Abstract
Background
Delivering uninterrupted cancer treatment to patients with musculoskeletal tumors has been essential during the rapidly evolving coronavirus 2019 (COVID-19) pandemic, as delays in management can be detrimental. Currently, the risk of contracting COVID-19 in hospitals when admitted for surgery and the susceptibility due to adjuvant therapies and associated mortality due to COVID-19 is unknown, but knowledge of these potential risks would help treating clinicians provide appropriate cancer care.
Questions/purposes
(1) What is the risk of hospital-acquired COVID-19 in patients with musculoskeletal tumors admitted for surgery during the initial period of the pandemic? (2) What is the associated mortality in patients with musculoskeletal tumors who have contracted COVID-19? (3) Are patients with musculoskeletal tumors who have had neoadjuvant therapy (chemotherapy or radiation) preoperatively at an increased risk of contracting COVID-19? (4) Is a higher American Society of Anesthesiologists (ASA) grade in patients with musculoskeletal tumors associated with an increased risk of contracting COVID-19 when admitted to the hospital for surgery?
Methods
This retrospective, observational study analyzed patients with musculoskeletal tumors who underwent surgery in one of eight specialist centers in the United Kingdom, which included the five designated cancer centers in England, one specialist soft tissue sarcoma center, and two centers from Scotland between March 12, 2020 and May 20, 2020. A total of 347 patients were included, with a median (range) age of 53 years (10 to 94); 60% (207 of 347) were men, and the median ASA grade was II (I to IV). These patients had a median hospital stay of 8 days (0 to 53). Eighteen percent (61 of 347) of patients had received neoadjuvant therapy (8% [27] chemotherapy, 8% [28] radiation, 2% [6] chemotherapy and radiation) preoperatively. The decision to undergo surgery was made in adherence with United Kingdom National Health Service and national orthopaedic oncology guidelines, but specific data with regard to the number of patients within each category are not known. Fifty-nine percent (204 of 347) were negative in PCR testing done 48 hours before the surgical procedure; the remaining 41% (143 of 347) were treated before preoperative PCR testing was made mandatory, but these patients were asymptomatic. All patients were followed for 30 days postoperatively, and none were lost to follow-up during that period. The primary outcome of the study was contracting COVID-19 in the hospital after admission. The secondary outcome was associated mortality after contracting COVID-19 within 30 days of the surgical procedure. In addition, we assessed whether there is any association between ASA grade or neoadjuvant treatment and the chances of contracting COVID-19 in the hospital. Electronic patient record system and simple descriptive statistics were used to analyze both outcomes.
Results
Four percent (12 of 347) of patients contracted COVID-19 in the hospital, and 1% (4 of 347) of patients died because of COVID-19-related complications. Patients with musculoskeletal tumors who contracted COVID-19 had increased mortality compared with patients who were asymptomatic or tested negative (odds ratio 55.33 [95% CI 10.60 to 289.01]; p < 0.001).With the numbers we had, we could not show that adjuvant therapy had any association with contracting COVID-19 while in the hospital (OR 0.94 [95% CI 0.20 to 4.38]; p = 0.93). Increased ASA grade was associated with an increased likelihood of contracting COVID-19 (OR 58 [95% CI 5 to 626]; p < 0.001)
Conclusion
Our results show that surgeons must be mindful and inform patients that those with musculoskeletal tumors are at risk of contracting COVID-19 while admitted to the hospital and some may succumb to it. Hospital administrators and governmental agencies should be aware that operations on patients with lower ASA grade appear to have lower risk and should consider restructuring service delivery to ensure that procedures are performed in designated COVID-19-restricted sites. These measures may reduce the likelihood of patients contracting the virus in the hospital, although we cannot confirm a benefit from this study. Future studies should seek to identify factors influencing these outcomes and also compare surgical complications in those patients with and without COVID-19.
Level of Evidence
Level III, therapeutic study.
