Abstract
Objectives
Increasing evidence suggests that SARS-CoV-2 infection may lead to severe and multi-site vascular involvement. Our study aimed at assessing the frequency of vascular and extravascular events’ distribution in a retrospective cohort of 42 COVID-19 patients.
Methods
Patients were evaluated by whole-body CT angiography between March 16 and April 30, 2020. Twenty-three out of the 42 patients evaluated were admitted to the intensive care unit (ICU). Vascular and extravascular findings were categorized into “relevant” or “other/incidental,” first referring to the need for immediate patient care and management. Student T-test, Mann-Whitney U test, or Fisher exact test was used to compare study groups, where appropriate.
Results
Relevant vascular events were recorded in 71.4% of cases (n = 30). Pulmonary embolism was the most frequent in both ICU and non-ICU cases (56.5% vs. 10.5%, p = 0.002). Ischemic infarctions at several sites such as the gut, spleen, liver, brain, and kidney were detected (n = 20), with multi-site involvement in some cases. Systemic venous thrombosis occurred in 30.9% of cases compared to 7.1% of systemic arterial events, the first being significantly higher in ICU patients (p = 0.002). Among incidental findings, small-sized splanchnic arterial aneurysms were reported in 21.4% of the study population, with no significant differences in ICU and non-ICU patients.
Conclusions
Vascular involvement is not negligible in COVID-19 and should be carefully investigated as it may significantly affect disease behavior and prognosis.
Key Points
• Relevant vascular events were recorded in 71.4% of the study population, with pulmonary embolism being the most frequent event in ICU and non-ICU cases.
• Apart from the lung, other organs such as the gut, spleen, liver, brain, and kidneys were involved with episodes of ischemic infarction. Systemic venous and arterial thrombosis occurred in 30.9% and 7.1% of cases, respectively, with venous events being significantly higher in ICU patients (p = 0.002).
• Among incidental findings, small-sized splanchnic arterial aneurysms were reported in 21.4% of the whole population.
Keywords: COVID-19, Thrombosis, Embolism, Infarction, CT angiography
Introduction
The rapid worldwide infection spreading of the emergent coronavirus SARS-CoV-2 and its related disease termed COVID-19 has dramatically challenged the medical community. This is because of the unexpectedly high degree of aggression of possible clinical scenarios, with the lung being the most frequently affected site [1–3]. Manifestations of COVID-19 range from asymptomatic infection and mild upper respiratory illness to severe bilateral pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, and death [4–6]. Since the first SARS-CoV-2 related pneumonia cases were reported in Wuhan, China [1–3], growing literature evidence suggests that COVID-19 is a systemic disease as it may affect almost all the human organs. In this issue, both venous and arterial thrombosis events have been reported as the main complications of COVID-19 in intensive care unit (ICU) patients, likely due to the uncontrolled cytokine storm in severely ill cases [7–9]. Pulmonary embolism (PE), deep vein thrombosis (DVT), systemic arterial embolism, myocardial infarction, and ischemic stroke are among the most frequently encountered manifestations that contribute to increased morbidity and mortality [8–15].
Whole-body CT angiography (WB-CTA) allows the investigation of all vascular sites of the body for diagnostics/prognostication and treatment purposes within a short amount of time with a single procedure. Commonly performed in multi-trauma patients, WB-CTA is used in a wide array of vascular diseases ranging from aneurysmal disease, arterial occlusive disease, thromboembolic disease, venous disease, vasculitides, nontraumatic hemorrhage, to vascular malformations. Given the increasing evidence of the severe and multi-site vascular involvement in COVID-19 patients, we investigated whether WB-CTA may have a role in the diagnostic workup in such a complex scenario.
Our study aimed at assessing the frequency distribution of systemic vascular events recorded using WB-CTA in a retrospective COVID-19 cohort of patients admitted to our hospital because of SARS-CoV-2 related pneumonia between March 16 and April 30, 2020. Extravascular findings were assessed as well.
Materials and methods
The study was approved by the local institutional Ethics Committee (registration number: 0021131/2020) and was conducted according to the Declaration of Helsinki principles.
