 describes the association of clinicopathological and treatment characteristics with the occurrence of primary progressive disease by means of univariable and multivariable logistic regression analyses. In details, poorer Eastern Cooperative Oncology Group (ECOG) performance status(PS), presence of peritoneal involvement and anti-PD-1 monotherapy were independent predictors of PD at first CT scan.
Table 2.
Association of clinicopathological and treatment characteristics with primary progressive disease by means of univariable and multivariable logistic regression analyses
| Characteristics | No primary PD N (%) N=132 |
Primary PD N (%) N=37 |
Univariable analysis | Multivariable model | ||
| OR (95% CI) | P value* | OR (95% CI) | P value* | |||
| Sex | ||||||
| Female | 63 (47.7) | 15 (40.5) | Ref | 0.439 | ||
| Male | 69 (52.3) | 22 (59.5) | 1.34 (0.64 to 2.81) | |||
| Age (years) | ||||||
| <70 | 92 (69.7) | 26 (70.3) | Ref | 0.946 | ||
| ≥70 | 40 (30.3) | 11 (29.7) | 0.97 (0.44 to 2.16) | |||
| ECOG PS | ||||||
| 0 | 90 (68.2) | 14 (37.8) | Ref | 0.001 | Ref | 0.002 |
| ≥1 | 42 (31.8) | 23 (62.2) | 3.52 (1.65 to 7.52) | 3.48 (1.53 to 7.90) | ||
| Primary tumor sidedness | ||||||
| Left | 34 (25.8) | 15 (40.5) | Ref | 0.083 | ||
| Right | 98 (74.2) | 22 (59.5) | 0.51 (0.24 to 1.09) | |||
| RAS and BRAF mutational status | 0.955 | |||||
| All wild-type | 50 (37.9) | 15 (40.6) | Ref | |||
| RAS mutated | 38 (28.8) | 10 (27.0) | 0.88 (0.36 to 2.17) | |||
| BRAF mutated | 44 (33.3) | 12 (32.4) | 0.91 (0.38 to 2.15) | |||
| Synchronous metastases | 0.540 | |||||
| No | 61 (46.2) | 15 (40.5) | Ref | |||
| Yes | 71 (53.8) | 22 (59.5) | 1.26 (0.60 to 2.64) | |||
| Liver metastases | 0.455 | |||||
| No | 84 (63.6) | 26 (70.3) | Ref | |||
| Yes | 48 (36.4) | 11 (29.7) | 0.74 (0.34 to 1.63) | |||
| Lung metastases | 0.587 | |||||
| No | 102 (77.3) | 27 (73.0) | Ref | |||
| Yes | 30 (22.3) | 10 (27.0) | 1.26 (0.55 to 2.89) | |||
| Lymph nodal metastases | 0.114 | |||||
| No | 42 (31.8) | 17 (45.9) | Ref | |||
| Yes | 90 (68.2) | 20 (54.1) | 0.55 (0.26 to 1.15) | |||
| Peritoneal metastases | 0.001 | |||||
| No | 87 (65.9) | 13 (35.1) | Ref | 0.001 | Ref | |
| Yes | 45 (34.1) | 24 (64.9) | 3.57 (1.66 to 7.67) | 3.88 (1.69 to 8.91) | ||
| Bone metastases | ||||||
| No | 126 (95.5) | 33 (89.2) | Ref | 0.166 | ||
| Yes | 6 (4.5) | 4 (10.8) | 2.55 (0.68 to 9.55) | |||
| No of metastatic sites | ||||||
| 1 | 56 (42.4) | 12 (32.4) | Ref | 0.275 | ||
| ≥2 | 76 (57.6) | 25 (67.6) | 1.54 (0.71 to 3.32) | |||
| Prior systemic treatment for metastatic disease | ||||||
| No | 33 (25.0) | 3 (8.1) | Ref | 0.034 | Ref | 0.122 |
| Yes | 99 (75.0) | 34 (91.9) | 3.78 (1.09 to 13.11) | 2.89 (0.75 to 11.08) | ||
| Time from metastatic condition to ICI treatment start | ||||||
| <18 months | 91 (68.9) | 23 (62.2) | Ref | 0.438 | ||
| ≥18 months | 41 (31.1) | 14 (37.8) | 1.35 (0.63 to 2.89) | |||
| ICI regimen | ||||||
| Anti-PD-1 | 84 (63.6) | 32 (86.5) | Ref | 0.012 | Ref | 0.015 |
| Anti-CTLA-4+ anti-PD-1 | 48 (36.4) | 5 (13.5) | 0.27 (0.10 to 0.75) | 0.26 (0.09 to 0.77) | ||
*Bold values denote statistical significance.