Introduction
After the outbreak of the coronavirus 2019 (COVID-19) pandemic, an exceptional burden has been placed on healthcare systems internationally to continue normal service delivery and mitigate the risk of hospital-acquired COVID-19 [1, 22]. However, with respect to surgical operations for cancer, timely service delivery is imperative. Delays in diagnosis and surgical management increase the likelihood of disease progression and metastasis and may be associated with increased morbidity and mortality, depending on the length of delay in care. Therefore, it is important that cancer surgery, in those patients where a delay would be detrimental, be conducted in a timely and safe manner during the pandemic.
Reports during the initial phase of the pandemic reflected an increased complication risk related to COVID-19 in patients with cancer [2, 3, 13, 20, 21]. Recommendations on restructuring departments and services to reduce the risks associated with hospitalization and surgical care for patients with cancer have been published [5, 10, 18]. Intentional postponement of elective cancer surgery to reduce patient traffic and therefore disease transmission, reallocation of resources such as personal protective equipment for clinically urgent surgical and nonsurgical services, and increasing inpatient bed capacity are examples of how organizations have restructured their services. Performing surgery during the pandemic also poses a risk to healthcare professionals because of the inevitable use of aerosol-generating procedures, even with extra safety protocols implemented, such as enhanced levels of personal protective equipment [6, 8]. It is important that cancer surgery be conducted with as few interruptions as possible while still attempting to provide protection from disease transmission during the pandemic. Nevertheless, there is a scarcity of data on the risk of patients with musculoskeletal tumors (bone and soft tissue tumors) undergoing surgical procedures contracting COVID-19 in the hospital and having associated mortality [11, 16]. The role of neoadjuvant therapy, including radiation therapy and chemotherapy, in patients with musculoskeletal tumors who undergo surgery during the pandemic also needs an analysis so we might tailor appropriate practices during the peak of the pandemic. Acquisition of large-scale data on a national level and analyzing outcomes would enable surgeons to assess the best practices for surgical procedures in patients with musculoskeletal tumors and assist in the development of evidence-based guidelines to deliver appropriate surgical care to patients with bone and soft tissue tumors by reducing their risk of contracting COVID-19 and its related complications.
This multicenter study from eight specialist centers managing musculoskeletal oncology across the United Kingdom during the pandemic aimed to address the following questions: (1) What is the risk of hospital-acquired COVID-19 in patients with musculoskeletal tumors admitted for surgery during the initial period of the pandemic? (2) What is the associated mortality in patients with musculoskeletal tumors who have contracted COVID-19? (3) Are patients with musculoskeletal tumors who have had neoadjuvant therapy (chemotherapy or radiation) preoperatively at an increased risk of contracting COVID-19? (4) Is a higher American Society of Anesthesiologists (ASA) grade in patients with musculoskeletal tumors associated with an increased risk of contracting COVID-19 when admitted to the hospital for surgery?
Patients and Methods
Study Design and Setting
On March 11, 2020, the WHO declared COVID-19 a pandemic [4], which resulted in the restructuring of how medical facilities provide care for patients with cardiac disease, cancer, and traumatic injuries; the restructuring included postponing elective surgery. This study is a retrospective, observational audit involving eight centers across the United Kingdom under the British Orthopaedic Oncology Society collaborative group. This included five designated centers that are nationally approved to manage both primary bone and soft tissue tumors in England; we also included two centers from Scotland and one specialist soft tissue sarcoma center to have a wider representation.
Between March 12, 2020 and May 20, 2020, data on patients with musculoskeletal tumors undergoing surgery in all eight specialized hospitals in the United Kingdom were collected and retrospectively analyzed. The decision to perform surgery at all centers was made with strict adherence to the guidelines of the National Health Service and the British Orthopaedic Oncology Society. Patients were categorized as follows, as per the guidelines [19]: Priority Level 1a: emergency surgery needed within 24 hours to save life, Priority Level 1b: urgent surgery needed within 72 hours, Priority Level 2: elective surgery to save life or prevent disease progression beyond operability, and Priority Level 3: elective surgery that can be delayed for 10 to 12 weeks and will have no predicted negative outcome. We do not have specific data regarding the number of patients included in each category. Considering the wide heterogeneous nature of musculoskeletal tumors, the decision as to whether patients were operated on was determined by the regional sarcoma multidisciplinary tumor board in adherence with the national guidelines. Each patient was discussed on a case-by-case basis considering not only their tumor status but also other comorbidities. We do not have more specific information on how these decisions were made.
