Abstract
The COVID-19 pandemic caused by the SARS-CoV-2 virus has affected millions of people around the globe. The most common presentation of COVID-19 is fever and upper and lower respiratory tract infection. Myalgia is fairly common in the prodromal phase of the viral illness which self-resolves. There is very scant literature on autoimmune myositis triggered by COVID-19 infection. We report a case of SARS-CoV-2 infection, who presented with progressive muscle weakness with rhabdomyolysis and necrotizing autoimmune myopathy on muscle biopsy. This case report imposes awareness of musculoskeletal autoimmune processes triggered by COVID-19 which requires clinical suspicion for early diagnosis and initiation of treatment.
Keywords: musculoskeletal and joint disorders, neurology (drugs and medicines), infections, muscle disease
Background
Since the emergence of the global pandemic of COVID-19, clinicians have been learning and exploring this new disease entity and managing the repercussions of the current health crisis. COVID-19 manifests with a wide range of presentations, most commonly with fever and upper and lower respiratory tract infection.1 2 The musculoskeletal manifestations include myalgia and fatigue, which are common in any prodromal phase of viral disease.3 The myositis associated with COVID-19 described as skeletal muscle injury and rhabdomyolysis have also been reported in up to 10% of infected patients.4 The COVID-19 disease process triggers an autoimmune inflammatory response which has been reported in the literature as few case reports.5–7 There is very scant literature on autoimmune myositis triggered by COVID-19 infection. We report a case of necrotizing autoimmune myopathy (NAM) associated with COVID-19. NAM is an unusual and rare subgroup of inflammatory myopathies which is confirmed by necrotic muscle fibres and absent or minimal inflammation on muscle biopsy. The etiology is usually idiopathic; however, it has also been associated with statin use, triggered by post-viral autoimmune antibodies, and sometimes has manifested as paraneoplastic presentation.8 The exact mechanism of muscle injury is not well understood, but the exaggerated inflammatory response and the viral infection of the skeletal cells are the suggested pathophysiology of myositis in these patients.9 It is important as a clinician to recognize the typical and atypical presentations of COVID-19 for timely intervention and to improve patient outcomes.
Case presentation
This is a case of a 57-year-old woman with medical history of hypertension, who had COVID-19-related mild upper respiratory infection, for which she self-quarantined at home and recovered gradually after 2 weeks. A month after the initial COVID-19 disease, she started to experience worsening dyspnoea and presented to the hospital. Initial examination and vital signs were reported within normal limits. She was noted to have positive SARS-CoV-2 immunoglobulin G (IgG) antibody and negative SARS-CoV-2 PCR, elevated creatine kinase (15 000 IU/L) and elevated troponin (1.4 μg/L) with normal kidney function. The ECG, echocardiogram and cardiac MRI were normal. The most likely diagnosis at the time of discharge was found to be rhabdomyolysis in the setting of COVID-19 disease. The patient was managed symptomatically and discharged home once stabilised.
Four months after the initial COVID-19 infection, she presented to the hospital again with worsening muscle weakness for 2 weeks. She was unable to get up from a seated position or lift arms above her head. On physical examination, the patient had vital signs reported within a normal range. On physical examination, she had 3/5 power in the proximal muscle group and 5/5 in the distal muscle group of bilateral upper and lower extremities and bilateral foot drop.
