Abstract
Background:
47,XYY syndrome (XYY) is a male sex chromosome disorder where subjects have one X chromosome and two copies of the Y chromosome. XYY is associated with a physical phenotype and carries increased risk of neurodevelopmental disorders such as autism spectrum disorder (ASD). Imbalance of excitation and inhibition has been proposed as a putative biological basis of disorders such as ASD [1–3] and several studies have reported atypical brain GABA levels in this population. Given the male preponderance in prevalence of ASD, the unique presence of the Y chromosome in males leads to the intriguing possibility of investigating boys with XYY syndrome as a model of excess Y chromosome genes.
Method:
In this study, we investigated the associations of genotype and clinical phenotype with levels of GABA, estimated by regionally localized edited magnetic resonance spectroscopy in boys with 47, XYY syndrome compared to age matched typically developing (XY) peers.
Results:
Overall, we observed a decrease in GABA levels in XYY vs XY, which appeared more significant in the left compared to the right hemisphere. There was no additional significant modulation of GABA levels in XYY according to presence/absence of ASD diagnosis. Interestingly, a positive correlation between bilateral GABA levels and testosterone levels was observed in pubescent XY boys that was not observed in XYY.
Conclusion:
The inhibitory neurotransmitter GABA appears to be reduced in boys with 47,XYY, especially in the left hemisphere. Further the typical association between GABA and testosterone levels, observed in older typically developing control boys was not evident in boys with 47,XYY.
Keywords: 47,XYY syndrome; XYY; Autism Spectrum Disorder; GABA; MRS
Introduction:
Although boys typically possess a single X and a single Y sex chromosome, approximately 1 in 1000 have an additional Y chromosome, and are described as having 47, XYY syndrome, a male sex chromosome aneuploidy (SCA) disorder [4]. 47, XYY syndrome (hereafter, XYY) has been associated with a physical phenotype (i.e. tall stature, hypotonia) but also increased risk for neurodevelopmental phenotypes, including developmental delays, difficulties with language, social-emotional difficulties, attention-deficit hyperactivity disorder and, of note, autism spectrum disorder (ASD) [5–9]. Learning difficulties are frequently experienced, although general intelligence may be typical, or slightly below average [7, 10]. In practice, XYY often goes undiagnosed, due to the mild and non-specific nature of the above characteristics. Interestingly, the prevalence of ASD diagnoses is significantly increased in XYY males, with reports in the range 19–50% [6, 11] compared with ~2.6% in the general male population [12]. Along with the general 4:1 male to female gender predominance in ASD, the high rate of ASD prevalence in XYY has led to the hypothesis that increased Y chromosome gene copy number in XYY leads to over-expression of Y-linked genes related to brain development and function, which increases risk of ASD [13, 14]. As an example, a recent XYY study by Ross, Tartaglia [14] found increased expression of NLGN4Y, a Y-chromosome gene coding for a trans-synaptic cell adhesion molecule, in boys with XYY versus XY controls, with higher expression of NLGN4Y associated with more severe autism symptoms. For this study, given the theory of excitation/inhibition imbalance in ASD [2, 3] and spectroscopic observations of reduced GABA in boys with ASD [15–17], especially in the superior temporal gyrus (STG), as well as the putative hypothesis linking the Y-chromosome to ASD, it is of interest to directly study STG GABA levels in boys with XYY.
Estimation of the inhibitory neurotransmitter GABA has recently been enabled via spectrally-edited magnetic resonance spectroscopy as an index of inhibitory tone [18]. The present study used magnetic resonance spectroscopy (MRS), spectrally-edited to yield estimates of GABA, employing a modified MEGA-PRESS sequence to minimize signal contamination with co-edited macromolecules [19, 20], to examine main effects of XY vs XYY group in male children and adolescents (6–18 years).
Further, given the focus on the male Y-chromosome, we additionally explored the association between brain-derived measures such as GABA MRS with gonadal hormone levels, specifically testosterone. Past studies have observed such a relationship preclinically, with the anxiolytic effects of testosterone being mediated by the GABA-a receptor [21, 22]. Human studies have observed changes in prefrontal GABA levels across the menstrual cycle and during the use of hormonal contraceptives [23] and relationships between testosterone and posterior cingulate have been observed in unmedicated depressed women [24]. Testosterone could mediate these effects via one or several mechanisms, including direct effect via the androgen receptor, aromatization of testosterone to estradiol and action through the estrogen receptor, or through conversion of testosterone to dihydrotestosterone or its metabolites 5α- androstane-3α,17β-diol (3α-diol) and to 5α-androstane-3β,17β-diol (3β-diol), or as neuroactive steroids possessing neuromodulatory activity [25].
