Abstract
BACKGROUND AND PURPOSE:
The IJVs are considered to be the main pathway draining the intracranial venous system. There is increasing evidence for the existence of alternative venous pathways. Studies using extracranial sonography techniques have demonstrated a nonjugular venous system. In the current study, we used MR images to investigate the NJV drainage system and its components (vertebral plexus, pterygopalatine plexus). The exact visualization and measurement of the intracranial NJVs could be of diagnostic importance and may have clinical importance.
MATERIALS AND METHODS:
A total of 64 participants with no history of neurologic disease were included in the study. All participants underwent scanning with a 2D time-of-flight, multisection sequence in the supine position. Image processing software was developed to identify and quantify the size of the IJVs and NJVs in the plane of the internal JF. For evaluation of software accuracy, all images were reviewed by a neuroradiologist experienced in neurovascular imaging preprocessing and postprocessing.
RESULTS:
The CSA of the NJVs correlated inversely with the CSA of the IJVs (r2 = 0.25; P < .0001). An inverse correlation was also significant when comparing IJV with NJV components (vertebral plexus: r2 = 0.19; P = .0004; pterygopalatine plexus: r2 = 0.11; P = .0069). Furthermore, only NJV cumulative CSA correlated inversely with participant age (r2 = 0.2; P = .0002).
CONCLUSIONS:
Our study indicates that the NJVs might serve as a compensatory drainage mechanism in the intracranial compartment. This mechanism appears less significant as the age of the patient progresses.
The IJVs are considered to be the main pathway of intracranial blood drainage. This hypothesis is supported by the anatomic findings of several cerebral venous vessels draining blood from the superficial as well as the deep cerebral venous system into the IJVs.1 However, the clinical observation that bilateral resection of the IJVs is usually well tolerated suggests the presence of alternative, nonjugular pathways.2
Studies using angiographic and extracranial sonography techniques have demonstrated an NJV system that is most prominent with the patient in the supine position.3,4 The vertebral plexus is considered a major component of this system.5,6 Another less-studied element is the pterygopalatine plexus.7 Precise quantitative measurements of cerebral veins are scarce. The current study uses 2D time-of-flight, multisection MR images at the level of the JF in conjunction with an image-processing algorithm that enabled the exact measurement of the IJV and NJV CSA. The purpose of our study was to explore the relationship between IJV and NJV sizes (CSA) as well as the relationship between the participant ages and drainage of NJV pathway sizes (CSA).
Materials and Methods
MR Image Acquisition
MR imaging was performed with a 1.5T whole-body scanner (Intera; Philips Healthcare, Best, the Netherlands). Venous angiography was obtained by use of a 2D time-of-flight, multisection sequence. The sections were acquired in a sequential single-section mode, by use of a spoiled fast-field echo with an inferiorly placed presaturation slab to eliminate the signal from the arterial blood. Scanning plane was set in parallel to the hard palate and was localized on midsagittal survey. The scan parameters were TR, 18.0 ms; TE, 4.0 ms; α, 40°; and section width, 2 mm with a 0.5-mm overlap. The field of view was 230 mm with an in-plane resolution of 0.89 mm. The water-fat shift was 1.264 pixels, equivalent to a bandwidth of 171.9 Hz/pixel. The acquisition time was 4.7 s/section (ie, 1 signal average), and typically, 50 sections were acquired in a study.
Volume reconstructions were carried out by using maximal intensity projections on the Extended Workspace workstation (Philips).
Image Processing
We used Matlab (version 7.0.4.365; January 29, 2005; MathWorks, Natick, Massachusetts) as the development platform for our image-processing software. Inputs to the software were axial MR images in DICOM format.
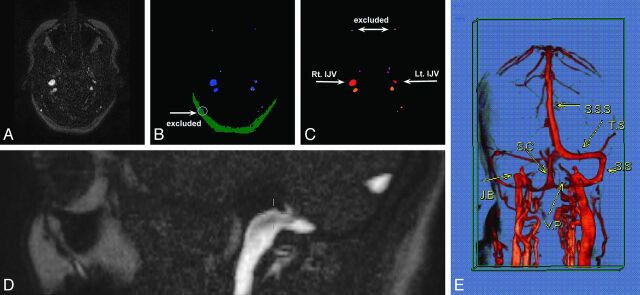
For each patient, 1 section was chosen at the level of the JF (Fig 1A). The sections were manually selected by a single experienced neuroradiologist (I.S.), who identified the JF and the jugular bulbs. The jugular bulbs were defined as the highest point of the IJVs. Illustrative contrast-enhanced MR venography with 3D reconstruction demonstrating venous drainage of the brain is presented in Fig 1E.
