Abstract
Previous studies identified a region on chromosome 1 associated with NG-nitro-L-arginine methyl ester (L-NAME) hypertension-induced renal disease in fawn-hooded hypertensive (FHH) rats. This region contains a mutant γ-adducin (Add3) gene that impairs renal blood flow (RBF) autoregulation, but its contribution to renal injury is unknown. The present study evaluated the hypothesis that knockout (KO) of Add3 impairs the renal vasoconstrictor response to the blockade of nitric oxide synthase and enhances hypertension-induced renal injury after chronic administration of L-NAME plus a high-salt diet. The acute hemodynamic effect of L-NAME and its chronic effects on hypertension and renal injury were compared in FHH 1Brown Norway (FHH 1BN) congenic rats (WT) expressing wild-type Add3 gene versus FHH 1BN Add3 KO rats. RBF was well autoregulated in WT rats but impaired in Add3 KO rats. Acute administration of L-NAME (10 mg/kg) raised mean arterial pressure (MAP) similarly in both strains, but RBF and glomerular filtration rate (GFR) fell by 38% in WT versus 15% in Add3 KO rats. MAP increased similarly in both strains after chronic administration of L-NAME and a high-salt diet; however, proteinuria and renal injury were greater in Add3 KO rats than in WT rats. Surprisingly, RBF, GFR, and glomerular capillary pressure were 41%, 82%, and 13% higher in L-NAME–treated Add3 KO rats than in WT rats. Hypertensive Add3 KO rats exhibited greater loss of podocytes and glomerular nephrin expression and increased interstitial fibrosis than in WT rats. These findings indicate that loss of ADD3 promotes L-NAME–induced renal injury by altering renal hemodynamics and enhancing the transmission of pressure to glomeruli.
SIGNIFICANCE STATEMENT
A mutation in the γ-adducin (Add3) gene in fawn-hooded hypertensive rats that impairs autoregulation of renal blood flow is in a region of rat chromosome 1 homologous to a locus on human chromosome 10 associated with diabetic nephropathy. The present results indicate that loss of ADD3 enhanced NG-nitro-L-arginine methyl ester–induced hypertensive renal injury by altering the transmission of pressure to the glomerulus.
Introduction
The fawn-hooded hypertensive (FHH) rat is a model of chronic kidney disease (CKD) that develops proteinuria, focal glomerulosclerosis, and mild hypertension as they age (Kreisberg and Karnovsky, 1978; Kuijpers and Gruys, 1984; Kuijpers and De Jong, 1987). The onset of renal disease in FHH rats is associated with reduced preglomerular vascular resistance, impaired myogenic response of renal arteries, elevated glomerular capillary pressure (PGC), and hyperfiltration relative to spontaneously hypertensive rats and other strains resistant to hypertension-induced renal injury (De Keijzer et al., 1988; Simons et al., 1993a; Verseput et al., 1998; Van Dokkum et al., 1999a,b). The progression of renal disease in FHH rats can be accelerated by uninephrectomy and by induction of deoxycorticosterone acetate (DOCA)-salt or NG-nitro-L-arginine methyl ester (L-NAME) hypertension, all of which raise glomerular capillary pressure (Simons et al., 1993b, 1994; Van Dokkum et al., 1998; Van Dijk et al., 2005; Fan et al., 2020a,b).
Previous linkage analysis identified five regions of the genome termed renal failure (Rf) 1–5 that cosegregate with the development of proteinuria and glomerulosclerosis with aging or after uninephrectomy in crosses of FHH rats and August Copenhagen Irish (ACI) or Brown Norway (BN) rats (Brown et al., 1996; Shiozawa et al., 2000). Subsequent studies using congenic strains identified a mutant Rab38 [Rat sarcoma (RAS) associated binding protein-3] , which downregulates albumin reuptake in proximal tubules, as the causal gene in the Rf-2 region (Rangel-Filho et al., 2013), and Shroom3 in the Rf-4 region was linked to proteinuria in FHH rats and CKD in humans by enhancing podocyte effacement (Yeo et al., 2015).
Additional studies confirmed that one or more genes in the Rf-1 region are associated with L-NAME–induced hypertensive renal disease in FHH congenic strains crossed with ACI or BN rats and narrowed the region locus to a 1.5∼2.4-Mbp segment on the distal arm of chromosome 1 (Jacob et al., 1995; Mattson et al., 2005, 2007; Van Dijk et al., 2005; Burke et al., 2013; Lazar et al., 2013). The Rf-1 region is homologous to a quantitative trait locus on human chromosome 10 associated with an increased incidence of diabetic nephropathy and CKD, especially in African Americans (Freedman et al., 2002; Hunt et al., 2002; Iyengar et al., 2003).
More recently, we identified a mutation in the γ-adducin (Add3) gene in the Rf-1 region that destabilizes its interaction with the actin-cytoskeleton and impairs the myogenic response of the afferent arteriole and autoregulation of renal blood flow (RBF) in FHH and Milan normotensive rats, which share this variant and are susceptible to hypertension-induced renal disease (López et al., 2006; Burke et al., 2013; Fan et al., 2020a,b). However, it remains to be determined whether the mutation in the Add3 gene is responsible for the increased susceptibility to L-NAME hypertension-induced renal disease originally linked to the Rf-1 region of FHH rats or if sequence variants in other genes in this region play the causal role. In this regard, Lazar et al. (2013) found that introgression of a 1.5-Mbp region of Rf-1 containing Sorcs1 but not Add3 in ACI 1FHH congenic strains increased proteinuria. Knockout of Sorcs1 in proximal tubular cells impaired protein trafficking and increased proteinuria in an FHH 1BN congenic strain. However, the role of Sorcs1 as the causal gene for hypertension-induced renal injury in FHH rats remains to be established since, unlike the clear loss-of-function mutation in Add3, no coding sequence variants or differences in expression of Sorcs1 were found in FHH rats versus ACI or BN rats.
The present study evaluated the hypothesis that knockout of Add3 impairs the renal vasoconstrictor response to the blockade of nitric oxide (NO) synthase (NOS) and enhances hypertension-induced renal injury after chronic administration of L-NAME plus a high-salt (HS) diet. The acute effects of L-NAME on blood and renal hemodynamics and its chronic actions on the development of hypertension and renal injury were compared in FHH 1BN congenic rats that express the wild-type Add3 gene in a 2.4-Mbp substitution of the Rf-1 region from BN rats versus FHH 1BN Add3 knockout (KO) (Add3 KO) rats to determine the role of Add3 in L-NAME–induced renal vasoconstriction and hypertension-induced renal injury.
Materials and Methods
Experimental Animals.
Experiments were performed on 12- to 15-week-old male congenic FHH 1BN-(D1Rat09-D1Rat225)/Mcwi rats that expressed the wild-type Add3 as the control strain [FHH 1BN congenic (WT)], FHH 1BN Add3 KO rats, and Sprague Dawley (SD) rats. The colonies were maintained in the Laboratory Animal Facility at the University of Mississippi Medical Center. The University of Mississippi Medical Center Institutional Animal Care and Use Committee approved all experimental protocols. The rats were maintained on Teklad 7034 (Envigo, Madison, WI) containing 0.3% NaCl and had free access to tap water before the experiment.
Blood Pressure and Renal Hemodynamic Responses to Acute Blockade of NOS with L-NAME.
