Abstract
Genetic studies have shifted to sequencing-based rare variants discovery after decades of success in identifying common disease variants by Genome-Wide Association Studies using Single Nucleotide Polymorphism chips. Sequencing-based studies require large sample sizes for statistical power and therefore often inadvertently introduce batch effects because samples are typically collected, processed, and sequenced at multiple centers. Conventionally, batch effects are first detected and visualized using Principal Components Analysis and then controlled by including batch covariates in the disease association models. For sequencing-based genetic studies, because all variants included in the association analyses have passed sequencing-related quality control measures, this conventional approach treats every variant as equal and ignores the substantial differences still remaining in variant qualities and characteristics such as genotype quality scores, alternative allele fractions (fraction of reads supporting alternative allele at a variant position) and sequencing depths. In the Alzheimer’s Disease Sequencing Project (ADSP) exome dataset of 9,904 cases and controls, we discovered hidden variant-level differences between sample batches of three sequencing centers and two exome capture kits. Although sequencing centers were included as a covariate in our association models, we observed differences at the variant level in genotype quality and alternative allele fraction between samples processed by different exome capture kits that significantly impacted both the confidence of variant detection and the identification of disease-associated variants. Furthermore, we found that a subset of top disease-risk variants came exclusively from samples processed by one exome capture kit that was more effective at capturing the alternative alleles compared to the other kit. Our findings highlight the importance of additional variant-level quality control for large sequencing-based genetic studies. More importantly, we demonstrate that automatically filtering out variants with batch differences may lead to false negatives if the batch discordances come largely from quality differences and if the batch-specific variants have better quality.
Introduction
Genetic studies have shifted from Single Nucleotide Polymorphism (SNP) chip-based genome-wide association study (GWAS) of common variants to exome and whole-genome sequencing-based associations of rare variants. The large samples required for statistical power in sequencing-based searches for rare disease-associated variants often inadvertently introduce batch effects and systematic biases. Batch effects refer to sources of variation arising not from the targeted biological differences between sample classes but from differences between experimental or technological groups of samples [1]. If not adequately addressed in the analysis, batch effects reduce statistical power and lead to both false-positive and false-negative associations. Practices in large sequencing studies that commonly introduce batch effects include dividing samples among multiple sequencing centers [2, 3], collecting or preparing samples under different protocols [4], and extracting exomes using different target capture kits [4, 5]. For example, the Alzheimer’s Disease Sequencing Project (ADSP) sequenced exomes of more than 10,000 cases and controls to identify genetic factors associated with Alzheimer’s disease (AD) [6]. Sequencing of ADSP samples took place at three centers. Center 1 prepared sequencing libraries using the Illumina Rapid Capture Exome kit, while Center 2 and Center 3 used the Roche NimbleGen VCRome v2.1 kit.
A standard approach to manage batch effects is to identify and visualize batch effects using Principal Components Analysis (PCA) of the variant genotypes, and then either to adjust association models using batch covariates or to exclude batch-associated variants from further analysis [7–11]. However, this approach, which was developed during the SNP chip GWAS era, may not be sufficient for large genetic studies by sequencing [12]. The qualities and characteristics of variants identified by sequencing vary substantially even when all variants included in the association analysis have passed quality control thresholds such as those defined by the Variant Quality Score Recalibration (VQSR) model from the Genome Analysis Toolkit (GATK) [13]. Some quality-related characteristics differ between samples at the single-variant level. For example, exome kit capture efficiency may differ between the reference and alternative alleles, and the template amplification and sequencing chemistry differ between variants located in GC-rich and AT-rich regions. These variations will lead to differences in depth of coverage (DP), genotype quality (GQ) and alternative allele fraction (AAF; fraction/percentage of the sequencing reads aligned to the variant positions that support the alternative allele), etc. Because the variants that pass VQSR are all treated equally for disease association analysis, the impact of differences in individual variant-level qualities and characteristics is often masked.
We studied the ADSP cohort of 9,904 exomes and found that conventional PCA of the genotypes did not reveal the magnitude of the batch differences between samples sequenced at three centers and processed by two exome target capture kits. Furthermore, genetic association analyses that adjusted for center batches did not sufficiently remove hidden batch differences at the variant level. We found differences between capture kits in both variant GQ and AAF that significantly impacted the identification of AD-associated risk variants. Our findings highlight the importance of additional variant-level quality control to help researchers find truly meaningful genetic variants that are masked by batch effects.
Results
Description of dataset
The Sequence Read Archive (SRA) files containing the raw sequencing data of 10,993 AD cases and controls were downloaded from dbGaP (https://www.ncbi.nlm.nih.gov/gap/) and converted to FASTQ read files using the SRA Toolkit (https://www.ncbi.nlm.nih.gov/books/NBK158899). Access to this public dataset was approved by dbGaP and the Institutional Review Boards of Mayo Clinic and the University of Illinois. The Alzheimer’s cases satisfied the National Institute on Aging and the Alzheimer’s Association criteria [14] for definite, possible or probable Alzheimer’s disease. These cases included patients with and without APOE [15] risk alleles. The controls were at least 60 years old, showed no sign of dementia based on cognitive testing, and scored low on risk assessment [6]. Of the 10,993 samples, 9,904 passed sample-level quality control (QC) [16] based on the following criteria: variant call rate >95% per sample for SNPs and >90% for INDELs; sequencing depths >10x for at least 90% of the variants; APOE genotype matching between cohort meta-data and sequenced genotypic data; average transition/transversion ratio > 2.75 in the exonic regions; FREEMIX sample contamination estimate < 0.02 [17]; sex check (PLINK F estimate > 0.7 for males and < 0.3 for females); no first-, second- or third-degree relatedness as defined by the KING-robust algorithm [18]; and removal of duplicate samples. Of the 9,904 samples passing sample-level QC, Center 1 contributed 4,427 samples; Center 2 contributed 3,260; and Center 3 contributed 2,217. No samples were sequenced by multiple centers. Of the 1,584,609 variants detected in 9,904 ADSP exomes, 166,947 variants passed VQSR and the additional filtering steps detailed in the methods section.