jitc-2021-002501supp003.pdf (69.2KB, pdf)
Tumor response dynamics in patients with clinical benefit
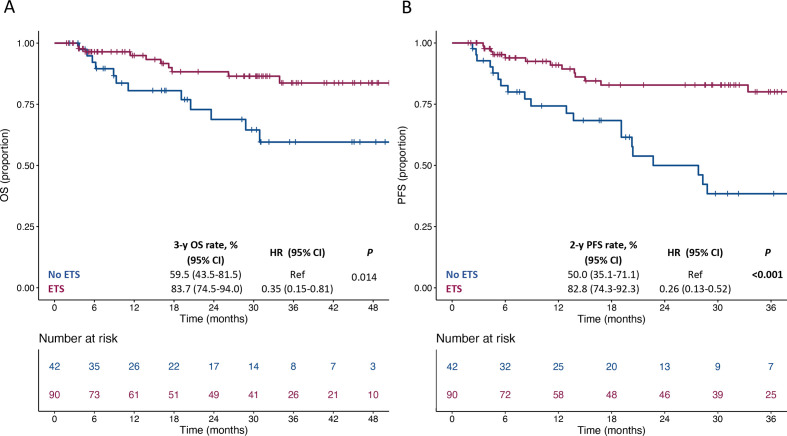
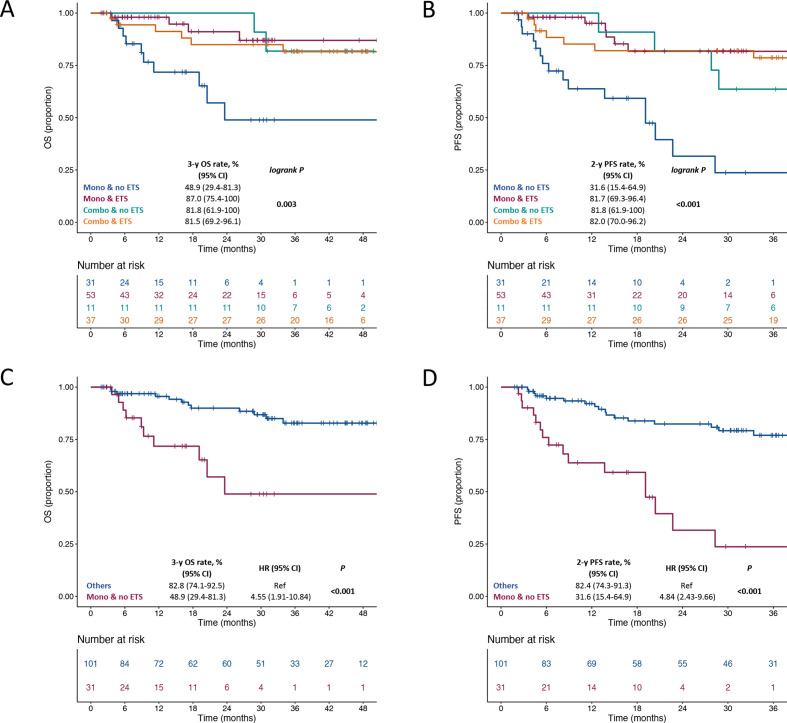
We then focused on patients with clinical benefit at least at the first disease reassessment (N=132). The distribution of ETS and DoR values in these patients according to their clinical and treatment characteristics is shown in online supplemental table 1. We first explored the association of survival outcomes with ETS as a continuous variable and we observed a significant association with both OS (HR per 20% increase: 1.53, 95% CI 1.01 to 2.32; p=0.046) and PFS (HR per 20% increase: 1.90, 95% CI 1.34 to 2.70; p<0.001). We then identified a relative reduction of at least 1% as the optimal cut-off for ETS (score test: 11.06, test statistic: 1.99, p<0.001). Thus, we defined ETS as whichever tumor reduction at the first disease reassessment. The presence of any ETS was associated with better OS (HR: 0.35, 95% CI 0.15 to 0.81; p=0.014) and PFS (HR: 0.26, 95% CI 0.13 to 0.52, p<0.001) (figure 1). (online supplemental figure 4) (A and B) shows the swimmer plots for OS and PFS according to the ETS status in patients with initial disease control. Figure 2 shows OS and PFS according to the combined assessment of ETS and the type of ICI regimen (anti-PD-1 vs anti-PD-1+anti-CTLA-4). Notably, patients treated with anti-PD-1 monotherapy and who did not experience ETS had a clearly worse OS (figure 2A) and PFS (figure 2B) compared with all other subgroups. The 2-year PFS and 3-year OS rates were 48.9% (95% CI 29.4% to 81.3%) and 31.6% (95% CI 15.4% to 64.9%) in patients treated with anti-PD-1 monotherapy and not achieving any ETS vs 82.8% (95% CI 74.1% to 92.5%) and 82.4% (95% CI 74.3% to 91.3%) in the remaining ones (HR for OS: 4.55, 95% CI 1.91 to 10.84; p<0.001, figure 2C; HR for PFS: 4.84, 95% CI 2.43 to 9.66; p<0.001, figure 2D).
