Abstract
Introduction and Objective
Ivermectin (IVM) and doxycycline (DOXY) have demonstrated in-vitro activity against SARS-CoV-2, and have a reasonable safety profile. The objective of this systematic review was to explore the evidence in the literature on the safety and efficacy of their use as monotherapy and combination therapy in COVID-19 management.
Methods
After prospectively registering the study protocol with the Open Science Framework, we searched PubMed, Google Scholar, clinicaltrials.gov, various pre-print servers and reference lists for relevant records published until 16 February, 2021 using appropriate search strategies. Baseline features and data pertaining to efficacy and safety outcomes were extracted separately for IVM monotherapy, DOXY monotherapy, and IVM + DOXY combination therapy. Methodological quality was assessed based on the study design.
Results
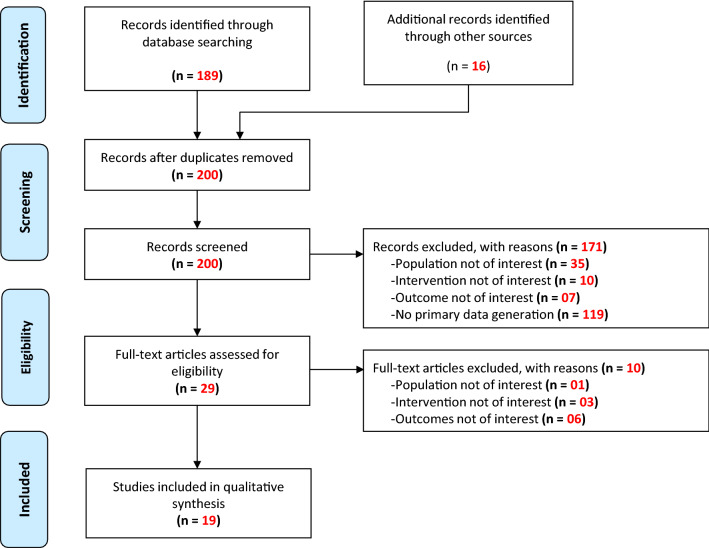
Out of 200 articles screened, 19 studies (six retrospective cohort studies, seven randomised controlled trials, five non-randomised trials, one case series) with 8754 unique patients with multiple stages of COVID-19 were included; four were pre-prints and one was an unpublished clinicaltrials.gov document. The comparator was standard care and ‘hydroxychloroquine + azithromycin’ in seven and three studies respectively, and two studies were placebo controlled; six studies did not have a comparator. IVM monotherapy, DOXY monotherapy and IVM + DOXY were explored in eight, five and five studies, respectively; one study compared IVM monotherapy and IVM + DOXY with placebo. While all studies described efficacy, the safety profile was described in only six studies. Efficacy outcomes were mixed with some studies concluding in favour of the intervention and some studies displaying no significant benefit; barring one study that described 9/183 patients with erosive esophagitis and non-ulcer dyspepsia with IVM + DOXY (without causality assessment details), there were no new safety signals of concern with any of the three interventions considered. The quality of studies varied widely, with five studies having a ‘good’ methodological quality.
Conclusions
Evidence is not sufficiently strong to either promote or refute the efficacy of IVM, DOXY, or their combination in COVID-19 management.
Systematic Review Protocol Registration Details
Open Science Framework: https://osf.io/n7r2j.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01066-y.
Key Points
| As of February 2021, apart from dexamethasone, no other drug or drug combination has proven to be unequivocally effective in reducing mortality due to COVID-19 |
| After analysing 19 different research papers that have described the outcomes of usage of ivermectin and doxycycline, either alone or together, we have observed that the evidence is not strong to either approve or disapprove the usage of these two drugs for COVID-19 treatment |
| Given that both these drugs are inexpensive, safe and have a potential role in the COVID-19 management, further studies are required to explore this in detail. |
Introduction
As of 5 December, 2020, the COVID-19 pandemic that started in November 2019 has affected over 64.6 million patients worldwide, leading to global deaths of over 1.5 million people. At present, the country most affected by the pandemic is the USA, both in terms of the number of cases (13.76 million) and the number of deaths (271,233) [1]. The onset of winter and a resurgence in the number of new cases hailing the arrival of a ‘second wave’ of the pandemic [2] further complicate matters, making the situation the most unprecedented global healthcare crisis in recent years. The news of successes with various COVID-19 vaccines appears to be the light at the end of the tunnel for COVID-19 prevention [3]. Reports of clinical improvements with drugs such as remdesivir [4] and dexamethasone [5] notwithstanding, there appears to be no drug that has unequivocally proven its efficacy and safety in reducing clinical symptoms or mortality due to COVID-19. Thus, a ‘drug of choice’ for treating COVID-19 is still an enigma.