Fig 1.
Example of vein detection and mapping algorithms in 1 participant. The original phase-contrast MR image at the level of the JF (A) was initially processed by the vein detection algorithm according to intensity levels (B). Blue represents pixels that surpassed a high-intensity threshold. Yellow stands for pixels between medium and high-intensity thresholds attached to high-intensity objects. The vein cross-sectional area was determined by the sum of both blue and yellow areas. Green corresponds to low-intensity subcutaneous fat tissue. Detected veins in this tissue drain the scalp and therefore were excluded (white arrow). The resulting image was further processed by the mapping algorithm according to size and location (C). The 2 most anterior veins were identified as facial veins and thus were excluded. Vessels anterior to the jugular veins (red) were considered to be the pterygopalatine plexus (purple). Vessels posterior to the jugular veins were regarded as the vertebral plexus (orange). The level of image acquisition (JF) is also presented (D). E, Illustrative contrast-enhanced MR venography with 3D reconstruction demonstrates venous drainage of the brain: S.S.S., superior sagittal sinus; T.S., transverse sinus; S.S., sigmoid sinus; S.C., sinus cerebella; J.B., jugular bulb; V.P., vertebral plexus.
Before image processing, comparison between JF CSA and jugular bulbs flow signal CSA was performed in the first 10 participants. There were no significant differences (results are not presented).
Matrices
The original image was transformed into high-, medium-, and low-intensity binary matrices, which were used later by the vein detection algorithm. Each matrix represented pixels above a predefined intensity threshold. The high-intensity matrix was used to identify the center of the venous lumen; the medium-intensity matrix, to identify the venous wall; and the low-intensity matrix, to identify fat tissue. Threshold levels were tuned until veins were detected correctly. Connected components analysis was performed on each matrix.
Vein Detection Algorithm
High- and medium-intensity matrices.
In 2D time-of-flight, MR image intensity level is directly associated with blood flow. CSA veins were detected as a high-intensity matrix.
Low-intensity matrix.
The low-intensity matrix was used to exclude artifacts and superficial veins that do not have a role in intracranial drainage. A vein was identified as a high-intensity object when larger than 3 pixels. For smaller objects, this observation was not always accurate because, in a few cases, low-intensity tissue included small regions of high-intensity artifacts. Therefore, before identifying a small object as a vein, the algorithm first examined whether this object was not located inside a large object in the low-intensity matrix.
Superficial veins were detected and were eliminated according to their attachment to subcutaneous fat, which was also detected in the low-intensity matrix.
An example of an outcome of the vein detection algorithm is depicted in Fig 1B.
Mapping Algorithm
IJVs.
The IJVs were measured at the level of the jugular bulb. Two zones were predefined: 1) zone L for the expected location of the left jugular bulb and 2) zone R for the expected location of the right jugular bulb. Because the jugular bulbs were usually the largest veins in the image, the mapping algorithm searched for the largest vein. If that vein was located inside zone L or R, then it was mapped as the left or the right jugular bulb, accordingly. In cases where the largest vessel was not located in any of the predefined zones, the algorithm continued searching as to whether the next largest vessel resided in any of the predefined zones. Once the first jugular bulb was found, the contralateral predefined area was narrowed to increase mapping accuracy. The search was completed when both the left and the right jugular bulbs were identified, or when the last vein was checked.
NJVs.
All vessels that were not excluded and were not mapped as IJVs were considered to be NJVs. The nonjugular system was further separated into its components. All NJVs anterior to the IJVs were considered to be the pterygopalatine plexus, whereas those posterior to the IJVs were regarded as the cephalic termination of the vertebral plexus.
Sides.
For comparison between the left and the right cerebral vessel size, the image was divided symmetrically by a sagittal line between the IJVs.
Vein exclusion.
The algorithm excluded veins that did not take part in intracranial drainage according to vessel location: 1) facial veins identified as the most anterior veins in a predefined area, and 2) veins at the level of the pterygopalatine fossa lateral to the IJVs (ie, retromandibular veins).
An example of the outcome of the vein-mapping algorithm is depicted in Fig 1C.
