Abstract
Objective
The aim of this study was to analyse the 2020 burden of Systemic Lupus Erythematosus (SLE) in Europe, from the patients’ perspective.
Methods
In May 2020, Lupus Europe, the European umbrella patient association for SLE, designed and disseminated a multilingual anonymous online survey to individuals with a self-reported physician’s diagnosis of SLE living in Europe.
Results
Data from 4375 SLE survey respondents (95.9% women, median age: 45 (IQR: 36–54) years, 70.7% Caucasians) from 35 European countries were analysed. The median age at SLE diagnosis was 30 years (IQR: 22–40) and the median diagnosis delay was 2 years (IQR: 0–6). The most commonly affected organ-systems included the joints (81.8%) and skin (59.4%), with renal involvement in 30%. Another diagnosis was given before that of SLE in 45.0%, including psychological/mental disorders in 9.1% and fibromyalgia in 5.9%. The median number of symptoms reported was 9 (IQR: 6–11) out of 21, with fatigue most common (85.3%) and most bothersome. The median number of SLE-related medications was 5 (IQR: 3–7), including antimalarials (75%), oral glucocorticoids (52.4%), immunosuppressants (39.8%) and biologics (10.9%). Respondents reported significant impact over their studies, career and emotional/sexual life in 50.7%, 57.9% and 38.2%, respectively. Appropriate access to care was highly variable across countries and care component.
Conclusion
This survey underlines the 2020 burden and strong heterogeneity in the care of SLE across Europe, from the patient’s perspective. Altogether, these data may prove crucial to physicians, patients and policy-makers to improve the diagnosis and management of this rare and complex disease.
Keywords: systemic lupus erythematosus, outcome assessment, health care, quality of life, epidemiology
Key messages.
What is already known about this subject?
Detailed information on the characteristics and burden of SLE at the European level are largely unknown to physicians, policy-makers and patients with lupus themselves.
What does this study add?
This study underlines the major burden and strong heterogeneity in the care of SLE across Europe, from the patient’s perspective, based on a very large sample of European patients with SLE (n=4375).
How might this impact on clinical practice or future developments?
These data may prove crucial to improve the diagnosis and management of SLE at the European level.
Introduction
SLE is an autoimmune systemic disease with an incidence of 0.3 to 5.1 per 100 000 per year in Europe and a prevalence of 6.5 to 85 per 100 000.1 This yields an estimated 200 000–250 000 prevalent cases of SLE across Europe. Of note, detailed information on the characteristics and burden of SLE at the European level are largely unknown to physicians, policy-makers and patients with lupus themselves.2 Also, due to differences in national regulations and health insurance policies, significant heterogeneity in the diagnosis and management strategy for SLE is remaining across the member states.3 In 2020, Lupus Europe, the European umbrella non-profit independent organisation that brings together national lupus patient organisations from across Europe, designed a survey which aimed at describing the impact of SLE on individuals with the disease, from the patient perspective. The last such survey was conducted in 2010.4
Methods
Survey design
From 9 May 2020 until 31 May 2020, Lupus Europe, the umbrella organisation federating European national lupus patient associations, conducted an on-line survey among people living with lupus in Europe, to better understand the reality of living with SLE, as viewed from a patient perspective. The questionnaire, built by members of Lupus Europe, contained a total of up to 33 questions (see online supplemental appendix 1). The original English-language questionnaire was translated by volunteers of national lupus member organisations, and verified back with on-line translation tools to identify possible areas of incorrect translation, which were then verified back with native speakers. In total, 20 different language versions were made available. The last translations (Romanian and Estonian) were only available for a week.