Methods:
Participants:
MRS data from 19 boys with XYY and 30 typically developing (XY) peers are reported. In a small fraction of participants, MRS was only obtained / evaluable from one of the two hemispheres.
XYY subjects were recruited through the eXtraordinarY Kids Clinics (for children with sex chromosome variations), at Nemours/Alfred I. DuPont Hospital for Children. Typically developing children were recruited via the Recruitment Enhancement Core (REC) of the Children’s Hospital of Philadelphia (CHOP), from the local and regional community and from CHOP primary care practices. No genetic testing was performed to confirm XY status; assuming a 1/1000 XYY prevalence the expected number of XYY subjects in our XY cohort of 19 is no more than 1 and thus was considered unlikely and only a minor possible contributor to statistical noise.
All participants were selected according to the following criteria: (1) no history of traumatic brain injury or other significant medical or neurological abnormality, (2) no active psychosis, (3) no MRI contraindications, and (4) no known drug or alcohol use prior to any study procedure. Members of the typically developing group had no current or past history of DSM-5 Axis I disorders noted by parents during initial screening and had no symptoms of ASD, as measured by autism screening questionnaires (Social Responsiveness Scale-2: SRS-2 [26] and Social Communication Questionnaire: SCQ [27]) and direct observation on the Autism Diagnostic Observation Schedule-2nd edition (ADOS-2 [28]). In the XYY+ASD sub-group, diagnosis of ASD was established by expert clinical consensus of two clinical psychologists (JM and LB) using the ADOS-2, DSM-5 criteria, review of available records, and parent questionnaires (SCQ and SRS-2). A measure of full-scale IQ (FSIQ) was obtained for all participants using the DAS-II [29]. The study was approved by the Children’s Hospital of Philadelphia Institutional Review Board, and by the Human Studies Committee at Nemours DuPont Hospital for Children. Written informed consent and assent (when age-appropriate) was obtained from all participating families.
Demographics (mean (SD)) are reported in Table 1 for the evaluable sample.
Table 1.
Group differences in demographics, assessments and testosterone levels
| XY | XYY | P-value | |
|---|---|---|---|
| N | 19 | 30 | - |
| Age (years) | 12.8 (3.1) | 13.0 (2.8) | 0.824 |
| SCQ [20] (raw scores) | 2 (2.5) | 9.7 (6.6) | <0.001 |
| SRS-2 [19] T-scores | 42.1 (6.8) | 70.1 (17.6) | <0.001 |
| FSIQ (DAS-II [22]) Standard scores | 113.8 (18.2) | 91.0 (15.9) | <0.001 |
| Handedness | L:0 Bi:0 R:19 | L:3 Bi:1 R:26 | 0.252 |
| Testosterone (ng/dl) | 179.3 (225.6) | 183.1 (196.2) | 0.953 |
| Pubescent | 259.1 (232.2) | 229.2 (199.5) | 0.697 |
| Prepubescent | 3.6 (1.5) | 6.3 (4.8) | 0.253 |
Additionally, venipuncture was performed to quantify the male gonadal hormone, testosterone. Serum samples were processed and frozen until the time of study completion. Quantification of total testosterone was performed by liquid chromatography tandem mass spectroscopy with a lower limit of quantification of 1.0 ng/dl; inter and intra-assay CV is <10%.
MRS data acquisition and analysis:
Macromolecular suppressed spectrally-edited MEGAPRESS sequence (Siemens WIP 529) were acquired from two single voxels sized 4×3×2cm placed bilaterally in STG with TR/TE=1500/80ms, 128 transient pairs (Figure 1) on a 3T Prisma MR scanner. Acquisition time for each voxel was approximately 6 minutes. For the MEGAPRESS sequence, suppression of potentially co-edited macromolecules is achieved by applying the “off” pulse at 1.5ppm, symmetric about 1.7ppm with the on pulse (at 1.9ppm) [20]. Manual high-order shimming was used to achieve unsuppressed water resonance linewidths, generally <20Hz.To minimize effects of field center-frequency drift during the MEGAPRESS acquisitions, MRS acquisitions always preceded other more gradient-demanding imaging sequences (such as DTI).