All contrast-enhanced WB-CTA scans performed in our center between March 16 and April 30, 2020, in patients tested positive for SARS CoV-2 were retrospectively obtained from the Picture Archiving and Communication System (PACS). WB-CTA was performed as follows: baseline brain and lung volumes were acquired before the double-phase high-speed injection of the contrast medium (arterial and venous phase acquisition) that allowed further sampling of the brain, neck, chest, abdomen, and pelvis. More in detail, 1.5–2 ml/kg of a non-ionic contrast agent (iopamidol 370 mg/ml) was injected at the flow rate of 3 ml/s, followed by 30 ml saline flush. The scanning delay was assessed using a contrast agent bolus tracking system with the region of interest (ROI) indicator placed on the aortic arch. The scan was initiated with a minimal delay after the signal attenuation in the ascending aorta reached the threshold of 100 Hounsfield units (HU) for the arterial phase acquisition. The venous phase followed 100 s later. Data acquisition was performed with a 128-MDCT Ingenuity scanner (Philips Healthcare) and a 64-MDCT LightSpeed VCT scanner (GE Healthcare) with the following parameters: 120 kV; 100–200 mAs; pitch 0.75–1.5; and collimation 0.625–1.25 mm. Images were reconstructed with a slice thickness of 1–1.25 mm using a high sharpness reconstruction algorithm (bone filter for lung evaluation). A dedicated soft tissue filter was used to assess the neck, abdomen, and pelvis; the same was performed for the brain study. WB-CTA images were acquired with the patient in the supine position with a scout view ranging from the vertex to the pubic symphysis. The arms were placed above the head in non-ICU patients and close to the body in ICU patients. For the lung study, full inspiration was required when feasible. Thorax imaging was analyzed with both lung (width 1500 HU; level −700 HU) and mediastinal (width 350 HU; level 40 HU) window settings. A dedicated reconstruction filter was adopted for the study of the extra-thoracic organs. Multi-planar reconstruction (MPR) was used to resolve any doubt interpretation.
Both vascular and alterations were recorded. According to Thompson RJ et al, findings were classified as relevant, that is related to the chief complaint, strictly pertinent to immediate patient care and management; or other/incidental, which are findings unrelated to the chief complaint and not relevant to the primary patient care [16]. All WB-CTA studies were first independently and blindly reviewed by four radiologists with 8–20 years of experience and then collectively discussed to reach a consensus. Inter-observer disagreement occurred in a few cases and concerned the scoring of lung disease severity. In these cases, the average value arisen from the evaluation of the four radiologists was used.
Statistical analysis
Numerical variables were described using the mean ± standard deviation (SD) in the symmetrical distribution or the median with interquartile range [25th; 75th percentile] in case of variables showing consistent skewness. Categorical variables were summarized using absolute frequencies and percentages. The Student T-test, the Mann-Whitney U test, or the Fisher exact test was used to compare study groups, where appropriate. All tests were two-tailed. A p value < 0.05 was considered significant.
Results
During the study period, 42 out of 126 hospitalized patients underwent contrast-enhanced WB-CTA, including 23 cases admitted to ICU. The main clinical indications in no-ICU patients included acute onset or unexplained worsening of dyspnea, sudden onset of edema of the limbs, and critical consciousness alterations. Worsening hypoxemia and D-dimer increase ( > 2-fold) were the main hallmarks in ICU cases. Overall, the mean time elapsed from fever onset and WB-CTA was 19.6 ± 6 days (including the pre-hospitalization period). Demographics and clinical features of the study population are reported in Table 1. As shown, there were no significant differences in gender or age between ICU and non-ICU patients, while active smokers’ frequency was higher in the former. Also, the prevalence of patients with comorbidities, like type II diabetes, was significantly higher among ICU cases, with most of them being also obese. The prevalence of systemic hypertension was similar in the two study groups, while no patients had an underlying chronic lung disease. SARS-CoV-2 related pneumonia was detected in all of them with an estimated mean CT disease extent of 13.7 according to the lung severity score by Zhao W et al [17]. Lung involvement was significantly greater in ICU patients, all of them requiring mechanical ventilation, as compared to non-ICU cases (16 ± 3.2 vs. 11 ± 4.1, p < 0.0001). Figure 1 shows representative bilateral pneumonia with ARDS-like features in an ICU patient. All patients were under anti-viral therapy with lopinavir/ritonavir and paracetamol as needed.