Priority Level 3 procedures were postponed across all centers to a suitable time after the pandemic. Surgical procedures categorized under Priority Levels 1a, 1b, and 2 and procedures for patients who were likely to benefit from a potentially curative cancer operation were planned after discussion in the regional sarcoma multidisciplinary team meeting tumor board. Across all centers, only patients who were deemed to have negative test results for COVID-19 were operated on. Because the testing policies and guidelines across respective tests varied and changed over time, a uniform method of preoperative testing for the disease was not possible, and in most instances, it was assumed that patients were COVID-19 negative if asymptomatic. The healthcare professionals involved in the surgical procedures used personal protection equipment across all centers. The study period included the peak of the COVID-19 pandemic in the United Kingdom. By policy, any patient who tested positive for COVID-19 before their operation would have been required to quarantine for 14 days and have a second, negative test to proceed unless this was a life-or-limb-threating operation. In this study, no such patients with musculoskeletal tumors fell into this category.
Patient Flow
Between March 12 and May 20, 2020, 4448 surgical procedures were performed in the eight participating centers. Of these, 4101 were excluded, including patients with trauma, infection, spine, arthroplasty, cardiac procedures, and cancers other than musculoskeletal tumors. In total, 347 patients with musculoskeletal tumors underwent surgery during this 9-week period at all eight centers, all of whom were followed up to 30 days postsurgery. No patient was lost to follow-up (Fig. 1).
Fig. 1.
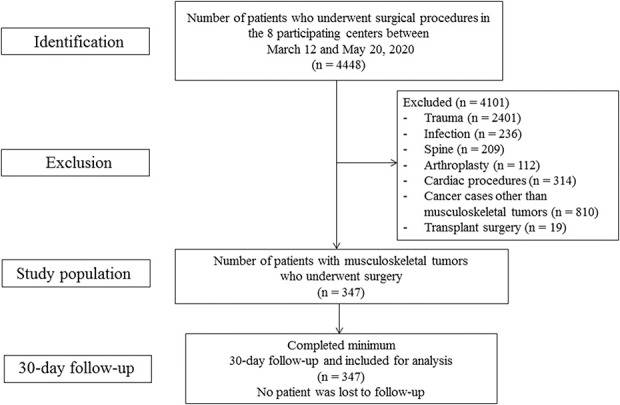
Flowchart depicting the selection of the study population from the eight participating centers during the 9-week study period.
Data Sources
Data were acquired from the electronic patient record system at each of the eight participating centers to ascertain the number of patients who contracted COVID-19 in the hospital and those who died. Relevant data were subsequently collated in Microsoft Excel (Redmond, WA, USA). Required data were available for all 347 patients in the study group.
Demographic variables including age, gender, and ASA physical status classification were collected for all patients (Table 1). The diagnosis of musculoskeletal tumor confirmed by the multidisciplinary tumor board was noted in all patients. Oncologic variables including diagnosis, details of neoadjuvant therapy if any, surgical procedure, and postoperative length of stay were recorded. A COVID-19 diagnosis during the hospital stay was made using nasal swabs and real-time PCR. Testing policies for patients varied across different hospitals during the initial period of the pandemic. Fifty-nine percent (204 of 347) were negative in PCR testing done 48 hours before surgery. Before mandatory testing, only asymptomatic patients were admitted for surgery. Forty-one percent (143 of 347) of patients who were of asymptomatic of COVID-19 before the introduction of mandatory PCR testing underwent surgery. Mortality within 30 days and its cause in patients with musculoskeletal tumors who underwent an operation were recorded.