These experiments were performed on WT and Add3 KO rats anesthetized with ketamine (30 mg/kg, i.m.) and Inactin (50 mg/kg, i.p.). The femoral artery and vein were cannulated to measure arterial pressure and for i.v. infusion. The left renal artery was exposed, and the kidney was denervated by applying a 10% phenol in ethanol. An ultrasonic flow probe (Transonic System Inc., Ithaca, NY) was placed around the left renal artery to measure RBF. After the surgery, a 6% solution of bovine serum albumin (BSA) in saline was given to replace surgical fluid losses and restore hematocrit to 43%∼45%. A 2% solution of fluorescein isothiocyanate (FITC)-inulin (F3272; Sigma-Aldrich, St. Louis, MO), and BSA in saline was infused at a rate of 1 ml/h per 100 g b.wt. to measure glomerular filtration rate (GFR). After a 30-minute equilibration period, urine flow, RBF, GFR, and mean arterial pressure (MAP) were measured during two 15-minute clearance periods. A bolus dose of L-NAME (10 mg/kg, i.v., N5751; Sigma-Aldrich) was given through the arterial line, and MAP, RBF, and GFR were redetermined during two 15-minute experimental periods. Then, a bolus dose of furosemide (10 mg/kg, i.v.) was given to block the influence of tubuloglomerular feedback (TGF) on the renal hemodynamic response to L-NAME.
Chronic Effect of L-NAME and a High-Salt Diet on the Development of Hypertension and Renal Injury.
Baseline protein excretion was measured overnight in a metabolic cage when rats were 12 weeks old. The rats were anesthetized with isoflurane, and a telemetry transmitter (HD-S10; Data Sciences International, St. Paul, MN) was inserted in the femoral artery for the measurement of MAP. The rats were permitted to recover for 3–5 days before the measurement of baseline MAP. Then the rats were switched to an HS diet (8.0% NaCl, TD.92012; Envigo), and L-NAME (12.5 mg/l) was added to the drinking water. Time-control WT and Add3 KO rats were maintained on the control diet and given tap water for 3 weeks. MAP and protein excretion were measured weekly. Urinary protein concentration was measured using the Bradford protein assay (5000006; Bio-Rad, Hercules, CA). At the end of the study, the rats were anesthetized using isoflurane, and kidneys were collected for histology and Western blot.
Assessment of GFR.
The rats were anesthetized using ketamine and Inactin. The femoral artery and vein were cannulated for the measurement of arterial pressure and intravenous infusions. The bladder was cannulated to collect urine. A 2% FITC-inulin and BSA solution in saline was infused intravenously at a rate of 1 ml/h per 100 g b.wt. After a 30-minute equilibration period, urine and plasma were collected during two 15-minute clearance periods. The concentration of FITC-inulin in the plasma and urine were measured using a plate reader (BioTek, Winooski, VT) at excitation and emission wavelength of 490 and 520 nm, and GFR was calculated from the clearance of FITC-inulin.
Autoregulation of RBF.
Autoregulation of RBF was compared in groups of WT and Add3 KO rats chronically treated with L-NAME and an HS diet for 3 weeks as previously described (Roman and Cowley, 1985). Time-control rats were maintained on the control diet and water alone. The rats were anesthetized with ketamine and Inactin. The femoral artery and vein were cannulated for measuring arterial pressure and i.v. infusions. RBF was measured using an ultrasonic flow probe. Renal perfusion pressure (RPP) was adjusted using clamps placed on the abdominal aorta above and below the left renal artery. After a 30-minute equilibration period, the mesenteric and celiac arteries were tied off to raise the arterial pressure, and the RBF was measured over the range of RPP from 50 to 180 mm Hg in steps of 10 mm Hg. At the end of the study, the kidney was collected for histology and Western blot experiments.
Measurement of PGC.
The rats were prepared as described for the measurement of RBF autoregulation. The left kidney was placed in a holder for micropuncture. PGC was estimated by the sum of the oncotic pressure of the plasma (π), and the stop-flow pressure (PSF) was measured from a wax-blocked proximal tubule using a servo-null micropressure device (model 900; World Precision Instruments, Sarasota, FL) as previously described (Van Dokkum et al., 1999b; Fan et al., 2020b). Plasma protein concentration was measured using a refractometer, and the oncotic pressure was determined using the Landis and Pappenheimer equation (Pappenheimer, 1963).
Histology.
The kidneys were immersion-fixed in 10% formalin and embedded in paraffin, and 3-µm sections were prepared. The sections were stained with Masson’s trichrome to quantitate the degree of glomerular injury, formation of tubular protein casts, and renal interstitial fibrosis. Thirty glomeruli per section were scored on a scale of glomerular injury score from 0 to 4, with 0 representing a normal glomerulus, 1 representing glomeruli with 1%∼25% loss of capillary area, 2 representing glomeruli with 26%∼50% loss of capillary area, 3 representing glomeruli with 51%∼75% loss of capillary area, and 4 representing glomeruli with 76%∼100% loss of capillary area (Raij et al., 1984). The degree of renal interstitial fibrosis was measured by the percentage of blue staining of collagen and fibrinogen per field using NIS-Element D 4.6 software (Nikon, Melville, NY). Protein casts were quantitated as a percentage of tubular area exhibiting red fluorescence.
Immunohistochemistry.
Paraffin sections were deparaffinized in xylene and rehydrated in 100%, 95%, and 70% ethanol. They were permeabilized by incubation with 0.1% trypsin in PBS at 37°C for 1 hour. They were subjected to epitope retrieval in 0.1% citrate buffer at 98°C for 30 minutes and then permeabilized by immersion in −20°C methanol for 5 minutes. The sections were blocked with 10% serum matching the host species of the secondary antibody. The sections were incubated with primary antibodies targeting the podocyte markers Wilm’s tumor suppressor (WT1) (1:200, sc-192; Santa Cruz, Dallas, TX) and nephrin (1:50, 20R-NP002; Fitzgerald, Acton, MA) and the capillary endothelial marker platelet/endothelial cell adhesion molecule-1 (PECAM-1) (1:200, sc-376764; Santa Cruz) at 4°C overnight. The sections were washed with PBS and incubated with Alexa Flour 488 or 555 secondary antibodies (Thermo Fisher Scientific, Waltham, MA) at 4°C for 1 hour. The sections were coverslipped using an antifade mounting medium containing 4,6-diamidino-2-phenylindole (H-1800; Vector Laboratories, Burlingame, CA). Images were taken using a Nikon Eclipse 55i microscope (Nikon) and a DS-Fi2 color camera. The number of WT1 positive podocytes was counted in individual glomeruli. The percentage of nephrin stained area was measured in each glomerulus. WT1 and nephrin staining in tubules were measured to compare uptake. PECAM-1 was costained with WT1 to highlight the endothelium and glomerular capillary area.
Western Blot.
The flash-frozen renal cortex was homogenized in radioimmunoprecipitation assay buffer (R0287; Sigma-Aldrich) supplemented with protease and phosphatase inhibitors (A32959; Thermo Fisher Scientific). The supernatant was collected after centrifugation at 9000g for 15 minutes at 4°C, aliquoted, and then electrophoresed on SDS-polyacrylamide gels. The proteins were transferred to nitrocellulose membranes with a Trans-Blot Turbo Transfer System (Bio-Rad). The membranes were blocked with 2.5% (w/v) blotting-grade blocker (1706404; Bio-Rad) and incubated with primary antibodies targeting matrix metallopeptidase (MMP) 9 (1:5000, ab76003; Abcam, Cambridge, MA), MMP2 (1:10,000, NB200-193; Novus Biologicals, Centennial, CO), transforming growth factor β1 (TGFβ1) (1:200, sc-146; Santa Cruz), E-cadherin (1:10,000, BD 610181; BD Biosciences, San Jose, CA), vimentin (1:1000, 3932; Cell Signaling, Danvers, MA), or α-smooth muscle actin (SMA) (1:1000, A5228; Sigma-Aldrich). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:2500, 2188; Cell Signaling) was used as a loading control. The membranes were incubated with horseradish peroxidase–coupled secondary antibodies (Abcam) and developed using an enhanced chemiluminescence reagent (32106; Thermo Fisher Scientific). The blots were imaged and analyzed using the Chemidoc XRS+ imaging system (Bio-Rad).