Figure 1.
Kaplan-Meier estimates for OS (panel A) and PFS (panel B) according to ETS. ETS, early tumor shrinkage; OS, overall survival; PFS, progression-free survival.
Figure 2.
Kaplan-Meier estimates for OS (A, C) and PFS (B, D) according to ETS and the type of ICI regimen. ETS, early tumor shrinkage; OS, overall survival; PFS, progression-free survival.
jitc-2021-002501supp004.pdf (62.5KB, pdf)
jitc-2021-002501supp005.pdf (91.4KB, pdf)
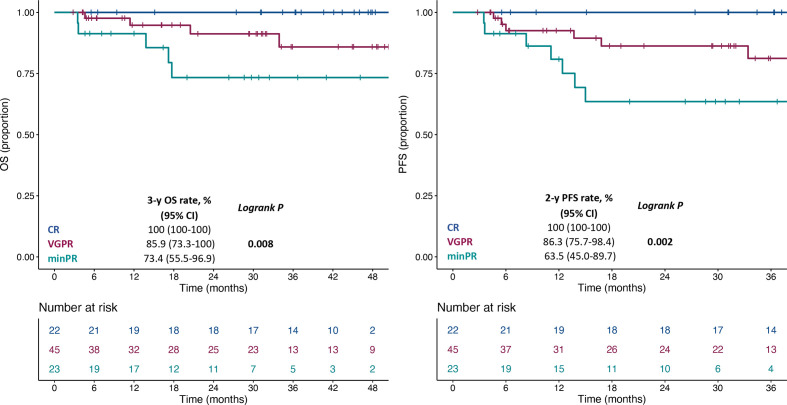
As expected, DoR categorized according to RECIST criteria v1.1 was associated with the survival outcomes (online supplemental figure 5A, B). In order to better stratify the outcomes according to DoR, we explored the association of survival outcomes with DoR as a continuous variable, and we observed a significant association with both OS (HR per 20% increase: 1.74, 95% CI 1.35 to 2.26; p<0.001) and PFS (HR per 20% increase: 1.88, 95% CI 1.51 to 2.33; p<0.001). We then identified a relative reduction of at least 50% as the optimal cut-off for the DoR (score test: 15.05, test statistic: 2.71, p<0.001). The presence of a DoR ≥50% was associated with better OS (HR: 0.14, 95% CI 0.05 to 0.41; p<0.001) and PFS (HR: 0.13, 95% CI 0.06 to 0.31, p<0.001) (online supplemental figure 6A, B). Table 3 shows the association of DoR with clinico-pathological and treatment characteristics. In details, older age, the presence of lymphnodal metastases, a shorter interval of time from metastatic condition to ICI treatment start and combination treatment with anti-PD-1+ anti-CTLA-4 were independent predictors of a DoR ≥50%. Exploiting the cut-off identified for DoR, we further defined minor partial response (minPR) as a DoR ≥30% and <50% and very good partial response (VGPR) as a DoR ≥50% and <100% and showed an incremental 3-year OS rate and 2-year PFS rate for patients with minPR, VGPR and complete response (CR) (figure 3).