Table 1.
Demographics and clinical features of the study population
| Parameter | ICU patients (n = 23) |
Non-ICU patients (n = 19) |
p |
|---|---|---|---|
| Age (yr) | 58 ± 12 | 64 ± 14 | 0.163 |
| Gender, M (%) | 16 (69) | 14 (74) | 1.000 |
| Smoking (%) | 11 (48) | 2 (10) | 0.017 |
| Type II diabetes (%) | 19 (83) | 5 (26) | < 0.001 |
| Systemic hypertension (%) | 8 (35) | 5 (26) | 0.739 |
| Obesity (%) | 11 (48) | 2 (10) | 0.017 |
| D-dimer (ng/ml) | 570 [368–2908] | 690 [336–1402] | 0.940 |
| C-reactive protein (mg/dl) | 17 [11.5–28.8] | 15 [7.4–22.3] | 0.172 |
| Ferritin (ng/ml) | 1052 [527–1212] | 718 [478–1211] | 0.312 |
| Interleukin-6 (ng/ml) | 356 [41–763] | 45 [33–64] | 0.003 |
| Blood lymphocytes/mm3 | 800 [600–1100] | 870 [720–1410] | 0.456 |
| Endotracheal intubation (%) | 23 (100) | 0 (0) | < 0.001 |
| Non-invasive ventilation (%) | 0 (0) | 8 (44) | 0.001 |
| Hydroxychloroquine (%) | 23 (100) | 19 (100) | 1 |
| Tocilizumab/sarilumab (%) | 9 (39) | 8 (42) | 1 |
| Azithromycin (%) | 0 (0) | 19 (100) | < 0.001 |
| Systemic steroids (beta- or dexamethasone) (%) | 18 (78) | 13 (68) | 0.504 |
| LMW prophylaxis (%) | 23 (100) | 8 (44) | < 0.001 |
| Death (%) | 6 (26) | 0 (0) | 0.024 |
Data are expressed as mean ± SD or as median [25th–75th], where appropriate. Statistically significant results are reported in bold
Fig. 1.
Axial thin-section baseline unenhanced lung CT scan in a 33-year-old patient who presented with fever and cough, ultimately requiring ICU admission due to significant worsening of respiratory failure. Diffuse bilateral confluent and patchy ground-glass and consolidative pulmonary opacities are evident from the lung apices (a, b) to the bases (c, d), with a total severity score of 20/24 (see text)
The frequency distribution of relevant vascular events detected using WB-CTA is depicted in Table 2A. Overall, they were reported in 71.4% (n = 30) of the study population, with a prevalence of 91% (n = 21) and 47% (n = 9) in ICU and non-ICU patients, respectively. Relevant vascular findings included both thrombotic/thromboembolic and hemorrhagic events. Pulmonary thromboembolism was the most frequent event in the whole population (35.7%), occurring in more than half of the ICU patients. It was associated with local or multi-focal detectable lung infarcts in 19% of cases (n = 8). A representative example of a massive PE occurring in a previously healthy young patient with no known risk factors is reported in Fig. 2. Ischemic infarction of other organs affected the gut, the spleen, the liver, the brain, and the kidneys with an increasing prevalence and a simultaneous ischemic occurrence in some patients, as shown in Fig. 3. Multi-focal venous thrombotic events were reported in 30.9% of the whole study population (n = 13). The majority of these events (11/13, 85%) occurred in the superior vena cava district with a frequency significantly higher in ICU patients, as detailed in Table 2A. Conversely, thrombosis of two main arteries (aorta and internal carotid artery) was reported in three cases with an overall prevalence of 7.1% and no differences between the two study groups. Active bleeding was recorded in 11.8% of patients (n = 5) with the involvement of skeletal muscles. Bleeding included both spontaneous (n = 2) and iatrogenic events (n = 3). A representative patient with multi-focal hemorrhages is shown in Fig. 4.