Table 1.
Demographics of the 347 musculoskeletal tumor patients operated on during the study period across eight centers in the UK (March 12, 2020 to May 20, 2020)
| Patient demographics | COVID-19 negative (n = 335) | COVID-19 positive (n = 12) | p value |
| Age in years, mean ± SD | 53 ± 21 | 63 ± 22 | 0.17 |
| Gender Men, n Women, n |
199 136 |
8 4 |
0.77 |
| Length of stay in days, mean ± SD | 7 ± 8 | 16 ± 15 | 0.19 |
A total of 347 patients with musculoskeletal tumors who underwent surgery during the 9-week study period at all eight centers formed the study group. The median (range) age was 53 years (10 to 94), 60% were men (207 of 347), the median ASA grade was II (I to IV), and the median hospital stay was 8 days (0 to 53). Of the 347 patients, 56% (194) had soft tissue tumors and 44% (153) had bone tumors. With relation to the ASA classification, 21% (74) had ASA Grade I, 47% (162) had ASA Grade II, 29% (102) had ASA Grade III, and 3% (9) had ASA Grade IV. Eighteen percent (61 of 347) of patients received neoadjuvant therapy (8% [27] had chemotherapy, 8% [28] underwent radiation, and 2% [6] had both chemotherapy and radiation) preoperatively (Table 2).
Table 2.
Details of distribution of ASA grade and neoadjuvant therapy among the study group (n = 347)
| Variable | Percentage (n) |
| ASA grade | |
| ASA I | 21 (74) |
| ASA II | 47 (162) |
| ASA III | 29 (102) |
| ASA IV | 3 (9) |
| Patients who received neoadjuvant treatment preoperatively | |
| Total | 18 (61) |
| Radiation therapy | 8 (28) |
| Chemotherapy | 8 (27) |
| Radiotherapy and chemotherapy | 2 (6) |
ASA = American Society of Anaesthesiologists.
The details of all patients were available for analysis at 30 days of follow-up. No patient was lost to follow-up.
Primary and Secondary Study Outcomes
The primary outcome of the study was contracting COVID-19 in the hospital after admission. The secondary outcome was associated mortality after contracting COVID-19 within 30 days of surgery. In addition, we assessed whether there was any association between ASA grade or neoadjuvant treatment and the chances of contracting COVID-19 in the hospital. The electronic patient record system and simple descriptive statistics were used to analyze both outcomes.
Statistical Analysis
Patient demographics and other clinical variables were statistically compared to ensure that their distribution was similar between the primary groups (COVID-19 and nonCOVID-19 patients) so that appropriate conclusions could be derived without any bias from clinical or demographic variables. Mortality, neoadjuvant status, and ASA grade were the main variables of interest and were assessed for their relationship with COVID-19 status. Apart from descriptive statistics, categorical variables were statistically assessed using a chi-square test or Fisher exact test, and continuous variables were compared using a t-test. Odds ratios and their confidence intervals were estimated when proportions of the variables were compared between the primary groups. Significance was considered at p < 0.05, and confidence intervals were estimated at 95%.
Results
Ninety-eight percent (340 of 347) of patients had recovered from surgery and were discharged home at a 30-day follow-up. Four percent (12 of 347) contracted COVID-19 in the hospital. Two percent (7 of 347) died within 30 days postoperatively. One percent (4 of 347) died because of complications associated with COVID-19, and 1% (3 of 347) died because of complications related to the underlying cancer.
Risk of Hospital-acquired COVID-19
Four percent (12 of 347) of patients with musculoskeletal tumors admitted for a surgical procedure and who were free of COVID-19, by either the absence of symptoms or negative COVID-19 test results, contracted COVID-19, confirmed by COVID-19 PCR testing, while admitted to one of our eight centers in the United Kingdom (Table 3). In addition, 71 other patients were tested by PCR and were negative. They had an uneventful recovery.
Table 3.