Table 3.
Association of clinicopathological and treatment characteristics with DoR ≥50% by means of univariable and multivariable logistic regression analyses
| Characteristics | DoR <50% N (%) N=65 |
DoR ≥50% N (%) N=67 |
Univariable analysis | Multivariable model | ||
| OR (95% CI) | P value* | OR (95% CI) | P value* | |||
| Sex | ||||||
| Female (n=63) | 27 (41.5) | 36 (53.7) | Ref | 0.162 | ||
| Male (n=69) | 38 (58.5) | 31 (46.3) | 0.61 (0.31 to 1.22) | |||
| Age (years) | ||||||
| <70 (N=92) | 51 (78.5) | 41 (61.2) | Ref | 0.033 | Ref | 0.041 |
| ≥70 (N=40) | 14 (21.5) | 26 (38.8) | 2.31 (1.07 to 4.98) | 2.46 (1.04 to 5.85) | ||
| ECOG PS | ||||||
| 0 (N=90) | 43 (66.2) | 47 (70.1) | Ref | 0.622 | ||
| ≥1 (N=42) | 22 (33.8) | 20 (29.9) | 0.83 (0.40 to 1.73) | |||
| Primary tumor sidedness | ||||||
| Left (N=34) | 18 (27.7) | 16 (23.9) | Ref | 0.617 | ||
| Right (N=98) | 47 (72.3) | 51 (76.1) | 1.22 (0.56 to 2.67) | |||
| RAS and BRAF mutational status | ||||||
| All wild-type (N=50) | 24 (36.9) | 26 (38.8) | Ref | 0.402 | ||
| RAS mutated (N=38) | 22 (33.9) | 16 (23.9) | 0.67 (0.29 to 1.57) | |||
| BRAF mutated (N=44) | 19 (29.2) | 25 (37.3) | 1.21 (0.54 to 2.74) | |||
| Synchronous metastases | ||||||
| No (N=61) | 33 (50.8) | 28 (41.8) | Ref | 0.302 | ||
| Yes (N=71) | 32 (49.2) | 39 (58.2) | 1.44 (0.72 to 2.86) | |||
| Liver metastases | ||||||
| No (N=84) | 44 (67.7) | 40 (59.7) | Ref | 0.341 | ||
| Yes (N=48) | 21 (32.3) | 27 (40.3) | 1.41 (0.69 to 2.89) | |||
| Lung metastases | ||||||
| No (N=102) | 46 (70.8) | 56 (83.6) | Ref | 0.082 | ||
| Yes (N=30) | 19 (29.2) | 11 (16.4) | 0.48 (0.21 to 1.10) | |||
| Lymph nodal metastases | ||||||
| No (N=42) | 26 (40.0) | 16 (23.9) | Ref | 0.049 | Ref | 0.009 |
| Yes (N=90) | 39 (60.0) | 51 (76.1) | 2.13 (1.01 to 4.50) | 3.15 (1.33 to 7.44) | ||
| Peritoneal metastases | ||||||
| No (N=87) | 43 (66.2) | 44 (65.7) | Ref | 0.953 | ||
| Yes (N=45) | 22 (33.8) | 23 (34.3) | 1.02 (0.50 to 2.10) | |||
| Bone metastases | ||||||
| No (N=126) | 62 (95.4) | 64 (95.5) | Ref | 0.970 | ||
| Yes (N=6) | 3 (4.6) | 3 (4.5) | 0.97 (0.19 to 4.98) | |||
| No of metastatic sites | ||||||
| 1 (N=56) | 27 (41.5) | 29 (43.3) | Ref | 0.839 | ||
| ≥2 (N=76) | 38 (58.5) | 38 (56.7) | 0.93 (0.47 to 1.86) | |||
| Prior systemic treatment for metastatic disease | ||||||