lupus-2020-000469supp001.pdf (194.5KB, pdf)
Survey dissemination and target population
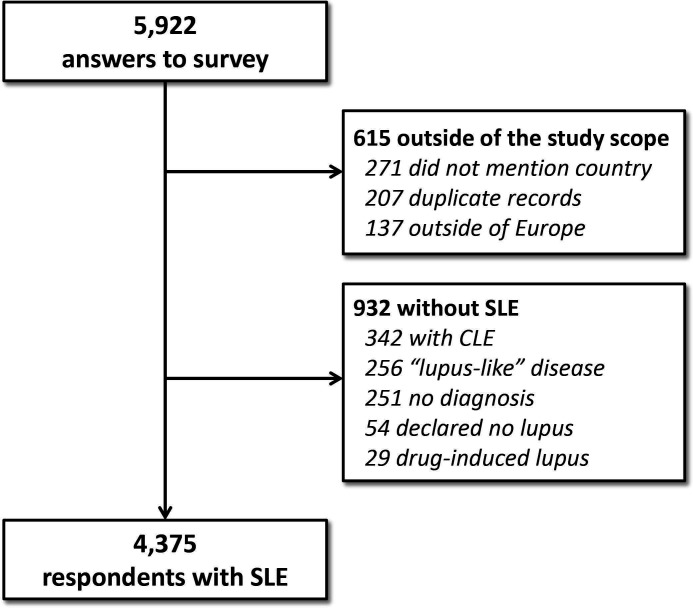
The survey was made available to European patients with lupus both through a unique link to a multilingual start page on lupus Europe’s website (www.lupus-europe.org) or through national language–specific direct access links. Data were collected via an anonymous online survey and included individuals with self-reported physician’s diagnosis of SLE. From a total of 5922 answers (figure 1), 137 were excluded because the country was out of the study scope, 271 did not state their country, 207 were identified as duplicate records, 54 declared they had no lupus, 29 reported drug-induced lupus,5 342 had cutaneous lupus erythematosus (CLE) only, 256 selected the “lupus-like disease” option (no formal lupus diagnosis) and 251 did not mention any specific diagnosis. The remaining 4375 participants reported having physician’s diagnosis of SLE and their data were retained for further analysis.
Figure 1.
Study flow chart. CLE, Cutaneous Lupus Erythematosus.
Statistical analysis
Data are presented as medians and their 25th–75th percentile IQR or counts and percentages. Comparisons between independent groups were made using the Mann-Whitney U test for continuous outcomes and the χ2 test (or Fisher’s exact test when appropriate) for quantitative data. Bonferroni correction was applied to correct for multiple testing, when appropriate. All tests were two-sided and p values <0.05 were considered statistically significant. Statistical analyses were performed with the software JMP13 (SAS Institute, Cary, NC, USA).
Results
General characteristics of respondents
Data from 4375 survey respondents were analysed (table 1), including 4181 (95.9%) women and a median age of 45 years (IQR: 36–54 years). Respondents originated from 35 European countries, mostly France (15.5%), UK (15.2%), Italy (12.7%) and Germany (6.9%) and self-identified as Caucasian or white in 70.7%, Hispanic or Latino in 6.3% and African, African-American or Caribbean in 2.2%. Detailed information regarding the countries of residence are shown in online supplemental appendix 2. Among respondents with available data, 65.6% were married or living with a partner and 56.3% were employed or self-employed. Educational levels are shown in table 1.
Table 1.
Respondents’ characteristics
| Respondents’ characteristics | Value |
| Gender (data availability: n=4358) | |
| Women, n (%) | 4181 (95.9) |
| Prefer not to say, n (%) | 15 (0.3) |
| Age (data availability: n=4303) | |
| Age of responders (in years), median (IQR 25–75) | 45 (36–54) |
| Ethnic background, n (%) (data availability: n=4290) | |
| Caucasian/White | 3035 (70.7) |
| Hispanic/Latino | 270 (6.3) |
| African/African American/Caribbean | 93 (2.2) |
| Mixed/multiple ethnic groups | 83 (1.9) |
| Asian/Pacific Islander/Indian | 68 (1.6) |
| Middle Easterner/North African | 48 (1.1) |
| Other | 374 (8.7) |
| Prefer not to say | 319 (7.4) |
| Civil status, n (%) (data availability: n=4287) | |
| Married/with partner | 2814 (65.6) |
| Single | 730 (17) |
| Divorced Child/young with family |
388 (9.1) 191 (4.5) |
| Widowed | 83 (1.9) |
| Other/prefer not to answer | 81 (1.9) |
| Employment status, n (%) (data availability: n=4247) | |
| Employed full time | 1468 (34.6) |
| Employed part time | 699 (16.5) |
| Stopped working for medical reason | 626 (14.7) |
| Retired | 491 (11.6) |
| Self-employed | 227 (5.4) |
| Looking for employment | 216 (5.1) |
| Not in paid employment/full time at home | 201 (4.7) |
| Student | 171 (4) |
| Other/prefer not to answer | 148 (3.5) |
| Educational level, n (%) (data availability: n=4276) | |
| High school/A level/international baccalaureate/vocational | 1642 (38.4) |
| Master (or higher) academic degree | 897 (21) |
| Bachelor (or equivalent) degree | 866 (20.3) |
| GCSE (or equivalent) | 593 (13.9) |
| Primary school | 152 (3.6) |
| Prefer not to answer | 126 (2.9) |
GCSE, General Certificate of Secondary Education.