Relationship between COVID-19 contraction and mortality and ASA Grade in musculoskeletal tumor patients admitted to the hospital for surgery
| Parameter | Group 1 COVID-19 negative (n = 335) | Group 2 COVID-19 positive (n = 12) | Odds ratio (95% CI) | p value |
| Mortality, % (n) | 1 (3) | 33 (4) | 55 (11-289) | < 0.001 |
| ASA Grade IV, % (n) | 2 (5) | 33 (4) | 58 (5-626)a | < 0.001 |
ASA Grade IV OR was calculated with respect to ASA Grade 1.
Mortality of Patients with Musculoskeletal Tumors Who Contracted COVID-19
One percent (4 of 347) of patients who contracted COVID-19 in the hospital died of COVID-19-related causes (Table 3). The mortality risk among patients who contracted COVID-19 after surgery was greater than among patients who did not have COVID-19 (OR 55.33 [95% CI 10.60 to 289.01]; p < 0.001)
Does Neoadjuvant Therapy (Chemotherapy or Radiation) Increased Risk of Contracting COVID-19?
With the numbers we had, we could not show an association between neoadjuvant therapy and the proportion of patients who acquired COVID-19 during their hospital stay (OR 0.94 [95% CI 0.20 to 4.38]; p = 0.93). A subgroup analysis indicated that neither chemotherapy (OR 0.2 [95% CI 0.40 to 9.02]; p = 0.42) nor radiation therapy was associated with acquiring COVID-19. We could not calculate an OR for radiotherapy since no patients who received radiation preoperatively became COVID-19 positive. This may be a phenomenon of small numbers.
ASA Grade and Risk of Contracting COVID-19
Higher ASA grade was associated with an increased likelihood of contracting COVID-19 (OR 58 [95% CI 5 to 626]; p < 0.001). The chance of patients with ASA Grade IV contracting COVID-19 was much greater compared with those who were ASA Grade I (Table 3). Mortality was twofold higher in patients with ASA Grade IV compared with those with ASA Grade III; there was no mortality in ASA Grade I or ASA Grade II.
Other Findings
Within the sample we had, there were differences between the COVID and nonCOVID groups for patient age (COVID-19 negative: 53 ± 21 years; COVID-19 positive: 63 ± 22 years; p = 0.17) and gender (men: COVID-19 negative 41% [136 of 335], COVID-19 positive 33% [4 of 12]; women: COVID-19 negative 59% [199 of 335], COVID-19 positive 67% [8 of 12]; p = 0.77).
Discussion
Minimizing the detrimental effects of delaying surgical treatment of musculoskeletal tumors, continued service delivery has been essential even during the COVID-19 pandemic. Therefore, knowledge about the risks of contracting COVID-19 and the associated mortality will help improve patient care. Further, large-scale data on these risks in patients with musculoskeletal tumors are required to enhance surgical care. This study aimed to address the above by analyzing data from eight centers across the United Kingdom during the early period of the COVID-19 pandemic (Fig. 2). Our data reflect that there is a small risk of patients contracting COVID-19 in the hospital, and when it occurs, it is associated with increased mortality. The risk of contracting COVID-19 increases with higher ASA grades. Neoadjuvant therapy did not increase the risk of contracting COVID-19. Centers treating patients with musculoskeletal tumors must therefore consider intentional postponement of surgery in patients with higher ASA grades where possible and aim to use COVID-19-restricted facilities to perform surgery.
Fig. 2.
Map of the United Kingdom depicting the locations of the eight specialist musculoskeletal tumor centers involved in the study.