Population substructure explains sample clusters in PCA
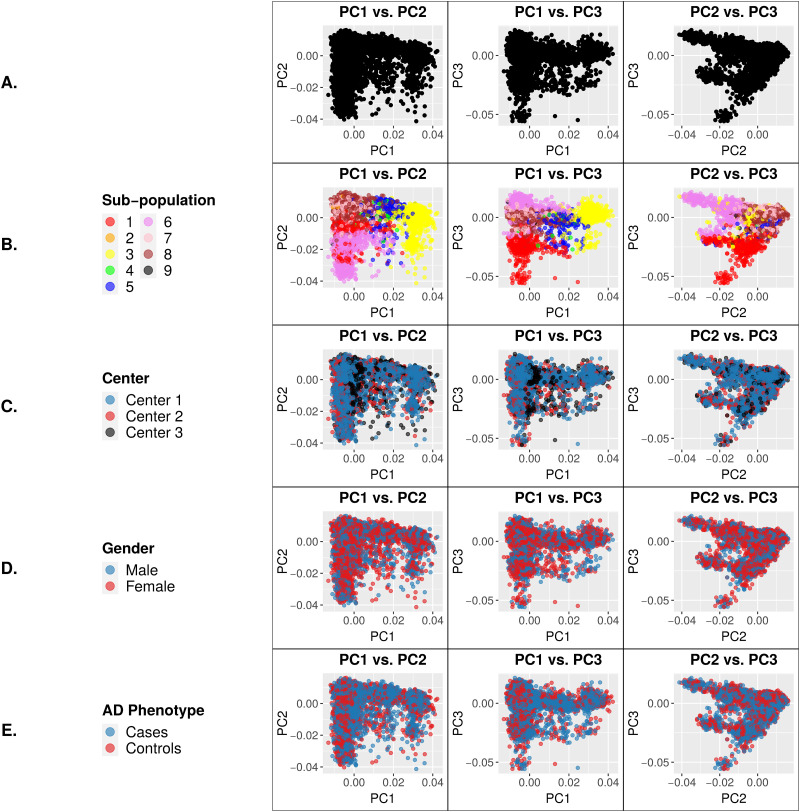
The 9,904 ADSP subjects that passed sample-level QC were homogeneous based on 99.8% reporting European ancestry [16, 19] and on comparison to 1000 Genomes reference samples (S1 Fig). However, PCA of the ADSP genotypes using a pruned set of high-quality common variants (see Methods section) identified multiple sample clusters (Fig 1A) despite the homogeneous European ancestry of the study subjects. To determine whether the observed sample clusters represented population substructure or batch effects, we performed a de novo estimate using Admixture [20] that identified 9 sub-populations. As shown in Fig 1B, these 9 sub-populations overlapped with and accounted for the sample clusters identified in PCA. The sample batches of three different sequencing centers were not clearly visible by PCA, nor were gender or case/control status (Fig 1C–1E). Therefore, the sample clusters visualized by PCA can be explained largely by population substructure alone, at first glance. To control for this substructure, eigenvectors from the first four principal components were included as covariates in the association analysis. All 9,904 subjects were included in both the PCA and the association analysis.
Fig 1. Principal Component (PC) eigenvector plots using genotypes of a pruned set of 16,187 high-quality common variants for 9,904 ADSP individuals.
Each data point represents a single individual. Clustering of samples for a particular variable signifies genotypic similarity between individuals for the trait represented by that color. (A) PCs of the genotypes. (B) PCs color coded based on sub-population. (C) PCs color coded based on center. (D) PCs color coded based on gender. (E) PCs color coded based on AD phenotype. As expected, clustering is apparent only by sub-population.
Association analysis
Of the 1,584,609 variants detected in 9,904 exomes, 166,947 variants passed the filtering criteria for association analysis detailed in the methods section (S2 Fig). Two association models were used. Model 1 adjusted for sequencing center and the first four principal components (PCs) from PCA underlying population substructure. Model 2 adjusted for sequencing center, the first 4 PCs, sex and APOE genotype. Neither model included age as a covariate because age confounds with disease status in this dataset by design. ADSP controls were deliberately selected to be younger than cases to favor the detection of disease-causal variants that are absent from older but cognitively normal individuals, and therefore previous ADSP association analyses have identified no significant variants under models adjusting for age [19], a finding that we replicated.
Combined, the association models identified 52 SNPs associated with AD with exome-wide significance, including 8 SNPs reported by the ADSP consortium [19] and 7 SNPs in the known AD genes APOE and TOMM40 (S3 Fig). In this paper, we focus on 1) the 29 “novel” SNPs that remained significant after adjustment for APOE and sex and that have not previously been linked to AD, as well as on 2) the 7 SNPs in APOE and TOMM40 as positive controls (Table 1).
Table 1. Seven SNPs in APOE and TOMM40 (indicated by * of the SNP IDs) and 29 novel SNPs reaching exome-wide significance (p < 3.0 x 10−7, Bonferroni-corrected cutoff of p < 0.05 / # tests): Population minor allele frequency (MAF) in cases and controls, MAF in controls processed by Illumina or NimbleGen exome capture kit, and MAF in Non-Finish European (NFE) cohort of the ExAC database (http://exac.broadinstitute.org/).