| No (N=33) | 13 (20.0) | 20 (29.9) | Ref | 0.194 | ||
| Yes (N=99) | 52 (80.0) | 47 (70.1) | 0.59 (0.26 to 1.31) | |||
| Time from metastatic condition to ICI treatment start | ||||||
| <18 months (N=91) | 38 (58.5) | 53 (79.1) | Ref | 0.012 | Ref | 0.001 |
| ≥18 months (N=41) | 27 (41.5) | 14 (20.9) | 0.37 (0.17 to 0.80) | 0.24 (0.10 to 0.57) | ||
| ICI regimen | ||||||
| Anti-PD-1 (N=84) | 49 (75.4) | 35 (52.2) | Ref | 0.006 | Ref | 0.005 |
| Anti-CTLA-4+ anti-PD-1 (N=48) | 16 (24.6) | 32 (47.8) | 2.80 (1.34 to 5.87) | 3.24 (1.42 to 7.37) | ||
*Bold values denote statistical significance.
ICI, immune checkpoint inhibitor.
Figure 3.
Kaplan-Meier estimates for OS (A) and PFS (B) according to DoR. DoR, depth of response; OS, overall survival; minPR, minor partial response; PFS, progression-free survival; VGPR, very good partial response.
jitc-2021-002501supp006.pdf (109KB, pdf)
jitc-2021-002501supp007.pdf (111.1KB, pdf)
Discussion
In this large, retrospective, cohort study, we provided new evidence on the prognostic impact of tumor response dynamics in patients with MSI-H/dMMR mCRC receiving ICIs. Of note, in the Keynote-177 first-line trial,3 the rate of patients randomized to pembrolizumab who experienced a PD at the first disease re-assessment was about 30% and similar to the rate of primary progression in our series.
Whereas the mechanisms of primary resistance to ICIs are not fully elucidated and may encompass a relatively lower tumor mutational burden,13 or even a misdiagnosis of dMMR status,14 we identified clinical characteristics independently associated with primary progressive disease, including poorer PS, peritoneal involvement and, notably, also the use of anti-PD-1 monotherapy. In line with these results, the uncontrolled trial of ipilimumab and nivolumab combination showed an extremely low rate of primary resistance in both first-line and pretreated cohorts, at the price of moderately increased rate of immune-related adverse events. Whether patients with specific clinical and molecular adverse characteristics may derive a relatively greater benefit from anti-PD-1 plus anti-CTLA-4 combinations or from the addition of chemotherapy and bevacizumab to an anti-PD-1 agent in the first line warrants further confirmation in subgroup analyses of the ongoing COMMIT and CheckMate 8HW trials (NCT02997228, NCT04008030).