Manual Intervention
After the veins were automatically detected and mapped, our software enabled us to perform manual intervention. This intervention allows the user to remove veins that do not take part in intracranial drainage and are not automatically excluded (eg, the software cannot identify a superficial vein if it is attached to a thin or fragmented layer of subcutaneous fat). It also enables the user to restore veins that were falsely removed because of location criteria, and add veins that were not initially detected because of the intensity level being lower than the high-intensity threshold. For example, a vessel that had direct contact with a major sinus was not considered as a posterior scalp vein and was added to the CSA of the NJVs manually.
Vein Size Measurements
Vein CSA was defined as the amount of pixels in the vein converted to millimeters. Vein size was calculated according to its CSA. The size of the IJVs was calculated as the CSA sum of the left and right internal jugular bulbs. The size of the NJVs was defined as the CSA sum of all included veins, excluding the jugular veins.
Patient Population
A total of 73 consecutive participants with no history of neurologic disease enrolled in the study. All MR imaging scans were performed as a part of a normal clinical work-up for various reasons. Of those, 5 participants were excluded because of low-quality MR images: 3 were excluded because the plane was not parallel to the hard palate, and 2 were excluded because the presaturation (rest) slab did not eliminate signals from the arteries. Four additional participants were excluded because the MR images were not acquired at the correct anatomic level. The final number of participants was 64 (22 men and 42 woman; age range, 16–76 years; mean age, 48.4 years).
All MR routine clinical images were reviewed by a neuroradiologist to exclude participants with intracranial abnormality, congenital anomaly, venous thrombosis, tumor, or previous craniotomy. The local ethical committee approved our study, and the participants gave their informed consent.
Statistical Analysis
We used GraphPad software (San Diego, California), version 4.01 (2004), to analyze the data. We used Pearson correlation analysis to investigate the correlation between the CSA of the IJVs and NJVs and to examine the effects of the left vs the right side and participant age. The effect of sex on vein CSA was analyzed with the unpaired t test.
Results
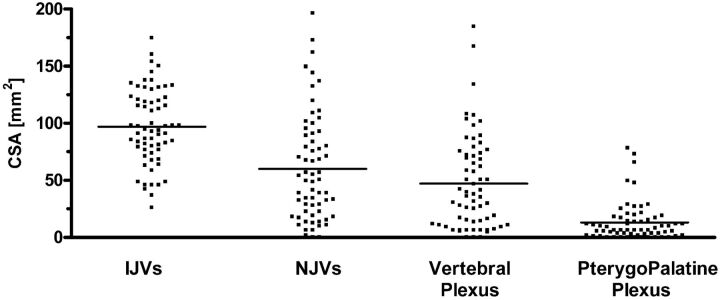
CSA measurements of the intracranial venous drainage system and its components were performed at the level of the JF (Fig 2). The mean IJV CSA was 96.99 mm2 (SD, 33.06 mm2), whereas the mean NJV CSA was 60.08 mm2 (SD, 46.95 mm2). Regarding the components of the NJVs, the mean CSA of the vertebral plexus was 47.01 mm2 (SD, 41.21 mm2), and the mean CSA of the pterygopalatine plexus was 13.06 (SD, 17.01 mm2).
Fig 2.
CSA measurements of the cerebral veins.
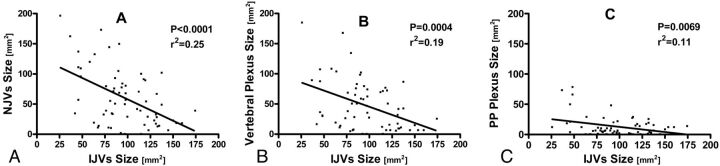
To explore the relationship between the IJVs and NJVs, we compared the CSA of both systems. An inverse correlation was identified between the IJVs and NJVs (r2 = 0.25; P < .0001) (Fig 3A). Then, the association between the IJVs and each component of the NJVs was further investigated. There was an inverse correlation between the IJVs and the vertebral plexus (r2 = 0.19; P = .0004) and between the IJVs and the pterygopalatine plexus (r2 = 0.11; P = .0069) (Figs 3B, -C).
Fig 3.
Relationship between the CSA of the IJVs and the CSA of the NJVs. Initially, the NJVs were regarded as a complete drainage system. This system was then further divided to its components (vertebral plexus and pterygopalatine plexus). The most significant correlation was found when the IJVs were compared with the NJVs as a whole system (A). When the NJVs were divided into their components — vertebral plexus (B) and pterygopalatine plexus (C) — the correlation was still significant though less prominent.