Figure 1:
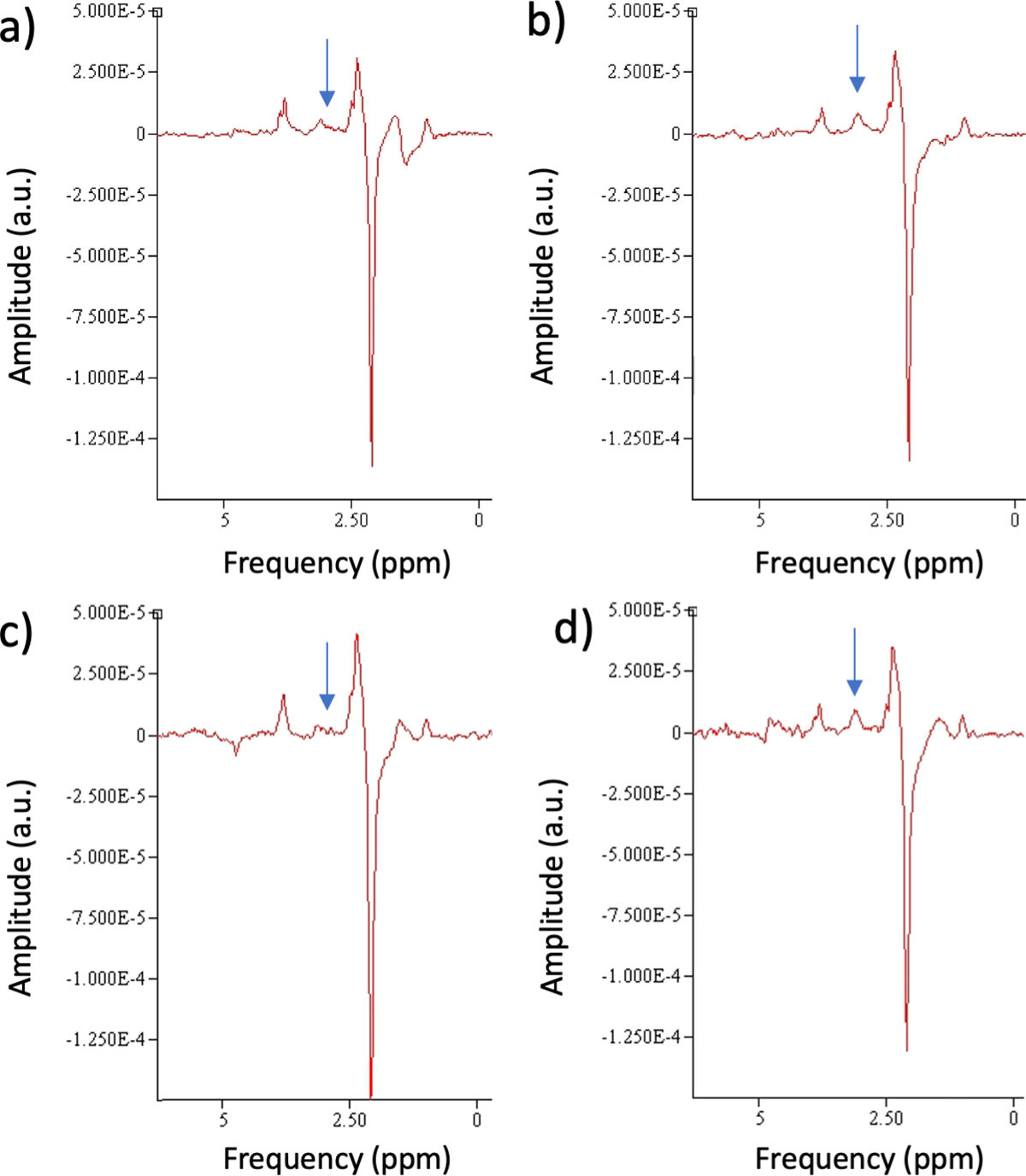
Edited MRS from representative participants with (a,b) XY(left and right hemisphere) and (c,d) XYY (left and right hemisphere). Arrows mark the GABA resonance in the subtracted spectra. Diminished left hemisphere GABA is evident compared to right hemisphere values. Also, a main effect of Group showed diminished GABA levels in XYY.
Spectra were pre-processed using a modification of the open-source FID-A toolbox [30] to 1) eliminate transients > +/− 2Hz from the nominal center frequency (mitigating effects of field drift, transient motion and inefficient RF “off-pulse” pulsing), 2) eliminate “bad” transients that did not match the average (to within 3 standard deviations), 3) to realign transients subject to slight (<2Hz) frequency variation and 4) to apply frequency and phase correction (FPC) of the “on” and “off” spectra to optimize subtraction. Sum and difference spectra were saved. There was no difference in the number of qualifying transient pairs between groups (XYY: 75.5 ± 3.7, XY: 81.0 ± 4.4; p=0.33).
Spectra were then quantified using jMRUI v6.0 beta. 5Hz Lorentzian line broadening was applied. An estimate of GABA was derived from the difference spectrum using the HLSVD fitting algorithm from the resonance in the subtracted spectra at 3ppm. When a small nearby resonance at ~3.1–3.2ppm (possibly attributable to phosphocholine) was observed this was fit separately and excluded from the GABA estimation. Estimation of Cr was obtained from similar HLSVD fitting of the “sum” spectrum. In general, between 10–20 HLSVD components were required to account for the full spectral range. Results are reported as the ratio of GABA to Cr (GABA/Cr) abbreviated to “GABA” in the analysis and discussion below. For both sum and difference fitting an estimate of goodness of fit was provided by the residual standard deviation. Notably the GABA levels tended to exceed this metric by a factor of ~10 or more.