| Chr | Position | Ref | Alt | p-value (Model 1) | p-value (Model 2) | Gene | SNP ID | ADSP Cases MAF | ADSP Controls MAF | ADSP Controls MAF, Illumina | ADSP Controls MAF, NimbleGen | ExAC AAF, NFE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 45411941 | T | C | 2.4E-185 | N/A | APOE | rs429358* | 0.2293 | 0.0701 | 0.074 | 0.067 | 0.1504 |
| 19 | 45396144 | C | T | 4.7E-103 | 0.7097 | TOMM40 | rs11556505* | 0.2023 | 0.0879 | 0.085 | 0.090 | 0.0875 |
| 19 | 45395714 | T | C | 2.4E-75 | 0.1439 | TOMM40 | rs157581* | 0.2766 | 0.1629 | 0.165 | 0.162 | 0.2326 |
| 19 | 45412079 | C | T | 1.8E-48 | N/A | APOE | rs7412* | 0.042 | 0.0988 | 0.116 | 0.087 | 0.0813 |
| 15 | 75913319 | T | G | 4.3E-45 | 2.7E-35 | SNUPN | rs1004285543 | 0.0825 | 0.0255 | 0.063 | 0.001 | NA |
| 17 | 25973604 | A | C | 2.6E-45 | 1.4E-34 | LGALS9 | rs761436847 | 0.0823 | 0.0256 | 0.056 | 0.006 | 0.0906 |
| 6 | 36979483 | T | G | 1.7E-39 | 1.6E-29 | FGD2 | rs769719224 | 0.0998 | 0.0404 | 0.072 | 0.019 | 0.0294 |
| 14 | 99976645 | A | C | 4.1E-36 | 7.5E-28 | CCNK | rs745936510 | 0.0899 | 0.0348 | 0.068 | 0.013 | 0.0713 |
| 13 | 114188430 | C | T | 4.7E-38 | 2.2E-27 | TMCO3 | rs77834374 | 0.1068 | 0.0459 | 0.080 | 0.024 | 0.1336 |
| 14 | 99976639 | G | C | 9.4E-36 | 3.7E-26 | CCNK | rs778243462 | 0.0932 | 0.0372 | 0.071 | 0.015 | 0.0808 |
| 17 | 25973598 | A | C | 4.3E-25 | 1.1E-21 | LGALS9 | rs760143837 | 0.0472 | 0.014 | 0.033 | 0.002 | 0.0243 |
| 14 | 77706020 | A | C | 9.7E-28 | 6.3E-21 | TMEM63C | rs774212969 | 0.0577 | 0.019 | 0.036 | 0.008 | 0.009 |
| 19 | 45397229 | G | A | 9.6E-21 | 0.3137 | TOMM40 | rs1160983* | 0.0173 | 0.0416 | 0.045 | 0.039 | 0.0718 |
| 11 | 117280516 | A | C | 7.9E-29 | 2.5E-20 | CEP164 | rs756182128 | 0.0748 | 0.0296 | 0.050 | 0.016 | 0.081 |
| 3 | 42739737 | T | G | 5.7E-25 | 9.7E-20 | HHATL | rs763168412 | 0.0539 | 0.0182 | 0.034 | 0.008 | 0.0919 |
| 3 | 48451952 | A | C | 3.2E-24 | 2.7E-19 | PLXNB1 | rs770786389 | 0.0562 | 0.02 | 0.037 | 0.009 | 0.0255 |
| 19 | 45397307 | C | T | 1.5E-18 | 0.928 | TOMM40 | rs112849259* | 0.0308 | 0.0107 | 0.005 | 0.014 | 0.0011 |
| 12 | 56622883 | A | C | 4.3E-24 | 1.2E-17 | NABP2 | rs757798976 | 0.0714 | 0.0301 | 0.054 | 0.014 | 0.0476 |
| 2 | 85662149 | A | C | 4.0E-21 | 4.3E-16 | SH2D6 | rs748669078 | 0.068 | 0.0309 | 0.044 | 0.022 | 0.0026 |
| 19 | 10946797 | G | C | 6.4E-21 | 2.5E-15 | TMED1 | rs767166604 | 0.0421 | 0.0128 | 0.029 | 0.002 | 0.0007 |
| 14 | 105932775 | G | C | 5.5E-21 | 3.1E-15 | MTA1 | rs782227993 | 0.0627 | 0.0259 | 0.047 | 0.012 | 0.0208 |
| 6 | 29429950 | A | C | 1.1E-19 | 3.6E-15 | OR2H1 | rs746691570 | 0.0402 | 0.0132 | 0.022 | 0.007 | 0.0207 |
| 11 | 117280522 | A | C | 6.5E-21 | 3.3E-14 | CEP164 | rs758240656 | 0.0529 | 0.0198 | 0.037 | 0.009 | 0.0768 |
| 2 | 85662154 | A | C | 4.9E-18 | 4.0E-14 | SH2D6 | rs760146451 | 0.0617 | 0.0288 | 0.040 | 0.021 | 0.0018 |
| 13 | 88330245 | A | C | 3.1E-17 | 1.1E-13 | SLITRK5 | rs773717935 | 0.0277 | 0.0065 | 0.014 | 0.002 | 3.1E-05 |
| 19 | 45409167 | C | G | 9.7E-13 | 0.3854 | APOE | rs440446* | 0.3332 | 0.3817 | 0.361 | 0.395 | 0.4346 |
| 19 | 10946802 | T | C | 1.2E-16 | 3.1E-12 | TMED1 | rs776909029 | 0.0366 | 0.0117 | 0.028 | 0.001 | 0.0009 |
| 9 | 34564740 | A | C | 6.5E-16 | 3.6E-12 | CNTFR | rs774039930 | 0.0516 | 0.0222 | 0.039 | 0.011 | 0.0008 |
| 3 | 108474687 | T | G | 1.4E-15 | 5.8E-12 | RETNLB | rs199707443 | 0.0328 | 0.0107 | 0.025 | 0.001 | 0.0493 |
| 19 | 43025485 | T | G | 2.6E-15 | 7.7E-12 | CEACAM1 | rs763190977 | 0.0921 | 0.0523 | 0.107 | 0.016 | 0.0026 |
| 3 | 31659462 | A | T | 4.8E-17 | 9.2E-12 | STT3B | rs74346226 | 0.0891 | 0.0514 | 0.076 | 0.035 | 0.131 |
| 12 | 109719316 | T | G | 9.1E-12 | 2.1E-10 | FOXN4 | rs760573591 | 0.0309 | 0.0115 | 0.025 | 0.003 | 1.5E-05 |
| 8 | 145112936 | T | C | 6.8E-14 | 5.0E-10 | OPLAH | rs781948612 | 0.0331 | 0.0114 | 0.026 | 0.002 | 0.0364 |
| 19 | 42799299 | T | C | 5.7E-12 | 1.4E-09 | CIC | rs745695673 | 0.019 | 0.0043 | 0.011 | 0.000 | 0 |
| 13 | 111164389 | A | C | 7.2E-12 | 1.6E-08 | COL4A2 | rs199702442 | 0.0517 | 0.0274 | 0.041 | 0.018 | 0.0285 |
| 22 | 30951295 | T | G | 2.0E-11 | 1.8E-08 | GAL3ST1 | rs762634521 | 0.0204 | 0.0056 | 0.013 | 0.001 | 0.028 |
Model 1 adjusted for sequencing center and the first four PCs underlying population substructure. Model 2 adjusted for sequencing center, the first 4 PCs, sex and APOE genotype.