Because new drug development is time consuming, the repurposing of drugs used for other conditions for COVID-19 management has been tried worldwide. As a result of this exercise, various drugs have gained interest as potential candidates for COVID-19 management. These drugs are expected to target different points in the disease pathophysiology, and belong to two major therapeutic categories, namely drugs inhibiting viral activity (including remdesivir, hydroxychloroquine [HCQ], favipiravir, azithromycin [AZT], lopinavir-ritonavir) and drugs modulating the antiviral immune response in the host (such as tocilizumab, interferons, corticosteroids) [6]. Among these drugs, two agents that have also been tried for COVID-19 management include the antiparasitic agent ivermectin (IVM) and the broad-spectrum tetracycline antibiotic doxycycline (DOXY).
The antiviral activity of IVM had been reported previously, wherein IVM was found to have in-vitro activity against both RNA and DNA viruses including human immunodeficiency virus type 1, influenza virus, Venezuelan equine encephalitis virus, dengue virus, yellow fever virus and Zika virus [7, 8]. In April 2020, the in-vitro activity of IVM against SARS-CoV-2 was reported: a single dose of the drug administered after 2 h of infecting cultured Vero/hSLAM cells reduced the viral load up to 5000 times within 48 h [9]. The possible antiviral mechanism of action of IVM in COVID-19 is by inhibiting transport of viral proteins in and out of the host nucleus by blocking the activity of importin α/β1 receptors, which are normally involved in the nucleo-cytoplasmic transport of multiple substrates [10]. As a part of similar drug repurposing efforts, DOXY was shown to have in-vitro activity against SARS-CoV-2 on infected Vero E6 cells [11]. The possible mechanism of antiviral action of DOXY may involve upregulation of the zinc finger antiviral protein, which binds to viral messenger RNAs and represses translation of viral RNAs [12]. Additionally, the in-vivo anti-inflammatory activity of DOXY was reported previously, which is through the capacity of DOXY to inhibit matrix metalloproteases and modulate serum levels of pro-inflammatory cytokines such as tumour necrosis factor-alpha, interleukin-6, and interleukin-8 [13]. Further, the anti-inflammatory properties of DOXY were thought to contribute to its efficacy in pulmonary inflammatory conditions including asthma, acute respiratory distress syndrome, cystic fibrosis, bronchiectasis, and chemical-induced lung damage [14]. DOXY is also proposed as an alternative to AZT for the treatment of COVID-19 in elderly patients [15]. Most importantly, both of these drugs have excellent safety profiles and are inexpensive [12, 16]. Both these drugs have been tried as combination therapy with varying degrees of success for managing parasitic infestations such as onchocerciasis [17] and bancroftian filariasis [18]. In the management of COVID-19, IVM + DOXY combination therapy theoretically has multiple merits, including a non-overlapping mechanism of action, targeting both the viral replication and pulmonary inflammation, a good tolerability profile and being economically viable. However, at the time of this writing, neither of these drugs nor their combination is approved by the US Food and Drug Administration for the management of COVID-19, and all such uses remain off-label [19].
Physician experiences of the efficacy and safety of these two drugs used alone and as a combination notwithstanding, it is required to establish whether there is actually a beneficial effect of such off-label usage in COVID-19 management. In this background, we performed the present scoping review to answer the research question: “What is the evidence available from published literature regarding the efficacy and safety of IVM and DOXY when used as monotherapy or in combination in the clinical management of patients with confirmed COVID-19?”
Materials and Methods
Study Protocol
The protocol for the scoping review was drafted in-house following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and revised by internal discussion. The final version of the protocol was registered prospectively with the Open Science Framework on 5 November, 2020, and is available from https://www.osf.io/n7r2j.
Eligibility Criteria
For identifying potentially relevant articles that answered our research question, we drafted the following eligibility criteria: ‘Population’ was all studies involving all patients with confirmed COVID-19; there was no restriction on age or sex of the patients, stage of the disease or presence of comorbidities. ‘Intervention’ included IVM and DOXY used alone or in combination for managing COVID-19; there was no restriction on dose or duration of treatment. Studies using either of these drugs in patients with COVID-19 for managing other concomitant conditions were excluded. We did not restrict studies based on the ‘comparator’, and included all studies irrespective of whether there was a comparator. ‘Outcomes’ included (1) efficacy outcomes, including impact on clinical course (duration of hospital stay, mortality, clinical progress or deterioration, requirement of oxygen or ventilatory support, and days to clinical or symptomatic recovery), and impact on viral load as determined by reverse-transcription polymerase chain reaction (RTPCR); and (2) safety outcomes, including the presence of adverse effects of any grade, and the presence of serious adverse effects. ‘Study design’ included all studies that described prospectively collected data, such as randomised controlled trials (RCTs), non-randomised trials (NRTs), cohort studies, retrospective cohort studies, observational studies, open-label studies, and case series and case reports involving at least five patients. The number of patients to be included in a case series to be eligible for inclusion in the review was amended from at least five patients in the review protocol to at least four patients after execution of the search strategy. Papers not describing primary data (such as narrative or systematic reviews, letters to editors, opinion pieces, commentaries, editorials, brief communications, news items) were excluded from our review. We also excluded studies describing non-human experiments (including in-vitro studies, in-silico experimentation) and studies involving only healthy volunteers. No restriction was applied in the search strategy with respect to date or language of publication.