Statistical Analysis:
Linear mixed models with fixed effects of hemisphere and XY vs. XYY status (“Group”) and random effect of subject, with age as a covariate, were used to assess the dependent variable GABA/Cr. Additionally, the relationship between GABA/Cr and testosterone was investigated in the older, pubescent (> 10.5 years) sub-cohort using linear mixed models, with fixed effects of age, hemisphere, testosterone and XY vs. XYY status (“Group”) along with their interactions and again using subject as a random effect. Of note 10.5 years was chosen as the cutoff as it presents a clear inflection point between low (near-zero) prepubescent testosterone production and increased testosterone production beginning in early puberty (see Figure 1 of [31]).
Results:
Demographics:
No significant age differences were observed between groups (XY: 12.8 ± 0.7 Yrs; XYY: 13.0 ± 0.5; p=0.82). In agreement with expected behavioral phenotypes, Group had a significant effect on DAS-II GCA (IQ) scores (XY: 113.8 ± 3.9; XYY: 91 ± 3.1; p<0.001), SRS scores (XY: 42.1 ± 3.3; XYY: 70.1 ± 2.6; p<0.001) and SCQ scores (XY: 2.0 ± 1.2; XYY: 9.7 ± 1.0; p<0.001). Thus, the XYY group differed from the XY group in measures of general cognitive ability and autism related behaviors.
Within the XYY group, no significant effect of concomitant ASD diagnosis (XYY+ASD vs XYY-ASD) was observed in either age (XYY-ASD: 13.8 ± 0.7 Yrs; XYY+ASD: 12.1 ± 0.7 Yrs; p=0.1), GCA scores (XYY-ASD: 95.3 ± 4.0; XYY+ASD: 86 ± 4.0; p=0.14), SRS scores (XYY-ASD: 69.1± 4.6; XYY+ASD: 71 ± 4.6; p=0.77) or SCQ scores (XYY-ASD: 7.6 ± 1.6; XYY+ASD: 11.8 ± 1.6; p=0.09). Thus, within the XYY group, the ASD diagnosis did not result in observed dichotomous group differences in general cognitive ability or autism related behaviors, nor was it conflated with age.
Rates of Evaluable Data:
The 19 XY participants yielded evaluable MRS data from 18 LH ROI’s and 18 RH ROI’s. The 30 XYY subjects yielded evaluable MRS data from 24 LH ROI’s and 26 RH ROI’s. Although there was slightly higher rate of unevaluable data in the XYY cohort, this difference did not reach significance (χ2 test, p=0.15 (LH), p=0.36 (RH)).
GABA/Cr
Representative bilateral spectra from typical individuals in each group (XY vs. XYY) are shown in Figure 1. The GABA resonances at ~3ppm in the subtracted spectra are marked by arrows.
Statistical analysis of STG GABA/Cr levels were performed using a linear mixed model with subject as a random effect to investigate main effects of hemisphere and group, covarying for age. Main effects were found for hemisphere (left: 0.051±0.005 vs right: 0.069±0.004, p<=0.002) and group (XY: 0.069±0.005; XYY: 0.051±0.004, p=0.014). Thus, left hemisphere was associated with lower GABA/Cr as was XYY vs XY group. No effect of age was observed nor any interactions between group, hemisphere and age. Although no significant interaction was observed between group and hemisphere, based on the main effect of hemisphere on GABA level, we further examined group differences in GABA levels for each hemisphere separately via additional post-hoc testing. In the left hemisphere (XY:0.060±0.007; XYY: 0.041±0.006) post-hoc t-testing showed significant difference between XY and XYY (p=0.038) while in the right hemisphere (XY: 0.077+/−0.007; XYY: 0.061+/−0.006) the XYY levels were less significantly reduced, compared to XY (p=0.061).
Since both ASD [15–17] and XYY appear to have an association with GABA levels, we examined the additional effect of ASD diagnosis in the XYY population. No significant difference was observed between boys with XYY meeting (XYY+ASD) vs. not-meeting diagnostic (XYY-ASD) criteria for ASD (p=0.43), although we note that both XYY cohorts, XYY+ASD and XYY-ASD, had significantly higher ASD metrics compared with their XY peers (SRS: XY 40.9 ± 3.3; XYY-ASD 64.5 ± 3.6, p<0.01; XYY+ASD 73.9 ± 3.5, p<0.01 and SCQ: XY 1.9 ± 1.2; XYY-ASD 7.2 ± 1.4, p<0.01; XYY+ASD 11.6 ± 1.3, p<0.01), reflecting ASD-like traits even in the cohort of XYY boys not meeting full diagnostic criteria for ASD.