Investigations
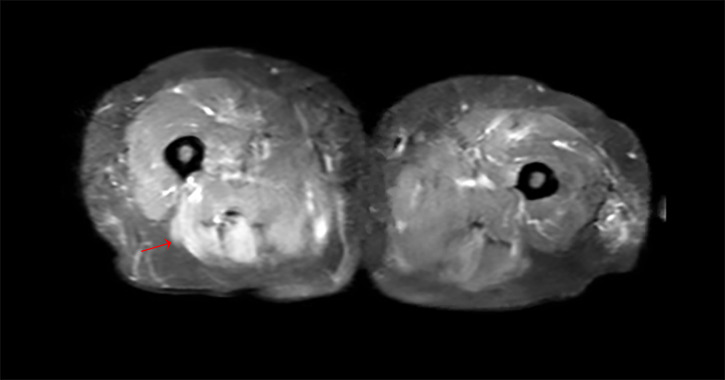
Bilateral thigh muscle MRI showed a diffuse signal abnormality in the bilateral adductors, hamstrings, gluteal muscles and obturator muscles with oedema along the myofascial layer, suggestive of myositis (figure 1). Electromyography showed an irritative myopathy pattern in the anterior tibialis muscle that would explain the foot drop.
Figure 1.
Images show inflammation of the muscles, kind of asymmetric distribution, mostly posterior and adductor compartment.
Differential diagnosis
The differential diagnosis for the myositis at this time was broad, which included autoimmune, infective, metabolic, paraneoplastic and post-viral inflammatory disease. A thorough infectious workup was negative for viral etiologies including IgM for cytomegalovirus, Epstein-Barr virus, coxsackie and human T-lymphotropic virus. The thyroid function was normal. Autoimmune serology was positive for antinuclear antibody (ANA) (1:320, specked pattern), and very low titre of anti-Smith antibody, which were deemed to be secondary to an acute infectious process.10 She had negative antiphospholipid antibodies and no symptoms of systemic lupus erythematosus.
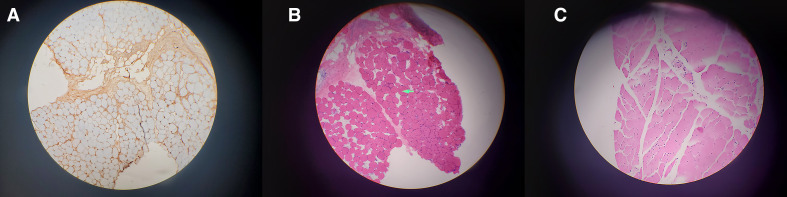
Anti-Jo-1, anti-signal recognition particle (SRP) and anti-3-hydroxy-3-methylglutaryl–coenzyme A reductase (HMGCR) autoantibodies, other common myositis-specific antibodies were negative. The muscle biopsy ruled out inflammatory myopathy conditions like polymyositis, dermatomyositis, inclusion body myositis and metabolic myopathies. The patient underwent muscle biopsy which showed a myopathic process with a few scattered necrotic myofibres and relatively minimal inflammatory cell infiltrates suggestive of necrotizing autoimmune myopathy (figure 2).
Figure 2.
(A) MHC class I stain of muscle biopsy where the muscles are not stained at all. (B, C) Muscle biopsy: mildly necrotic muscle bundles with minimal inflammation. MHC, major histocompatibility complex.
Treatment
After pathological confirmation of necrotizing autoimmune myositis, the patient was started on high-dose prednisone (1 mg/kg) daily and aggressive physical therapy.
Outcome and follow-up
Initiation of steroids resulted in the improvement of muscle strength. Creatine kinase gradually down trended to 900 IU/L. The patient was discharged to a rehabilitation facility. The patient was evaluated after a month when she continued to report progressive improvement. About 3 months after the initiation of steroids, she felt at baseline, was able to stand and ambulate without any assistance. Prednisone was gradually tapered along with the initiation of methotrexate as a steroid-sparing agent. The patient continued to report feeling much better in the following months. She almost regained back all her strength and now is able to carry out her daily activities.