Our observation about the association of ETS with survival outcomes is in line with previously reported data in patients with other tumor types treated with ICIs.15–17 Of note, some of these previous studies are limited by the inclusion of patients with primary progressive disease—who have an extremely poor survival—in the subgroup without ETS, thus magnifying the prognostic impact of ETS itself. Here, we decided to properly restrict our focus on patients with disease control at the first radiological reassessment, as in the work of Kawachi et al.16 From a clinical perspective, we observed that patients treated with anti-PD-1 monotherapy and not achieving ETS at the 8/9 weeks time point had a clearly and significantly worse outcome as compared with other patients. Based on the potential clinical usefulness of ETS as an immediate marker of treatment efficacy, a dynamic trial investigating the addition of an anti-CTLA-4 agent to PD-1 blockade or the continuation of anti-PD-1 monotherapy based on the absence or presence of ETS, respectively, would be justified. In fact, the clinical validation of such dynamic strategy could increase long-term disease control in patients with poorer predicted outcomes to single-agent treatment and spare the increased toxicity of combinations in patients with the highest susceptibility to single-agent therapy. However, although the surrogacy analysis of ETS for survival is warranted, this would typically require pooled datasets of prospective clinical trials and is therefore not feasible at present.
Regarding the DoR, a recent study encompassing data from 43 trials with anti-PD-1 or anti-PD-L1 agents in patients with solid tumors showed a week surrogacy between RECIST criteria-based endpoints and OS,18 similar to what observed at the trial level in another retrospective analysis of clinical trials with ICI.19 Therefore, being aware that the 30% cut-off for defining RECIST response may be associated with loss of power in prognostic stratification, we showed that a higher cut-off for DoR (ie, 50%) had a better discriminative ability in the subgroup of patients with clinical benefit. This result indicates that patients with a deep—but still not complete—response have a high chance of long-term disease control. Consistently, MSI-high mCRC patients with pathological CR after ICI treatment and secondary resection of metastases had almost always evidence of residual disease on imaging,20 corresponding to the immune cell infiltrate or to a combination of mucin and necrosis. Such speculation is also supported by the evidence of a persistent clinical benefit in patients with MSI-H/dMMR solid tumors who discontinued pembrolizumab with evidence of residual disease by imaging after 2 years of treatment.21 Given the excellent survival outcomes observed in patients experiencing a DoR ≥50%, we suggest that DoR may be used to select patients that may be eligible for trials investigating a shorter treatment duration and early deintensification, in order to both spare financial toxicity and reduce the burden of adverse events.
The main limitations of our study are the lack of validation of the identified cut-offs and the retrospective nature of the study, even if the quite large number of patients included and the multicenter contribution to our effort partially mitigate such limitations. Moreover, we are aware that tumor response is clearly expected to be associated with survival, but the role of parameters related to the rapidity and DoR is new in this field.
In conclusion, we propose ETS and DoR as important prognostic factors in patients with MSI-H/dMMR mCRC treated with ICIs that might help in the design of treatment intensification/deintensification strategies. We are firmly convinced that their prospective validation should be achieved mainly thanks to the investigation of such dynamic activity endpoints in preplanned analyses of clinical trials.
Footnotes
Contributors: Conception and design: GF, SL, FP. Acquisition of data: FC, MA, RI, SM, EF, PM, CM, FD, AR, FM, SC, MP, AS, VQ, CB, MV, GC, CC and VZ. Analysis and interpretation of data: GF, FP, PM, FDB, MDB, SL and FP. Manuscript drafting: GF, FC, MA, FP and PM. Manuscript revision: MA, RI, SM, EF, CM, FD, AR, FM, SC, MP, AS, VQ, CB, MV, CC, FDB, MDB, VZ and SL. Final approval: GF, FC, MA, RI, SM, EF, PM, CM, FD, AR, FM, SC, MP, AS, VQ, CB, MV, GC, CC, FDB, MDB, VZ, SL and FP.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: GC reports the following competing interests outside the present work: advisory board member of Novartis, Roche, Lilly, Daichi-Sankyo, Astra Zeneca, Veracyte and Genomic Health; scientific advisor for Ellipsis. All the remaining authors declared no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Fondazione IRCCS Istituto Nazionale dei Tumori of Milan (INT 117/15).