SLE diagnosis and reported organ involvement
The median age at SLE diagnosis was 30 years (IQR: 22–40) and 5.6% of participants reported childhood-onset SLE (table 2). The median reported delay between the first symptom of the disease and SLE diagnosis was 2 years (IQR: 0–6), with 26.5% being diagnosed within 1 year of first symptoms. A majority reported involvement of joints (81.8%, n=3515), skin (59.4%, n=2551) and muscles (41.6%, n=1787), with renal involvement in 30% (detailed organ involvements are shown in table 2). In addition, 20.9% (n=899) reported a diagnosis of antiphospholipid syndrome (APS).
Table 2.
Age, diagnosis delay, organ manifestations and prior diagnoses
| Respondents’ characteristics | Value |
| Age at diagnosis (in years), median (IQR25–75) (data availability: n=4184) | 30 (22–40) |
| Diagnosis delay (years), median (IQR25–75) (data availability: n=4154) | 2 (0–6) |
| Within first year of first symptoms onset, n (%) | 1102 (26.5) |
| Within 2 years, n (%) | 1979 (47.6) |
| Within 5 years, n (%) | 2883 (69.4) |
| Within 10 years, n (%) | 3492 (81.1) |
| Disease manifestations, n (%) (data availability: n=4298) | |
| Joints | 3515 (81.8) |
| Skin | 2551 (59.4) |
| Muscles | 1787 (41.6) |
| Kidney | 1290 (30) |
| Bloodstream (cytopenia) | 1173 (27.3) |
| Lungs | 767 (17.8) |
| Heart | 731 (17) |
| CNS | 696 (16.2) |
| Muscles | 1787 (41.6) |
| Prior diagnosis before that of SLE, n (%) (data availability: n=4275) | |
| Psychological or mental disease | 388 (9.1) |
| UCTD or MCTD | 293 (6.9) |
| Fibromyalgia | 254 (5.9) |
| Sjögren’s disease | 207 (4.8) |
| Antiphospholipid syndrome | 104 (2.4) |
| Other diagnoses (non-autoimmune or rheumatic) | 724 (16.9) |
| Other autoimmune or rheumatic disease | 605 (14.2) |
MCTD, mixed connective tissue disease; UCTD, undifferentiated connective tissue disease.
Importantly, 45.0% (n=1925) received another diagnosis before that of SLE, typically another rheumatic condition such as undifferentiated connective tissue disease (UCTD), mixed connective tissue disease (MCTD), Sjögren’s or APS (table 2). However, 9.1% (n=388) reported being initially diagnosed with a psychological or mental disorder and 5.9% (n=254) with fibromyalgia. As expected, the diagnosis delay was significantly increased in patients who reported another diagnosis before that of SLE (3 years vs 1 year, p<0.0001). When the first diagnosis given was fibromyalgia, the median diagnosis delay for SLE increased from 2 years (IQR: 0–5) to 7 (2–14) years, p<0.0001. Conversely, the median diagnosis delay was significantly shorter for patients who reported renal involvement (1 year (IQR: 0–4)) versus 2 years without (IQR: 1–7), p<0.0001.
