Abstract
Circulating concentrations of the pleiotropic cytokine interleukin-6 (IL-6) are known to be increased in pro-inflammatory critical care syndromes, such as sepsis and acute respiratory distress syndrome. Elevations in serum IL-6 concentrations in patients with severe COVID-19 have led to renewed interest in the cytokine as a therapeutic target. However, although the pro-inflammatory properties of IL-6 are widely known, the cytokine also has a series of important physiological and anti-inflammatory functions. An adequate understanding of the complex processes by which IL-6 signalling occurs is crucial for the correct interpretation of IL-6 concentrations in the blood or lung, the use of IL-6 as a critical care biomarker, or the design of effective anti-IL-6 strategies. Here, we outline the role of IL-6 in health and disease, explain the different types of IL-6 signalling and their contribution to the net biological effect of the cytokine, describe the approaches to IL-6 inhibition that are currently available, and discuss implications for the future use of treatments such as tocilizumab in the critical care setting.
This is the second in a Series of four papers about COVID-19
Introduction
The molecule now known as interleukin-6 (IL-6) was initially named B-cell stimulating factor, having first been discovered in the mid-1980s by Hirano and colleagues1 as a product of T cells that appeared to stimulate B cells and enhance antibody production. IL-6 has since emerged as a master regulator of inflammation and one of the few truly pleiomorphic cytokines, alongside IL-1 and tumour necrosis factor (TNF).
The three-dimensional structure of the cytokine consists of a four-helix bundle linked by loops and an additional mini-helix. IL-6 is secreted by a wide range of cell types under the conditions of infection, inflammation, or neoplastic disease.2, 3 The gene expression of this cytokine is controlled by activating nuclear factors such as nuclear factor (NF)-κB,4, 5, 6 NF-IL6,7 and hypoxia-inducible factor-1α.8 The stimuli driving the transcription and release of IL-6 are variable, and include the engagement of Toll-like receptors by bacterial lipopolysaccharides,9 changes in cellular metabolism,10 pro-inflammatory cytokines such as TNF and IL-1,11, 12 and viral infection.13
Elevations in IL-6 have been identified in critical illnesses such as sepsis, acute respiratory distress syndrome (ARDS), and, most recently, COVID-19, prompting consideration of the use of therapies designed to inhibit the biological effects of the cytokine. Although the pro-inflammatory effects of IL-6 are well recognised, there are many instances in which it functions as an anti-inflammatory or protective molecule. IL-6 is essential for innate and adaptive immunity, is required for efficient pathogen clearance, and has an important physiological role in humans, regulating the acute-phase response, haematopoiesis, metabolic rate, lipid homoeostasis, and neural development (figure 1 ).2 It would seem reasonable, therefore, that any attempt to target the pathological effects of IL-6-mediated inflammation should aim to avoid the unintentional concomitant abolition of its anti-inflammatory and pro-resolution effects. To achieve this balance—and to properly interpret IL-6 concentrations in the blood or lung as biomarkers of disease status—an adequate understanding of IL-6 signalling is crucial.
Figure 1.
The pleiotropic effects of IL-6
IL-6, which consists of a four-helix bundle, is a master cytokine that exerts various biological effects. In addition to driving inflammation, fever, cytokinaemia, and tumorigenesis, IL-6 regulates metabolism, bone turnover, and haematopoiesis, and is essential for innate and adaptive immunity. In the liver, IL-6 contributes to tissue regeneration, lipid balance, and induction of the acute-phase response. This cytokine also promotes macrophage polarisation from the pro-inflammatory M1 state to an anti-inflammatory M2 phenotype and is required for the proliferation of the intestinal epithelium. IL-6=interleukin-6. RANKL=receptor activator of nuclear factor-κB ligand. VEGF=vascular endothelial growth factor.
IL-6 signalling
The soluble receptor
Three principles are central to understanding IL-6 signalling. The first is that, for signal transduction to occur, IL-6 must first bind the IL-6 receptor (IL-6R), after which the resultant complex must associate with a second protein called glycoprotein 130 (gp130).14, 15 The second principle is that IL-6R is a membrane-bound receptor expressed by only a few cell types, most notably hepatocytes and some leucocytes such as macrophages and T-cell subsets, whereas gp130 is expressed by all cell types.16, 17, 18 Finally, IL-6 has an affinity for IL-6R but not for gp130,14 which means that IL-6 will not bind gp130 directly and must instead be part of a complex with IL-6R to do so.
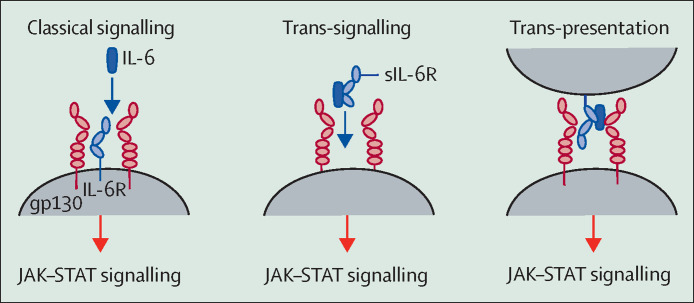
In healthy states, these principles mean that there are essentially two categories of cells: those that express both IL-6R and gp130 and are naturally IL-6-responsive, and those that express gp130 but not IL-6R and therefore cannot respond to the cytokine. IL-6 signalling via its membrane-bound cognate receptor is termed classical signalling (figure 2 ).
Figure 2.
The different types of IL-6 signalling
In classical signalling, IL-6 binds the membrane-bound IL-6R to form an IL-6–IL-6R complex, which subsequently associates with gp130 to generate signal transduction via the JAK–STAT pathway. IL-6 can also bind to soluble forms of the IL-6R before converging on gp130 (trans-signalling), or be trans-presented from dendritic cells via their membrane-bound IL-6R to T cells (trans-presentation). In general terms, classical signalling causes the physiological, anti-inflammatory, and pro-resolution effects of IL-6, with the pathological effects of the cytokine mediated by trans-signalling and trans-presentation. Importantly, monoclonal antibodies against IL-6R, such as tocilizumab, do not discriminate between these signalling types, and instead block all IL-6 signalling. gp130=glycoprotein 130. IL-6=interleukin-6. IL-6R=interleukin-6 receptor. JAK=Janus kinase. sIL-6R=soluble interleukin-6 receptor. STAT=signal transducer and activator of transcription.