Table 2.
Frequency distribution of relevant (A) and other/incidental (B) findings of vascular events by contrast-enhanced whole-body CT angiography
| Parameter | Total patients (n = 42) | ICU patients (n = 23) |
Non-ICU patients (n = 19) |
p |
|---|---|---|---|---|
| A | ||||
| Ischemic brain injury | 6 (14.2%) | 4 (17.4%) | 2 (10.5%) | 0.672 |
| Pulmonary thrombo-embolism | 15 (35.7%) | 13 (56.5%) | 2 (10.5%) | 0.003 |
| Pulmonary infarction | 8 (19%) | 5 (21.7%) | 3 (15.7%) | 0.709 |
| Venous thrombosis of the superior vena cava district (jugular/subclavian/axillary/superior vena cava) | 11 (26%) | 10 (43.4%) | 1 (5.2%) | 0.005 |
| Venous thrombosis of the inferior vena cava district (ileo-femoral) | 2 (4.7%) | 2 (8.7%) | 0 | 0.492 |
| Aorta thrombosis | 1 (2.3%) | 1 (4.3%) | 0 | 1 |
| Internal carotid artery thrombosis | 2 (4.7%) | 2 (8.7%) | 0 | 0.492 |
| Hepatic infarction | 2 (4.7%) | 2 (8.7%) | 0 | 0.492 |
| Splenic infarction | 3 (7.1%) | 1 (4.3%) | 2 (10.5%) | 0.581 |
| Renal infarction | 8 (19%) | 6 (26%) | 2 (10.5%) | 1 |
| Mesenteric ischemia | 1 (2.3%) | 1 (4.3%) | 0 | 0258 |
| Iliopsoas hematoma with active bleeding | 4 (9.5%) | 2 (8.7%) | 2 (10.5%) | 1 |
| Rectus abdominis/quadriceps femoris hematoma with active bleeding | 1 (2.3%) | 1 (4.3%) | 0 | 1 |
| B | ||||
| Main pulmonary artery enlargement (> 30 mm) | 2 (4.7%) | 2 (8.7%) | 2 (10.5%) | 1 |
| Portal vein enlargement (> 16 mm) | 8 (19%) | 4 (17.4%) | 4 (21%) | 1 |
| Small-sized splanchnic arterial aneurysms | 9 (21.4%) | 7 (30%) | 2 (10.5%) | 0.149 |
Data are reported as absolute number (%). Statistically significant results are reported in bold
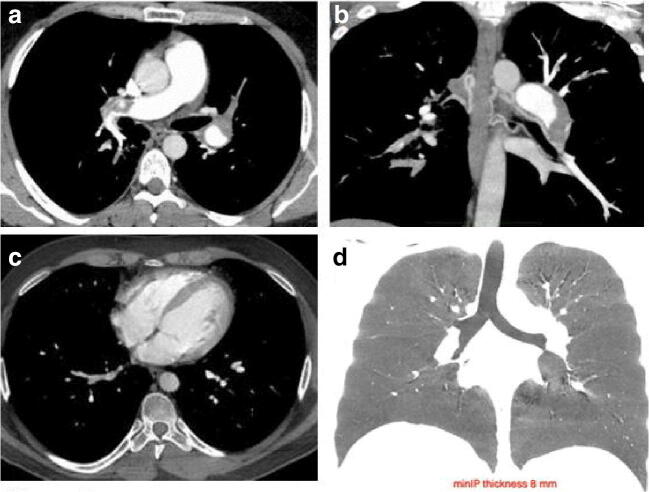
Fig. 2.