Batch effects between exome capture kits among AD-associated variants
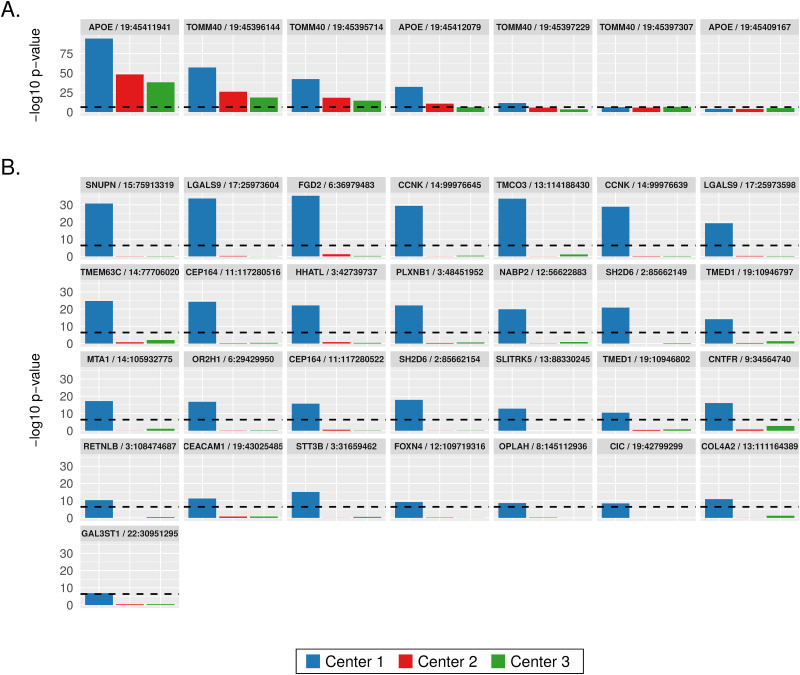
Additional analyses of the 36 SNPs listed in Table 1 revealed that the significance of association came more from the individuals sequenced at Center 1 using the Illumina exome capture kit than from those sequenced at Center 2 and Center 3 using the NimbleGen exome capture kit. Association analyses of center-specific cohorts demonstrated that this center bias occurred in previously known AD SNPs in APOE and TOMM40 (Fig 2A) as well as in the 29 novel SNPs (Fig 2B). The significance of AD association of the 29 novel SNPs came exclusively from the Center 1 cohort processed using the Illumina exome kit (Fig 2B), which may explain why the ADSP consortium did not report these SNPs.
Fig 2. Sequencing center specific association p-values of SNPs that reached exome-wide significance (denoted by the dashed horizontal lines) in the full-dataset analysis.
(A) Seven SNPs in TOMM40 and APOE. (B) Twenty-nine novel SNPs.
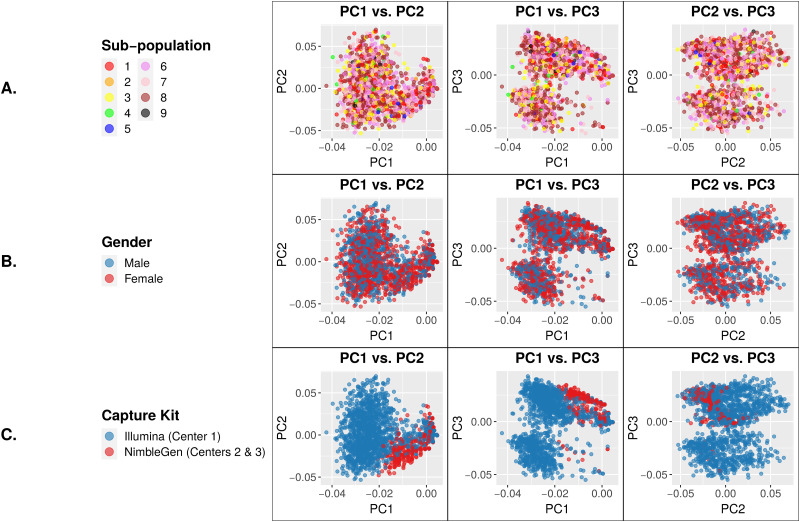
We next studied these 29 novel SNPs using PCA. The SNP genotypes showed no differences between sub-populations or genders (Fig 3A and 3B). However, we observed a clear and significant difference between samples captured by the Illumina kit (Center 1) vs. those captured by the Roche NimbleGen kit (Center 2 and Center 3) (Fig 3C), consistent with the observation that these SNPs are only associated with AD in Center 1 samples as shown in Fig 2B. Since our association models included sequencing center as a covariate, the batch differences visualized in Fig 3C clearly were due to other factors, probably at the individual-variant level.
Fig 3. PC eigenvector plots of genotypes at 29 exome-wide significant SNPs.
Each data point represents a single individual. Clustering of samples for a particular variable indicates genotypic similarity between individuals for the trait represented by that color. (A) PCs color coded based on sub-population. (B) PCs color coded based on gender. (C) PCs color coded based on capture kit. The NimbleGen-captured samples cluster tightly together, indicating their genotypic similarity that is distinct from the Illumina-captured samples.