Literature Search, Data Extraction and Quality Assessment
Following the eligibility criteria described above, the literature search strategy was drafted by in-house experts, which was modified through internal discussion. Using the refined search strategy, a systematic literature search was performed in PubMed/MEDLINE, Google Scholar and clinicaltrials.gov, from their inception till 16 February, 2021, using a combination of search terms and Boolean operators; the detailed PubMed search strategy is presented as Table 1 of the Electronic Supplementary Material (ESM). In addition to published studies, we also searched pre-print servers to identify potentially relevant but unpublished papers that are yet to undergo peer review.
The titles and abstracts of all retrieved articles were screened for eligibility, and after deduplication and excluding ineligible articles after providing reasons for exclusion, full texts of potentially relevant articles were assessed for including in review. We additionally hand searched the bibliographic sections of relevant articles and excluded systematic reviews to identify other eligible records that might have been missed by the database search. We excluded non-English articles from the review because our team of reviewers was proficient with the English language. Once all the eligible records were pooled, relevant data from the papers were extracted and entered into a predefined data extraction grid, after reading through the full texts of the selected studies. Extracted data included data pertaining to the details of the study (year of publication, country of the first author, study design, details of the intervention and comparator), participant details (number of patients, age, sex and patient profile), intervention details (whether monotherapy or combination therapy of IVM and DOXY, and dose details) and outcome details (efficacy and safety). For the purpose of this scoping review, no assumptions or simplifications were employed, and all results were extracted descriptively. A meta-analysis was not performed.
For assessing the methodological quality and/or risk of bias of the included studies, we used tools as per the study design. We used the RoB-2 tool for assessing the risk of bias in RCTs [20]. The official Microsoft Excel tool provided by the Cochrane foundation was used for implementing RoB-2 [21]. For NRTs, we used the ROBINS-I tool [22]. For retrospective cohort studies, we used the tool provided by the National Institutes of Health [23]. For case series, we used the evaluation tool proposed by Murad et al. in 2018 [24]. The final overall risk of bias/methodological quality of each paper was described as per the interpretation of the respective tool, without changing the terms or nomenclature. The literature search, determining articles for their inclusion in the study, data extraction and risk of bias assessment was performed by two independent authors (AD and VBN), and any disagreements were resolved by discussion and via moderation by another author (SB, SD).
Statistical Analysis
Study selection and data extraction were performed electronically in Microsoft Excel. Cohen’s kappa value for judging inter-rater reliability of study inclusion and methodological quality assessment of included papers was calculated using SPSS Version 20, with the p value of ≤ 0.05 considered statistically significant. The cut-offs for the kappa statistic were interpreted as ≤ 0.20 = slight agreement; 0.21–0.40 = fair agreement; 0.41–0.60 = moderate agreement; 0.61–0.80 = substantial agreement; 0.81–0.99 = near-perfect agreement; and 1.00 = perfect agreement [25].
Data Availability
All datasets used to derive conclusions in this study are available from the corresponding author on reasonable request.
Results
Study Selection, Baseline Characteristics
From a total of 200 records screened, 19 studies were included for data extraction and review. Figure 1 depicts the study selection process.
Fig. 1.
Study selection process
Out of the 19 included studies, six were retrospective cohort studies, seven were RCTs, five were NRTs, and one was a case series involving four patients. Four of the included studies (three RCTs and one NRT) were pre-prints. The first authors of the 19 studies came from eight different countries, of which the most frequent was Bangladesh with seven papers, followed by three papers from the USA.
Twelve of the studies involved patients with non-severe (mild or mild-to-moderate) COVID-19; three studies included hospitalised patients with COVID-19 without specifying the stage of COVID-19; one study each included patients with severe COVID-19, high-risk patients with COVID-19 in long-term care facilities, and all stages of COVID-19, and the case series described four patients with COVID-19 with comorbid conditions. The criteria for defining the severity of COVID-19 were similar in all the included studies. The intervention was IVM monotherapy in eight studies, DOXY monotherapy in five studies, and IVM + DOXY combination therapy in five studies; one study compared IVM monotherapy and IVM + DOXY combination therapy with placebo. There was no comparator in 6/19 studies; of the remaining 13 studies, seven studies had ‘standard care’, three studies had ‘combination of HCQ + AZT’ and two studies had placebo as the comparator. One study was a four-armed study with DOXY monotherapy in one arm, the remaining three arms being AZT monotherapy, combination of DOXY with nitazoxanide and combination of AZT with nitazoxanide. Standard care was not uniform in all the involved studies, owing to the novel nature of the disease and the lack of uniform treatment guidelines. Standard care was generally based on clinical discretion, which depended upon the treatment protocol established by the hospital, which was in turn based on the guidelines given by the regulatory/competent authority of the geographical area where the hospital was located. Standard care generally involved symptomatic and supportive management through the use of antipyretics, anti-histamines, antibiotics for secondary infection and other supportive measures. Some studies also mentioned having used drugs such as HCQ and AZT as a part of standard care, and some studies did not specify the nature of standard care. Out of the 8754 unique patients in the 19 included studies, 851 patients received IVM monotherapy, 288 patients received DOXY monotherapy, 636 received the IVM + DOXY combination, and 3574 patients received comparator therapy. While all 19 studies described the efficacy of the intervention, only nine studies described the safety profile adequately. The baseline characteristics of all included studies and the demographic details are provided as Tables 2 and 3 of the ESM, and the main outcomes (efficacy and safety) of the studies are summarised in Table 4 of the ESM.