Limitations
Our study has several limitations. Although the study was retrospective, all necessary variables to analyze these patients postoperatively were collected from the databases of the participating centers. Generally, with multicenter studies, there is reduced adherence to protocols during data collection, but in our study, pertinent data from each center were collected by clinical fellows and verified by consultants. Another major limitation was that each center had different guidelines with regard to patient testing and the surgical pathway in admitting patients preoperatively. Nevertheless, this was to be expected and would be analogous in all healthcare systems globally, because the pandemic has evolved rapidly across various geographic regions based on the disease prevalence. The diverse and heterogeneous nature of patients with musculoskeletal tumors was also a limiting factor, but their treatment was based on the standard principles of cancer care across specialized centers involving experienced multidisciplinary teams. Healthcare professionals involved with musculoskeletal tumor care during the initial stages of the pandemic were not routinely tested, and these providers may have been asymptomatic carriers of COVID-19. Nonetheless, all healthcare professionals were eventually tested across all eight centers, and only those with negative test results were involved in patient care. Those who tested positive were quarantined at home for at least 14 days before needing a negative test to return to work. We do not have data for the number of healthcare workers who contracted COVID-19 during this time, nor do we know anything about an association with transmission to patients. Although this study shows that there is no apparent association between neoadjuvant treatment and contracting COVID-19, this could be falsely reassuring due to the study being underpowered, especially given that this group of patients could be more susceptible to COVID-19 due to immunosuppression. Each of the eight centers involved in this study responded differently to the pandemic based on their infrastructure and available resources. This lack of uniformity could have influenced results. However, as the pandemic was in the initial period, each hospital was responsible for local infection control guidelines but with time, nationally approved protocols may help address this lack of uniformity. One further limitation of this study is the lack of standardized testing across the eight centers. Due to resource constraints, continuous testing of asymptomatic individuals was not possible, and this could conceivably affect results. Nationally approved guidelines for standardized testing of asymptomatic individuals may help mitigate this limitation. Finally, the COVID-19 pandemic remains a fluid situation, with new data and guidelines being published regularly. Global multicenter studies will help in understanding the exact relevance of our results with respect to musculoskeletal tumors. Analyzing greater numbers of patients with musculoskeletal tumors who underwent surgery will enable us to answer more-nuanced questions and guide further research.
Risk of Hospital-acquired COVID-19 and Mortality After a COVID-19 Diagnosis
We observed that 4% (12 of 347) of our patients with musculoskeletal tumors contracted COVID-19 during their hospital stay, and 1% (4 of 347) of patients died. These findings underscore that hospitals are potential sources of COVID-19 infection. Similar results were reported by two centers participating in this multicenter study; an analysis of a small series of patients during a shorter study period showed that patients with musculoskeletal tumors were at a considerable risk of contracting COVID-19 [11, 16]. This fact is even more concerning to patients with cancer awaiting surgery, whose immunity is compromised because of the underlying cancer and more so if they receive adjuvant treatment. Several hospitals in the United Kingdom reported that nearly 20% of patients with a diagnosis of COVID-19 had contracted it nosocomially [7]. These findings highlight the fact that nonurgent surgery should be postponed to a more suitable time after the pandemic. However, this may not be possible with cancer surgery, including musculoskeletal tumors, because deferring surgery could result in disease progression with potentially profound adverse effects, including mortality. Thus, delaying treatment may not be a reasonable option. Therefore, we believe that COVID-19-restricted facilities must be seriously considered to perform cancer surgery, and these centers should follow strict steps to mitigate disease transmission. Measures including restrictions to visitors, temperature checks before hospital entry, mandatory donning of personal protective equipment, and strict social distancing must be implemented as preventive methods of reducing disease transmission.
The fact that patients contracted COVID-19 in the hospital implies that healthcare staff could act as unknowing, asymptomatic virus carriers, emphasizing the need for routine real-time PCR testing of all healthcare staff. Designated COVID-19-restricted centers might potentially be better for performing cancer surgery, and periodic testing of involved healthcare staff is also warranted to mitigate disease transmission between hospital staff and patients. The need for designated COVID-19-free hospitals to offer continued cancer care was recommended by Restivo et al. [14] after their initial observation of the pandemic in Italy and has also been recommended by others [12, 15]. These facilities must implement standardized, mandatory preoperative testing pathways before patient admission. Furthermore, continuous testing of asymptomatic healthcare professionals is likely to be beneficial in reducing transmission from healthcare staff to patients. Once these protocols are in place, we need to study the effects of these interventions. The efficacy of COVID-19-restricted centers in cancer care needs to be analyzed and, if associated with positive outcomes, could be the way forward during any future COVID-19 spikes.