References
- 1.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch Repair-Deficient/Microsatellite Instability-High metastatic colorectal cancer. J Clin Oncol 2018;36:773–9. 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 2.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.André T, Shiu K-K, Kim TW, et al. Pembrolizumab in Microsatellite-Instability-High advanced colorectal cancer. N Engl J Med 2020;383:2207–18. 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 4.NCCN . Clinical practice Guidelies in oncology, version 2, 2021. Available: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 5.Cremolini C, Loupakis F, Antoniotti C, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III tribe trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol 2015;26:1188–94. 10.1093/annonc/mdv112 [DOI] [PubMed] [Google Scholar]
- 6.Stintzing S, Modest DP, Rossius L, et al. Folfiri plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final Ras wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016;17:1426–34. 10.1016/S1470-2045(16)30269-8 [DOI] [PubMed] [Google Scholar]
- 7.Claret L, Gupta M, Han K, et al. Evaluation of tumor-size response metrics to predict overall survival in Western and Chinese patients with first-line metastatic colorectal cancer. J Clin Oncol 2013;31:2110–4. 10.1200/JCO.2012.45.0973 [DOI] [PubMed] [Google Scholar]
- 8.Manca P, Corallo S, Randon G, et al. Impact of early tumor shrinkage and depth of response on the outcomes of panitumumab-based maintenance in patients with Ras wild-type metastatic colorectal cancer. Eur J Cancer 2021;144:31–40. 10.1016/j.ejca.2020.11.017 [DOI] [PubMed] [Google Scholar]
- 9.Petrelli F, Pietrantonio F, Cremolini C, et al. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer 2015;51:800–7. 10.1016/j.ejca.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 10.Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232–43. 10.1093/annonc/mdz116 [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2013;31:3764–75. 10.1200/JCO.2012.42.8532 [DOI] [PubMed] [Google Scholar]
- 13.Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol 2019;30:1096–103. 10.1093/annonc/mdz134 [DOI] [PubMed] [Google Scholar]
- 14.Cohen R, Hain E, Buhard O, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol 2019;5:551–5. 10.1001/jamaoncol.2018.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins AM, Kichenadasse G, Karapetis CS, et al. Early tumor shrinkage identifies long-term disease control and survival in patients with lung cancer treated with atezolizumab. J Immunother Cancer 2020;8:e000500. 10.1136/jitc-2019-000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawachi H, Fujimoto D, Morimoto T, et al. Early depth of tumor shrinkage and treatment outcomes in non-small cell lung cancer treated using nivolumab. Invest New Drugs 2019;37:1257–65. 10.1007/s10637-019-00770-y [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Chen C, Jemielita T, et al. Are tumor size changes predictive of survival for checkpoint blockade based immunotherapy in metastatic melanoma? J Immunother Cancer 2019;7:39. 10.1186/s40425-019-0513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie R-C, Chen F-P, Yuan S-Q, et al. Evaluation of objective response, disease control and progression-free survival as surrogate end-points for overall survival in anti-programmed death-1 and anti-programmed death ligand 1 trials. Eur J Cancer 2019;106:1–11. 10.1016/j.ejca.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 19.Mushti SL, Mulkey F, Sridhara R. Evaluation of overall response rate and progression-free survival as potential surrogate endpoints for overall survival in immunotherapy trials. Clin Cancer Res 2018;24:2268–75. 10.1158/1078-0432.CCR-17-1902 [DOI] [PubMed] [Google Scholar]
- 20.Ludford K, Cohen R, Svrcek M. Pathological tumor response following immune checkpoint blockade for deficient mismatch repair advanced colorectal cancer. J Natl Cancer Inst 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002501supp001.pdf (78.8KB, pdf)
jitc-2021-002501supp002.pdf (96.5KB, pdf)
jitc-2021-002501supp003.pdf (69.2KB, pdf)
jitc-2021-002501supp004.pdf (62.5KB, pdf)
jitc-2021-002501supp005.pdf (91.4KB, pdf)
jitc-2021-002501supp006.pdf (109KB, pdf)
jitc-2021-002501supp007.pdf (111.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.