To the Editor:
Type I interferons (IFNs) are crucial in protecting against severe coronavirus disease 2019 (COVID-19), and a high percentage of patients with life-threatening COVID-19 either carry loss-of-function variants in the genes for type I IFNs or neutralizing autoantibodies (aAbs) against type I IFNs.1 , 2 This is in agreement with observations that the IFN response is important in limiting the early viremic phase of the disease, as well as for the subsequent resolution of inflammation.3 Removal of aAbs through plasmapheresis has been suggested as a targeted treatment in severe COVID-19 with concomitant type I IFN aAbs.1 , 4
Autoimmune polyendocrine syndrome type 1 (APS1) is characterized by neutralizing aAbs to type I IFNs.5 As a proof-of-concept, we present the case of a child with APS1 and aAbs to type I IFN who developed life-threatening COVID-19 and responded rapidly to treatment with plasmapheresis, intravenous immunoglobulin (IVIg), and high-dosage corticosteroids. The patient is an 8-year-old girl with APS1, verified by homozygous AIRE [c.1616C>T (p.Pro539Leu)] mutations, typical clinical presentation including Addison disease, hypoparathyroidism, and vitiligo. She carried aAbs characteristic for APS1, among them high-titer aAbs against IFN-ω and IL-22 (Fig 1 , C).
Fig 1.
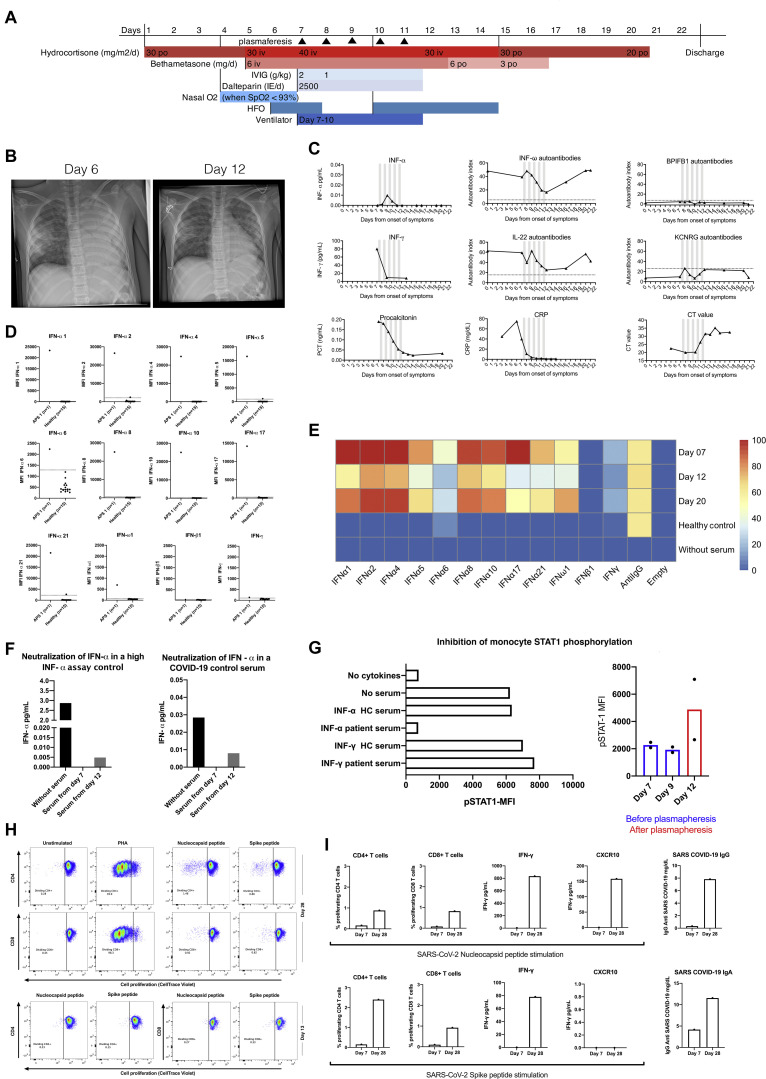
A, Timeline showing interventions in our patient. B, Chest x-radiographs before start of plasmapheresis and after treatment. C, Trajectories for serum aAbs, inflammatory markers, and SARS-CoV-2 Ct values throughout the disease course. Gray bars indicate plasmapheresis. D, IFN aAbs in sera from the patient and healthy controls assessed using a bead-based array. The patient is positive for all IFN aAbs tested except for aAbs against IFN-γ and IFN-β1. E, Heatmap illustrating that IFN aAb concentrations decrease during plasmapheresis (day 12) and return to high levels after discontinuation of plasmapheresis (day 20). F, Neutralization assay for IFN-α. High-level IFN-α control and serum from a patient with COVID-19 with 0.1% serum from our patient before and after plasmapheresis. G, Effects of patient sera diluted 1:10, on the phosphorylation of STAT1 in monocytes after stimulation with either IFN-α or IFN-γ. Phosphorylation after IFN-α stimulation in presence of 0.4% patient serum during treatment. H, Proliferation of CD4+ and CD8+ T cells stimulated, separately, with SARS-CoV-2 nucleocapsid (N) and spike (S) peptides and PHA. I, T-cell proliferation and production of cytokines, after peptide N and peptide S stimulation of PBMCs, and serum concentrations of specific IgG and IgA anti–SARS-CoV-2 antibodies on days 7 and 28. CRP, C-reactive protein; HC, healthy control; HFO, high-flow oxygen; iv, intravenous, MFI, mean fluorescence intensity; po, per os (by mouth); STAT1, signal transducer and activator of transcription 1.