IVM Monotherapy
Eight studies explored the outcomes of IVM monotherapy [26–33]. Two of these six studies are RCTs, but the RCT by Podder et al. is a pre-print whose results have not been peer reviewed and published [30]. Four studies administered IVM as a single oral dose of 200 µg per kg, one study each administered a 400-µg/kg and a 12-mg single dose, one study administered a higher dose of IVM at 12 mg once daily for 12 days, while one study did not specify the dose (Table 2 of the ESM).
The efficacy outcomes varied across the studies (Table 4 of the ESM). Some studies reported results favouring IVM monotherapy. The overall duration of hospital stay with IVM treatment was observed to be significantly lower compared with HCQ + AZT by Gorial et al. [26]. The overall mortality with IVM treatment was observed to be significantly lower compared with standard care by Rajter et al. [27]. Other results favouring IVM in COVID-19 management included a lower number of median days with positive RTPCR for SARS-CoV2 compared with HCQ + AZT [26], and a higher proportion of survival without intensive care unit transfer compared with standard care [28]. Pre-treatment of patients with suspected COVID-19 with IVM on an outpatient basis was not shown to reduce the odds of eventual hospitalisation in a multi-intervention retrospective study [31]. Compared with placebo, IVM was found to have non-statistically significant lower median viral loads after 4 and 7 days of treatment and a lower number of patient-days for overall symptoms, anosmia and cough [32]. IVM monotherapy was also found to have significantly lower days to viral clearance, significantly lower days of hospitalisation and significantly lower deaths compared to standard care [33].
However, some results were not in favour of IVM monotherapy. The overall duration of hospital stay was found to be not significantly lower with IVM monotherapy compared to standard care by Rajter et al. [27] and the overall mortality with IVM treatment was not significantly different from standard care by Soto-Becerra et al. [28]. Other efficacy outcomes that were not in favour of IVM included no significant improvements seen in parameters such as intensive care unit admission, clinical features (after 10 days), RTPCR positive result (after 3–5 days) [29]; extubation rate [27]; survival without oxygen supplementation [28]; days to recovery and RTPCR negative result on day 10 [30]. Reverse-transcription polymerase chain reaction positivity with IVM monotherapy was also found to be similar to that with placebo [32].
Only four studies had explored safety parameters, and none of them reported any new safety signals or concerns with respect to IVM [26, 29, 32, 33]. Thus, while the studies did not reveal any new safety signals after using IVM for COVID-19 management, the benefits observed with IVM (or lack thereof) in COVID-19 management were not consistent across all studies.
DOXY Monotherapy
Five studies explored the efficacy of DOXY monotherapy in COVID-19. One of these was a case series of four patients with COVID-19 having various comorbid conditions treated with varying doses of DOXY monotherapy (Table 2 of the ESM). All included patients were symptom free after a maximum of 10 days, with no adverse reactions reported [34]. The second study was a retrospective cohort study involving 89 high-risk patients with COVID-19 in a long-term care facility who were administered DOXY 100 mg for 7 days. There was clinical improvement (as defined by resolution of fever, shortness of breath or improvement in oxygen saturation) in 76/89 patients, while ten of the patients died. No new safety signals were noted in this study as well. In the absence of a comparator, it is challenging to determine if these findings suggest an improved efficacy of DOXY over standard care [35]. DOXY monotherapy was found to have no benefit among hospitalised patients with COVID-19 with respect to the risk of death, severe acute respiratory distress syndrome or all-cause mortality. However, a study by Falcone et al. focussed on the role of low-molecular-weight heparin in patients with COVID-19, and a small group of included patients had received DOXY monotherapy [36]. Another observational study by Gironi et al. reported a prompt resolution of symptoms including body temperature, cough and dyspnoea. However, this study included patients with COVID-19 with some previous dermatological condition for which systemic tetracycline treatment was given, and hence DOXY was not actively prescribed for COVID-19 management. Further, the absence of a comparator makes these findings difficult to interpret [37]. Finally, an RCT by Sayed et al. compared the outcome of DOXY monotherapy, AZT monotherapy, DOXY with nitazoxanide, and AZT with nitazoxanide when given to patients with COVID-19 and found that symptomatic improvement started in 5 days when nitazoxanide was given either with DOXY or with AZT, but with DOXY monotherapy, it started in 7 days. The information about AZT was not clearly given [38].