Most common and bothersome symptoms
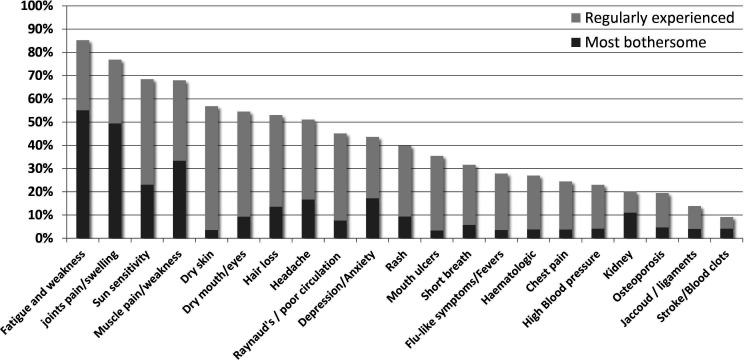
Out of 4347 respondents, 1228 (28.2%) felt that SLE had not been “under control” during the 3 months before the survey. The reported prevalence of fibromyalgia was 10.5% in patients without disease control versus 4.1% when SLE was “under control”, p<0.0001. A total of 4197 participants identified the symptoms or features of lupus that they regularly experience (figure 2) from a list of 21 options (online supplemental appendix 3). Out of a maximum of 21, the median number of SLE symptoms reported by respondents (n=4197) was 9 (IQR:6–11). Fatigue was the most common (85.3%), followed by pain and/or swelling in joints (76.9%), photosensitivity (68.5%), muscle pain and weakness (68.0%), dryness of the skin (56.9%), dryness in the mouth or eyes (54.5%), hair loss (53.0%), and headaches or migraine (51.1%). The main three symptoms that respondents would like the most to go away (figure 2 and online supplemental appendix 3) were “fatigue and weakness” (n=2311, 55.1%), “joints pain and swelling” (n=2076, 49.5%) and “muscle pain and weakness” (n=1400, 33.4%). To note, of the 683 (16.7%) that identified anxiety or depression as one of their most bothersome symptoms, only 315 (46.1%) reported using antidepressant or anxiolytics medication.
Figure 2.
Most common and bothersome SLE symptoms, as reported by respondents. Proportion of responders reporting each symptom as regularly experienced (light grey) or most bothersome (dark grey).
Access to care and treatments
Across all responses, 8.6% of participants reported a limited access to prescribed medications, ranging from 5% in Spain to 74.3% in Bulgaria (online supplemental appendix 4). They reported using a median of 5 (IQR: 3–7) different types of medication related to SLE (data available for 4099 respondents). Prescribed medications included antimalarials in 75%, oral glucocorticoids in 52.4%, immunosuppressive agents in 39.8% and biologics in 10.9% (table 3). Taking into account countries with more than 100 responders, the overall use of biologics ranged from ≈2%–4% in Belgium, Poland and Croatia to >15% in Bulgaria and Spain (online supplemental appendix 4). Overall, 68.0% of participants agreed that they had appropriate access to affordable treatments, with France and Spain achieving the highest scores (84.6% and 83.5%, respectively) and Bulgaria and Poland the lowest (26.2% and 38.7%, respectively). Among respondents, 69.3% reported having an access they estimate was appropriate to an “experienced lupus doctor”, 49.1% to a multidisciplinary team, 34.8% to a “specialised nurse that knows lupus”, 30.2% to physiotherapy, rehabilitation or occupational therapy, 29.7% to adequate social support and only 26.2% to professional psychological support.
Table 3.
Reported treatments
| Reported treatments (data available in 4099) | n (%) |
| Antimalarials | 3076 (75) |
| Oral steroids (dose available in 3978) | 2147 (52.4) |
| <5 mg/day | 1029 (25.9) |
| 5 to 15 mg/day | 804 (20.2) |
| >15 mg/day | 155 (3.9) |
| Injections in past 3 months | 38 (1) |
| Immunosuppressive agents | 1632 (39.8) |
| Biologics | 446 (10.9) |
| Other treatments | |
| Vitamin D | 2804 (68.4) |
| Analgesics | 2053 (50.1) |
| NSAIDs | 1348 (32.9) |
| Calcium | 1219 (29.7) |
| Antidepressant | 698 (17) |
| Anxiolytic | 480 (11.7) |
| Statins | 241 (5.9) |
NSAIDs, non-steroidal anti-inflammatory drugs.