Discussion
The COVID-19 pandemic has affected millions of people around the globe, with high rates of morbidity and mortality. The most common presentation includes fever and upper and lower respiratory symptoms.1 The prevalence of generalised myalgia, which is defined as muscle ache without muscle damage, can be seen in up to 50% of cases, but the progression of myopathy to myositis has been rarely reported.4 Viral diseases have also been associated with rhabdomyolysis, which causes disruption of skeletal muscle integrity that leads to the direct release of intracellular muscle components, including myoglobin, creatine kinase, and in severe cases can cause electrolyte imbalances and acute renal failure.9 11–13 Infective myositis presenting as localised muscle group weakness has been associated with viruses such as coxsackievirus, parainfluenza, adenovirus, echovirus, Epstein-Barr virus, varicella‐zoster virus, cytomegalovirus, herpes simplex and HIV.14
As per the literature review, there have been a few cases of rhabdomyolysis related to SARS-CoV-2 but all were reported as acute and concurrent with pneumonia within the first 2 weeks of infection onset.5–7 14–19 However, this case report is unique with its subacute course and no temporality with the lung involvement when the patient presented with myositis.
NAM is a rare subgroup of autoimmune inflammatory myositis. The aetiology is usually idiopathic, but it has also been associated with statin use, triggered by post-viral autoimmune antibodies and sometimes has manifested as paraneoplastic presentation.8 It is characterised clinically based on the subacute onset of severe generalised muscle weakness and very high levels of creatine kinase and is histologically differentiated from another inflammatory myositis by the absence or minimal inflammation.20 Anti-Jo-1, anti-SRP and anti-HMGCR autoantibodies are associated with triggering NAM.21
In this case, the patient had SARS-CoV-2 IgG, a month later after the initial COVID-19 infection, she presented with rhabdomyolysis and the symptoms gradually progressed to myositis. All the possible causes that could trigger myositis like acute viral infections, electrolyte imbalances, endocrinopathies and statin were evaluated and ruled out. Autoimmune serology was positive for ANA (1:320, speckled pattern) and very low titre anti-Smith antibody, which were deemed to be secondary to an acute infectious process.10 The scattered necrotic myofibres with relatively minimal inflammatory cell infiltrates are suggestive of necrotizing autoimmune myopathy. The absence of anti-Jo-1, HMGCR and SRP antibodies made NAM less likely to be entirely an idiopathic autoimmune phenomenon. Henceforth, after a thorough workup, and detailed review of the sequence of the symptoms, COVID-19 IgG-related myositis was the final diagnosis of exclusion.
The pathophysiology of the viral-associated myositis is not well understood. The complement system activated from the deposition of virus–antibody complexes can result in collateral muscle damage, and the circulating viral toxins can also cause direct muscle injury.9 The T-cell or macrophage-mediated injury was also shown on muscle biopsy in some cases of viral myositis.21–24 Interestingly, direct viral muscle invasion is not well established as viral particles are rarely found in a muscle biopsy in cases of acute viral myositis.14 The ACE 2 receptors, the entry site of SARS-CoV-2, are present on skeletal muscles which necessitate further investigation to identify SARS-CoV-2 as the first virus that can potentially cause direct muscle injury.22
A timely diagnosis of viral antibody-mediated NAM is critical as it can improve outcomes. In the early presentations, the symptoms improved with high-dose corticosteroids and supportive physical therapy.25 The steroids should gradually be tapered over the due course of time. In case there is a relapse of symptoms, then steroid-sparing disease-modifying anti-rheumatic drugs (DMARD) therapy would be considered in the future.26 In conclusion, we present a rare case of inflammatory necrotizing myositis associated with SARS-CoV-2 IgG, timely diagnosis and treatment with high-dose steroids improved the outcome of the patient.
Learning points.
This is the first case reported of COVID-19 immunoglobulin G (IgG)-related autoimmune myositis, confirmed by muscle biopsy and MRI, which improved with the initiation of corticosteroids.
COVID-19 can present with atypical musculoskeletal manifestations and autoimmunity such as myositis.
Our case imposes awareness on musculoskeletal autoimmune processes triggered by COVID-19 which requires clinical suspicion for early diagnosis and initiation of treatment.