In the older, pubertal, subcohort (>10.5yrs), linear mixed models of STG GABA/Cr using subject as a random effect and fixed effects of hemisphere, age, Group (XY. vs. XYY status), testosterone and their interactions found significant effects of Age (−0.009 per year p=0.0032), Group (XY:0.086+/−0.010, XYY: 0.046+/−0.006, p=0.0029), Testosterone (9.3e-5 ng/dl p=0.017), and Group x Testosterone interaction (p=0.01). The Group x Testosterone interaction was further investigated using post hoc linear modeling separately in each group. In the XY group, a significant positive GABA/Cr to Testosterone relationship was observed (2.0e-4 per ng/dl, p = 0.037). This relationship was not observed in the XYY group (p > 0.7). Due to the linear relationship between Testosterone and age (levels of testosterone increase with both pubertal development and age which are highly correlated), we considered a potential confound of testosterone by adopting a nonlinear age dependence in step-wise linear models. A base model of Age, Group and Age x Group explained 27% of GABA/Cr variance. Adding testosterone and Group x Testosterone explained a significant amount of additional variance (ΔR2=10%, p=0.024) whereas adding either 1/Age and 1/Age X Group or Age2 and Age2 X group did not explain significant variance (both ΔR2=3%, ps > 0.2), suggesting that the observed relationship with testosterone is not merely an effect of nonlinear maturation of GABA/Cr.
Discussion and Conclusion:
The main finding of this study is a bilateral decrease in superior temporal gyrus (STG) levels of inhibitory neurotransmitter GABA in boys with 47,XYY compared to age-matched peers. Such findings of decreased STG GABA have previously been reported in ASD, although primarily in the left hemisphere [15–17, 19]. An overall statistical main effect indicates the finding of reduced GABA in XYY should be interpreted bilaterally, although there is a trend towards a hemispheric bias (with left hemisphere effects being somewhat more pronounced). Interestingly, previous studies of auditory cortex function in XYY (with partial sample overlap with the present cohort) have implicated predominantly left hemispheric dysfunction [32]. Potential hemispheric asymmetries should be considered in future studies.
Previous studies [15–17, 19] have shown diminished temporal lobe estimates of GABA in idiopathic ASD, particularly in the left hemisphere. Given the high prevalence of ASD in 47,XYY syndrome it is notable that an analogous GABA depletion is observed in 47,XYY. Although there are no differences in GABA levels in XYY between individuals meeting ASD diagnostic criteria from those not meeting diagnostic criteria, it should be borne in mind (and is evident from the clinical assessments) that even XYY individuals not meeting formal diagnostic criteria for ASD nonetheless exhibited significant ASD traits and that the XYY cohort as a whole had significantly higher SCQ and SRS scores compared to their typically-developing XY peers, suggesting autism-like traits.
Importantly, in older boys, with non-zero levels of the gonadal hormone, testosterone, GABA levels appear linearly associated with testosterone levels in the XY cohort, but do not exhibit this relationship in XYY although there is no significant difference in the mean testosterone levels between XY and XYY. Previously we have reported subtle differences in testicular function in a large cohort of boys with XYY [31], with lower inhibin b, but no detectable differences in testosterone with age. It is tempting to speculate that the age/testosterone-related increase in GABA, observed typically in adolescent boys, is inhibited in XYY by over-expression of key Y-chromosome genes, and that this effect overshadows the age/testosterone GABA relationship.
The limitations of this study include the relatively smaller sample of post-pubertal individuals, in whom testosterone levels might be higher, as well as the lack of quantitative evidence of the effects of XYY on Y-chromosome gene expression. Furthermore, given the large tissue volume contributing to the GABA estimation, it is difficult to interpret these values more specifically than “basal inhibitory tone”.
In summary, boys with XYY show bilaterally depleted levels of the inhibitory neurotransmitter, GABA compared to typically developing boys. This finding is also described in boys with autism spectrum disorder, raising the question of XYY being a genetic autism model (if genes on the Y chromosome are in part responsible for the ~fourfold increased prevalence of ASD among boys compared to girls, then perhaps increased expression of these genes in 47,XYY may be associated with the yet further increased (~30–40% vs. ~3%) prevalence of ASD in XYY compared to XY). Furthermore, an association of GABA with testosterone levels, separate from age effects, observed in typically developing boys was not observed in boys with XYY, suggesting subtle alterations in testicular function and gonadal brain interactions in XYY. These results suggest further investigation into the interaction of gonadal sex hormones and GABA levels, as well as the modulatory influence of expression of Y-chromosome genes on brain and behavior.