IVM + DOXY Combination Therapy
Five studies, none of which were obtained from the database search, explored the outcomes of the IVM + DOXY combination in COVID-19. Three of these were RCTs, of which two were pre-prints, and one was a clinicaltrials.gov document, thereby marking all three included RCTs as non-peer-reviewed and unpublished records. The dosing schedule used by each study is different (Table 2 of the ESM). Alam et al. report 100% RTPCR negativity and no intensive care unit admissions with the IVM + DOXY combination; however, there is no comparator in this study [39]. Rahman et al. report earlier viral clearance with IVM + DOXY compared with HCQ + AZT; however, the description of this finding in this study is difficult to interpret given the lack of clarity [40]. The findings of the RCT by Mahmud posted in clnicaltrials.gov are strongly in favour of the IVM + DOXY combination wherein the combination shows a significantly higher proportion of patients with early clinical improvement within 7 days, with a corresponding lower proportion of patients having late clinical improvement in 12 days, persistent RTPCR positivity after 14 days and clinical deterioration in 1 month, compared with standard care. This study suggests that the IVM + DOXY combination was associated with unique adverse drug reactions including erosive esophagitis and non-ulcer dyspepsia in two and seven patients, respectively, among the 183 patients (combined adverse event incidence of 4.91%) who received the combination; however, there was no mention of a causality assessment in the results posted [41].
Average days to symptom resolution and clinical recovery with the IVM + DOXY combination was found to be lower compared with the standard care by Hashim et al., and compared to the HCQ + AZT combination by Chowdhury et al. The study by Hashim et al. found no benefit with the IVM + DOXY combination in patients with severe COVID-19 compared to standard care [42]. The study by Chowdhury et al. reported that the average number of days to RTPCR negativity was similar with the IVM + DOXY combination and the HCQ + AZT combination [43]. Neither of these studies indicated any new safety concerns with the IVM + DOXY combination. Finally, it should be noted that both these studies are pre-prints of RCTs.
Thus, apart from the one study that suggested an increased incidence of erosive esophagitis and non-ulcer dyspepsia in 9/183 patients, whose causality assessment is uncertain, there appears to be no significant safety concerns with the IVM + DOXY combination; however, the efficacy of the combination in COVID-19 is also not unequivocally established.
Other Designs
Ahmed et al. reported a three-arm RCT comparing IVM monotherapy, IVM + DOXY combination therapy, and placebo and found that the mean duration to viral clearance was significantly lower with IVM monotherapy compared with placebo (9.7 days vs 12.7 days with placebo, p = 0.02), but not significantly lower with IVM + DOXY combination therapy (11.5 days vs 12.7 days with placebo, p = 0.27). However, the duration of hospital stay was comparable between the three groups (p = 0.93). It is important to note here that the dose of IVM used in this study was significantly high, at 12 mg once daily for 5 days [44].
Methodological Quality/Risk of Bias Assessment of Included Studies
Out of the six retrospective cohort studies, four studies had ‘good quality’ [27, 28, 31, 33], and two had ‘fair quality’ of methodology [29, 35], as per the National Institutes of Health tool. Out of the seven RCTs in our review, five RCTs had a high risk of bias as per the ROB-2 tool [30, 38, 42–44]; three of these five RCTs are pre-prints [30, 42, 43]. The RCT by Chaccour et al. had a low risk of bias [32], and the Mahmud RCT, which was sourced from clinicaltrials.gov, had some concerns in the methodology as per the ROB-2 tool [41]. Out of the five NRTs, two studies had a moderate risk of bias [26, 36], and the remaining three studies had a serious risk of bias [37, 39, 40], as per the ROBINS tool. Finally, the case study by Yates et al. [34] had moderate methodological quality as per the Murad et al. quality assessment tool (Table 3 of the ESM).
Inter-Rater Reliability
The inter-rater reliability for the selection of papers for review between the two reviewers was substantial, with a Cohen’s kappa value of 0.687 (95% confidence interval 0.658–0.716) and 0.613 (95% confidence interval 0.584–0.642) for title-abstract screening and full-text screening, respectively; mediation was required in 21/200 papers for title-abstract screening and 4/29 papers for full-text screening. For the methodological quality assessment, as multiple tools were used based on the study design, an inter-rater reliability calculation was not feasible. There was agreement between the two reviewers with respect to the methodological quality of 15/19 papers, with only four papers needing mediation, leading to an inter-rater agreement of 78.95%.
Discussion
After evaluating 19 relevant papers from sources for this scoping review, it was not possible for us to establish a strong evidence either in favour of or against IVM, DOXY, and the IVM + DOXY combination when used in the management of patients with COVID-19. The results of efficacy outcomes were mixed, and only one study indicated that the IVM + DOXY combination resulted in non-ulcer dyspepsia and erosive esophagitis in nearly 5% of the patients who were administered the combination. Considering that the association of esophagitis with DOXY therapy has been previously reported in the literature [45], our review did not identify any new safety signals concerning the use of either drug alone or in combination.