Statistical Analysis.
Mean values ± S.D. are presented. A two-way repeated-measure ANOVA followed by a Holm-Sidak test for preplanned comparisons was used to evaluate the significance of differences in corresponding values between and within groups. An unpaired Student’s t test was used to determine the significance of differences in mean values between only two groups. A P value <0.05 was considered statistically significant.
Results
Acute MAP and Renal Hemodynamic Response to L-NAME.
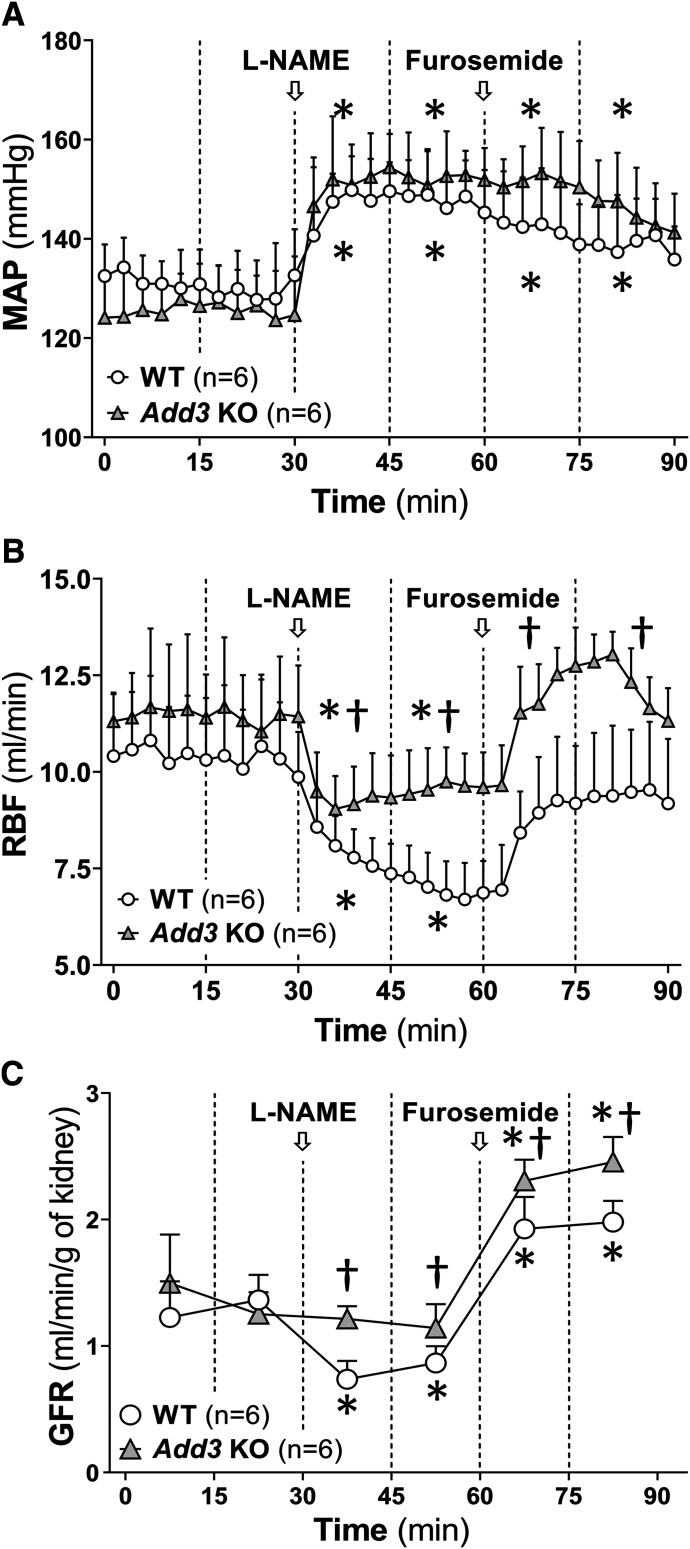
Baseline MAP, RBF, and GFR were not significantly different in WT and Add3 KO rats. Acute administration of L-NAME produced a similar 25∼30–mm Hg increase in MAP in both WT and Add3 KO rats (Fig. 1A). RBF fell by 37% in WT rats versus a 15% reduction in Add3 KO rats (Fig. 1B). GFR decreased to a greater extent by 39% in WT rats compared with 15% in Add3 KO rats (Fig. 1C). Furosemide was then given to block the contribution of TGF in the renal hemodynamic response to L-NAME. It returned RBF to values not significantly different from control in both strains without altering MAP. However, RBF was significantly greater in Add3 KO rats than in WT rats after the combined administration of L-NAME and furosemide. GFR also increased to values significantly greater than control in both strains after furosemide, and the GFR was significantly higher in Add3 KO rats than in WT rats treated with L-NAME and furosemide.
Fig. 1.
Comparison of the renal hemodynamic response to acute administration of L-NAME in FHH 1BN (WT) and FHH 1BN Add3 KO. The time course of changes in (A) MAP, (B) RBF, and (C) GFR after a bolus administration of 10 mg/kg L-NAME and in response to a subsequent bolus of 10 mg/kg furosemide. Statistical analysis was performed comparing the averages of each 15-minute clearance period indicated by the vertical dashed lines. Arrows indicate the times when L-NAME or furosemide was injected. *P < 0.05 vs. baseline value of the corresponding strain. †P < 0.05 vs. the corresponding value in WT rats.
Effects of the Chronic Administration of L-NAME and an HS Diet on Blood Pressure and Protein Excretion in SD, WT, and Add3 KO Rats.
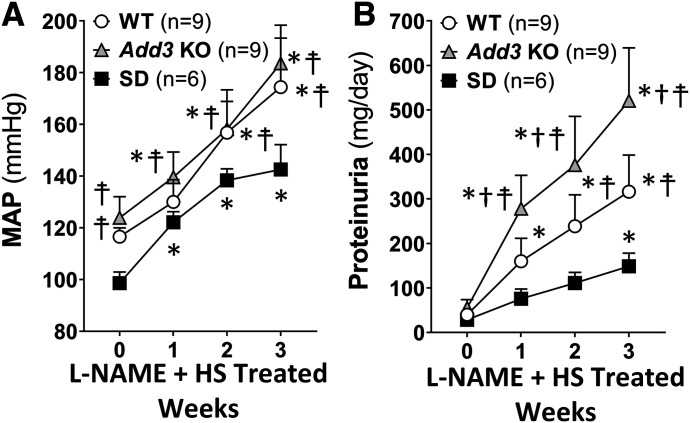
Baseline MAP were similar in SD, WT, and Add3 KO rats (Fig. 2A). MAP of SD rats increased from 98.6 ± 4.3 to 142.6 ± 9.6 mm Hg after 3 weeks of L-NAME and HS diet treatment. In contrast, MAP of WT and Add3 KO rats rose to 174.4 ± 18.9 and 183.6 ± 14.8 mm Hg, respectively. Proteinuria increased to a significantly greater extent in Add3 KO (520.5 ± 118.9 mg/day) rats than in WT rats (316.4 ± 82.3 mg/day, Fig. 2B) after the administration of L-NAME and HS diet. The increase in proteinuria was much less in SD rats (148.6 ± 29.3 mg/day) than in WT or Add3 KO rats.
Fig. 2.