Figure 2:
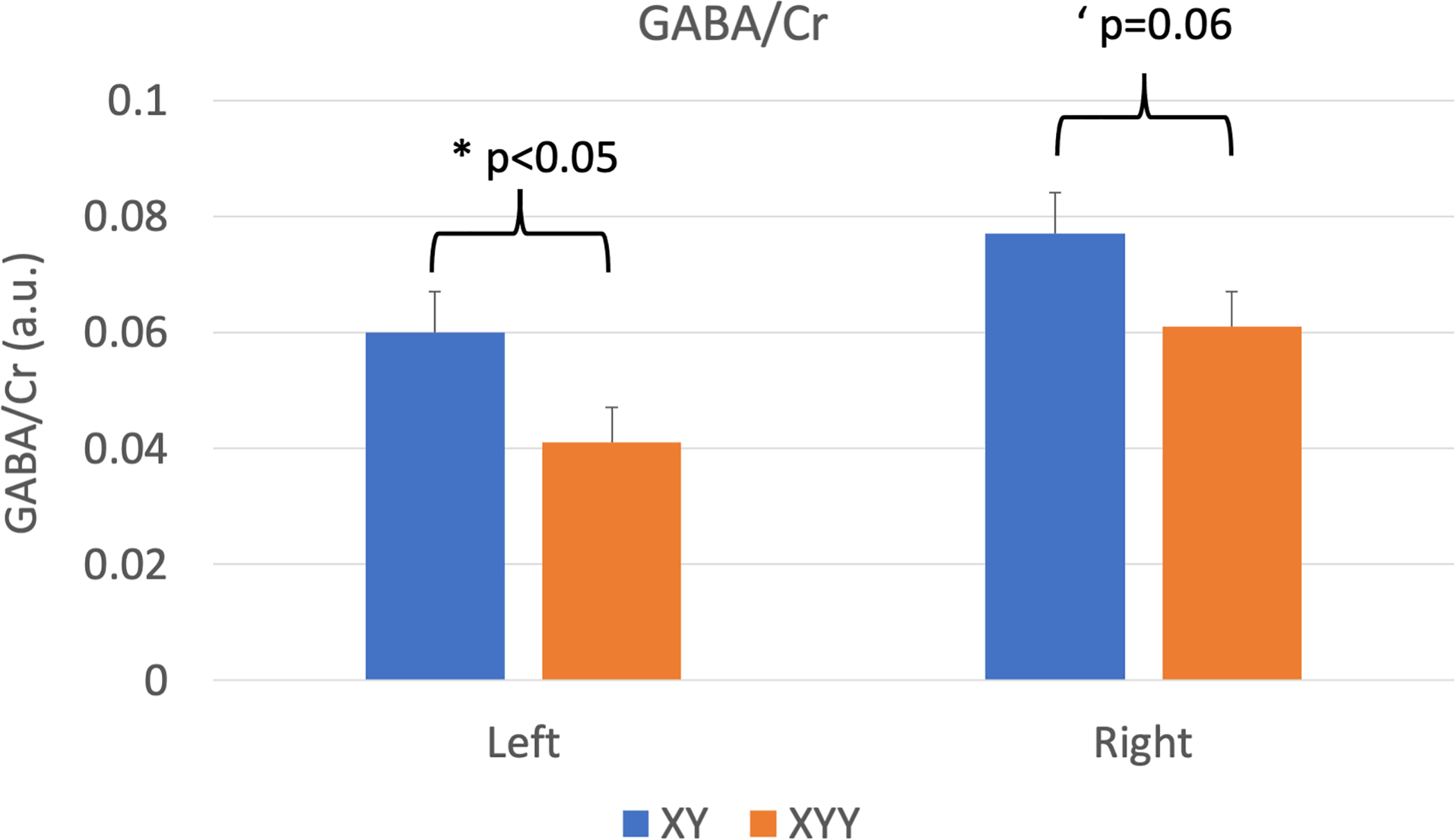
MRS estimates (mean +/− s.e.m.) of GABA/Cr by group and hemisphere. Significant main effects of Group and Hemisphere were observed. Within hemispheres, group differences were significant in the left hemisphere (*p<0.05) and strongly trended in the right hemisphere (‘p=0.06)
Figure 3:
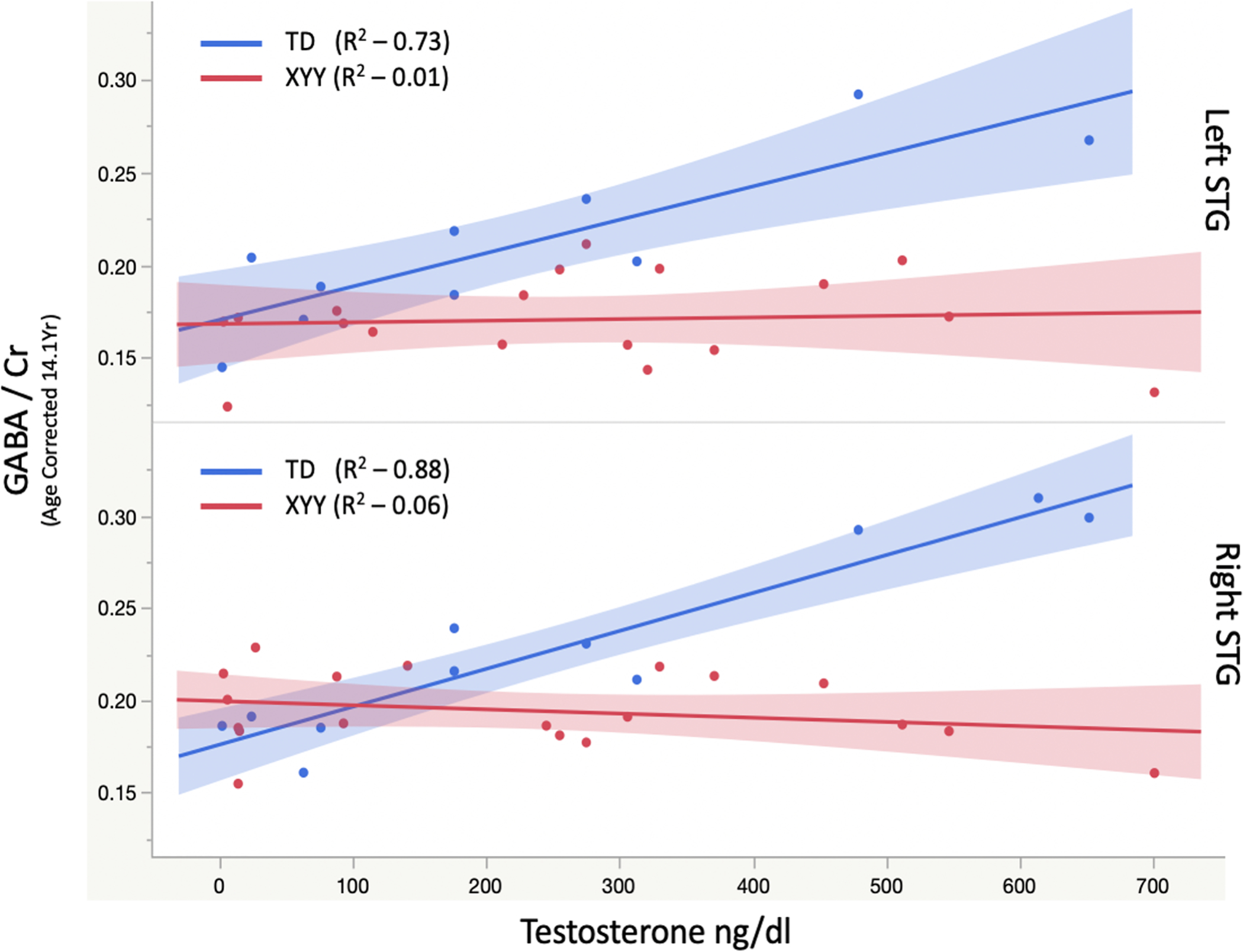
Association of GABA/Cr with testosterone in boys > 10.5 years of age, shows a significant positive association with bilateral GABA levels in XY that is conspicuously absent in XYY. The group X testosterone interaction was significant (p<0.05).
Acknowledgements:
Dr. Roberts would like to thank the Oberkircher family for the Oberkircher Family Chair in Pediatric Radiology at CHOP.
Funding Sources:
NIH R21-MH109158 (TR), NIH R01-DC008871 (TR), NIH K01-MH108762 (LB), NIH U54- HD086984 (institutional IDDRC), DoD CDMRP IDEA AR140197 (TR/JR)
Footnotes
Conflicts of Interests:
Dr. Roberts reports consulting agreements with CTF MEG, Ricoh, Spago Nanomedicine, Avexis, Acadia Pharmaceuticals, Proteus Neurodynamics and Prism Clinical Imaging. Dr. Blaskey reports a consulting agreement with Koronis Biomedical Technologies. No other authors report disclosures.