The patient presented with a cough, fever, and a positive PCR result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Her clinical condition deteriorated, leading to transfer to the intensive care unit where she was put on a ventilator on day 7 from the onset of symptoms. Because the patient had high concentrations of aAbs to type I IFN before infection, we hypothesized that removal of these aAbs might improve her severe clinical manifestation of COVID-19. Therefore, daily plasmapheresis, followed by IVIg, was applied for 5 consecutive days (days 7-11). After 4 days on the ventilator, the patient could be extubated on day 10, and on day 22, the patient was discharged in good condition without any sequelae (Fig 1, A).
Monitoring of aAbs to a broad range of type I IFNs during the course of disease showed that the initially high concentrations decreased during plasmapheresis and returned to high levels again when plasmapheresis was discontinued (Fig 1, D and E). In parallel with the decline in aAb levels, a small surge in IFN-α was detected, which was undetectable before plasmapheresis (Fig 1, C). Furthermore, after plasmapheresis, the patient’s serum was less neutralizing (Fig 1, F) and to a lower extent inhibiting IFN-α–induced signal transducer and activator of transcription 1 phosphorylation (Fig 1, G) in monocytes in in vitro assays.
The patient developed adaptive immunity to SARS-CoV-2 with high titers of IgA and IgG SARS-CoV-2 antibodies as well as proliferation of CD4 and CD8 T cells and production of IFN-γ and CXCL10/IP10 after S and N protein stimulation (Fig 1, H and I).
The present case underlines the importance of considering inborn errors of immunity in patients with severe COVID-19. Furthermore, although a single case cannot demonstrate causality, we hypothesize that the removal of type I IFN–specific aAbs by plasmapheresis, in addition to high-dose corticosteroids and IVIg, represents a targeted treatment with benefits for patients who have aAbs against type I IFN and severe COVID-19. Formal studies are needed to test this hypothesis.
Acknowledgments
We thank the National Facility for Autoimmunity and Serology Profiling at SciLifeLab and Jennie Olofsson for excellent technical support related to the bead-based array studies. Finally, we thank the patient, family members, and healthy controls for participating in this study, without whom it would not have been possible to conduct this research.
Footnotes
This study was supported by grants from the Swedish Research Council (grant no. 2018-02752) and from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (grant nos. 76800 and 76210).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Zarzoso M., Gómez-Seguí I., de la Rubia J. Therapeutic plasma exchange: review of current indications. Transfus Apher Sci. 2019;58:247–253. doi: 10.1016/j.transci.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Meager A., Visvalingam K., Peterson P., Möll K., Murumägi A., Krohn K. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]