Identification of variant-level differences in genotype quality and alternative allele fraction between two exome capture kits
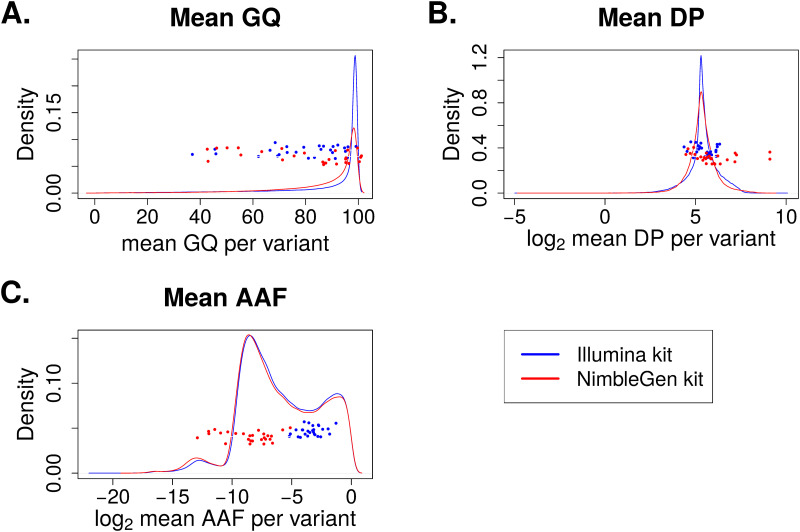
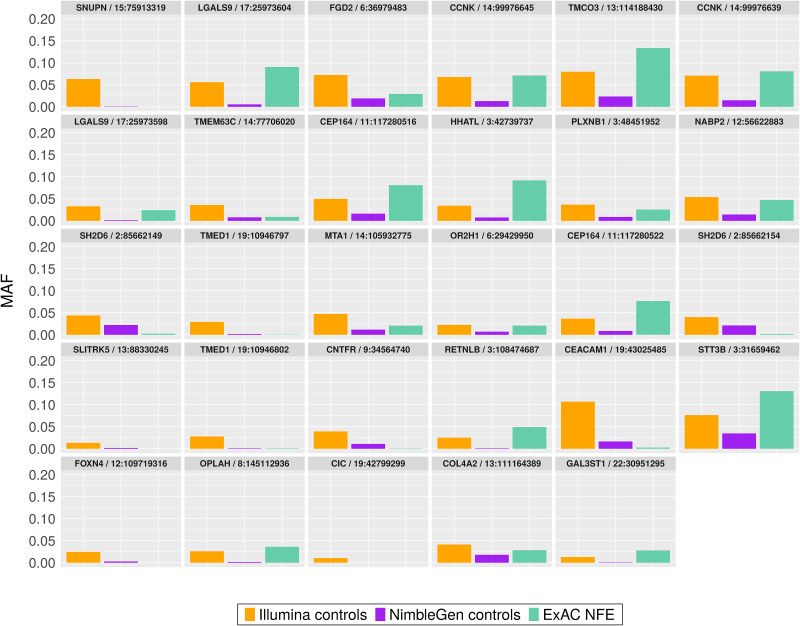
As the PCA plot of the 29 novel SNPs showed significant batch differences of genotypes between samples processed by two exome capture kits, we next examined variant-level factors that could explain why exclusively the Illumina kit-captured exomes yielded this set of highly significant novel SNPs. Although all analyzed SNPs passed VQSR quality thresholds, we speculated that there remained significant differences in qualities and characteristics at the individual-variant level between exome kits that might explain the greater significance of association in samples captured by one exome kit vs. the other. First, we compared several quality measures of the variants called in cohorts captured by the two kits. For the 166,947 SNPs used in the association analyses, we studied the distribution of the three most common variant quality parameters: GQ, DP and AAF (Fig 4). The distributions of mean GQ, DP, and AAF from 166,947 SNPs were very similar between capture kits. For the 29 SNPs of interest, the GQ (Fig 4A) and DP (Fig 4B) were also similar between capture kits. Interestingly, we observed a bi-modal distribution of AAF values from both capture kits, with the first mode closer to zero indicating approximately equal percentage of reads supporting reference and alternative alleles at the variant positions, and a second mode significantly deviated from zero and centered around log2 value of -6. This second mode represented variants with significantly lower percentage of reads supporting the alternative alleles than the reference alleles. More importantly, the AAFs of the 29 SNPs captured by the Illumina exome kit are mostly located close to the first mode and have a more balanced ratio of reference to alternative allele-supporting reads, while the AAFs of the 29 novel SNPs captured by the NimbleGen kit are located closer to the second mode. This observation implies that the NimbleGen kit lost most of the alternative alleles during exome capture, leading to the lack of variant calls in the NimbleGen cohort. Indeed, in the ADSP control group, the population minor allele frequencies (MAFs) of these 29 SNPs in the Illumina-captured cohort are generally higher compared to those in the NimbleGen-captured cohort, and the MAFs from the Illumina-captured cohort more closely resemble those in the Non-Finish European (NFE) population of the ExAC database (http://exac.broadinstitute.org/) (Table 1; Fig 5). This observation supports our hypothesis of more effective capture of the alternative alleles by the Illumina kit.
Fig 4. Density plots of variant quality parameters between two exome capture kits.
Mean values were computed across all samples for each variant. The solid lines show the distributions of all 166,947 variants used in the association analyses, and the scattered dots represent the 29 novel SNPs. (A) Density plot for mean genotype quality (GQ). (B) Density plot for mean read depth (DP). (C) Density plot for mean alternative allele fraction (AAF).
Fig 5. Minor Allele Frequency (MAF) of 29 exome-wide significant SNPs in AD control exomes processed by two capture kits and in the ExAC Non-Finnish European (NFE) population.
To further investigate variant quality differences between capture kits beyond the top 29 SNPs, we calculated distributions of log2 ratios of mean GQ, DP, and AAF between capture kits for all 166,947 variants (S4 Fig). We divided these variants into two groups: (1) the 10% of variants with the largest differences in GQ, DP, or AAF between two capture kits (5% at each of the two tails); and (2) the remaining 90% of variants. The PCA plots show that the 10% of variants with the biggest quality differences clearly contributed to the batch differences between the two exome capture kits (Fig 6A). Intriguingly, dividing these SNPs into those with better quality in Illumina-captured samples (right tail of the 5% variants; Fig 6B) and those with better quality in NimbleGen-captured samples (left tail of the 5% variants; Fig 6C) demonstrated that the batch differences came mostly from the latter. We observed no obvious batch contribution from the 90% of variants with similar GQ, DP, or AAF (Fig 6D).
Fig 6. PC eigenvector plots of genotypes at variants lying in different sections of quality-metric ratio distributions.
Each data point represents a single individual, color coded according to capture kit. (A) PCs of variants in either 5% tail. (B) PCs of variants in right 5% tail. (C) PCs of variants in left 5% tail. (D) Variants in middle 90% of distributions. Variants in the tails, in particular the left 5% tail (better quality in NimbleGen kit), show clear separation by capture kit in both cases and controls.