WB-CTA in a 33-year-old patient (same case of Fig. 1) showing the presence of extensive embolic obstruction of the right (a) and left (b, multi-planar reconstruction) main branch of the pulmonary artery (a and b, respectively). Dilatation of the bronchial arteries is also appreciable (b, MPR reconstruction). This finding was likely suggestive of unrecognized subclinical recurrent or chronic PE (unremarkable clinical history, no previous CT imaging available). However, no indirect features compatible with chronic thromboembolic pulmonary hypertension, like deviation of the interventricular septum and mosaic perfusion, were detected, as respectively shown in panels c (axial section on the heart chambers) and d (min-IP coronal view of the lungs)
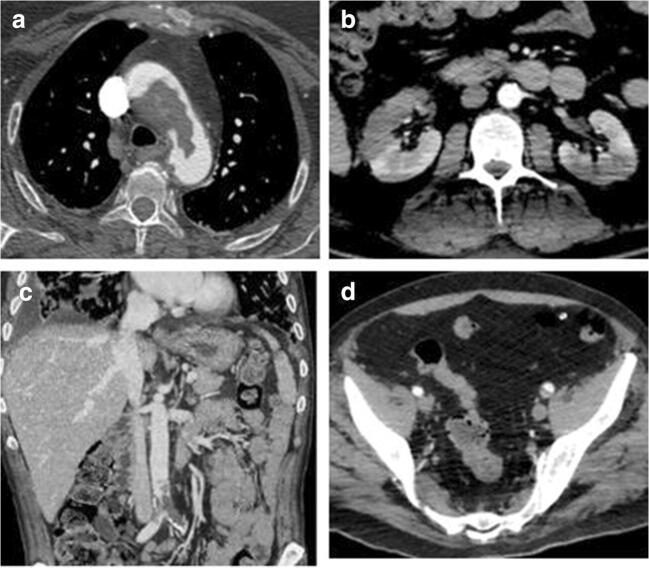
Fig. 3.
WB-CTA showing an extensive thrombotic filling defect within the aortic arch (a) and bilateral kidney infarction (b) in a 57-year-old no-ICU patient with acute chest pain and sudden onset of discoloration of the upper limbs. Thrombosis of the distal inferior cava and iliac veins (c) along with the concomitance of left iliac artery partial thrombotic occlusion in a 68-year-old ICU patient with acute onset of lower limb edema
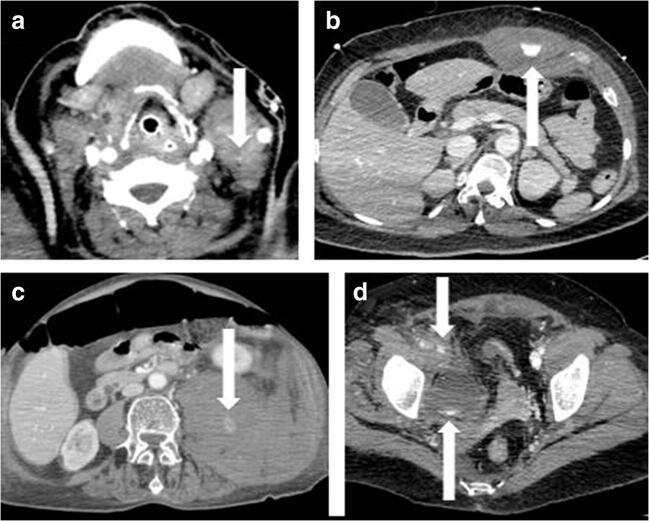
Fig. 4.
Multi-site active bleeding in a 63-year-old ICU patient with sudden onset of dyspnea along with severe acute anemia. Arrows show contrast medium extravasation in the left carotid space (a), in the left rectus abdominis muscle (b), in the left psoas muscle and homolateral perirenal/posterior pararenal space (c), and the right iliac extra-peritoneal space (d)
Other/incidental vascular-related findings are reported in Table 2B. They included portal vein enlargement and small-sized splanchnic arterial aneurysms (21.4% of the whole population), with no significant differences in ICU and non-ICU patients. The enlargement of the main pulmonary artery was also evident in both study subgroups.