Impact of SLE on studies, work and family life
Among the 1492 respondents diagnosed with SLE before the age of 25, 50.7% (n=757) felt that SLE had impacted their studies. Among participants having identified their employment status, 57.9% reported a negative impact of SLE over their career and 14.7% declared that they stopped working for medical reasons (online supplemental appendix 5). Regarding the “ability to perform normal daily activities such as studying, working, housework, leisure or participation to family life”, 49.7% of respondents highlighted either a medium, high or very high burden, while an additional 1.8% reported being fully unable to perform daily activities. In their opinion, 72.4% reported being less active than people of the same age without SLE and 76.1% said lupus had a significant impact on their emotional and sexual life. This impact was viewed as negative for 1608 (38.2%), mixed for 1523 (36.2%) and positive for only 67 participants (1.6%). A total of 4042 respondents answered the question “With regards to the mid to long term future, how worried are you about your lupus progressing?” providing a score from 1 (not worried at all) to 10 (extremely worried). The median score was 7 (IQR 25–75=5–8). A comparison of European countries is provided in online supplemental appendix 6.
Discussion
Lupus Europe, a major European lupus patient association, has performed a survey about the burden of SLE in Europe, involving a large sample of 4375 respondents from 35 European countries who reported having physician-confirmed SLE.
While the median age at SLE diagnosis of 30 years and the proportion childhood-onset SLE was in line with most epidemiological studies in Europe,5 one key finding of the survey is the median reported diagnosis delay of 2 years (IQR: 0–6) with SLE diagnosed within 1 year of first symptoms in only about a quarter of respondents. This is significantly less than previously reported in a large patient survey from the UK.6 While another rheumatic condition such as UCTD, MCTD, Sjögren’s or APS was initially diagnosed in a significant proportion of respondents, it is worthy to note that 9.1% reported being initially diagnosed with a purely psychological or mental disorder and 5.9% with fibromyalgia, increasing significantly the median diagnosis delay from 2 to 7 years.
The predominant manifestations reported by respondents (fatigue, articular and skin manifestations) are in line with most epidemiological studies.7 8 Among respondents, 85.3% reported fatigue in 2020, versus 82.5% in the previous 2010 study by Lupus Europe.4 Also, 54.9% reported fatigue as among the three main bothersome symptom in 2020, versus 45.8% in 2010. It is striking to note the high prevalence of symptoms compatible with Sjögren’s syndrome as well as the high prevalence of headaches (51.1%) which is neither in line with the general population nor the typical frequency of medically reported lupus headache.9 Interestingly, the main manifestations patients with SLE would like to get rid of are fatigue, which is in line with several studies,6–8 as well as painful manifestations such as joint and muscle pain.6 10 11
The study highlights strong differences in access to care between countries.3 12 Several factors may account for the wide variation across Europe (see online supplemental document 1). Those include national policies and national SLE recommendations, access to specialised care and SLE expertise, pharmaceuticals pricing and medicines reimbursement policies (both from the patient as well as from the health system perspective, including that of private payers). Initiatives such as European Reference Networks and European Transborder Care may help partly reduce these inequities but country-specific health insurance and reimbursement policies may increase the overall economic burden for patients with SLE.13 Data regarding medications revealed the use of antimalarials in only 75% of patients, and the large sample size allows for the first time a very large-scale estimation of the use of glucocorticoids (52.4%), immunosuppressive agents (39.8%) and biologics (10.9%), with a strong variability between European countries.14 Of note, the reported use of hydroxychloroquine was lower than reported in a previous survey from the USA.15 Finally, psychological support was available to <35% of participants across Europe, which is of outstanding importance given the fact that SLE is a multisystemic disease which affects young women predominantly.