Comparison of the time course of the development of hypertension and proteinuria in FHH 1BN (WT), FHH 1BN Add3 KO, and SD rats in response to L-NAME (12.5 mg/l) in drinking water and a high-salt diet. (A) The time course of development of L-NAME hypertension in WT, Add3 KO, and SD rats. (B) The time course of development of proteinuria in WT, Add3 KO, and SD rats after the development of L-NAME hypertension. The number in the parentheses indicates the number of animals per group. *P < 0.05 vs. baseline value of the corresponding strain at week 0. †P < 0.05 vs. the corresponding value in WT rats. ☨P < 0.05 vs. the corresponding value in SD rats.
Effects of L-NAME and an HS Diet on Renal Hemodynamics and Injury.
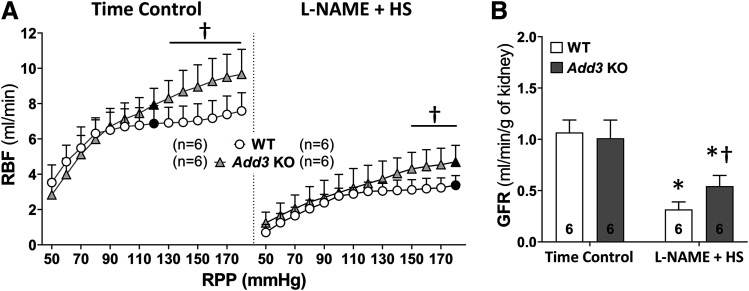
Baseline RBF (Fig. 3A, indicated by solid symbols) was similar in time-control WT and Add3 KO rats. RBF was well autoregulated in WT rats and only increased by 17% when RPP increased from 80 to 170 mm Hg. In contrast, autoregulation of RBF was significantly impaired in Add3 KO rats, and RBF increased by 59% in response to the same change in RPP (Fig. 3A).
Fig. 3.
Comparison of the renal hemodynamics in FHH 1BN (WT) and FHH 1BN Add3 KO rats treated with L-NAME and an HS diet for 3 weeks vs. time controls. (A) Comparison of autoregulation of RBF in WT and Add3 KO rats treated with L-NAME for 3 weeks in comparison with time-control groups. Solid symbols indicate the baseline RBF corresponding to the measured MAP within a group. (B) GFR of WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. The number in the bar indicates the number of rats studied per group. †P < 0.05 vs. the corresponding value in WT rats. *P < 0.05 vs. the time-control group within the corresponding strain.
Baseline RBF fell by 51% and 41% in WT and Add3 KO rats treated with L-NAME and an HS diet (Fig. 3A, indicated by solid symbols) despite the 50–mm Hg increase in MAP in both strains. RBF was still well autoregulated in the hypertensive WT rats and increased by 17% when pressure was altered from 110 to 180 mm Hg. Autoregulation of RBF remained impaired in Add3 KO rats after the development of L-NAME hypertension. RBF increased by 46% in response to the same change in RPP. GFR decreased from 1.1 ± 0.1 to 0.3 ± 0.1 ml/min per gram kidney weight in WT rats versus a lesser reduction from 1.0 ± 0.2 to 0.5 ± 0.1 ml/min per gram kidney weight in Add3 KO rats chronically treated with L-NAME and an HS diet (Fig. 3B). Baseline PGC was similar in time-control WT and Add3 KO rats. PGC was significantly higher in both WT and Add3 KO rats after 3 weeks of treatment with L-NAME and an HS diet versus their respective time-control groups, and PGC rose to a greater extent in Add3 KO rats than WT rats in L-NAME–treated groups (Table 1).
TABLE 1.
Comparison of the glomerular capillary pressure in FHH 1BN (WT) and FHH 1BN Add3 KO rats treated with L-NAME and an HS diet vs. time-control groups
| Treatment | Strain | n of Rats | n of PSF Measured | PSF (mm Hg) | π (mm Hg) | PGC (mm Hg) |
|---|---|---|---|---|---|---|
| Time control | WT | 6 | 43 | 29.8 ± 2.2 | 20.3 | 50.1 ± 2.2 |
| Add3 KO | 6 | 40 | 30.9 ± 3.8 | 20.3 ± 1.7 | 51.2 ± 4.3 | |
| L-NAME + HS | WT | 4 | 30 | 35.7 ± 5.1* | 20.6 ± 0.5 | 56.2 ± 4.9* |
| Add3 KO | 4 | 26 | 45.5 ± 2.9† * | 18.9 ± 1.2 | 64.3 ± 2.7† * |
Mean ± S.D. (if available). Statistical analysis was performed using Student’s unpaired t test. PGC, glomerular capillary pressure; π, systemic oncotic pressure.
P < 0.05 vs. corresponding values in WT rats.
P < 0.05 vs. the control values within the strain.
Comparison of the Renal Injury in WT and Add3 KO Rats after the Development of L-NAME Hypertension.
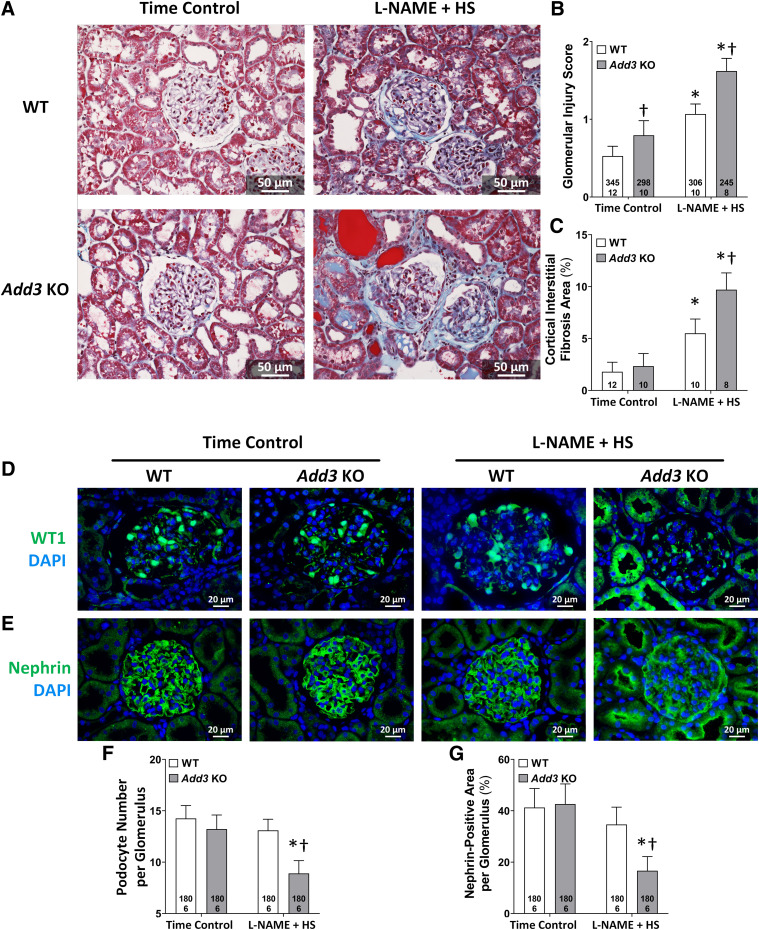
The kidneys of time-control WT rats exhibited very little glomerulosclerosis or renal fibrosis at the age of 15 weeks. Glomeruli of the time-control Add3 KO rats exhibited some mesangial matrix expansion and occluded capillaries (Fig. 4A), and the glomerular injury score was slightly but significantly greater in Add3 KO rats than that seen in WT rats (Fig. 4B). The degree of interstitial fibrosis was not significantly different between the strains in time-control groups (Fig. 4C). WT rats exhibited increased mesangial matrix expansion, loss of capillary area, and renal interstitial fibrosis after the 3-week treatment with L-NAME and an HS diet. The degree of glomerular injury and fibrosis increased to a greater extent and was significantly higher in Add3 KO rats than in WT rats 3 weeks after the development of L-NAME hypertension.