The most prevalent relevant extravascular findings are summarized in Table 3A. Pneumothorax and pneumomediastinum accounted for 14% of events, with no differences among ICU and non-ICU cases. Acute pancreatitis was detected in 3 ICU patients with no relation to previous chronic alcohol consumption or gallbladder or bilious tract diseases. According to the revised Atlanta classification [18], pancreatitis was mild in 2 cases and moderate to severe in the other one.
Table 3.
Frequency distribution of relevant (A) and other/incidental (B) findings of extravascular events by contrast-enhanced whole body CT angiography
| Parameter | Total patients (n = 42) | ICU patients (n = 23) | Non-ICU patients (n = 19) | p |
|---|---|---|---|---|
| A | ||||
| Pneumothorax | 3 (7.1%) | 2 (8.7%) | 1 (5.2%) | 1 |
| Pneumomediastinum | 3 (7.1%) | 3 (13%) | 0 | 0.238 |
| Acute pancreatitis | 3 (7.1%) | 3 (13%) | 0 | 0.238 |
| B | ||||
| Cervical subcutaneous emphysema | 3 (7.1%) | 3 (13%) | 0 | 0.238 |
| Hydropericardium | 2 (4.7%) | 2 (8.7%) | 0 | 0.492 |
| Pneumoretroperitoneum | 2 (4.7%) | 2 (8.7%) | 0 | 0.492 |
| Mediastinal lymphadenopathy | 2 (4.7%) | 1 (4.3%) | 1 (5.2%) | 1 |
| Ascites | 11 (26%) | 9 (39%) | 2 (10.5%) | 0.075 |
| Hepatomegaly | 12 (28.5%) | 8 (34.7%) | 4 (21%) | 0.494 |
| Splenomegaly (> 15 cm) | 7 (16.6%) | 5 (21.7%) | 2 (10.5%) | 0.427 |
| Hydropic gallbladder | 1 (2.3%) | 1 (4.3%) | 0 | 1 |
Data are reported as absolute number (%)
Other/incidental extravascular findings included a wide array of alterations depicted in Table 3B. Overall, there were no significant differences between ICU and non-ICU patients. The most prevalent findings were hepatomegaly, ascites, and splenomegaly, with a decreasing frequency ranging from 28.5 to 16.6%.
Six ICU patients died, accounting for an overall mortality rate of 26%.
Discussion
To the best of our knowledge, this is the first report addressing the prevalence distribution of systemic vascular alterations evaluated using whole-body CT angiography. This retrospective case series refers to a selected cohort of COVID-19 patients that we observed during the first pandemic wave in our department when treatment strategies were not yet efficiently tailored. The choice of performing WB-CTA was clinically guided but was also evaluated from a logistical point of view as it avoided the need for repeated patient examinations that would require transportation of the patient from the ward to the radiology service with a potential increased risk of infection spreading among hospital staff and other patients.
Vascular CT findings were recorded both in ICU and non-ICU patients and were differentiated into either clinically relevant or incidental. They were most frequently represented in ICU patients, in whom more advanced lung disease, comorbidities, obesity, and increased IL-6 levels played as triggering factors. In agreement with previous observations, pulmonary embolism was the most frequent event (35.7% of the total patient population), with 86% of ICU patients. None of them had a previous history of deep vein thrombosis or PE. Venous thrombosis occurred in different sites and accounted for 26% of total clinically relevant events, with 62.5% of cases admitted to ICU. This finding is in line with data by Santoliquido et al, who reported an 11.9% incidence of deep vein thrombosis in a cohort of 84 non-ICU COVID-19 patients receiving thromboprophylaxis [19]. Additional vascular events widely ranged from pulmonary infarction and ischemic brain injury to infarction of abdominal parenchymal organs. The frequency distribution of these events was similar in ICU and non-ICU patients, suggesting a sort of dysregulation of the coagulation process even in less severe cases. Unlike vein thrombosis, only a few reports have been focused on systemic arterial embolism in COVID-19 patients. Among these, Klok FA et al reported a 3.7% prevalence of arterial thrombotic events in 184 ICU patients [20], while Kashi M et al more recently described a case series of additional 7 cases [21]. Increasing evidence suggests infection of endothelial cells by SARS-COV-2 to be relevant to pathogenesis [22]. In our case series, the prevalence of systemic vein thrombosis was 30.9% compared to the 7.1% of arterial thrombosis, with venous events mostly occurring in ICU patients compared to non-ICU cases. Altogether, these observations are of utmost clinical relevance as early anticoagulation therapy has been shown to significantly improve patient outcomes in moderate–high-risk patients [23–26]. Incidental vascular findings were also similarly distributed in both ICU and non-ICU cases, with small-sized splanchnic arterial aneurysms being present in 30% of ICU patients (21.4% of the whole population).