References:
- 1.Port RG, Oberman LM, and Roberts TP, Revisiting the excitation/inhibition imbalance hypothesis of ASD through a clinical lens. Br J Radiol, 2019. 92(1101): p. 20180944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenstein JL and Merzenich MM, Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav, 2003. 2(5): p. 255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohal VS and Rubenstein JLR, Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry, 2019. 24(9): p. 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stochholm K, Juul S, and Gravholt CH, Diagnosis and mortality in 47,XYY persons: a registry study. Orphanet J Rare Dis, 2010. 5: p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardsley MZ, et al. , 47,XYY Syndrome: Clinical Phenotype and Timing of Ascertainment. J Pediatr, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop DV and Scerif G, Klinefelter syndrome as a window on the aetiology of language and communication impairments in children: the neuroligin-neurexin hypothesis. Acta Paediatr, 2011. 100(6): p. 903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson A, et al. , Sex chromosome aneuploidy: the Denver Prospective Study. Birth Defects Orig Artic Ser, 1990. 26(4): p. 59–115. [PubMed] [Google Scholar]
- 8.Ross J and Hoeft F, Introduction: cognitive profiles in sex chromosome disorders. Dev Disabil Res Rev, 2009. 15(4): p. 269. [DOI] [PubMed] [Google Scholar]
- 9.Ross JL, et al. , Behavioral and Social Phenotypes in Boys With 47,XYY Syndrome or 47,XXY Klinefelter Syndrome. Pediatrics, 2012. 129(4): p. 769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netley CT, Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy. Birth Defects Orig Artic Ser, 1986. 22(3): p. 293–306. [PubMed] [Google Scholar]
- 11.Tartaglia NR, et al. , Autism Spectrum Disorder in Males with Sex Chromosome Aneuploidy: XXY/Klinefelter Syndrome, XYY, and XXYY. J Dev Behav Pediatr, 2017. 38(3): p. 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baio J, et al. , Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ, 2018. 67(6): p. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop DV, et al. , Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child, 2011. 96(10): p. 954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross JL, et al. , Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes Brain Behav, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaetz W, et al. , GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage, 2014. 86: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Port RG, et al. , Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Autism Res, 2017. 10(4): p. 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojas DC, et al. , Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage, 2014. 86: p. 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikkelsen M, et al. , Big GABA: Edited MR spectroscopy at 24 research sites. Neuroimage, 2017. 159: p. 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts TPL, et al. , A Multimodal Study of the Contributions of Conduction Velocity to the Auditory Evoked Neuromagnetic Response: Anomalies in Autism Spectrum Disorder. Autism Research, 2020. 13(10): p. 1730–1745. [DOI] [PubMed] [Google Scholar]
- 20.Edden RAE, Puts NAJ, and Barker PB, Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magnetic resonance in medicine, 2012. 68(3): p. 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Guasti A and Martínez-Mota L, Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology, 2005. 30(8): p. 762–70. [DOI] [PubMed] [Google Scholar]
- 22.Aikey JL, et al. , Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm Behav, 2002. 42(4): p. 448–60. [DOI] [PubMed] [Google Scholar]
- 23.De Bondt T, et al. , Prefrontal GABA concentration changes in women-Influence of menstrual cycle phase, hormonal contraceptive use, and correlation with premenstrual symptoms. Brain Res, 2015. 1597: p. 129–38. [DOI] [PubMed] [Google Scholar]
- 24.Flores-Ramos M, et al. , Testosterone is related to GABA+ levels in the posterior-cingulate in unmedicated depressed women during reproductive life. J Affect Disord, 2019. 242: p. 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durdiokova j., Ostatnikova D, and Celec P, Testosterone and its metabolites - modulators of brain functions. Acta Neurobiol Exp (Wars), 2011. 71(434–54). [DOI] [PubMed] [Google Scholar]
- 26.Constantino JN, et al. , Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of autism and developmental disorders, 2003. 33(4): p. 427–433. [DOI] [PubMed] [Google Scholar]
- 27.Rutter M, Bailey A, and Lord C, The social communication questionnaire: Manual. 2003: Western Psychological Services. [Google Scholar]
- 28.Lord C, et al. , Autism Diagnostic Observation Schedule- 2nd ed. 2012, Torance (CA): Western Psychological Services. [Google Scholar]
- 29.Elliott CD, Differential Ability Scales - Second Edition. 2007, San Antonio, TX: Harcourt. [Google Scholar]
- 30.Simpson R, et al. , Advanced processing and simulation of MRS data using the FID appliance (FID-A)—An open source, MATLAB-based toolkit. Magnetic Resonance in Medicine, 2017. 77(1): p. 23–33. [DOI] [PubMed] [Google Scholar]
- 31.Davis SM, et al. , Testicular function in boys with 47,XYY and relationship to phenotype. Am J Med Genet C Semin Med Genet, 2020. 184(2): p. 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloy L, et al. , AuditoryEvoked Response Delays in Children with 47,XYY Syndrome. Neuroreport, 2019. 30(7): p. 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]