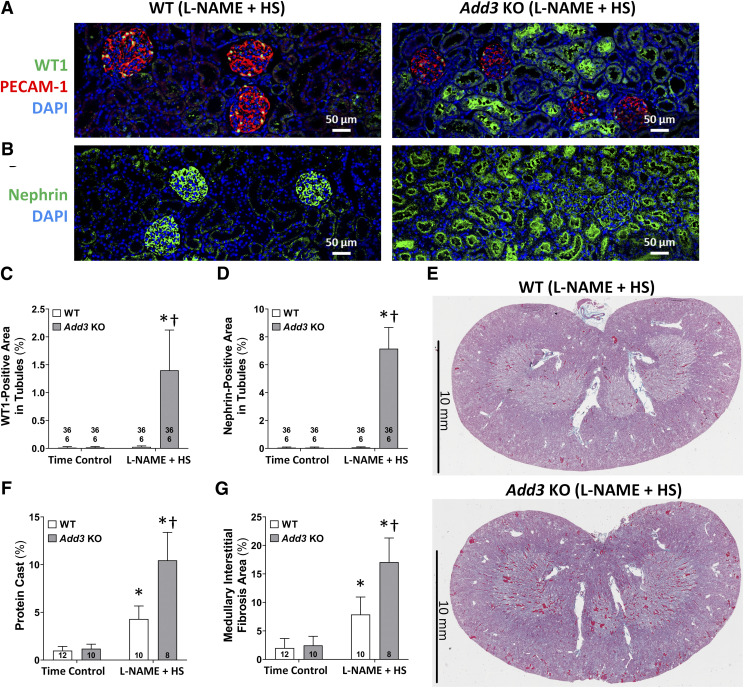
Fig. 4.
Comparison of the degree of glomerular injury and renal interstitial fibrosis in FHH 1BN (WT) and FHH 1BN Add3 KO rats treated with L-NAME and an HS diet for 3 weeks vs. time controls. (A) Representative images of Masson’s trichrome–stained renal sections from WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. (B) Glomerular injury scores of WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. (C) Quantitation of cortical interstitial fibrosis in WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. Representative images of immunostained glomeruli with (D) WT1 and (E) nephrin antibodies in WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. Quantitation of (F) WT1-positive podocytes and (G) nephrin expression in a glomerulus of WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. The number in the bar indicates the number of glomeruli and rats studied per group. Thirty glomeruli were counted for each rat. †P < 0.05 vs. the corresponding value in WT rats. *P < 0.05 vs. the time-control group within the corresponding strain.
Immunohistochemical analysis with antibodies targeting WT1 (Fig. 4D) and nephrin (Fig. 4E) was performed to examine the effects of L-NAME on podocyte loss in WT and Add3 KO rats. The number of podocytes (Fig. 4F) and glomerular nephrin area (Fig. 4G) were not significantly different in the glomeruli of WT versus Add3 KO rats in time-control groups. After 3-week exposure to L-NAME and an HS diet, the number of podocytes per glomerulus and the percentage of glomerular nephrin staining were not significantly altered in WT rats. However, the number of WT1 positive podocytes and glomerular nephrin area fell by 33% and 61%, respectively, in Add3 KO rats. The loss of podocytes and nephrin expression was accompanied by increased staining of WT1 and nephrin in the proximal tubules of Add3 KO rats. WT1 and nephrin staining was not seen in the tubules of L-NAME–treated WT rats (Fig. 5, A and B, left panels) but accounted for 1.4% ± 0.7% and 7.2% ± 1.5% of the tubular area in Add3 KO rats (Fig. 5, A and B, right panels).
Fig. 5.
Immunostaining of WT1 and nephrin in the renal cortex and evaluation of protein cast formation and renal interstitial fibrosis in FHH 1BN (WT) rats and FHH 1BN Add3 KO rats treated with L-NAME and an HS diet for 3 weeks in comparison with time controls. Representative images at low magnification of sections immunostained for (A) WT1 and (B) nephrin in the renal cortex of WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. PECAM-1 was stained to outline the endothelium of the glomerulus. Quantitation of immunostained (C) WT1 and (D) nephrin in the tubules of WT and Add3 KO rats treated with L-NAME for 3 weeks vs. time-control groups. The number in the bar indicates the number of fields and rats studied per group. Six fields were randomly chosen within the cortex of each rat. (E) Representative images of the kidney of L-NAME–treated WT and Add3 KO rats stained with Masson’s trichrome. Blue indicates areas of fibrosis, and red indicates tubular protein casts. Quantitation of (F) protein casts are expressed as the percentage of the red fluorescent area, and (G) medullary interstitial fibrosis is expressed as the percentage of the area stained blue. †P < 0.05 vs. the corresponding value in WT rats. *P < 0.05 vs. the vehicle-treated group within the corresponding strain.
The degree of protein casts in the medullary tubules and renal interstitial fibrosis increased significantly in WT rats (Fig. 5E, upper panel; quantitation in Fig. 5, F and G) 3 weeks after the administration of L-NAME versus the time-control groups. The increase in protein cast area and renal interstitial fibrosis was also significantly greater in the kidney of Add3 KO rats in comparison with WT rats treated with L-NAME and an HS diet for 3 weeks (Fig. 5E, lower panel).
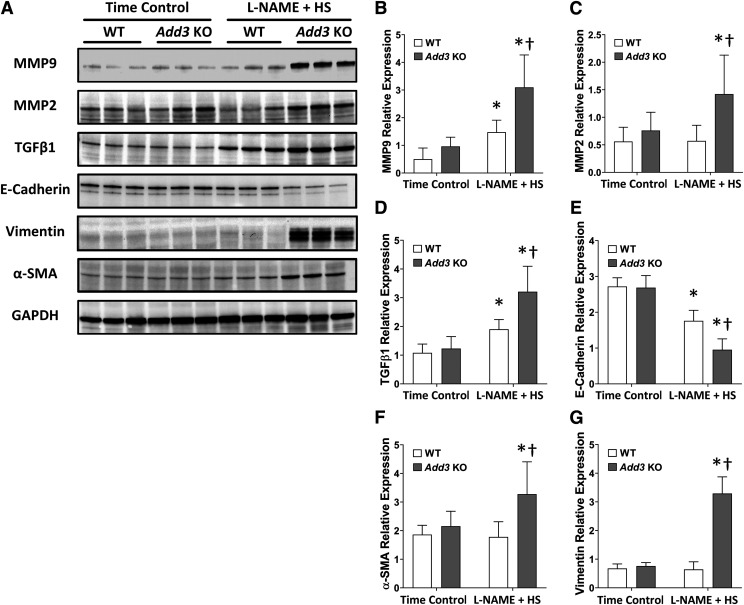
A comparison of the effects of L-NAME on the expression of MMP9, MMP2, and TGFβ1 in the renal cortex of WT and Add3 KO rats are presented in Fig. 6. The expression of these profibrotic markers was not different in the renal cortex of time-control groups. The expression of MMP9 and TGFβ1 increased in WT rats after 3 weeks of L-NAME hypertension. The expression of MMP9, MMP2, and TGFβ1 increased to a greater extent in L-NAME–treated Add3 KO rats than in the WT rats. The expression of the tubular tight junction marker E-cadherin and the mesenchymal markers vimentin, and α-SMA was similar in the time-control WT and Add3 KO rats. After a 3-week treatment with L-NAME and an HS diet, the expression of E-cadherin fell, whereas the level of the mesenchymal markers remained unaltered in WT rats. However, the expression of tight junction protein E-cadherin decreased to a greater extent in Add3 KO rats in association with elevated expression of mesenchymal markers, vimentin, and α-SMA.