However, in pro-inflammatory states, the framework shifts. Cleavage of IL-6R from the cell surface, and to a minor extent alternative splicing, generates a soluble form, sIL-6R. The key enzyme that undertakes this cleavage event is disintegrin and metalloprotease domain-containing protein 17 (ADAM-17), which is activated in response to inflammation and infection.19 Crucially, sIL-6R retains the ability to bind its ligand IL-6. Moreover, the IL-6–sIL-6R complex that ensues is capable of binding gp130. Under these circumstances, since gp130 is ubiquitously expressed, IL-6 can bind cells that are otherwise unresponsive to the cytokine and induce signalling. This process, termed trans-signalling (figure 2),20 explains why epithelial cells, smooth muscle cells, and endothelial cells have a pronounced and rapid response to IL-6 in critical illness, and identifies ADAM-17 as a potential therapeutic target.
Key messages.
IL-6 is best known for its pro-inflammatory effects. However, this pleiomorphic cytokine also has anti-inflammatory, pro-resolution, and regenerative properties, is important for pathogen clearance, and triggers the release of acute-phase proteins via the liver.
IL-6 signalling
-
•
IL-6 binds the IL-6R, after which the IL-6–IL-6R complex must associate with a second protein, gp130, before signal transduction can occur; gp130 is present in all cells
-
•
Signalling via membrane-bound IL-6R is termed classical signalling; only the few cell types that express IL-6R, most notably hepatocytes and macrophages, can respond to IL-6 under normal circumstances
-
•
In trans-signalling, ADAM-17-mediated cleavage of IL-6R during acute inflammation generates a soluble receptor that readily binds IL-6 away from the cell surface; the resultant IL-6–sIL-6R complex can bind cellular gp130, enabling cells that do not express IL-6R to become responsive to IL-6
-
•
Anti-inflammatory and antibacterial activities of IL-6 are mediated by classical signalling, whereas pro-inflammatory effects are mediated by trans-signalling
Strategies for blocking IL-6
-
•
Monoclonal antibodies against IL-6R, such as tocilizumab, do not discriminate between classical signalling and trans-signalling, but instead block both
-
•
Potential pharmacological alternatives are in development to selectively inhibit trans-signalling or modulate the JAK–STAT pathway through which IL-6 signals
Interpreting IL-6 measurements
-
•
The net biological effect of IL-6 is established by multiple factors beyond its absolute concentration; understanding of the balance of these factors is crucial for the accurate interpretation of serum IL-6 measurements
-
•
Attempts to compare diseases or syndromes on the basis of isolated IL-6 measurements are limited by differences in assay type used, timing of sampling, sample processing, and concomitant pharmacotherapy, all of which influence the IL-6 concentration detected
Implications for future studies
-
•
Clinical trials of interventions should target specific phenotypes and be informed by mechanistic studies
-
•
Treatment focused on changing absolute concentrations of cytokines such as IL-6 might represent a double-edged sword; therapeutic approaches that address underlying cause of cytokine elevations are more likely to be beneficial
-
•
This approach might also lead to the development of new therapies for critical care syndromes, as opposed to the repurposing of pre-existing therapies designed for other conditions
ADAM-17=disintegrin and metalloprotease domain-containing protein 17. gp130=glycoprotein 130. IL-6=interleukin-6. IL-6R=interleukin-6 receptor. JAK=Janus kinase. sIL-6R=soluble interleukin-6 receptor. STAT=signal transducer and activator of transcription.
ADAM-17 orchestrates many signalling pathways other than those pertaining to IL-6. In cancer, its proteolytic effects provide a supply of previously membrane-bound ligands for the epidermal growth factor receptor; it also drives autoimmunity by cleaving the transmembrane protein TNF to make it systemically available.
There is one further non-classical means by which IL-6 signalling can occur. This method, known as trans-presentation, involves the antigen-specific interaction of a dendritic cell (which produces the IL-6 signal) and a T cell (which receives it), resulting in the commitment of the T cell to a highly tissue-destructive phenotype (figure 2).2, 21, 22
As a rule, the anti-inflammatory, pro-resolution, and anti-bacterial activities of IL-6 are mediated by classical signalling, whereas the pro-inflammatory activities of IL-6 are mediated by trans-signalling, and at times by trans-presentation.2, 21
The buffer in the blood
The next key concept regarding the in vivo biology of IL-6 is that of the buffer in the blood. IL-6 binds to the IL-6R with a medium to high affinity on the order of 1 nM. However, the affinity of the IL-6–IL-6R complex for gp130, be it membrane-bound or soluble, is substantially higher, at approximately 10 pM or 100 times more than that of IL-6 for its cognate receptor. Consequently, when all three components are present, IL-6 will preferentially bind IL-6R and the IL-6–IL-6R complex will then bind gp130. Notably, soluble forms of gp130 (sgp130) also exist, generated by alternative RNA splicing.2 Therefore, although the interaction between the IL-6–sIL-6R complex and membrane-bound gp130 forms the basis for trans-signalling and its attendant hazards, the interaction of sgp130 with this complex presents an opportunity to neutralise its effects.