When looking at extravascular findings, ventilation-related complications, like pneumothorax and pneumomediastinum, were detected in a small proportion of ICU cases. Previously reported as COVID-19 presenting event by Aloysius et al [27], acute pancreatitis was detected in 3 ICU cases, thus allowing clinicians to start targeted therapies.
Our study has some limitations that include the small sample size, the single-center setting, the short-term observation period, and its retrospective nature. The inclusion of a more significant number of patients would undoubtedly have improved the power of the study. Unfortunately, this was not realistic as our study population already included all patients evaluated with whole-body angiography during the first wave of the COVID-19 pandemic. Also, we had to rule out patients evaluated with organ-selective angiography to avoid any interpretation bias. Certainly, despite a not negligible rate of deaths (26%), our observations have helped our clinicians facilitate patient management, reinforcing the role of a multi-disciplinary team in an acute setting like this. The study period was also concise and limited to early disease occurrence when therapy strategies were still empirical. Comparison with larger cohorts including COVID-19 patients receiving tailored treatments should have been useful to address how the disease appearance has changed over time and the improvement of medical interventions. According to literature data, the prevalence of thrombotic events in ICU patients can be up to 60%, with incidental thromboembolic alterations detected by CT in 31.9% of ICU cases [28, 29]. About this finding, it is not possible to exclude that the high frequency of such alterations in our ICU COVID-19 case series was independent of the SARS-CoV-2 infection but related to ICU hospitalization itself. Again, comparison with no COVID-19 ICU patients should have been interesting but unfortunately not reliable because of the emergency working conditions.
In conclusion, vascular involvement is not negligible in COVID-19 patients. WB-CTA proves to be useful in this setting as it helps to map the vasculature at the systemic level and simultaneously offers the opportunity to catch extravascular events, either relevant or not. Radiologists are critical players in COVID-19 management as early imaging identification of both clinically relevant and incidental findings may significantly affect disease behavior and prognosis.
Acknowledgements
The authors wish to thank the whole medical and nursing staff of the Azienda Ospedaliera dei Colli (Cotugno, Monaldi, and CTO Hospitals) for the tireless effort done to fight COVID-19.
Abbreviations
- DVT
Deep vein thrombosis
- HU
Hounsfield unit
- ICU
Intensive care unit
- Min-IP
Minimum intensity projection
- MPR
Multi-planar reconstruction
- PACS
Picture Archiving and Communication System
- PE
Pulmonary embolism
- ROI
Region of interest
- WB-CTA
Whole-body CT angiography
Funding
The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is:
Dr Gaetano Rea, MD
Department of Radiology
Azienda dei Colli, Monaldi Hospital
Via L. Bianchi, 5 80131 Naples, Italy
email: gaetano.rea71@gmail.com
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr. Daniela Pacella (Department of Public Health, University of Naples Federico II, Naples, Italy) kindly provided statistical advice for this manuscript.
Informed consent
Informed consent was waived due to the retrospective nature of our study and the emergency context.
Ethical approval
Local Institutional Ethics Committee approval was obtained (Protocol no. 0021131/2020).