Discussion
We analyzed 9,904 exomes from ADSP to study the impact of batch effects on variant calling and association analysis. We focused on the known batches of three different sequencing centers and two exome capture kits. At first, no batch differences between sequencing centers or exome capture kits were visible from the PCA plots, and visually we were able to attribute all sample clusters in PCA to population substructure (Fig 1B). Sequencing center was included as a covariate in our association analyses; however, exome capture kit was not included in the association models because it confounds with sequencing center and therefore is not an independent variable. Besides the two models described in this paper, we analyzed the data using a third model which included age as a covariate. This third model identified no AD-associated variants because age confounds with disease status by design. Our results from Model 3 are similar to those reported by the ADSP consortium. Our association analyses identified 29 novel SNPs in addition to variants in previously known AD genes including APOE and TOMM40 as well as variants reported by the ADSP consortium [19]. These 29 SNPs exemplified the impact of batch effects that were later attributed to the differences between the two exome capture kits. First, the significance of association with AD came exclusively from the samples processed by the Illumina exome kit at Center 1, while those processed by the NimbleGen kit at either Center 2 or Center 3 lacked significance (Fig 2B). Second, PCA of these 29 SNPs clearly showed separation of the samples according to capture kit (Fig 3C). The qualities of the variants between the two captured kits were similar overall at first glance but significantly different for a substantial number of variants (Figs 4 and 6). The fact that the majority of the variants had similar quality measures suggested that batch effects impacted individual variants rather than all variants.
Further analyses of the 29 SNPs of interest (Fig 4) pinpointed the difference in AAF (Alternative Allele Fraction; percent of reads supporting alternative allele) as a source of batch differences between the Illumina- and NimbleGen- processed cohorts. Although the distributions of the quality parameters GQ, DP, and AAF among all variants showed no differences between capture kits, the AAF of the 29 novel SNPs in particular were strikingly higher within the Illumina-processed cohort (Fig 4C), suggesting a batch effect that impacted individual variants rather than all variants. In addition, we observed a batch difference in GQ among the top 10% most different variants between the two capture kits (Fig 6A) and among the variants with better overall quality in the NimbleGen kit (Fig 6C). It is clear that the NimbleGen kit did not effectively capture the alternative alleles for these 29 SNPs, which implies that the variant calling of Illumina-captured was more reliable, and that the AD association of these 29 SNPs unique to the Illumina exome kit is likely real. We speculate that in previous publications the ADSP consortium applied an undisclosed filtering step to exclude variants with discordant significance between cohorts processed at different sequencing centers and/or by different exome capture kits, which would explain why these 29 SNPs have not been reported (e.g. [19]). Additional investigation of the genes connected to these 29 SNPs supports this speculation. Multiple genes underlying these 29 SNPs have previously been linked to neural function or pathology, including Transmembrane P24 Trafficking Protein 1 (TMED1; rs767166604, association p = 7.22 x 10−15 for Illumina kit cohort; rs776909029, p = 3.25 x 10−11), Plexin B1 (PLXNB1; rs770786389, p = 7.49 x 10−23), Capicua Transcriptional Repressor (CIC; rs745695673, p = 3.70 x 10−9), Centrosomal Protein 164 (CEP164; rs756182128, p = 5.66 x 10−25; rs758240656, p = 1.94 x 10−16), and Cyclin K (CCNK; rs745936510, p = 3.98 x 10−30; rs778243462, p = 1.50 x 10−29). TMED1 is reported to interact with Amyloid Precursor Protein (APP) [21], whose cleavage into amyloid beta generates one of the key components of AD-associated pathological protein aggregation [22]. PLXNB1 influences amyloid beta load [23], and CIC is a transcriptional repressor whose inactivation promotes gliomagenesis, the formation of glial tumors in the brain [24]. CEP164 binds to TTBK2, a kinase that phosphorylates tau [25] and contributes to neurodegeneration in frontotemporal dementia [26]. Finally, copy-number mutations in the transcriptional regulator CCNK have been associated with neurodevelopmental abnormalities [27].
Because not all batch effects are created equal, no universal approach can manage all batch effects in every study. For large sequencing-based genetic studies, differences from sample storage/preparation methods, sequencing library construction protocols such as the choice of exome capture kits, and bioinformatics pipelines all result in batch differences that are potentially associated with data quality differences. The default approach today is to require concordant variant significances from all major batches and to ignore disease-associated variants if the signals were observed in one batch only. This practice will lead to potential false negatives. Instead, the key is to search for the source of batch-specific variant significance by checking variant-level quality parameters including, but not limited to, variant genotyping quality, mapping quality of the reads, sequencing depth, alternative allele fractions, base qualities, and the concordance of the MAF in the control cohort with public variant databases. As we demonstrated in our study, if the batch effects can be attributed to quality differences, the batch-associated variants should not be automatically discarded. We recommend that variants impacted by batch effects be included in validation studies using a different dataset or by wet lab functional assays.
Conclusions
In summary, we discovered batch effects at the individual-variant level resulting from differences in GQ and AAF between different exome capture kits. In particular, we found that the signal of some variants most significantly associated with AD came exclusively from samples processed by one exome capture kit that was more effective at capturing the alternative alleles compared to the other kit. In general, a subset of variants with the biggest disparities in GQ and AAF values between the two exome capture kits contributed to the remaining batch effects. Our findings highlight the importance of additional variant-level quality control for large sequencing-based genetic studies. More importantly, we demonstrated that automatically filtering out variants with batch differences may lead to false negatives if the batch discordances come largely from quality differences and if the variants from one batch have better quality scores.
Methods
Ethics approval and consent to participate
All aspects of the study were approved the Institutional Review Boards of both Mayo Clinic and University of Illinois at Urbana-Champaign. This study was also approved by dbGAP. Written informed consent was obtained from all participants and surrogates by the ADSP consortium.
Variant calling
The paired-end sequence reads were aligned to the human reference genome build 37 using Novoalign (http://www.novocraft.com) (default parameters), which was selected on the basis of its greater accuracy in read placement relative to other methods [28, 29] and its lack of prior application to this dataset for association testing (e.g. [19, 30]). The alignment files were then sorted by read position using Novosort (http://www.novocraft.com), realigned around small insertions and deletions (INDELs) using Picard (https://broadinstitute.github.io/picard/), and subjected to base recalibration using the Genome Analysis Toolkit (GATK) version 3.4 [31]. Variant calling followed GATK’s best practices guidelines for germline variants (https://gatk.broadinstitute.org/hc/en-us): per-sample variant calling on the realigned, recalibrated BAM files was performed using HaplotypeCaller, and multi-sample joint genotyping of all 9,904 samples was performed using GenotypeGVCFs. Variant calling was conducted only on the exome regions common between the two exome capture kits (Illumina Rapid Capture Exome kit and NimbleGen VCRome v2.1 kit). Variants were annotated by SnpEff [32] and ANNOVAR [33].