The serum concentrations of IL-6 found in healthy individuals are low, around 1–5 pg/mL. These amounts are only slightly more than the lower limit of detection for most commercially available assays. Curiously, circulating concentrations of sIL-6R are much higher, approximately 40–75 ng/mL, with sgp130 even higher again, at 250–400 ng/mL. The molecular weight of IL-6 is 20 kDa, sIL-6R is 55 kDa, and sgp130 is 100 kDa. On the basis of the circulating concentrations of these proteins, a 3·5-times molar excess of sgp130 over sIL-6R is sometimes inferred, but since sgp130 exists predominantly as a dimer in vivo, the true excess is closer to 2 times. Therefore, it is apparent that the capacity provided by this buffering system is limited and is determined by the sIL-6R concentration.23 Conventional dendritic cells are a major source of circulating sIL-6R and set the in-solution persistence of IL-6, thereby regulating its signalling systemically.24
The buffer offers a reasonable amount of protection from acute or chronic low-level elevations in IL-6 and sIL-6R, but in the acute phase of some inflammatory states, such as meningococcal sepsis or cytokine release syndrome, the buffer is overwhelmed. This is not due to increases in IL-6—which can reach even microgram concentrations—per se, but rather a parallel surge in sIL-6R, which increases as much as 10 times in these conditions and easily exceeds the more modest increases in sgp130.
Inhibiting the biological effects of IL-6
Although specific receptors for individual ligands do exist, most receptors—especially those involved in signal transduction—are bound by multiple ligands, meaning that there are fewer receptors than there are signalling proteins. The implication of this imbalance is that directly inhibiting a given cytokine should offer increased precision and fewer off-target effects than the blockade of its receptor, since the approach of direct cytokine inhibition does not prevent other cytokines that use the same receptor from initiating their own signalling cascades and exerting their biological effects. As the inhibition target moves further downstream to kinases and transcription factors, the loss of specificity increases.25
The difficulty with IL-6 is its substantial pleiotropy, a characteristic that presents the unenviable task of trying to inhibit the bad while preserving the good. This challenge is reflected in the many different anti-IL-6 agents developed to date, and their various mechanisms of action (table ; figure 3 ). Many of the anti-IL-6 therapies that are available can only be administered orally, which represents a limitation, since the absorption of enteral drugs is highly variable in patients with critical illness.
Table.
Anti-IL-6 agents in the clinical drug development pipeline
| Route | Target | Conditions and phase of development | Comment | |
|---|---|---|---|---|
| Biologics | ||||
| Clazakizumab | Intravenous | IL-6 (site 1) | Rheumatoid arthritis and antibody-mediated rejection (renal transplantation; phase 2) | Multiple phase 2 COVID-19 studies recruiting, most not placebo-controlled; phase 2 study in psoriatic arthritis showed no dose effect |
| Sirukumab | Subcutaneous | IL-6 (site 1) | Depression (phase 2) | Phase 3 study in rheumatoid arthritis discontinued (increased rate of respiratory tract infections); phase 2 study in lupus nephritis discontinued |
| Siltuximab | Intravenous | IL-6 (site 1) | Castleman disease (approved); multiple myeloma and amyloid light-chain amyloidosis (phase 2) | Multiple phase 2 COVID-19 studies recruiting |
| Olokizumab | Subcutaneous | IL-6 (site 3) | Rheumatoid arthritis (phase 3) and COVID-19 (phase 2 and 3) | Phase 2 study in Crohn's disease discontinued because of delay in timelines |
| Tocilizumab | Subcutaneous and intravenous | IL-6R | Rheumatoid arthritis, juvenile idiopathic arthritis, Castleman disease, giant cell arteritis, cytokine release syndrome, and Takayasu's arteritis (approved); COVID-19 and neuromyelitis optica (phase 2) | Prospective, double-blind RCTs showed no mortality benefit in COVID-19; mixed results in open-label trials in COVID-19; high-dose group of phase 2 study in systemic lupus erythematosus stopped on safety grounds |
| Sarilumab | Subcutaneous | IL-6R | Rheumatoid arthritis (approved); COVID-19 (phase 3) | Unsuccessful in COVID-19 so far |
| Vobarilizumab | Subcutaneous | IL-6R | Rheumatoid arthritis and systemic lupus erythematosus (phase 2) | Progressing to phase 3 in rheumatoid arthritis despite missing primary endpoint (difference in ACR20 response at week 12) in phase 2 |
| Olamkicept | Intravenous | IL-6 and sIL-6R | Inflammatory bowel disease (phase 2) | Inhibitor of trans-signalling |
| Small-molecule inhibitors | ||||
| Tofacitinib | Oral | JAK1, JAK2, and JAK3 | Rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, and Crohn's disease (approved); psoriatic arthritis, psoriasis, alopecia areata, and ulcerative colitis (phase 3); scleroderma (phase 2) | Multiple phase 2 COVID-19 studies recruiting; clinical benefit might reflect inhibition of cytokines other than IL-6 |
| Ruxolitinib | Oral | JAK1 and JAK2 | Myelofibrosis and polycythaemia rubra vera (approved); graft-versus-host disease, polycythaemia rubra vera, vitiligo, and atopic dermatitis (phase 3); acute myeloid leukaemia, chronic lymphocytic leukaemia, essential thrombocythaemia, haemophagocytic syndrome, and bronchiolitis obliterans syndrome (phase 2) | Multiple phase 1 and 2 and one phase 3 COVID-19 studies recruiting; clinical benefit might reflect inhibition of cytokines other than IL-6 |
| Baricitinib | Oral | JAK1 and JAK2 | Rheumatoid arthritis (approved); juvenile idiopathic arthritis, systemic lupus erythematosus, alopecia areata, psoriaisis, and uveitis (phase 3); COVID-19 (phase 2 and 3); giant cell arteritis (phase 2) | Phase 2 trials in COVID-19 completed; prospective RCT data for COVID-19 not yet available |
| Filgotinib | Oral | JAK1 | Rheumatoid arthritis, Crohn's disease, and ulcerative colitis (phase 3); psoriatic arthritis, ankylosing spondylitis, lupus nephritis, and Sjogren's syndrome (phase 2) | Selective for JAK1; approval by the US Food and Drug Administration for use in rheumatoid arthritis denied on safety grounds |
| Upadacitinib | Oral | JAK1 | Rheumatoid arthritis, juvenile rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Takayasu's arteritis, giant cell arteritis, Crohn's disease, ulcerative colitis, and atopic dermatitis (phase 3) | Selective for JAK1 |
| Abrocitinib | Oral | JAK1 | Atopic dermatitis (phase 3) | Selective for JAK1 |
| Decernotinib | Oral | JAK3 | Rheumatoid arthritis (as VX-509; phase 3) | Specific for JAK3 |
Status as of Jan 31, 2021. Phase 1 studies not listed. IL-6=interleukin-6. IL-6R=interleukin-6 receptor. RCT=randomised controlled trial. sIL-6R=soluble interleukin-6 receptor.