Both IVM and DOXY can be considered ‘latecomers’ in the bandwagon of drugs used for COVID-19 management. This might be one reason why neither of these drugs has received as much public and media attention as drugs such as remdesivir, HCQ, or favipiravir. Perhaps fuelled by the increased use of IVM to treat endemic filariasis, and in part because of the inexpensive nature of either drug, the usage of these two drugs is seen more in Asian countries, especially Bangladesh, as evidenced by the distribution of country of the first authors of the studies included in this review. In a recently published paper from India, the prophylactic potential of IVM was explored in a case-control study, which found that when IVM was given as two doses of 300 μg/kg with a gap of 72 h, the incidence of SARS-CoV-2 infection among healthcare workers in the subsequent month was reduced by 73% [46]. For these reasons, regardless of the reason for the apparent lack of interest, we believe that these drugs deserve a more comprehensive evaluation of their role in COVID-19 management.
As described previously, both these drugs have non-overlapping and viable in-vitro (and in the case of DOXY, in vivo as well) mechanisms of action in the pathophysiology of the disease [10, 12–14]. However, through the course of the current review, we have observed that this has not completely translated into clinical benefits. Two possible reasons for this observation are non-compatible pharmacokinetics and an inadequate dosing/route of administration.
The pharmacokinetics of DOXY suggests that it is distributed in the pulmonary tissue after oral administration, and the concentration of the drug in the lung is reported to be 18–23% of the serum concentration in humans [47]. Further, oral DOXY is reported to achieve moderate pulmonary penetration when administered to patients with cystic fibrosis [48]. Finally, oral DOXY has shown efficacy when used to treat patients with chronic obstructive pulmonary disease [49]. However, the as far as IVM is concerned, by virtue of having a high plasma protein binding of 93%, its uptake by endothelial cells is limited [50]. With regard to the pulmonary distribution of IVM, while adequate literature is not available describing the accumulation of IVM in the lungs when administered to human subjects, the concentrations of IVM reaching cattle lungs after injecting the drug at a dose of 200 μg/kg was approximately 0.1 μM, which is significantly less than the 5-μM IVM concentration that was used in the Caly study to inhibit the growth of SARS-CoV-2 in vitro [9, 51, 52]. Considering that the conventional dose of IVM for treating strongyloidiasis is a single 200-μg/kg oral dose, there is no evidence that this dosing schedule would result in IVM reaching an antiviral concentration in the lungs [51]. Consequently, alternative administration approaches should be explored for IVM in COVID-19, such as an alternative route of administration (e.g. inhalational), a higher dose, a multiple dosing schedule, and using in combination with drugs that enhance the activity of IVM by mechanisms such as improving the pulmonary penetration of IVM.
As the usage of both IVM and DOXY and their combination for COVID-19 is off-label, throughout this study we have observed an inconsistency in the dosing schedule, with respect to the dose, frequency, and duration of administration, of both IVM and DOXY. This might have contributed to the lack of consistent efficacy of the drugs. Next, barring one study, there have been no safety signals of concern with the use of these drugs in their current doses; however, the safety aspect should be explored when alternative administration schedules are considered. Future studies should also take into consideration other aspects such as the interaction between IVM and DOXY, the safety and efficacy of these drugs in patients experiencing different clinical stages of COVID-19, and different patient populations (such as extremes of ages, patients with comorbidities and pregnancy).
Our study is not without limitations. We did not search for additional databases such as SCOPUS and EMBASE, and for unpublished conference abstracts; thus, additional relevant studies indexed in these databases or unpublished studies presented in conferences might have been missed. Though two independent reviewers were involved in data curation, extraction, and quality assessment, the risk of bias in reviewing and reporting cannot be completely ruled out. We did not assess the role of IVM or DOXY in COVID-19 prophylaxis. Finally, the inherent publication bias wherein only studies with positive findings are reported cannot be completely ruled out.
Conclusions
Existing evidence is not sufficient to strongly advocate the usage of IVM and DOXY, either as monotherapy or as a combination therapy, in the treatment of COVID-19. However, it should be considered that both of these drugs have several advantages associated with them, such as viable mechanism of action, easy availability, acceptable safety profile, experience of long-term use and inexpensiveness. Because a lack of evidence for efficacy does not necessarily mean evidence for lack of efficacy, and also because the prophylactic role of IVM has been recently reported in the literature, further studies are essential to explore if this combination has the potential to manage patients with COVID-19, and to find out if this combination is a solution for the long-sought drug of choice for COVID-19 management.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No external funding was received for the preparation of this article.
Conflicts of interest/competing interests
Subhrojyoti Bhowmick, Amit Dang, Vallish BN and Sumit Dang have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
This is a systematic review, and because there is no direct data collection from patients, ethics committee approval is not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All datasets used to derive conclusions in this study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
SB provided the concept and ideation for the article; acquisition of data, analysis and interpretation of data; and revised the article critically for important intellectual content. AD was responsible for the acquisition of data, analysis and interpretation of data and revising the article critically for important intellectual content. VBN was responsible for the acquisition of data, analysis and interpretation of data; formal analysis; drafting the article, and revising the article critically for important intellectual content. SD was responsible for the acquisition of data, analysis and interpretation of data; and revising the article critically for important intellectual content. All authors approved the final version of the manuscript.
Contributor Information
Subhrojyoti Bhowmick, Email: drsubhro@gmail.com.
Amit Dang, Email: amit.d@marksmanhealthcare.com.