Fig. 6.
Western blots of the expression of MMP-TGFβ1 pathway and EMT markers in FHH 1BN (WT) and FHH 1BN Add3 KO rats treated with L-NAME and an HS diet for 3 weeks vs. time controls. (A) Western blots of the expression of MMP-TGFβ1 pathway markers, including MMP9, MMP2, and TGFβ1, the tight junction marker E-cadherin and mesenchymal markers vimentin and α-SMA as well as the load control GAPDH. Densitometric analyses of the expression of (B) MMP9, (C) MMP2, (D) TGFβ1, (E) E-cadherin, (F) vimentin, and (G) α-SMA relative to the expression of GAPDH. Mean values ± S.D. from six rats per group are presented. †P < 0.05 vs. the corresponding value in WT rats. *P < 0.05 vs. the vehicle-treated group within the corresponding strain.
Discussion
Chromosomal substitution studies confirmed that one or more genes in the Rf-1 region are associated with L-NAME–induced hypertensive renal disease in congenic FHH rats crossed with ACI or BN rats (Jacob et al., 1995; Mattson et al., 2005, 2007; Van Dijk et al., 2005). More recently, we identified a loss-of-function mutation in Add3 that is responsible for impaired myogenic response in FHH rats (Fan et al., 2017, 2020a,b). However, it remains to be determined whether this same mutation is responsible for the increased susceptibility to renal injury in FHH rats, especially using the L-NAME hypertensive model, which elevates renal vascular resistance similar to spontaneously hypertensive rats, angiotensin-II hypertensive models, and patients with salt-resistant, essential hypertension that are more resistant to renal injury than salt-sensitive models (Griffin, 2017).
Acute blockade of NOS produces vasoconstriction that elevates MAP and lowers RBF and GFR. The increase in renal vascular resistance is thought to be mediated by autoregulatory responses (Zatz and Baylis, 1998) secondary to enhanced myogenic response and TGF-mediated constriction of the afferent arteriole triggered by pressure natriuresis and increased delivery of sodium to macula densa. We found that knockout of Add3 did not alter the MAP response to L-NAME (Fig. 1A), but RBF and GFR fell to a greater extent in WT rats than in Add3 KO rats (Fig. 1, B and C). This finding suggests that loss of ADD3 reduces the renal vasoconstrictor response to NOS blockade.
We then determined the role of TGF to the renal vasoconstrictor response to L-NAME in WT and Add3 KO rats using furosemide. Furosemide blocks TGF and increases renin release by inhibiting sodium reabsorption in macula densa (Carlström et al., 2015). Our finding that furosemide reversed the L-NAME–induced fall in RBF and GFR in both strains is consistent with the view that NO produced by macula densa tonically attenuates TGF responsiveness (Wei et al., 2019) and that activation of TGF plays an important role in renal vasoconstrictor response to NOS blockade (Zhang et al., 2019b). Moreover, the increases in RBF and GFR above the control in L-NAME–treated Add3 KO rats given furosemide are likely due to the loss of the myogenic response in this strain. In contrast, RBF did not increase above control in L-NAME–treated WT rats with intact myogenic response given furosemide.
Previous studies mapping the genes for L-NAME–induced renal disease in FHH congenic strains did not consider whether the results were influenced by strain differences in blood pressure in response to L-NAME. For example, Mattson et al. (2007) reported that MAP was 30 mm Hg higher in FHH rats than in BN rats after the same dose of L-NAME and an HS diet used in the present study. Others reported a higher dose of L-NAME was needed to induce the same degree of hypertension in ACI versus FHH rats (Van Dokkum et al., 1998, 2000). In the present study, chronic administration of L-NAME and an HS diet increased MAP similarly in WT, Add3 KO, and SD rats (Fig. 2A). MAP was lower in L-NAME–treated SD rats than WT rats. However, this is likely due to a 20–mm Hg difference in baseline MAP in SD rats versus WT and Add3 KO rats on the FHH 1BN genetic background.
Proteinuria was lower in SD rats than in WT and Add3 KO rats after L-NAME plus an HS diet, which is consistent with previous reports that FHH rats are more susceptible to hypertension-induced renal diseases than other strains (Simons et al., 1993a; Van Dokkum et al., 1997, 1998; Fan et al., 2020a). Indeed, five genomic regions have been linked to renal disease in FHH rats. WT and Add3 KO rats are genetically identical at all of these loci except for Add3, whereas SD rats do not carry any of these variants. The present results also indicate that proteinuria was 2-fold higher in L-NAME–treated Add3 KO rats than in WT rats throughout the study. The degree of kidney injury was also greater in Add3 KO rats than in WT rats. These results establish that the loss-of-function mutation in Add3 is the causal gene for the increased susceptibility to L-NAME hypertension-induced renal injury in FHH rats as previously identified in the Rf-1 region. They are also consistent with our previous finding that knockout of Add3 transiently enhanced proteinuria during the development of DOCA-salt hypertension (Fan et al., 2020b). However, sustained renoprotective effects of the wild-type ADD3 could not be demonstrated using the DOCA-salt model because the renal hypertrophy associated with uninephrectomy and the suppression of the renin-angiotensin system has the same effect as knockout of Add3 to impair RBF autoregulation and produces renal injury in the control rats.
The present study also examined the role of renal hemodynamics changes in enhancing renal injury in L-NAME–treated Add3 KO rats. The baseline RBF of time-control WT and Add3 KO rats on a normal-salt diet was not different at their MAP of 110∼120 mm Hg. As expected, RBF was autoregulated in time-control WT rats but was impaired in Add3 KO rats (Fig. 3A). As typically seen in many models of chronic hypertension-induced renal disease (Bidani et al., 2009), RBF and GFR fell in both strains, and RBF autoregulatory curves were shifted to higher pressures 3 weeks after the development of L-NAME hypertension. However, RBF and GFR were higher in Add3 KO than WT rats, with greater proteinuria and renal injury. Add3 KO rats also exhibited elevated PGC, which is likely a consequence of impaired myogenic response of the afferent arteriole.
The increase in PGC in L-NAME–treated Add3 KO rats would be expected to distend glomerular capillaries and promote podocyte detachment. This prediction was confirmed by the decreased number of WT1 positive podocytes and glomerular nephrin expression (Fig. 4, D and E), whereas WT1 and nephrin reabsorbed in proximal tubules were elevated (Fig. 5, A and B). These results suggest that increased transmission of pressure to glomeruli promotes podocyte effacement and damages the glomerular filtration barrier, which is associated with more severe proteinuria in Add3 KO rats (Fig. 2B).
Glomerular hyperfiltration and excessive protein delivery to proximal tubules promote EMT and renal interstitial fibrosis (Dahly-Vernon et al., 2005; Bolbrinker et al., 2006; Williams et al., 2011a). Cheng and Lovett, (2003) and Cheng et al. (2006) presented evidence that increases in renal MMP2 level disrupt tubular basement membranes, and Zeisberg et al. (2001) reported that TGFβ1 stimulates the proliferation of fibroblasts and EMT. The present findings that the renal expression of MMP2 and 9, TGFβ1, and EMT markers were elevated to a greater extent in L-NAME–treated Add3 KO rats than in WT rats are consistent with a role for protein overload and MMPs in enhancing the renal interstitial fibrosis in the Add3 KO rats.