Figure 3.
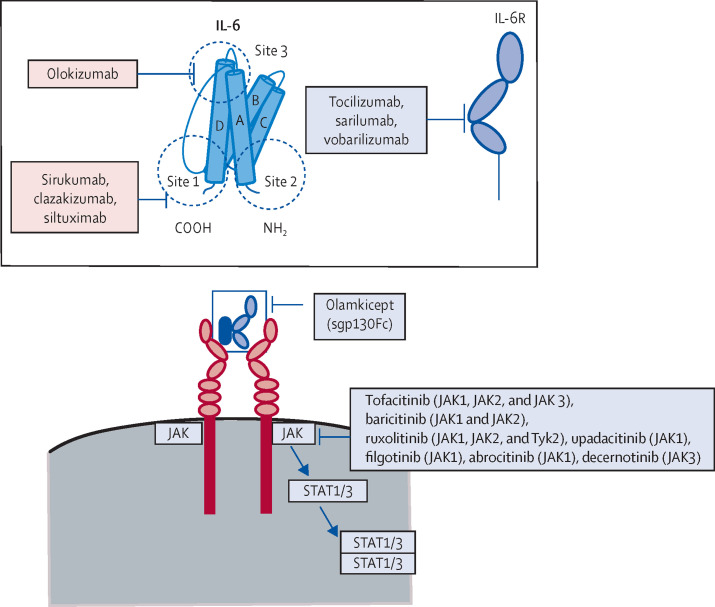
Pharmacological approaches to inhibiting the biological effects of IL-6
IL-6 can be inhibited directly at site 1, site 2, or site 3. The IL-6R can be blocked at the IL-6-binding site by the monoclonal antibodies tocilizumab, sarilumab, and vobarilizumab. The sgp130Fc olamkicept blocks IL-6 trans-signalling by neutralising the IL-6–sIL-6R complex before it can interact with cellular gp130. Within the cell, JAKs can be blocked by small-molecule kinase inhibitors of variable selectivity. IL-6=interleukin-6. IL-6R=interleukin-6 receptor. JAK=Janus kinase. STAT=signal transducer and activator of transcription. Tyk=non-receptor tyrosine-protein kinase.
Blocking IL-6R
By far the most widely studied method of inhibiting the effects of IL-6 is the use of monoclonal antibodies, such as tocilizumab, that are directed against the IL-6R. Tocilizumab neutralises IL-6 activity by binding the IL-6R at the IL-6-binding epitope.26 Since the initial approval of tocilizumab for the treatment of Castleman disease (a rare lymphoproliferative disorder),27 it has also been licensed for use in rheumatoid arthritis, systemic arthritis, juvenile idiopathic arthritis, adult-onset Still's disease, Takayasu's arteritis, giant cell arteritis, and chimeric antigen receptor T-cell-induced cytokine release syndrome.2, 21, 26, 28, 29 Additionally, a second monoclonal antibody, sarilumab, has received approval for the treatment of rheumatoid arthritis and Castleman disease.
The main limitation of tocilizumab is that it does not discriminate between classical signalling or trans-signalling, but instead blocks all IL-6R signalling, thereby inhibiting the aforementioned protective and reparative functions of IL-6 mediated by classical signalling.26, 30 In patients given tocilizumab, an increased incidence of bacterial infection has been observed, as have elevated concentrations of serum transaminases and dyslipidaemia, pancreatitis, and intestinal perforation, particularly in those who are also receiving corticosteroids.
Of additional concern, the effects of tocilizumab cannot be reversed after administration, and at high serum concentrations, it has a terminal half-life of approximately 16 days. Although its half-life does not necessarily preclude the use of this drug, an inability to change course in patients more prone to sudden fluctuation (eg, in clinical, biochemical, haemodynamic, or ventilatory variables) than stable outpatients receiving the therapy for rheumatological disease might prove problematic. Furthermore, the safest way to wean acutely unwell patients from this therapy has yet to be agreed.
Blocking IL-6 directly
Early attempts to directly inhibit IL-6 involved the use of neutralising antibodies against the cytokine. Although these antibodies had a degree of efficacy in multiple myeloma,31 the size of the complexes that ensued meant that they could not be adequately excreted by the kidney, with circulating IL-6 increasing to milligram concentrations. At this point, IL-6 began to dissociate off the antibody.26, 32 The severe side-effects that ensued, which included persistent fever, fatigue, bone pain, and hypercalcaemia, reduced the enthusiasm for this approach.
IL-6 has three distinct binding sites (sites 1, 2, and 3), each of which represents a target for monoclonal antibodies (figure 3). IL-6 binds the IL-6R via site 1, with the establishment of the IL-6–IL-6R complex causing gp130 to attain a dimer conformation. IL-6 then binds both dimerised gp130 molecules, one via site 2 and the other via site 3.33, 34 The same binding sites and sequence of events apply when the soluble forms of IL-6R and gp130 are involved.
Neutralising gp130
gp130 is present in all cell types, and because it functions as a signalling receptor for many cytokines other than IL-6,35 neutralising antibodies against gp130 stand to interfere with multiple signalling pathways.36 Results from in vivo animal models have not been encouraging: mice deficient for gp130 do not survive after birth, and gp130 ablation in cardiac myocytes is a critical event in the onset of heart failure during biomechanical stress.37, 38 No pharmacological gp130 blockers are in clinical studies at present.