Finally, the sample size allowed for a large-scale estimation of the burden of the disease on daily life,8 with approximately half of respondents who felt that SLE had impacted their studies or their employment status,8 16 17 as well as their ability to perform normal daily activities.18 19 In 2010,4 69.5% reported that lupus had affected their career versus 65.8% in 2020; of those, 29.4% reported the need to work flexible hours in 2010 versus 31.9% in 2020. Similar results have been shown in large US studies,20–22 also showing a relationship between disease activity and work productivity loss, as well as with activity impairment. This yielded generally high levels of anxiety about the future in this survey.23
Among the main limitations of the study is its largely declarative nature, as we cannot ascertain that all respondents had a physician-confirmed diagnosis of SLE. However, the survey was disseminated by a well-established patient association, and the questions were designed to capture (and exclude) several alternative diagnoses other than medically confirmed SLE, including CLE only, or drug-induced lupus. Also, respondents may not be able to fully differentiate joint pain due to SLE from that due to other causes, including osteoarthritis and fibromyalgia. Of note, the study was set during the COVID-19 crisis in Europe, after almost 2 months of confinement for many. This may have influenced some of the answers, for example around anxiety about the future. Finally, the involvement of physicians has been very limited in the design of the questionnaire. This may have limited the interpretation of some findings as patients commonly used daily language terminology which may differ from medically recognised terms, occasionally resulting in ambiguity from a medical perspective.
Conclusion
This large survey reveals the European landscape of SLE from the patients’ perspective in Europe in 2020. The long diagnosis delay highlights the need for increased training of physicians in the field of autoimmune diseases and the lack of proper patient pathways in most countries, despite the role of EULAR and of European Reference Networks such as ReCONNET. Significant differences in access to care and treatment strategies remain within Europe, as illustrated by the broad variability in the proportion of patients treated with biologics. Altogether, these data may prove crucial to physicians, patients and policy-makers to improve the diagnosis and management of this rare and complex disease.
Acknowledgments
LUPUS EUROPE is very thankful to the volunteers that helped build and translate the survey, and then disseminate it throughout Europe. It is also grateful to the 5922 persons living with lupus that have given their time and data to help us better understand the disease. The authors wish to thank Ms Sylvie Thuong for her invaluable help in the preparation of the manuscript.
Footnotes
Twitter: @kikkams, @@Lupusreference
Contributors: Study design: AC, JA, KM, AE, LA. Data acquisition: AC, JA, KM, AE. Data analysis: LA, AC. Drafting: LA. Critically revising for important content: AC, JA, KM, AE, LA. Final approval by all authors.
Funding: The study has been funded by LUPUS EUROPE. LUPUS EUROPE is itself largely funded from unrestricted grants from industry, where no company exceeded 17% of total funds raised.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Ethic Committee of Strasbourg Medical School (#CE-2020-109).
References
- 1.Arnaud L, Fagot J-P, Mathian A, et al. Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation-wide population-based study. Autoimmun Rev 2014;13:1082–9. 10.1016/j.autrev.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 2.Felten R, Sagez F, Gavand P-E, et al. 10 most important contemporary challenges in the management of SLE. Lupus Sci Med 2019;6:e000303. 10.1136/lupus-2018-000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamirou F, Arnaud L, Talarico R, et al. Systemic lupus erythematosus: state of the art on clinical practice guidelines. RMD Open 2019;4:e000793. 10.1136/rmdopen-2018-000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon C, Isenberg D, Lerstrom K, et al. The substantial burden of systemic lupus erythematosus on the productivity and careers of patients: a European patient-driven online survey. Rheumatology 2013;52:2292–301. 10.1093/rheumatology/ket300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnaud L, Mertz P, Gavand P-E, et al. Drug-induced systemic lupus: revisiting the ever-changing spectrum of the disease using the WHO pharmacovigilance database. Ann Rheum Dis 2019;78:504–8. 10.1136/annrheumdis-2018-214598 [DOI] [PubMed] [Google Scholar]