Does Neoadjuvant Therapy (Chemotherapy or Radiation) Increase Risk of Contracting COVID-19?
We found that with the numbers available, radiation therapy and chemotherapy were not associated with an increased risk of contracting COVID-19 disease among patients with musculoskeletal tumors who underwent surgery. Neoadjuvant therapy is integral in musculoskeletal oncology care. In a prospective, observational study analyzing 800 patients with cancer and COVID-19 who were treated with systemic anticancer treatment, the authors found no difference in mortality compared with patients with cancer who had not received chemotherapy [9]. In spite of these results, we do not really know whether patients receiving neoadjuvant therapies for sarcoma are at increased risk for COVID-19 infection, but it is certainly possible that they are since they are immunocompromised. Additional large-scale data are warranted on this key area in musculoskeletal oncological care to draw conclusions about the association between neoadjuvant therapy and COVID-19 transmission.
ASA Grade and Risk of Contracting COVID-19
An increased ASA grade was associated with an increased risk of contracting COVID-19 in patients with musculoskeletal tumors while admitted for surgery. The COVIDSurg Collaborative, which reported 30-day mortality and pulmonary complications postoperatively in patients with COVID-19 across 235 hospitals in 24 countries, showed that an ASA grade of III to V was a factor in predicting mortality compared with ASA grades I and II [3]. In an adjusted analysis, even the 7-day mortality in patients with ASA grades III to V was higher. This is an important factor to consider when performing surgery in patients with cancer who have comorbidities or higher ASA grades. For patients with musculoskeletal tumors undergoing surgery who have higher ASA grades, a prudent approach involving a frank discussion between the clinical team and the patient and his or her relatives is essential. It is important to consider the aggressiveness of the cancer and the risk-benefit ratio. Intentional postponement of surgery to a suitable time after the pandemic can be considered in some such patients [17].
Conclusion
The results of our study illustrate that there was a small risk of contracting COVID-19 in hospitals and associated mortality in patients with musculoskeletal tumors admitted for surgical procedures during the initial part of the pandemic. Higher ASA grades were associated with increased risk of contracting COVID-19. Therefore, to reduce COVID-19 transmission, it would be prudent to consider intentionally postponing surgery in patients with higher ASA grade where possible. Furthermore, the use of COVID-19-restricted facilities for surgical procedures, preoperative testing of all patients before their procedure, and frequent testing of hospital staff must be considered in the event of a second COVID-19 peak or another future pandemic. These are logical steps, but the practicality and effectiveness of these measures remains to be tested. The acquisition of additional large-scale data are warranted in future studies to identify the factors influencing these results in addition to comparing surgical complications in those patients with and without COVID-19.
Group Authors
British Orthopaedic Oncology Society Collaborative Group: Adesegun Abudu, Will Aston, Corey D. Chan, Scott Evans, Sanjay Gupta, Sanjeev Kotecha, Danielle Maes, Ashish Mahendra, Guy Morris, Michael Parry, Muhammad Sarmad Tamimy, Sofia Thoma, Roger Tillman
Acknowledgments
We thank Mr. Janardhan Yerramshetty PhD for his help with the statistical analysis of data. We thank the following healthcare professionals across all centers who helped with data collection and who were involved in the surgical care of patients with musculoskeletal tumors during the COVID-19 pandemic: Harriet Branford White FRCS, M. Ather Siddiqi FCPS, Richard Myatt MBBS, Ermias Hailemeskel MS, Viswanath Jayasankar MS, Vineet Kurisunakal MS, Floortje Verspoor PhD, Gillian Cribb FRCS, and Karen Shepherd PhD.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
*Members of the British Orthopaedic Oncology Society Collaborative Group are listed in an Appendix at the end of this article.