Ancestry estimation
We implemented a series of filtering steps to select a pruned set of high-quality variants from the 1,584,609 variants detected across the cohort. Variants were excluded if they (1) failed VQSR; (2) lay within the highly variable HLA, LCT, 8p and 17q regions; (3) had MAF below 5% or deviation from Hardy-Weinberg Equilibrium below p < 1.0 E-4; or (4) had linkage disequilibrium r2 value above 0.2 within 0.1 megabase sliding windows. In total, 16,187 variants passed all filters, and 12,100 variants overlapped with variants detected in the 1000 Genomes samples of known ethnicities. The genotypes of the ADSP samples were combined with those of the 1000 Genomes samples at these 12,100 loci. PCs of the genotypes were then computed and plotted using the software plinkQC (https://meyer-lab-cshl.github.io/plinkQC/). The first four PCs of the ADSP genotypes had eigenvalues above 1 and were retained as covariates in the association analyses.
Sub-population estimation
The software Admixture [20] was used to estimate sub-population number from the ADSP data de novo, using the 16,187 variants described above. Sub-population k values between 1 and 20 were tested using default settings. A value of 9 produced the lowest cross-validation standard error, indicating that 9 sub-populations best fit the data.
Variant-level quality control for association analyses
Several steps were undertaken to minimize the number of false-positive variant calls prior to running the association models. The Variant Quality Score Recalibration (VQSR) step implemented in GATK uses machine learning algorithms to compute new, well-calibrated quality scores for each variant based on the annotations of a high-quality subset of the analyzed data. In accordance with GATK Best Practices for whole-exome data, the variables included in the VQSR model consisted of QD, MQ, MQRankSum, ReadPosRankSum, FS, SOR and InbreedingCoeff for SNPs; and QD, MQRankSum, ReadPOsRankSum, FS, SOR and InbreedingCoeff for INDELs [31]. A sensitivity threshold of 99.5 was used for SNPs and 99.0 for INDELs. Detected variants were excluded from association analysis if they failed VQSR, deviated significantly (p < 1.0 x 10−6) from Hardy-Weinberg equilibrium (HWE) in the control samples, or had an alternative allele call supported by fewer than 10 reads across the cohort (S2 Fig).
Association tests, statistical model and variant filtering
After quality control, association testing using disease status (case or control) as the phenotype was performed on the variants under an additive logistic regression model implemented in Plink 1.9 [34]. PCs were calculated using Plink. The association tests were conducted on all 9,904 samples together for the full-cohort analysis, as well as on sets of samples stratified by sequencing center for the center-based association analyses. Variants were considered exome-wide statistically significant at the Bonferroni-corrected threshold of p < 0.05 / # tests, as in [19]. Only bi-allelic SNPs with a recalibrated variant quality score (VQSLOD) > 0 were retained for further analysis.
Supporting information
Each data point represents a single individual. 1000 Genomes reference individuals are color-coded by ancestry. ADSP samples are shown in black. The position of ADSP samples relative to the 1000 Genomes reference samples indicates their genotypic similarity, which reflects ancestry. Most ADSP samples cluster near European reference samples (e.g. Finland and Spain).
(DOCX)
These variants totaled 120,572 from the Center 1 samples; 108,390 from the Center 2 samples; and 98,542 from the Center 3 samples. Approximately 70% of variants were shared among samples from all three sequencing centers. The larger number of variants detected in Center 3 samples was likely due to the larger number of individuals sequenced by Center 1 compared to the other two centers.
(DOCX)
The red vertical line denotes exome-wide statistical significance (p < 3.0 x 10−7).
(DOCX)
Mean values were computed across all samples for each variant. Solid vertical lines demarcate the boundaries separating the 5% tails from the middle 90% of the distribution. The scattered dots represent the positions of the top 29 SNPs within the distributions. Almost all top SNPs lie far to the right of the mean AAF ratio distribution, indicating that these variants are highly discrepant between capture kits.
(DOCX)
Acknowledgments
We thank the Mayo Clinic Bioinformatics Core for their help downloading and processing all exome sequencing data.
Abbreviations
- AAF
Alternative Allele Fraction
- ADSP
Alzheimer’s Disease Sequencing Project
- DP
Sequencing Depth of Coverage
- GATK
Genome Analysis Toolkit
- GQ
Genotype Quality
- GWAS
Genome-Wide Association Study
- MAF
Minor Allele Frequency
- PCA
Principal Components Analysis
- QC
Quality Control
- SNP
Single Nucleotide Polymorphism
- VQSR
Variant Quality Score Recalibration
Data Availability
All exome sequencing data used in this paper are available from dbGAP (accession number phs000572): https://www.ncbi.nlm.nih.gov/gap/.