Overall, the present study supports the view that impaired myogenic response in Add3 KO rats attenuates the renal vasoconstrictor response to L-NAME and contributes to the increased PGC and podocyte loss and the rapid development of proteinuria and glomerulosclerosis, which is similar to that seen in genetic salt-sensitive hypertension and some diabetic models in which autoregulation of RBF is impaired (Burke et al., 2014).
The link between ADD3 variants and CKD in humans remains tenuous. The Rf-1 region is homologous to a quantitative trait locus associated with end-stage renal diseases (Freedman et al., 2002; Hunt et al., 2002; Wuttke et al., 2019) and diabetic nephropathy (Iyengar et al., 2003) on human chromosome 10. There is also a rare variant in ADD3 associated with cognitive dysfunction and nephrotic syndrome. However, the most recent genome-wide association study (GWAS) meta-analysis for CKD suggested that ADD3 is 5∼6 Mbp from the closest-linked genetic marker (Wuttke et al., 2019). On the other hand, the Genome Aggregation Database (https://macarthurlab.org/2019/10/16/gnomad-v3-0/) indicates 492 nonsynonymous variants in ADD3, with 227 associated with the disease. All of these are rare variants (frequency <1%) that were excluded in the previous GWAS of CKD. Thus, additional GWASs that pool results from subjects with damaging genotypes are needed to discern whether rare variants in ADD3 increase the susceptibility to hypertension or diabetic nephropathy.
The results of the present study and others (Bidani et al., 2009) highlight that impaired autoregulation of RBF and elevated PGC are the driving force for renal injury in different hypertensive and diabetic models, which provides insights for the development of new therapeutic approaches to prevent the progression of CKD. In this regard, we have reported that enhanced large conductance potassium channel activity in FHH rats and administration of iberiotoxin rescued the impaired myogenic response (Pabbidi and Roman, 2017). We also confirmed that the myogenic response is dependent on the phosphorylation activity of extracellular signal–related kinase 1/2 and protein kinase C, in which knockout of their phosphatase (Zhang et al., 2019a) or administration of the phosphatase inhibitor (Murphy et al., 2002) promotes myogenic response. Similarly, administration of a 20-hydroxyeicosatetraenoic acid agonist or protein kinase C activators increases myogenic tone and potentially may be renoprotective. We also identified upregulation of the expression of proinflammatory cytokines and MMPs as downstream effectors of impaired renal hemodynamics, and they are potential druggable targets to prevent the development and progression of CKD (Williams et al., 2011b).
Acknowledgments
We thank Goldie M. Faircloth and Baoying Zheng for maintaining animal colonies.
Abbreviations
- ACI
August Copenhagen Irish
- Add3
γ-adducin
- BN
Brown Norway
- BSA
bovine serum albumin
- CKD
chronic kidney disease
- DOCA
deoxycorticosterone acetate
- EMT
endothelial-mesenchymal transition
- FHH
fawn-hooded hypertensive
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFR
glomerular filtration rate
- GWAS
genome-wide association study
- KO
knockout
- L-NAME
NG-nitro-L-arginine methyl ester
- MAP
mean arterial pressure
- MMP
matrix metallopeptidase
- NO
nitric oxide
- NOS
NO synthase
- PECAM-1
platelet/endothelial cell adhesion molecule-1
- PGC
glomerular capillary pressure
- PSF
stop-flow pressure
- RBF
renal blood flow
- Rf
renal failure
- RPP
renal perfusion pressure
- SD
Sprague Dawley
- SMA
smooth muscle actin
- Sorcs1
sortilin-related vacuolar protein sorting protein 10 domain containing receptor 1
- TGF
tubuloglomerular feedback
- TGFβ1
transforming growth factor β1
- WT
FHH 1BN congenic
- WT1
Wilm’s tumor suppressor
Authorship Contributions
Participated in research design: L. Fan, F. Fan, Roman.
Conducted experiments: L. Fan, Gao, Liu, Jefferson.
Performed data analysis: L. Fan, Roman.
Wrote or contributed to the writing of the manuscript: L. Fan, Roman.
Footnotes
This work was supported in part from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK104184], National Heart, Lung, and Blood Institute [Grant HL138685], National Institute on Aging [Grants AG057842 and AG060049], and National Institute of General Medical Sciences [Grant P20-GM104357].
Disclosure
No author has an actual or perceived conflict of interest with the contents of this article.
References
- Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R (2009) Protective importance of the myogenic response in the renal circulation. Hypertension 54:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbrinker J, Markovic S, Wehland M, Melenhorst WB, van Goor H, Kreutz R (2006) Expression and response to angiotensin-converting enzyme inhibition of matrix metalloproteinases 2 and 9 in renal glomerular damage in young transgenic rats with renin-dependent hypertension. J Pharmacol Exp Ther 316:8–16. [DOI] [PubMed] [Google Scholar]
- Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ (1996) Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12:44–51. [DOI] [PubMed] [Google Scholar]
- Burke M, Pabbidi M, Fan F, Ge Y, Liu R, Williams JM, Sarkis A, Lazar J, Jacob HJ, Roman RJ (2013) Genetic basis of the impaired renal myogenic response in FHH rats. Am J Physiol Renal Physiol 304:F565–F577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M, Pabbidi MR, Farley J, Roman RJ (2014) Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlström M, Wilcox CS, Arendshorst WJ (2015) Renal autoregulation in health and disease. Physiol Rev 95:405–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Lovett DH (2003) Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol 162:1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH (2006) Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 20:1898–1900. [DOI] [PubMed] [Google Scholar]
- Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ (2005) Transforming growth factor-β, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45:643–648. [DOI] [PubMed] [Google Scholar]
- de Keijzer MH, Provoost AP, Molenaar JC (1988) Glomerular hyperfiltration in hypertensive fawn-hooded rats. Ren Physiol Biochem 11:103–108. [DOI] [PubMed] [Google Scholar]
- Fan F, Geurts AM, Pabbidi MR, Ge Y, Zhang C, Wang S, Liu Y, Gao W, Guo Y, Li L, et al. (2020a) A mutation in γ-adducin impairs autoregulation of renal blood flow and promotes the development of kidney disease. J Am Soc Nephrol 31:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Pabbidi MR, Ge Y, Li L, Wang S, Mims PN, Roman RJ (2017) Knockdown of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. Am J Physiol Renal Physiol 312:F971–F981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Gao W, Nguyen BV, Jefferson JR, Liu Y, Fan F, Roman RJ (2020b) Impaired renal hemodynamics and glomerular hyperfiltration contribute to hypertension-induced renal injury. Am J Physiol Renal Physiol 319:F624–F635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BI, Rich SS, Yu H, Roh BH, Bowden DW (2002) Linkage heterogeneity of end-stage renal disease on human chromosome 10. Kidney Int 62:770–774. [DOI] [PubMed] [Google Scholar]
- Griffin KA (2017) Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension 70:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Hasstedt SJ, Coon H, Camp NJ, Cawthon RM, Wu LL, Hopkins PN (2002) Linkage of creatinine clearance to chromosome 10 in Utah pedigrees replicates a locus for end-stage renal disease in humans and renal failure in the fawn-hooded rat. Kidney Int 62:1143–1148. [DOI] [PubMed] [Google Scholar]