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Science, University of Oxford, Oxford, UK.
Contributor Information
Collaborators: Adesegun Abudu, Will Aston, Corey D Chan, Scott Evans, Sanjay Gupta, Sanjeev Kotecha, Danielle Maes, Ashish Mahendra, Guy Morris, Michael Parry, Muhammad Sarmad, Muhammad Sarmad Tamimy, Sofia Thoma, and Roger Tillman
References
- 1.Al-Jabir A, Kerwan A, Nicola M, et al. Impact of the coronavirus (COVID-19) pandemic on surgical practice - part 1. Int J Surg . 2020;79:168-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brindle EM, Gawande A. Managing COVID-19 in surgical systems. Ann Surg . 2020;272:e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed . 2020;91:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulia A, Arora RS, Panda PK, et al. Adapting management of sarcomas in COVID-19: an evidence-based review. Indian J Orthop. Published online May 30, 2020. DOI: 10.1007/s43465-020-00143-1. [DOI] [PMC free article] [PubMed]
- 6.Herron JBT, Hay-David AGC, Gilliam AD, Brennan PA. Personal protective equipment and Covid 19- a risk to healthcare staff? Br J Oral Maxillofac Surg. 2020;58:500-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iacobucci G. Covid-19: Doctors sound alarm over hospital transmissions. BMJ . 2020;369:m2013. [DOI] [PubMed] [Google Scholar]
- 8.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study [published correction appears in Lancet. 2020;39:758]. Lancet. 2020;395:1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study [published correction appears in Lancet. 2020;396:534]. Lancet. 2020;395:1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Service. Clinical guide for the management of non-coronavirus patients requiring acute treatment: cancer. Available at: https://covid19.aischannel.com/guidelines-nhs/files/clinical-guide-for-the-management-of-non-coronavirus-patients-requiring-acute-treatment-cancer. Accessed March 23, 2020.
- 11.Rajasekaran RB, Kotecha S, Whitwell D, et al. Patient safety associated with the surgical treatment of bone and soft tissue tumours during the COVID-19 pandemic-results from an observational study at the Oxford Sarcoma Service. Int Orthop. 2020;44:1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajasekaran RB, Whitwell D, Cosker TDA, Gibbons CLMH. Service delivery during the COVID-19 pandemic: experience from The Oxford Bone Tumour and Soft Tissue Sarcoma service. J Clin Orthop Trauma. 2020;11(suppl 4):S419-S422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond E, Thieblemont C, Alran S, Faivre S. Impact of the COVID-19 outbreak on the management of patients with cancer. Target Oncol. 2020;15:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Restivo A, De Luca R, Spolverato G, et al. The need of COVID19 free hospitals to maintain cancer care. Eur J Surg Oncol. 2020;46:1186-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shubber N, Sheppard J, Alradhawi M, Ali Y. The impacts of the novel SARS-CoV-2 outbreak on surgical oncology [letter]. Int J Surg. 2020;79:109-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson JD, Evans S, Morris G, et al. Mortality of high-risk orthopaedic oncology patients during the COVID-19 pandemic: a prospective cohort study. J Surg Oncol. Published online July 15, 2020. DOI: 10.1002/jso.26127. [DOI] [PMC free article] [PubMed]
- 17.Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31:1065-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsamakis K, Gavriatopoulou M, Schizas D, et al. Oncology during the COVID-19 pandemic: challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20:441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West Midlands Cancer Alliance. Joint statement from the British Sarcoma Group, British Orthopaedic Oncology Society, and the Chairs of the Regional Sarcoma Advisory groups relating to COVID-19. https://wmcanceralliance.nhs.uk/images/Documents/Covid-19_2020/Joint_Statement_from_BSG_BOOS_and_the_Chairs_of_the_Regional_Sarcoma_Adpdf.pdf. Accessed July 23, 2020.
- 20.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol . 2020;21:904-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020;395:1038]. Lancet. 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]