Methodology
• retrospective
• observational
• performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joob B, Wiwanitkit V (2020) Chest CT findings and clinical conditions of coronavirus disease (COVID-19). AJR Am J Roentgenol 215(1):W5. 10.2214/AJR.20.23064 [DOI] [PubMed]
- 5.Jalaber C, Lapotre T, Morcet-Delattre T, Ribet F, Jouneau S, Lederlin M. Chest CT in COVID-19 pneumonia: a review of current knowledge. Diagn Interv Imaging. 2020;101:431–437. doi: 10.1016/j.diii.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bösmüller H, Traxler S, Bitzer M, et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;30:1–9. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu B, Huang S, Yin L (2020) The cytokine storm and COVID-19. J Med Virol. 10.1002/jmv.26232 [DOI] [PMC free article] [PubMed]
- 8.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poyiadji N , Cormier P , Patel PY et al. (2020) Acute pulmonary embolism and COVID-19. Radiology 201955. 10.1148/radiol.2020201955 [DOI] [PMC free article] [PubMed]
- 10.Zamboni P. COVID-19 as a vascular disease: lesson learned from imaging and blood biomarkers. Diagnostics. 2020;10:440–450. doi: 10.3390/diagnostics10070440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. JACC- State of the art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan KH, Slim J, Shaaban HS (2020) Pulmonary embolism and increased levels of d-dimer in patients with coronavirus disease. Emerg Infect Dis 26(10). 10.3201/eid2610.202127 [DOI] [PMC free article] [PubMed]
- 13.Bavaro DF, Poliseno M, Scardapane A et al (2020) Occurrence of acute pulmonary embolism in COVID-19 - a case series. Int J Infect Dis S1201-9712(20)30501-4. 10.1016/j.ijid.2020.06.066 [DOI] [PMC free article] [PubMed]
- 14.Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127–1131. doi: 10.1136/heartjnl-2020-317186. [DOI] [PubMed] [Google Scholar]
- 15.Kremer S, Lersy F, de Sèze J et al (2020) Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology 202222. 10.1148/radiol.2020202222
- 16.Thompson RJ, Wojcik SM, Grant WD, Ko PY. Incidental findings on CT scans in the emergency department. Emerg Med Intern. 2011;2011:624847. doi: 10.1155/2011/624847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 3:1–6. 10.2214/AJR.20.22976 [DOI] [PubMed]
- 18.Maldonado I, Shetty A, Estay MC et al (2020) Acute pancreatitis imaging in MDCT: state of the art of usual and unusual local complications. 2012 Atlanta Classification Revisited.Curr Probl Diagn Radiol S0363-0188(20)30057-8. 10.1067/j.cpradiol.2020.04.002 [DOI] [PubMed]
- 19.Santoliquido A, Porfidia A, Nesci A et al (2020) Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 10.1111/jth.14992 [DOI] [PMC free article] [PubMed]
- 20.Klok FA, Kruip MJH, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashi M, Jacquin A, Daknil B, et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costanzo L, Failla G, Antignani PL et al (2020) The vascular side of COVID-19 disease. Position paper of the International Union of Angiology. Int Angiol. 10.23736/S0392-9590.20.04539-3 [DOI] [PubMed]
- 23.Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paar V, Wernly B, Zhou Z et al (2020) Anticoagulation for COVID-19 treatment: both antithrombotic and anti-inflammatory? J Thromb Thrombolysis 1–6. 10.1007/s11239-020-02212-6
- 25.Khan IH, Savarimuthu S, Leung MST, Harky A (2020) The need to manage the risk of thromboembolism in COVID-19 patients J VascSurg S0741-5214(20)31157-5. 10.1016/j.jvs.2020.05.015 [DOI] [PMC free article] [PubMed]
- 26.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulation treatment is associated with decreased mortality in severe coronavirus disease 19 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H (2020) COVID-19 presenting as acute pancreatitis. Pancreatology S1424-3903(20)30154-X. 10.1016/j.pan.2020.05.003 [DOI] [PMC free article] [PubMed]
- 28.Minet C, Potton L, Bonadona A, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. 2015;19:287. doi: 10.1186/s13054-015-1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schramm D, Bach AG, Meyer HJ, Surov A. Thrombotic events as an incidental finding on computed tomography in intensive care unit patients. Thromb Res. 2016;141:171–174. doi: 10.1016/j.thromres.2016.03.030. [DOI] [PubMed] [Google Scholar]