Funding Statement
This research was supported in part by the Mayo Clinic Center for Individualized Medicine and Illinois Alliance Fellowships for Technology-Based Healthcare Research program awarded to DWW. This research is also part of the Blue Waters sustained-petascale computing project, which is supported by the National Science Foundation grants (Nos. OCI-0725070 and ACI-1238993) awarded to MEH, LSM, and YWA, and the state of Illinois. Blue Waters is a joint effort of the University of Illinois at Urbana-Champaign and its National Center for Supercomputing Applications. This study was also supported in the form of funding awarded to YWA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goh WW, Wang W, Wong L. Why batch effects matter in omics data, and how to avoid them. Trends Biotechnol. 2017;35(6):498–507. 10.1016/j.tibtech.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 2.Koire A, Katsonis P, Lichtarge O. Repurposing germline exomes of the Cancer Genome Atlas demands a cautious approach and sample-specific variant filtering. Pacific Symp Biocomput. 2016;21:207–18. [PMC free article] [PubMed] [Google Scholar]
- 3.Rasnic R, Brandes N, Zuk O, Linial M. Substantial batch effects in TCGA exome sequences undermine pan-cancer analysis of germline variants. BMC Cancer. 2019;19(1):1–10. 10.1186/s12885-018-5219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley AR, Standish KA, Bhutani K, Ideker T, Lasken RS, Carter H, et al. Pan-cancer analysis reveals technical artifacts in TCGA germline variant calls. BMC Genomics. 2017;18(1):1–15. 10.1186/s12864-016-3406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang VG, Kim H, Chuang JH. Whole-exome sequencing capture kit biases yield false negative mutation calls in TCGA cohorts. PLoS One. 2018;13(10):1–14. 10.1371/journal.pone.0204912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beecham GW, Bis JC, Martin ER, Choi SH, DeStefano AL, Van Duijn CM, et al. Clinical/Scientific Notes: The Alzheimer’s disease sequencing project: Study design and sample selection. Neurol Genet. 2017;3(5): 10.1212/NXG.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2(12):2074–93. 10.1371/journal.pgen.0020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 9.Vansteelandt S, Goetgeluk S, Lutz S, Waldman I, Lyon H, Schadt EE, et al. On the adjustment for covariates in genetic association analysis: A novel, simple principle to infer direct causal effects. Genet Epidemiol. 2009;33:394–405. 10.1002/gepi.20393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, Mitra N, Kanetsky PA, Nathanson KL, Rebbeck TR. A practical approach to adjusting for population stratification in genome-wide association studies: Principal components and propensity scores (PCAPS). Stat Appl Genet Mol Biol. 2018;17(6): 10.1515/sagmb-2017-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varma M, Paskov KM, Jung JY, Chrisman BS, Stockham NT, Washington PY, et al. Outgroup machine learning approach identifies single nucleotide variants in noncoding DNA associated with autism spectrum disorder. Pacific Symp Biocomput. 2019;24:260–71. [PMC free article] [PubMed] [Google Scholar]
- 12.Nygaard V, Rødland EA, Hovig E. Methods that remove batch effects while retaining group differences may lead to exaggerated confidence in downstream analyses. Biostatistics. 2016;17(1):29–39. 10.1093/biostatistics/kxv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna A, Hanna M, Banks E, Sivacheko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science (80-). 1993. August;261:921–3. 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 16.Ren Y, Reddy J, Pottier C, Sarangi V, Tian S, Sinnwell J, et al. Identification of missing variants by combining multiple analytic pipelines. BMC Bioinformatics. 2018;19: 10.1186/s12859-018-2151-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun G, Flickinger M, Hetrick K, Romm J, Doheny K, Abecasis G, et al. Detecting and estimating contamination of human DNA samples in sequencing and array-based genotype data. Am J Hum Genet. 2012. November;91:839–48. 10.1016/j.ajhg.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26(22):2867–73. 10.1093/bioinformatics/btq559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bis JC, Jian X, Kunkle BW, Chen Y, Hamilton-Nelson KL, Bush WS, et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-Associated variants involved in immune response and transcriptional regulation. Mol Psychiatry. 2018; 10.1038/s41380-018-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–64. 10.1101/gr.094052.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Prete D, Suski JM, Oulès B, Debayle D, Gay AS, Lacas-Gervais S, et al. Localization and processing of the amyloid-β protein precursor in mitochondria-associated membranes. J Alzheimer’s Dis. 2017;55:1549–70. 10.3233/JAD-160953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penke B, Bogár F, Fülöp L. β-amyloid and the pathomechanisms of Alzheimer’s disease: A comprehensive view. Molecules. 2017;22: 10.3390/molecules22101692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mostafavi S, Gaiteri C, Sullivan SE, White CC, Tasaki S, Xu J, et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat Neurosci. 2018;21:811–9. 10.1038/s41593-018-0154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang R, Chen LH, Hansen LJ, Carpenter AB, Moure CJ, Liu H, et al. Cic loss promotes gliomagenesis via aberrant neural stem cell proliferation and differentiation. Cancer Res. 2017;77(22):6097–108. 10.1158/0008-5472.CAN-17-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao J, Yang T, Weng R, Kuo C, Chang C. TTBK2: A tau protein kinase beyond tau phosphorylation. Biomed Res Int. 2015; 10.1155/2015/575170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor LM, McMillan PJ, Liachko NF, Strovas T, Ghetti B, Bird T, et al. Pathological phosphorylation of tau and TDP-43 by TTBK1 and TTBK2 drives neurodegeneration. Mol Neurodegener. 2018;13(7): 10.1186/s13024-018-0237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Yin W, Hu B, Kline AD, Zhang VW, Liang D, et al. De novo mutations of CCNK cause a syndromic neurodevelopmental disorder with distinctive facial dysmorphism. Am J Hum Genet. 2018;103(3):448–55. 10.1016/j.ajhg.2018.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv. 2013;1303.3997(00):http://arxiv.org/abs/1303.3997.
- 29.Thankaswamy-Kosalai S, Sen P, Nookaew I. Evaluation and assessment of read-mapping by multiple next-generation sequencing aligners based on genome-wide characteristics. Genomics. 2017;109:186–91. 10.1016/j.ygeno.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Patel T, Brookes KJ, Turton J, Chaudhury S, Guetta-Baranes T, Guerreiro R, et al. Whole-exome sequencing of the BDR cohort: evidence to support the role of the PILRA gene in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2018;44:506–21. 10.1111/nan.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. From fastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Current Protocols in Bioinformatics. 2013. 10.1002/0471250953.bi1110s43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010. September;38(16): 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang C, Chow C, Tellier L, Vattikuti S, Purcell S, Lee J. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each data point represents a single individual. 1000 Genomes reference individuals are color-coded by ancestry. ADSP samples are shown in black. The position of ADSP samples relative to the 1000 Genomes reference samples indicates their genotypic similarity, which reflects ancestry. Most ADSP samples cluster near European reference samples (e.g. Finland and Spain).
(DOCX)
These variants totaled 120,572 from the Center 1 samples; 108,390 from the Center 2 samples; and 98,542 from the Center 3 samples. Approximately 70% of variants were shared among samples from all three sequencing centers. The larger number of variants detected in Center 3 samples was likely due to the larger number of individuals sequenced by Center 1 compared to the other two centers.
(DOCX)
The red vertical line denotes exome-wide statistical significance (p < 3.0 x 10−7).
(DOCX)
Mean values were computed across all samples for each variant. Solid vertical lines demarcate the boundaries separating the 5% tails from the middle 90% of the distribution. The scattered dots represent the positions of the top 29 SNPs within the distributions. Almost all top SNPs lie far to the right of the mean AAF ratio distribution, indicating that these variants are highly discrepant between capture kits.
(DOCX)
Data Availability Statement
All exome sequencing data used in this paper are available from dbGAP (accession number phs000572): https://www.ncbi.nlm.nih.gov/gap/.