Interfering with JAK–STAT signalling
JAK1, JAK2, JAK3, and TYK2 make up the Janus kinase (JAK) family of cytoplasmic tyrosine kinases,39 with JAK1 thought to be the dominant kinase activated by IL-6 in vivo.40, 41 However, the development of small molecules capable of selectively blocking JAK1 while preserving the other three kinases has been hampered by the extensive amino acid sequence homology among these kinases.42
Despite this challenge, a small number of JAK1-specific inhibitors, including filgotinib—which displays a 30-times greater selectivity for JAK1 than JAK2,43 and is orally administered—and upadacitinib, have made it to phase 3 clinical trials in rheumatoid arthritis and inflammatory bowel disease.44, 45, 46 Other agents, such as ruxolitinib, tofacitinib, and baricitinib, competitively inhibit JAK1 but do not have the same degree of selectivity, and are associated with an increased risk of viral respiratory tract infection, presumably because of compromised interferon responses.47
The transcription factor signal transducer and activator of transcription 3 (STAT3) is phosphorylated by JAKs following their activation by IL-6. Once phosphorylated, STAT3 dimerises and translocates to the nucleus, where it binds to specific DNA tandem motifs and induces the transcription of target genes (figure 3). The inhibition of STAT3 is difficult.2 The protein is essential for mammalian development, and its deletion in mice is lethal.48 Furthermore, because STAT3 is an intracellular protein, it is impervious to antibodies, since they are unable to permeate the cell membrane. To date, efforts to inhibit STAT3 have focused primarily on peptides that prevent STAT3 phosphorylation or dimerisation before its nuclear translocation.49
Inhibiting trans-signalling
In the event that targeted inhibition of the inflammation driven by IL-6 is required, specific inhibition of IL-6 trans-signalling should preserve the anti-inflammatory and antibacterial properties of the cytokine, and might therefore represent a more beneficial and safer strategy. The sgp130Fc protein olamkicept,50 which is currently in phase 2 trials in inflammatory bowel disease, acts to supplement the sgp130 component of the blood buffer described earlier, leaving IL-6 signalling via membrane-bound IL-6R unaffected.23 The concept of inhibiting trans-signalling with Fc proteins is informed by head-to-head animal studies comparing global IL-6R blockade versus sgp130Fc, in which sgp130Fc negated the pathological effects of IL-6 without compromising pathogen clearance.51, 52 Similarly, transgenic mice that overexpressed sgp130Fc were protected from inflammation and displayed resistance to IL-6-mediated disease.53, 54
IL-6 as a biomarker in critical illness
Measuring IL-6 concentrations
Measuring circulating concentrations of IL-6, and interpreting the results, is not straightforward. The cytokine peaks at different times in different illnesses, making the timing of sampling especially important. In the ProCESS sepsis trial, for example, plasma IL-6 concentrations were 400–1100 pg/mL at the first blood draw after randomisation, but decreased to approximately 55 pg/mL at 72 h.55
The magnitude of the IL-6 response to infection is, in absolute terms, also variable between patients and is age-dependent.56 Furthermore, exercise, alterations to the circadian rhythm, concomitant pharmacotherapy, and immunometabolic comorbidities such as obesity can also influence circulating IL-6 concentrations and IL-6 release.57, 58, 59, 60 Factors relating to the processing and handling of samples also stand to influence assay measurements.61, 62, 63, 64, 65 Delays in processing are particularly relevant, since IL-6 and other cytokines are released spontaneously from blood cells over time or after the priming of these cells in samples that are agitated or shaken when being transported to the laboratory.61, 62 This makes the practice of processing blood samples in batches after long periods of time in storage at room temperature unsuitable for cytokine studies.64 Circulating immune cells are additionally susceptible to activation by temperature shifts during sample processing, and longer centrifugation at high speeds. Cytokine measurements in samples that are substantially diluted in assay buffers also stand to be affected before analysis.65 This effect is problematic, since many of the clinical lab assays currently available have been optimised for the concentration range observed in chronic inflammatory diseases, rather than the concentrations present in critical illness. Methodological differences in the measurement of IL-6, coupled with changes in test precision as manufacturers revise assays over time, have made comparisons between COVID-19 studies and historical data from ARDS and sepsis more difficult to interpret.
Ultimately, given what is known about the biology of IL-6, to compare IL-6 concentrations between syndromes in isolation, without consideration of the factors that determine its biological effects, might lead to conclusions that are somewhat misleading. There are additional limitations to these analyses, however, that merit further consideration. Some of the comparisons between COVID-19 and ARDS,66, 67, 68, 69 for example, focused on large clinical COVID-19 studies70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87 done at the outset of the pandemic, while omitting studies that, despite smaller numbers, concentrated more on inflammatory mediators than clinical characteristics. Of the COVID-19 studies evaluated, many had incomplete data, used varying criteria for admission to hospital or an intensive care unit (ICU), applied arbitrary definitions of disease severity, were retrospective, and included many patients already on therapies known to influence cytokine concentrations.70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87
That the prevailing practice in many centres at the time was to pre-emptively intubate deteriorating patients with COVID-1988, 89 is also consequential. Not all of these patients had ARDS. This fact is important, since one of the hallmarks of ARDS is an increase in alveolar permeability,90 which amplifies the leakage of cytokines from the lung. Furthermore, translocation of the inciting inflammatory stimulus might also occur: patients with ARDS who have positive blood cultures are, by definition, more likely to have higher concentrations of circulating cytokines, since their blood and vascular cells are exposed directly to lipopolysaccharides and other bacterial or fungal components; the same principle applies to patients with sepsis. Inclusion of these individuals in comparisons with patients who have viral pneumonia might therefore be ill advised. It is worth noting that historical mean serum IL-6 concentrations quoted by large ARDS randomised control trials (RCTs) such as ALVEOLI, ARMA, FACCT, and SAILS also vary considerably.67, 91, 92, 93, 94, 95, 96, 97
Interpretation of these initial COVID-19 IL-6 data is further clouded by the timing of sampling, which varied across most studies and was undefined in others.66, 67, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87 In some cases, the IL-6 measurements quoted for patients who went on to require ICU support were obtained at their admission to hospital. As a result, it could be speculated that the serum IL-6 values quoted for some patients categorised as having “critical” COVID-19 in these studies might actually apply to a timepoint that preceded their deterioration to the point of becoming critically unwell.