Next, we evaluated the correlation between CSA of the left and right cerebral vessels. Most participants had asymmetric IJVs. The right jugular bulb was larger than the left in 64% of participants (mean size, 57.38 mm2 for right jugular vs 39.61 mm2 for left jugular; P = .0019). Furthermore, an inverse correlation was found between the dominant jugular bulb and its contralateral equivalent (r2 = 0.088; P = .017). In contrast to the IJVs, nonsignificant asymmetry was demonstrated between the left and the right NJVs (right NJV mean size, 31.85 mm2; left NJV mean size, 28.22 mm2). Moreover, a positive correlation was identified between the left and the right NJV CSA size (r2 = 0.356; P < .0001).
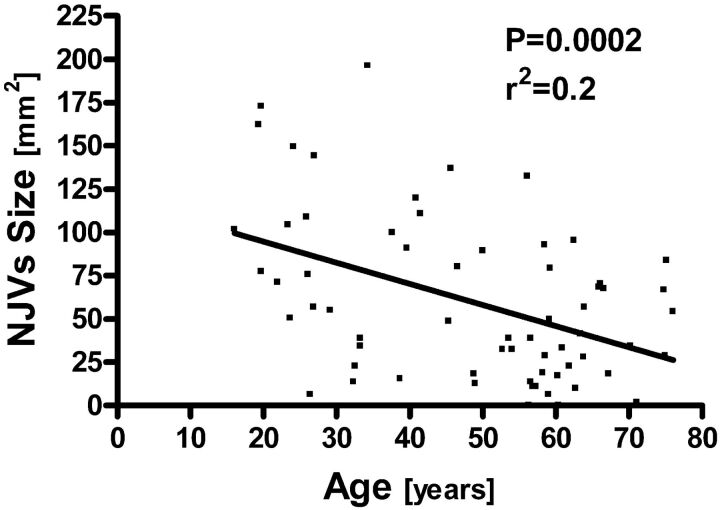
We further examined the effects of age and sex on CSA of the IJVs and NJVs. Correlation between age and NJV size was significant and showed CSA reduction with advancing age (r2 = 0.2; P = .0002) (Fig 4). In contrast, IJV size was not associated with age. Also, IJV and NJV CSA yielded no significant differences between men and women.
Fig 4.
Scatterplot demonstrating the relationship between age and NJV cumulative CSAs.
We explored the accuracy of vein detection by the automated algorithm before manual intervention. To calculate false-positive and false-negative rates, we referred to the neuroradiologist's vein selection as the reference standard. A false-positive rate was 1.64%, and a false-negative rate was 3.02%.
Discussion
Our study indicates that an inverse correlation exists between the CSA of the IJVs vs the surrounding intracranial NJVs measured at the level of the JF.
In the classic sense, cerebral venous drainage occurs mostly through the cortical system and the deep cerebral veins. The 2 systems have a final common pathway directed via the confluence sinus toward the sigmoid sinuses and into the jugular veins.1,8 Although the IJVs are considered to be the main venous outflow system, increasing evidence in the literature suggests the existence of alternative nonjugular pathways.9 The NJVs at the level of the JF consist of veins mainly at the cephalic termination of the vertebral plexus and, to a lesser extent, at the pterygopalatine plexus. The vertebral plexus drains blood from the condyloid veins, emissary veins, and through segmental connections.10 It is important to keep in mind that the anterior and lateral condyloid veins may also be connected to the jugular bulb via the anterior condyloid confluent, preferentially in the upright position.10 Blood from the cephalic termination of the vertebral plexus can continue its flow through the vertebral plexus or be directed to the posterior paravertebral veins, which are located in the soft tissue of the neck. The pterygopalatine plexus receives blood from the cavernous sinuses and from the superior longitudinal sinus and drains into the extraspinal system.11 Quantification of jugular and nonjugular venous vessels has focused mainly on extracranial venous flow measurements.12–14 The deep regions of the intracranial nonjugular venous system are difficult to visualize. Subsequently, study and quantization of this additional system have been reportedly sparse. However, we were able to visualize and measure the CSA of the IJVs and the intracranial NJVs at the level of the JF. We quantitated the CSA by using an image-processing tool developed by our team. Our results demonstrated that the smaller the IJVs, the larger the CSA of the NJVs. Furthermore, comparison of IJV CSA to the vertebral and pterygopalatine plexuses separately demonstrated an inverse correlation, which was significant for each component individually. It is noteworthy that correlation with the IJVs was most prominent when the NJVs were regarded as a whole system. These observations may imply that the NJVs are a drainage mechanism counterbalancing the IJVs and that each of their components contributes to blood outflow separately.