B. N. Vallish, Email: vallish.bn@marksmanhealthcare.com
Sumit Dang, Email: sumitdang@uky.edu.
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. https://www.covid19.who.int/. Accessed 25 Mar 2021.
- 2.Middleton J, Lopes H, Michelson K, Reid J. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65(9):1525–1527. doi: 10.1007/s00038-020-01455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaway E. COVID vaccine excitement builds as Moderna reports third positive result. Nature. 2020;587(7834):337–338. doi: 10.1038/d41586-020-03248-7. [DOI] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19: final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fatima SA, Asif M, Khan KA, Siddique N, Khan AZ. Comparison of efficacy of dexamethasone and methylprednisolone in moderate to severe Covid 19 disease. Ann Med Surg. 2020;60:413–416. doi: 10.1016/j.amsu.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam S, Lombardi A, Ouanounou A. COVID-19: a review of the proposed pharmacological treatments. Eur J Pharmacol. 2020;886:173451. doi: 10.1016/j.ejphar.2020.173451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixit A, Yadav R, Singh AV. Ivermectin: potential role as repurposed drug for COVID-19. Malays J Med Sci. 2020;27(4):154–158. doi: 10.21315/mjms2020.27.4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui AJ, Jahan S, Ashraf SA, Alreshidi M, Ashraf MS, Patel M, et al. Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1802345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta D, Sahoo AK, Singh A. Ivermectin: potential candidate for the treatment of Covid 19. Braz J Infect Dis. 2020;24(4):369–371. doi: 10.1016/j.bjid.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gendrot M, Andreani J, Jardot P, Hutter S, Delandre O, Boxberger M, et al. In vitro antiviral activity of doxycycline against SARS-CoV-2. Molecules. 2020;25(21):5064. doi: 10.3390/molecules25215064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malek AE, Granwehr BP, Kontoyiannis DP. Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21:e00864. doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Caprio R, Lembo S, Di Costanzo L, Balato A, Monfrecola G. Anti-inflammatory properties of low and high doxycycline doses: an in vitro study. Mediat Inflamm. 2015;2015:329418. doi: 10.1155/2015/329418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rempe S, Hayden JM, Robbins RA, Hoyt JC. Tetracyclines and pulmonary inflammation. Endocr Metab Immune Disord Drug Targets. 2007;7(4):232–236. doi: 10.2174/187153007782794344. [DOI] [PubMed] [Google Scholar]
- 15.Malek AE, Granwehr BP. Doxycycline as an alternative to azithromycin in elderly patients. Int J Antimicrob Agents. 2021;57(1):106168. doi: 10.1016/j.ijantimicag.2020.106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vora A, Arora VK, Behera D, Tripathy SK. White paper on ivermectin as a potential therapy for COVID-19. Indian J Tuberc. 2020;67(3):448–451. doi: 10.1016/j.ijtb.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masud H, Qureshi TQ, Dukley M. Effects of ivermectin with and without doxycycline on clinical symptoms of onchocerciasis. J Coll Physicians Surg Pak. 2009;19(1):34–38. [PubMed] [Google Scholar]
- 18.Turner JD, Mand S, Debrah AY, Muehlfeld J, Pfarr K, McGarry HF, et al. A randomized, double-blind clinical trial of a 3-week course of doxycycline plus albendazole and ivermectin for the treatment of Wuchereria bancrofti infection. Clin Infect Dis. 2006;42(8):1081–1089. doi: 10.1086/501351. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. Antiviral drugs that are approved or under evaluation for the treatment of COVID-19. 2020. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/. Accessed 25 Mar 2021.
- 20.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Current version of RoB 2. https://www.sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2. Accessed 25 Mar 2021.
- 22.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institutes of Health. Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 25 Mar 2021.