Even allowing for these factors, however, the serum concentrations of IL-6 described by several of these early COVID-19 studies were unusually low given the C-reactive protein (CRP) concentrations reported in the same patients.70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87 Circulating IL-6 is the primary inducer of CRP from the liver, acting via the membrane-bound IL-6R. This discrepancy might be partly explained by the use of clinically based assays to measure IL-6, which are known to be less sensitive than currently available research-grade assays. Indeed, a series of more recent cytokine-focused prospective studies of critically unwell patients with COVID-19 reported substantially higher IL-6 concentrations than those previously described using clinical IL-6 measurements, both in absolute terms and relative to other inflammatory airway conditions such as ARDS or severe community-acquired pneumonia.98, 99, 100, 101, 102, 103, 104, 105
Interpreting IL-6 concentrations
It should be emphasised that for any cytokine, isolated elevations after a pathogenic stimulus do not automatically identify a patient whose clinical condition is deteriorating and might instead be indicative of a vigorous inflammatory response that is proportionate to the magnitude of the insult. By corollary, the possibility that elevated concentrations of IL-6 in critical illness are compensatory should also be considered, with additional upregulation of IL-6 seen in patients who go on to have a poor outcome representing a futile attempt to overcome a signalling pathway that is insufficiently responsive. In polymicrobial sepsis, for example, it has been shown that the phosphorylation of gp130 and STAT3 is decreased, with subsequent impairment of IL-6 signal transduction despite markedly increased serum IL-6.106 Although this decrease could be viewed as a protective adaptation, it would also be consistent with the development of sepsis-induced immunosuppression, an occurrence often invoked as a mechanism of sepsis-associated mortality and morbidity.
The cytokine storm and IL-6R blockade in COVID-19
At the outset of the SARS-CoV-2 pandemic, the combination of elevated concentrations of circulating IL-6, lactate dehydrogenase, CRP, and ferritin in febrile and critically unwell patients with COVID-19 fuelled comparisons with cytokine release syndrome, and the adoption of the term cytokine storm.66, 67 Unlike cytokine release syndrome, a cytokine storm is an undefined descriptor that does not have a clear pathophysiology and does not constitute a diagnosis; it is, therefore, essentially meaningless in the context of COVID-19.
Unfortunately, the widespread acceptance of the term prompted the off-label use of tocilizumab and other immunomodulators in many centres.107, 108, 109, 110, 111 It is understandable that in the absence of any approved COVID-specific therapeutics, clinicians faced with an overwhelming surge of severe cases felt compelled to intervene. However, the use of tocilizumab in these instances, although undoubtedly well intentioned, was undertaken in the absence of definitive evidence of survival benefit.
Despite an effect on mortality being initially suggested by several retrospective observational studies,107, 108, 109, 111 prospective clinical trial data have been less convincing.110, 112, 113, 114, 115 Of particular interest, in two randomised, double-blind, placebo-controlled trials done by the drug's manufacturer—COVACTA115 and EMPACTA116—no significant effect on 28-day survival was observed, with a further randomised double-blind, placebo-controlled study of tocilizumab administered early in the course of disease corroborating these findings.114 A US-based trial investigating sarilumab in COVID-19 was also stopped early,117 as was a Brazilian trial studying tocilizumab in COVID-19, after an increased number of deaths in the tocilizumab group.118
Another potential pitfall of applying monoclonals designed for chronic diseases to acute conditions with a more pronounced inflammatory response is that general IL-6R blockade also inhibits the acute-phase response, preventing the production and release of protective endogenous antiproteases such as α1-antitrypsin (AAT) by the liver.119 AAT is best known as an irreversible inhibitor of neutrophil elastase,120 an omnivorous serine protease released by activated or disintegrating neutrophils and a recognised cause of lung tissue damage and airway inflammation in acute pulmonary infection and ARDS.121, 122, 123 In addition to its antiprotease effects,124, 125 AAT is a potent anti-inflammatory and immunomodulator.126, 127, 128, 129, 130 AAT binds the potent neutrophil chemoattractant IL-8 to prevent excessive neutrophil infiltration,127 blocks the in vivo biological effects of TNF,126 regulates complement,129 and inhibits ADAM-17.127 Of particular relevance to critical illness, AAT also modulates the production and activity of several key pro-inflammatory cytokines, including the aforementioned IL-1β, IL-6, IL-8, and TNF,65, 123, 126, 127, 131 while preserving the production of the anti-inflammatory and pro-resolution cytokine IL-10.132
In forecasting the potential sequelae of abolishing this protective response via blanket inhibition of IL-6 signalling, the clinical and biochemical consequences of abrupt cessation of AAT replacement therapy in patients with a severe hereditary deficiency of the protein are informative. Withdrawal of treatment in these individuals resulted in marked increases in the concentrations of IL-1, IL-6, IL-8, and TNF, the loss of IL-10, and increased exacerbations, hospital admissions, and progression to respiratory failure.133 In patients with COVID-19, an increased ratio of IL-6 to AAT is observed in individuals with more severe disease, and failure to normalise this ratio is associated with poor outcome in those requiring intubation and mechanical ventilation.101 In this regard, the use of tocilizumab or sarilumab as an acute therapeutic in the critical care setting should be approached with caution in some individuals. Based on the available data, clinical practice guidelines from the US National Institutes of Health134 and the Infectious Diseases Society of America135 have advised against the use of tocilizumab monotherapy in COVID-19 outside of a clinical trial setting.
This advice notwithstanding, it would be premature to write off the use of tocilizumab entirely. The results of the RECOVERY and REMAP-CAP trials were published in February, 2021, shortly after we completed the literature search for this Series paper. Although neither study demonstrated a mortality benefit from tocilizumab alone, both suggest a benefit from the addition of tocilizumab to high-dose dexamethasone. Preliminary results from the RECOVERY trial136 identified a modest reduction in 28-day mortality in patients treated with the combination of steroid and tocilizumab versus steroid alone. Mortality was 4–5% higher in patients who received tocilizumab monotherapy compared with those who did not receive tocilizumab or steroids, although this difference was not statistically significant. Of particular interest is the question of whether the mortality effect of tocilizumab plus dexamethasone identified in RECOVERY is sustained beyond 28 days, representing a true reduction, rather than merely a delay, in mortality.