Footnotes
Contributors: MV and SK identified the case and wrote the manuscript. BA supervised and managed the case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.W-j G, Z-y N, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382:2372–4. 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217573. [Epub ahead of print: 23 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 6.Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus 2020;12:e7561. 10.7759/cureus.7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 2020;26:1618–20. 10.3201/eid2607.200445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan NAJ, Khalid S, Ullah S, et al. Necrotizing autoimmune myopathy: a rare variant of idiopathic inflammatory myopathies. J Investig Med High Impact Case Rep 2017;5:232470961770903. 10.1177/2324709617709031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadila MF, Wool KJ. Rhabdomyolysis secondary to influenza A infection: a case report and review of the literature. N Am J Med Sci 2015;7:122–4. 10.4103/1947-2714.153926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali Y. Rheumatologic tests: a primer for family physicians. Am Fam Physician 2018;98:164–70. [PubMed] [Google Scholar]
- 11.Hu J-J, Kao C-L, Lee P-I, et al. Clinical features of influenza A and B in children and association with myositis. J Microbiol Immunol Infect 2004;37:95–8. [PubMed] [Google Scholar]
- 12.Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J 2015;15:58–69. [PMC free article] [PubMed] [Google Scholar]
- 13.Nance JR, Mammen AL. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve 2015;51:793–810. 10.1002/mus.24606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L-L, Hsu C-W, Tian Y-C, et al. Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome. Int J Clin Pract 2005;59:1162–6. 10.1111/j.1368-5031.2005.00540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husain R, Corcuera-Solano I, Dayan E, et al. Rhabdomyolysis as a manifestation of a severe case of COVID-19: a case report. Radiol Case Rep 2020;15:1633–7. 10.1016/j.radcr.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee A, Ghosh R, Aftab G. Rhabdomyolysis in a patient with coronavirus disease 2019. Cureus 2020;12:e8956. 10.7759/cureus.8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borku Uysal B, Ikitimur H, Yavuzer S, et al. Case report: a COVID-19 patient presenting with mild rhabdomyolysis. Am J Trop Med Hyg 2020;103:847–50. 10.4269/ajtmh.20-0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Shan KS, Minalyan A, et al. A rare presentation of coronavirus disease 2019 (COVID-19) induced viral myositis with subsequent rhabdomyolysis. Cureus 2020;12:e8074. 10.7759/cureus.8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KH, Farouji I, Abu Hanoud A, et al. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19). Am J Emerg Med 2020;38:1548.e1–1548.e3. 10.1016/j.ajem.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalakas MC. Myositis: are autoantibodies pathogenic in necrotizing myopathy? Nat Rev Rheumatol 2018;14:251–2. 10.1038/nrrheum.2018.54 [DOI] [PubMed] [Google Scholar]
- 21.Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;373:1734–47. 10.1056/NEJMra1402225 [DOI] [PubMed] [Google Scholar]
- 22.Dalakas MC. Guillain-Barré syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm 2020;7. 10.1212/NXI.0000000000000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler BL, Christopher-Stine L. Triggers of inflammatory myopathy: insights into pathogenesis. Discov Med 2018;25:75–83. [PMC free article] [PubMed] [Google Scholar]
- 24.Illa I, Nath A, Dalakas M. Immunocytochemical and virological characteristics of HIV-associated inflammatory myopathies: similarities with seronegative polymyositis. Ann Neurol 1991;29:474–81. 10.1002/ana.410290505 [DOI] [PubMed] [Google Scholar]
- 25.Mastaglia FL, Zilko PJ. Inflammatory myopathies: how to treat the difficult cases. J Clin Neurosci 2003;10:99–101. 10.1016/S0967-5868(02)00271-0 [DOI] [PubMed] [Google Scholar]
- 26.Cordeiro AC, Isenberg DA. Treatment of inflammatory myopathies. Postgrad Med J 2006;82:417–24. 10.1136/pgmj.2005.038455 [DOI] [PMC free article] [PubMed] [Google Scholar]