The choice of drainage predominance is position dependent. Although the jugular veins are the predominant system with the person in the supine position, the vertebral plexus is the major outflow pathway in the erect position.3,15,16 Our study involved participants only in the supine position in which the expected major outflow is through the jugular veins. Thus, our finding of an inverse correlation between IJV size with the participants in the supine position and NJV CSA is even more remarkable. Our finding may imply that the NJVs are used not only in the upright position but also in the supine position. It should be considered that the vertebral veins and IJV flows were measured extracranially in previous studies,3,13,14 but our study evaluated CSA of the intracranial vessels. Thus, our explanation remains speculative.
Another point of discussion is that we used an axial image at the level of the JF where the IJVs are attached to the surrounding bone.17 Thus, we chose this locus to eliminate jugular size variation because of the Valsalva effect or respiratory influence. The acquisition plane was axial to surmount measurement inaccuracy resulting from vascular flow in parallel planes to that of MR imaging sections. Therefore, to accurately measure the CSA of the vein, we measured veins in which the flow was rostrocaudal. Measurements of the cerebral vessels whose major anatomy is in the horizontal plane represent length rather than surface due to posteroanterior flow.
The venous drainage system of the brain might use compensatory mechanisms to assure blood outflow. One of them could possibly stem from asymmetry between sides.18 Our observation concerning the right IJV being usually the dominant side confirms previous observations.19,20 We observed an inverse correlation between the CSA of the right and left jugular veins. This inverse association may imply a lateralization effect in which one side compensates for the other. Whether the lateralization effect also exists in the NJVs was further explored. We found a positive correlation between the CSA of the left and right NJVs, which can imply that this drainage system works as a whole and not necessarily in a lateralized fashion.
Documented morphologic changes in the cerebral vasculature of the aging brain include, among other things, thinning of the endothelium, reduction in the diameter of the capillary lumen, and a decreasing number of capillary endothelial cells. The different behavior of the cerebral venous system in advanced age compared with that of the young brain is not yet understood.11,21 By means of different techniques, several studies have shown that blood flow and velocity in the sinuses of the brain decrease with age.22–24 Our results demonstrated that NJV CSA decreased with age. However, no reduction was noted in IJV CSA. To establish a relationship between age and cumulative drainage vessel size, future prospective studies are needed.
It is noteworthy that our measurements assessed CSA and not flow volume. Although a correlation exists between vessel CSA and blood volume, it is not an accurate measurement of blood flow. Better-combined techniques are needed to quantify blood flow to improve our understanding of the complexity of this system.
Larger cohorts are needed to verify the widespread applicability of our software because of the heterogeneity in vein locations and other confounding abnormal structures that are mapped by the software. It is unclear whether our findings reflect a spectrum of normal anatomic variation. An interesting next topic of research would be the quantification of NJV CSA in patients with pathologic conditions vs our findings in healthy participants. This setting would enable us to elucidate the extent of the NJVs potentially compensatory abilities in situations such as sinus vein thrombosis, normal pressure hydrocephalus, etc. It may also help us favor more aggressive treatment options in participants who have small compensatory reserves vs more conservative options for patients with large reserves.
Conclusions
The current study indicates that NJVs might serve as a compensatory drainage mechanism to the main IJVs system in the intracranial compartment. The results demonstrate that the CSA of the NJVs are inversely correlated with the CSA of the IJVs. This compensatory drainage mechanism appears to be less significant as age progresses. A failure of this compensatory mechanism might be an important co-factor for increased ICP.