- 6.Morgan C, Bland AR, Maker C, et al. Individuals living with lupus: findings from the LUPUS UK Members Survey 2014. Lupus 2018;27:681–7. 10.1177/0961203317749746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnaud L, Gavand PE, Voll R, et al. Predictors of fatigue and severe fatigue in a large international cohort of patients with systemic lupus erythematosus and a systematic review of the literature. Rheumatology 2019;58:987–96. 10.1093/rheumatology/key398 [DOI] [PubMed] [Google Scholar]
- 8.Kent T, Davidson A, Newman D, et al. Burden of illness in systemic lupus erythematosus: results from a UK patient and carer online survey. Lupus 2017;26:1095–100. 10.1177/0961203317698594 [DOI] [PubMed] [Google Scholar]
- 9.Urowitz MB, Gladman DD, Ibañez D, et al. American College of Rheumatology criteria at inception, and accrual over 5 years in the SLICC inception cohort. J Rheumatol 2014;41:875–80. 10.3899/jrheum.130704 [DOI] [PubMed] [Google Scholar]
- 10.Fischin J, Chehab G, Richter JG, et al. Factors associated with pain coping and catastrophising in patients with systemic lupus erythematosus: a cross-sectional study of the LuLa-cohort. Lupus Sci Med 2015;2:e000113. 10.1136/lupus-2015-000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldheim E, Ajeganova S, Bergman S, et al. Variation in pain related to systemic lupus erythematosus (SLE): a 7-year follow-up study. Clin Rheumatol 2018;37:1825–34. 10.1007/s10067-018-4079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherlinger M, Mertz P, Sagez F, et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun Rev 2020;19:102531. 10.1016/j.autrev.2020.102531 [DOI] [PubMed] [Google Scholar]
- 13.Doria A, Amoura Z, Cervera R, et al. Annual direct medical cost of active systemic lupus erythematosus in five European countries. Ann Rheum Dis 2014;73:154–60. 10.1136/annrheumdis-2012-202443 [DOI] [PubMed] [Google Scholar]
- 14.Rydén-Aulin M, Boumpas D, Bultink I, et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med 2016;3:e000163. 10.1136/lupus-2016-000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace DJ, Tse K, Hanrahan L, et al. Hydroxychloroquine usage in US patients, their experiences of tolerability and adherence, and implications for treatment: survey results from 3127 patients with SLE conducted by the Lupus Foundation of America. Lupus Sci Med 2019;6:e000317. 10.1136/lupus-2019-000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth S, Price E, Walker E. Fluctuation, invisibility, fatigue – the barriers to maintaining employment with systemic lupus erythematosus: results of an online survey. Lupus 2018;27:2284–91. 10.1177/0961203318808593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekblom-Kullberg S, Kautiainen H, Alha P, et al. Education, employment, absenteeism, and work disability in women with systemic lupus erythematosus. Scand J Rheumatol 2015;44:157–62. 10.3109/03009742.2014.953200 [DOI] [PubMed] [Google Scholar]
- 18.Corneloup M, Maurier F, Wahl D, et al. Disease-specific quality of life following a flare in systemic lupus erythematosus: an item response theory analysis of the French EQUAL cohort. Rheumatology 2020;59:1398–406. 10.1093/rheumatology/kez451 [DOI] [PubMed] [Google Scholar]
- 19.Stevens MJ, Walker-Bone K, Culliford DJ, et al. Work participation, mobility and foot symptoms in people with systemic lupus erythematosus: findings of a UK national survey. J Foot Ankle Res 2019;12:26. 10.1186/s13047-019-0335-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz P, Nelson WW, Daly RP, et al. Patient-reported lupus flare symptoms are associated with worsened patient outcomes and increased economic burden. J Manag Care Spec Pharm 2020;26:275–83. 10.18553/jmcp.2020.26.3.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke AE, Yazdany J, Kabadi SM, et al. The economic burden of systemic lupus erythematosus in commercially- and Medicaid-insured populations in the United States. Semin Arthritis Rheum 2020;50:759–68. 10.1016/j.semarthrit.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Sawah S, Daly RP, Foster SA, et al. The caregiver burden in lupus: findings from UNVEIL, a national online lupus survey in the United States. Lupus 2017;26:54–61. 10.1177/0961203316651743 [DOI] [PubMed] [Google Scholar]
- 23.Moustafa AT, Moazzami M, Engel L, et al. Prevalence and metric of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. Semin Arthritis Rheum 2020;50:84–94. 10.1016/j.semarthrit.2019.06.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2020-000469supp001.pdf (194.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.