- 24.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang A, Chidirala S, Veeranki P, Vallish BN. A critical overview of systematic reviews of chemotherapy for advanced and locally advanced pancreatic cancer using both AMSTAR2 and ROBIS as quality assessment tools. Rev Recent Clin Trials. 2020 doi: 10.2174/1574887115666200902111510. [DOI] [PubMed] [Google Scholar]
- 26.Gorial F, Mashhadani S, Sayaly H, Dakhil B, AlMashhadani M, Aljabory A, et al. Effectiveness of ivermectin as add-on therapy in COVID-19 management (pilot trial) medRxiv. 2020 doi: 10.1101/2020.07.07.20145979. [DOI] [Google Scholar]
- 27.Rajter JC, Sherman M, Fatteh N, Vogel F, Sacks J, Rajter J-J. ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19. medRxiv. 2020 doi: 10.1101/2020.06.06.20124461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soto-Becerra P, Culquichicon C, Hurtado-Roca Y, Araujo-Castillo R. Real-world effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: results of a target trial emulation using observational data from a nationwide healthcare system in Peru. medRxiv. 2020 doi: 10.1101/2020.10.06.20208066. [DOI] [Google Scholar]
- 29.Camprubí D, Almuedo-Riera A, Martí-Soler H, Soriano A, Hurtado J, Subirà C, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PLoS ONE. 2020;15(11):e0242184. doi: 10.1371/journal.pone.0242184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podder C, Chowdhury N, Sina M, Haque W. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC J Med Sci. 2020;14(2):002. [Google Scholar]
- 31.Szente Fonseca S, de Queiroz SA, Wolkoff A, Moreira M, Pinto B, Valente Takeda C, et al. Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: comparative analysis. Travel Med Infect Dis. 2020;31(38):101906. doi: 10.1016/j.tmaid.2020.101906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32:100720. doi: 10.1016/j.eclinm.2020.100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan MSI, Khan MSI, Debnath CR, Nath PN, Mahtab MA, Nabeka H, et al. Ivermectin treatment may improve the prognosis of patients with COVID-19. Arch Bronconeumol. 2020;56(12):828–830. doi: 10.1016/j.arbres.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yates PA, Newman SA, Oshry LJ, Glassman RH, Leone AM, Reichel E. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther Adv Respir Dis. 2020;14:1753466620951053. doi: 10.1177/1753466620951053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam MM, Mahmud S, Rahman MM, Simpson J, Aggarwal S, Ahmed Z. Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Cureus. 2020;12(8):e9658. doi: 10.7759/cureus.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falcone M, Tiseo G, Barbieri G, Galfo V, Russo A, Virdis A, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7(12):ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gironi LC, Damiani G, Zavattaro E, Pacifico A, Santus P, Pigatto PDM, et al. Tetracyclines in COVID-19 patients quarantined at home: literature evidence supporting real-world data from a multicenter observational study targeting inflammatory and infectious dermatoses. Dermatol Ther. 2020;34(1):e14694. doi: 10.1111/dth.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sayed AM, Khalaf AM, Abdelrahim MEA, Elgendy MO. Repurposing of some anti-infective drugs for COVID-19 treatment: a surveillance study supported by an in silico investigation. Int J Clin Pract. 2020;75(4):e13877. doi: 10.1111/ijcp.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alam MT, Murshed R, Bhiuyan E, Saber S, Alam R, Choudhury RR. A case series of 100 COVID-19 positive patients treated with combination of ivermectin and doxycycline. J Bangl Coll Physicians Surg. 2020;38:10–15. doi: 10.3329/jbcps.v38i0.47512. [DOI] [Google Scholar]
- 40.Rahman MA, Iqbal S, Islam M, Niaz M, Hussain T, Siddiquee T. Comparison of viral clearance between ivermectin with doxycycline and hydroxychloroquine with azithromycin in COVID-19 patients. J Bangl Coll Physicians Surg. 2020 doi: 10.3329/jbcps.v38i0.47514. [DOI] [Google Scholar]
- 41.Mahmud R. Clinical trial of ivermectin plus doxycycline for the treatment of confirmed Covid-19 infection. 2020. https://clinicaltrials.gov/ct2/show/NCT04523831. Accessed 26 Mar 2021.
- 42.Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv. 2020 doi: 10.1101/2020.10.26.20219345. [DOI] [Google Scholar]
- 43.Chowdhury ATMM, Mohammad S, Md Rezaul K, Johirul I, Dan G, Shuixiang H. Research Square. 2020 doi: 10.21203/rs.3.rs-38896/v1. [DOI] [Google Scholar]
- 44.Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelat VG, Seah M, Lim KH. Doxycycline induced acute erosive oesophagitis and presenting as acute dysphagia. J Assoc Physicians India. 2011;59:57–59. [PubMed] [Google Scholar]
- 46.Behera P, Patro BK, Singh AK, Chandanshive PD, Ravikumar SR, Pradhan SK, et al. Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: a matched case–control study. PLoS ONE. 2021;16(2):e0247163. doi: 10.1371/journal.pone.0247163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15(6):355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- 48.Beringer PM, Owens H, Nguyen A, Benitez D, Rao A, D'Argenio DZ. Pharmacokinetics of doxycycline in adults with cystic fibrosis. Antimicrob Agents Chemother. 2012;56(1):70–74. doi: 10.1128/aac.05710-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalvi PS, Singh A, Trivedi HR, Ghanchi FD, Parmar DM, Mistry SD. Effect of doxycycline in patients of moderate to severe chronic obstructive pulmonary disease with stable symptoms. Ann Thorac Med. 2011;6(4):221–226. doi: 10.4103/1817-1737.84777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Audus KL, Knaub SR, Guillot FL, Schaeffer JM. The effect of protein binding on ivermectin uptake by bovine brain microvessel endothelial cells. Vet Res Commun. 1992;16(5):365–377. doi: 10.1007/bf01839186. [DOI] [PubMed] [Google Scholar]
- 51.Peña-Silva R, Duffull SB, Steer AC, Jaramillo-Rincon SX, Gwee A, Zhu X. Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19. Br J Clin Pharmacol. 2021;87(3):1589–1590. doi: 10.1111/bcp.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lifschitz A, Virkel G, Sallovitz J, Sutra JF, Galtier P, Alvinerie M, et al. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet Parasitol. 2000;87(4):327–338. doi: 10.1016/s0304-4017(99)00175-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets used to derive conclusions in this study are available from the corresponding author on reasonable request.