ABBREVIATIONS:
- CSA
cross-sectional area
- IJV
internal jugular vein
- JF
jugular foramen
- NJV
nonjugular vein
REFERENCES
- 1. Kretschmann HJ, Weinrich W. Cranial Neuroimaging and Clinical Neuroanatomy. New York: Thieme; 2004 [Google Scholar]
- 2. Gius JA, Grier DH. Venous adaptation following bilateral radical neck dissection with excision of the jugular veins. Surgery 1950;28:305–21 [PubMed] [Google Scholar]
- 3. Valdueza JM, von Münster T, Hoffman O, et al. Postural dependency of the cerebral venous outflow. Lancet 2000;355:200–01 [DOI] [PubMed] [Google Scholar]
- 4. Newton TH, Potts DG, eds. Veins. St Louis: Mosby; 1974 [Google Scholar]
- 5. Epstein HM, Linde HW, Crampton AR, et al. The vertebral venous plexus as a major cerebral venous outflow tract. Anesthesiology 1970;32:332–37 [DOI] [PubMed] [Google Scholar]
- 6. Eckenhoff JE. The physiologic significance of the vertebral venous plexus. Surg Gynecol Obstet 1970;131:72–78 [PubMed] [Google Scholar]
- 7. Lanzieri CF, Duchesneau PM, Rosenbloom SA, et al. The significance of asymmetry of the foramen of Vesalius. AJNR Am J Neuroradiol 1988;9:1201–04 [PMC free article] [PubMed] [Google Scholar]
- 8. Meder JF, Chiras J, Roland J, et al. Venous territories of the brain. J Neuroradiol 1994;21:118–33 [Article in English, French] [PubMed] [Google Scholar]
- 9. Doepp F, Schreiber SJ, von Münster T, et al. How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns. Neuroradiology 2004;46:565–70 [DOI] [PubMed] [Google Scholar]
- 10. San Millan Ruiz D, Gailloud P, Rufenacht DA, et al. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol 2002;23:1500–08 [PMC free article] [PubMed] [Google Scholar]
- 11. Schaller B. Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain Res Brain Res Rev 2004;46:243–60 [DOI] [PubMed] [Google Scholar]
- 12. Schreiber SJ, Lurtzing F, Gotze R, et al. Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound. J Appl Physiol 2003;94:1802–05 [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann O, Weih M, von Münster T, et al. Blood flow velocities in the vertebral veins of healthy subjects: a duplex sonographic study. J Neuroimaging 1999;9:198–200 [DOI] [PubMed] [Google Scholar]
- 14. Müller HR, Hinn G, Buser MW. Internal jugular venous flow measurement by means of a duplex scanner. J Ultrasound Med 1990;9:261–65 [DOI] [PubMed] [Google Scholar]
- 15. Gisolf J, van Lieshout JJ, van Heusden K, et al. Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol 2004;560:317–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cirovic S, Walsh C, Fraser WD, et al. The effect of posture and positive pressure breathing on the hemodynamics of the internal jugular vein. Aviat Space Environ Med 2003;74:125–31 [PubMed] [Google Scholar]
- 17. Piffer CR. Microscopic studies on the transition between the sigmoid sinus, the superior bulb of the jugular vein and the first portion of the internal jugular vein. Acta Anat (Basel) 1979;105:121–33 [DOI] [PubMed] [Google Scholar]
- 18. Ichijo H, Hosokawa M, Shinkawa H. Differences in size and shape between the right and left sigmoid sinuses. Eur Arch Otorhinolaryngol 1993;250:297–99 [DOI] [PubMed] [Google Scholar]
- 19. Lobato EB, Sulek CA, Moody RL, et al. Cross-sectional area of theright and left internal jugular veins. J Cardiothorac Vasc Anesth 1999;13:136–38 [DOI] [PubMed] [Google Scholar]
- 20. Lichtenstein D, Saifi R, Augarde R, et al. The internal jugular veins are asymmetric. Usefulness of ultrasound before catheterization. Intensive Care Med 2001;27:301–05 [DOI] [PubMed] [Google Scholar]
- 21. Otsuka H, Nakase H, Nagata K, et al. Effect of age on cerebral venous circulation disturbances in the rat. J Neurosurg 2000;93:298–304 [DOI] [PubMed] [Google Scholar]
- 22. Mattle H, Edelman RR, Reis MA, et al. Flow quantification in the superior sagittal sinus using magnetic resonance. Neurology 1990;40:813–15 [DOI] [PubMed] [Google Scholar]
- 23. Baumgartner RW, Gönner F, Arnold M, et al. Transtemporal power- and frequency-based color-coded duplex sonography of cerebral veins and sinuses. AJNR Am J Neuroradiol 1997;18:1771–81 [PMC free article] [PubMed] [Google Scholar]
- 24. Stolz E, Kaps M, Kern A, et al. Transcranial color-coded duplex sonography of intracranial veins and sinuses in adults. Reference data from 130 volunteers. Stroke 1999;30:1070–75 [DOI] [PubMed] [Google Scholar]