- Iyengar SK, Fox KA, Schachere M, Manzoor F, Slaughter ME, Covic AM, Orloff SM, Hayden PS, Olson JM, Schelling JR, et al. (2003) Linkage analysis of candidate loci for end-stage renal disease due to diabetic nephropathy. J Am Soc Nephrol 14(7 Suppl 2):S195–S201. [DOI] [PubMed] [Google Scholar]
- Jacob HJ, Brown DM, Bunker RK, Daly MJ, Dzau VJ, Goodman A, Koike G, Kren V, Kurtz T, Lernmark A, et al. (1995) A genetic linkage map of the laboratory rat, Rattus norvegicus. Nat Genet 9:63–69. [DOI] [PubMed] [Google Scholar]
- Kreisberg JI, Karnovsky MJ (1978) Focal glomerular sclerosis in the fawn-hooded rat. Am J Pathol 92:637–652. [PMC free article] [PubMed] [Google Scholar]
- Kuijpers MH, de Jong W (1987) Relationship between blood pressure level, renal histopathological lesions and plasma renin activity in fawn-hooded rats. Br J Exp Pathol 68:179–187. [PMC free article] [PubMed] [Google Scholar]
- Kuijpers MH, Gruys E (1984) Spontaneous hypertension and hypertensive renal disease in the fawn-hooded rat. Br J Exp Pathol 65:181–190. [PMC free article] [PubMed] [Google Scholar]
- Lazar J, O’Meara CC, Sarkis AB, Prisco SZ, Xu H, Fox CS, Chen M-H, Broeckel U, Arnett DK, Moreno C, et al. (2013) SORCS1 contributes to the development of renal disease in rats and humans. Physiol Genomics 45:720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López B, Ryan RP, Moreno C, Sarkis A, Lazar J, Provoost AP, Jacob HJ, Roman RJ (2006) Identification of a QTL on chromosome 1 for impaired autoregulation of RBF in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol 290:F1213–F1221. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW Jr, Jacob HJ (2007) Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol 293:F1905–F1914. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Kunert MP, Roman RJ, Jacob HJ, Cowley AW Jr (2005) Substitution of chromosome 1 ameliorates L-NAME hypertension and renal disease in the fawn-hooded hypertensive rat. Am J Physiol Renal Physiol 288:F1015–F1022. [DOI] [PubMed] [Google Scholar]
- Murphy TV, Spurrell BE, Hill MA (2002) Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways. Clin Exp Pharmacol Physiol 29:612–619. [DOI] [PubMed] [Google Scholar]
- Pabbidi MR, Roman RJ (2017) Elevated K+ channel activity opposes vasoconstrictor response to serotonin in cerebral arteries of the Fawn Hooded Hypertensive rat. Physiol Genomics 49:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer LE Jr (1963) Exchange of substances through capillary walls, in Handbook of Physiology (Hamilton WF ed) vol 2, pp 961–1034, American Physiological Society, Washington, DC. [Google Scholar]
- Raij L, Azar S, Keane W (1984) Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26:137–143. [DOI] [PubMed] [Google Scholar]
- Rangel-Filho A, Lazar J, Moreno C, Geurts A, Jacob HJ (2013) Rab38 modulates proteinuria in model of hypertension-associated renal disease. J Am Soc Nephrol 24:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ, Cowley AW Jr (1985) Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol 248:F190–F198. [DOI] [PubMed] [Google Scholar]
- Shiozawa M, Provoost AP, van Dokkum RP, Majewski RR, Jacob HJ (2000) Evidence of gene-gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J Am Soc Nephrol 11:2068–2078. [DOI] [PubMed] [Google Scholar]
- Simons JL, Provoost AP, Anderson S, Rennke HG, Troy JL, Brenner BM (1994) Modulation of glomerular hypertension defines susceptibility to progressive glomerular injury. Kidney Int 46:396–404. [DOI] [PubMed] [Google Scholar]
- Simons JL, Provoost AP, Anderson S, Troy JL, Rennke HG, Sandstrom DJ, Brenner BM (1993a) Pathogenesis of glomerular injury in the fawn-hooded rat: early glomerular capillary hypertension predicts glomerular sclerosis. J Am Soc Nephrol 3:1775–1782. [DOI] [PubMed] [Google Scholar]
- Simons JL, Provoost AP, De Keijzer MH, Anderson S, Rennke HG, Brenner BM (1993b) Pathogenesis of glomerular injury in the fawn-hooded rat: effect of unilateral nephrectomy. J Am Soc Nephrol 4:1362–1370. [DOI] [PubMed] [Google Scholar]
- Van Dijk SJ, Specht PA, Lazar J, Jacob HJ, Provoost AP (2005) Renal damage susceptibility and autoregulation in RF-1 and RF-5 congenic rats. Nephron, Exp Nephrol 101:e59–e66. [DOI] [PubMed] [Google Scholar]
- Van Dokkum RP, Alonso-Galicia M, Provoost AP, Jacob HJ, Roman RJ (1999a) Impaired autoregulation of renal blood flow in the fawn-hooded rat. Am J Physiol 276:R189–R196. [DOI] [PubMed] [Google Scholar]
- van Dokkum RP, Jacob HJ, Provoost AP (1997) Difference in susceptibility of developing renal damage in normotensive fawn-hooded (FHL) and August x Copenhagen Irish (ACI) rats after N(ω)-nitro-L-arginine methyl ester induced hypertension. Am J Hypertens 10:1109–1116. [DOI] [PubMed] [Google Scholar]
- Van Dokkum RP, Jacob HJ, Provoost AP (1998) Genetic differences define severity of renal damage after L-NAME-induced hypertension in rats. J Am Soc Nephrol 9:363–371. [DOI] [PubMed] [Google Scholar]
- van Dokkum RP, Jacob HJ, Provoost AP (2000) Blood pressure and the susceptibility to renal damage after unilateral nephrectomy and L-NAME-induced hypertension in rats. Nephrol Dial Transplant 15:1337–1343. [DOI] [PubMed] [Google Scholar]
- van Dokkum RP, Sun C-W, Provoost AP, Jacob HJ, Roman RJ (1999b) Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol 276:R855–R863. [DOI] [PubMed] [Google Scholar]
- Verseput GH, Braam B, Provoost AP, Koomans HA (1998) Tubuloglomerular feedback and prolonged ACE-inhibitor treatment in the hypertensive fawn-hooded rat. Nephrol Dial Transplant 13:893–899. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhang J, Jiang S, Wang L, Persson AEG, Liu R (2019) High-protein diet-induced glomerular hyperfiltration is dependent on neuronal nitric oxide synthase β in the macula densa via tubuloglomerular feedback response. Hypertension 74:864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Burke M, Lazar J, Jacob HJ, Roman RJ (2011a) Temporal characterization of the development of renal injury in FHH rats and FHH.1BN congenic strains. Am J Physiol Renal Physiol 300:F330–F338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Zhang J, North P, Lacy S, Yakes M, Dahly-Vernon A, Roman RJ (2011b) Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. Am J Physiol Renal Physiol 300:F983–F998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M, Tin A, Wang L, Chu AY, Hoppmann A, et al. Lifelines Cohort Study; V. A. Million Veteran Program (2019) A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51:957–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo NC, O’Meara CC, Bonomo JA, Veth KN, Tomar R, Flister MJ, Drummond IA, Bowden DW, Freedman BI, Lazar J, et al. (2015) Shroom3 contributes to the maintenance of the glomerular filtration barrier integrity. Genome Res 25:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz R, Baylis C (1998) Chronic nitric oxide inhibition model six years on. Hypertension 32:958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Bonner G, Maeshima Y, Colorado P, Müller GA, Strutz F, Kalluri R (2001) Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol 159:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, He X, Murphy SR, Zhang H, Wang S, Ge Y, Gao W, Williams JM, Geurts AM, Roman RJ, et al. (2019a) Knockout of dual-specificity protein phosphatase 5 protects against hypertension-induced renal injury. J Pharmacol Exp Ther 370:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R (2019b) Macula densa SGLT1-NOS1-tubuloglomerular feedback pathway, a new mechanism for glomerular hyperfiltration during hyperglycemia. J Am Soc Nephrol 30:578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]