The REMAP-CAP study137 of tocilizumab or sarilumab versus standard care in critically ill patients with COVID-19 receiving organ support in the ICU found improved outcomes (organ support-free days) to 21 days and improved 90-day survival in patients receiving both an IL-6R antagonist and high-dose steroids. Whether this effect would have been observed had the steroid dose been increased further, rather than supplemented with tocilizumab, has yet to be fully determined. Further studies that characterise the precise subgroup of patients that is most likely to benefit from the combination of dexamethasone and tocilizumab, as well as the optimal time to institute such a strategy, are eagerly awaited.
It should be noted that the absence of an effect on mortality does not necessarily mean that a drug is ineffective. The long-term sequelae of COVID-19 have yet to be fully determined, but worrying descriptions of COVID-related syndromes have already begun to emerge. IL-6 is known for its involvement in prolonged fever, hypercoagulability, depressive disorder, and arthralgia, for example, the incidences of which appear to be increased during or after COVID-19.138, 139, 140, 141 It is possible that, for those who survive, an effect on morbidity in response to a reduction in the IL-6 burden might yet be identified.
When analysing data from placebo-controlled studies of tocilizumab in COVID-19, the role of IL-6 as a CRP inducer should be considered, since the abolition of serum CRP caused by IL-6 blockade risks unblinding the study. By the same token, studies evaluating tocilizumab for COVID-19 that do not match for IL-6 concentrations at enrolment should match cohorts for CRP, since groups with a high CRP—and, by extension, high concentrations of circulating IL-6—might have more to gain from an anti-IL-6 therapy. Furthermore, the timing of studies relative to one another should be factored in, not only because of changes in the criteria for hospital or ICU admission during the pandemic, but also because of the widespread adoption of steroids as standard of care for critically unwell patients with COVID-19. This change should be considered when interpreting data from the tocilizumab arms of the open-label REMAP-CAP and RECOVERY trials, for example, where recruitment coincided with the publication of outcome data from the steroid arm of RECOVERY.142 Although the assessment of tocilizumab monotherapy might be unclear as a result, and despite the inherent limitations of an open-label trial design, the results of REMAP-CAP and the tocilizumab group of RECOVERY nevertheless provide useful insights into the role of tocilizumab as an adjunctive therapy to steroids.
Implications for non-COVID-19 ARDS
Although initial COVID-19 treatment algorithms might have been extrapolated from syndromes such as ARDS and sepsis, the experience and knowledge gained from the COVID-19 pandemic should be applied to future studies of these syndromes in the ICU. The application of this knowledge is needed especially for studies of inflammatory processes involved in critical illness.
Although highly powered studies are clearly desirable, the accurate measurement of cytokines and identification of signalling cascades is a delicate process. It is possible that smaller and more detailed mechanistic, cellular, and biomarker-focused studies might yield more informative results than larger studies that rely on banked samples from previous RCTs. Substantial work has already been undertaken to identify ARDS subphenotypes that might enrich such studies.92 In the event that future biomarker studies in ARDS are still linked to clinical trials, the inclusion of additional parallel control groups as points of reference would improve the applicability of the data generated.
Despite the fact that biomarkers are promising to clinicians and researchers, they need to be interpreted in context. In the case of IL-6, an absolute concentration of this cytokine in isolation is largely unhelpful, given the complex interplay between circulating concentrations of the cytokine itself, the soluble receptor, gp130, and ADAM-17, and the variation in each of these contributors between different inflammatory states and even different people. In the COVID-19 pandemic, it has become increasingly clear that the concept of cytokine balance is a superior predictor of outcome than are measures of IL-6 or other cytokines alone, with a blunting or loss of anti-inflammatory protection associated with a worse outcome.98, 143, 144
It is possible that anti-IL-6 strategies might prove to be more effective in some inflammatory ARDS subgroups, or in ARDS with a specific cause. However, cytokines such as IL-6 are ultimately byproducts of an underlying inflammatory process. To avoid falling into the trap of treating a number rather than a person, and to better identify the patients who stand to benefit, it is necessary to redouble efforts to understand the mechanisms driving this inflammation.
Conclusions and future directions
The COVID-19 pandemic has provided many clear examples of what not to do when it comes to generating new knowledge and integrating this into practice, from uncontrolled case series, opinion being touted as evidence in high-impact journals, retracted studies, and questionable medical advice via social media. However, the challenges encountered might have a net positive effect on the study of critical illness syndromes such as ARDS by providing the impetus required to properly incorporate the concept of precision medicine into clinical trials, so as to get the right treatment to the right patient at the right time.
The present cornerstone of ARDS management is meticulous supportive care, such as low tidal volume ventilation and prone positioning in moderate-to-severe ARDS, both of which have been shown to reduce mortality.91, 145 However, ARDS is a heterogeneous syndrome with different subphenotypes that are characterised by different clinical features, inflammatory cytokine profiles, physiological mechanisms, and responses to interventions.146 Rather than searching for a magic bullet, clinical trials of interventions should be targeted at specific phenotypes using knowledge gained from painstaking mechanistic studies.
In terms of pharmacological intervention, treatment aimed at changing the concentration or activity of a specific cytokine such as IL-6 might represent a double-edged sword. Although IL-6R blockade might have a role in some patients, therapeutic approaches that address the underlying cause of changes in IL-6 and other mediators more likely to bear fruit.
Search strategy and selection criteria
We searched MEDLINE and PubMed for reviews, meta-analyses, clinical trials, and original research articles on interleukin-6 (IL-6) biology, signalling, and inhibition relevant to critical illness syndromes, published in English between Jan 1, 1986, and Jan 31, 2021, using the terms “interleukin-6”, “IL-6”, “IL-6R”, “beta 2-interferon/hepatocyte-stimulating factor/interleukin 6”, “BSF-2”, “Janus kinase”, “JAK”, “JAK/STAT”, “gp130”, “cytokines”, “cytokinemia”, “ARDS”, “acute respiratory distress syndrome”, “sepsis”, “coronavirus”, “SARS-CoV-2”, “COVID-19”, “COVID”, “pneumonia”, and “tocilizumab”. Other relevant articles published between 1986 and 2021 were identified through searches of the authors' personal files and in Google Scholar and Springer Online Archives Collection. Articles generated by these searches and relevant references cited therein were reviewed. ClinicalTrials.gov and other databases were searched for ongoing trials. Peer-reviewed papers were favoured, but key preprints were reviewed and cited in this Series paper if no alternative was available. The final list of references was selected with the aim of providing a broad overview of IL-6 signalling, the potential roles of IL-6 in health and disease, and the challenges of targeting IL-6 in critical illness. Reports of findings from the RECOVERY and REMAP‑CAP studies, which were published in February, 2021, shortly after we completed the literature search for this Series paper, were included.
Declaration of interests
SR-J has acted as a consultant and speaker for AbbVie, Amgen, Janssen, Chugai, Roche, Genentech Roche, Pfizer, Eli Lilly, and Sanofi; he is a co-owner of Conaris Research Institute, and an inventor on a patent for the sgp130Fc protein olamkicept now owned by Conaris Research Institute (PCT/EP2008/008736). NGM reports grant funding unrelated to the current work from Chiesi, Grifols, and pH Pharma; he has been an investigator and served on advisory boards for Chiesi, CSL Behring, Grifols, pH Pharma, and Vertex. OJM and GFC declare no competing interests.
Acknowledgments
Acknowledgments
We confirm that no funding was received for the writing of this manuscript.
Contributors
OJM and NGM conceptualised the manuscript and decided on the final list of topics for discussion. SR-J and OJM wrote the introductory overview of IL-6 biology and the section on IL-6 signalling. OJM and NGM wrote the section describing potential pharmacological approaches to inhibiting the biological effects of IL-6. OJM and GFC wrote the sections discussing IL-6 as a biomarker, the role of tocilizumab in COVID-19, and the concept of a cytokine storm. SR-J and OJM edited sections pertaining to IL-6 biology. OJM, GFC and NGM edited sections pertaining to drug targets, drug development, and pharmacotherapeutics. All authors were involved in the editing process and read the final version of the manuscript.
References
- 1.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 2.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu H, Mitomo K, Watanabe T, Okamoto S, Yamamoto K. Involvement of a NF-κB-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990;10:561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray A, Tatter SB, May LT, Sehgal PB. Activation of the human “β2-interferon/hepatocyte-stimulating factor/interleukin 6” promoter by cytokines, viruses, and second messenger agonists. Proc Natl Acad Sci USA. 1988;85:6701–6705. doi: 10.1073/pnas.85.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran SE, O'Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y, Moldawer LL, Marano M, et al. Endotoxemia elicits increased circulating β2-IFN/IL-6 in man. J Immunol. 1989;142:2321–2324. [PubMed] [Google Scholar]
- 10.Palsson-McDermott EM, O'Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. BioEssays. 2013;35:965–973. doi: 10.1002/bies.201300084. [DOI] [PubMed] [Google Scholar]
- 11.Kohase M, Henriksen-DeStefano D, May LT, Vilcek J, Sehgal PB. Induction of β2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986;45:659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- 12.Kohase M, May LT, Tamm I, Vilcek J, Sehgal PB. A cytokine network in human diploid fibroblasts: interactions of β-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987;7:273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sehgal PB, Helfgott DC, Santhanam U, et al. Regulation of the acute phase and immune responses in viral disease. Enhanced expression of the β2-interferon/hepatocyte-stimulating factor/interleukin 6 gene in virus-infected human fibroblasts. J Exp Med. 1988;167:1951–1956. doi: 10.1084/jem.167.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taga T, Hibi M, Hirata Y, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki K, Taga T, Hirata Y, et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN β2) receptor. Science. 1988;241:825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 16.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon β2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 18.Oberg HH, Wesch D, Grüssel S, Rose-John S, Kabelitz D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int Immunol. 2006;18:555–563. doi: 10.1093/intimm/dxh396. [DOI] [PubMed] [Google Scholar]
- 19.Riethmueller S, Somasundaram P, Ehlers JC, et al. Proteolytic origin of the soluble human IL-6R in vivo and a decisive role of N-glycosylation. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300:281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 22.Heink S, Yogev N, Garbers C, et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol. 2017;18:74–85. doi: 10.1038/ni.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jostock T, Müllberg J, Ozbek S, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 24.Yousif AS, Ronsard L, Shah P, et al. The persistence of interleukin-6 is regulated by a blood buffer system derived from dendritic cells. Immunity. 2020 doi: 10.1016/j.immuni.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schooltink H, Rose-John S. Cytokines as therapeutic drugs. J Interferon Cytokine Res. 2002;22:505–516. doi: 10.1089/10799900252981981. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi T, Nakanishi T, Takada K, et al. A case of multicentric Castleman's disease having lung lesion successfully treated with humanized anti-interleukin-6 receptor antibody, tocilizumab. J Korean Med Sci. 2010;25:1364–1367. doi: 10.3346/jkms.2010.25.9.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Medicines Agency RoActemra: Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_en.pdf
- 30.Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13:399–409. doi: 10.1038/nrrheum.2017.83. [DOI] [PubMed] [Google Scholar]
- 31.Klein B, Lu ZY, Gaillard JP, Harousseau JL, Bataille R. Inhibiting IL-6 in human multiple myeloma. Curr Top Microbiol Immunol. 1992;182:237–244. doi: 10.1007/978-3-642-77633-5_29. [DOI] [PubMed] [Google Scholar]
- 32.Klein B, Wijdenes J, Zhang XG, et al. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood. 1991;78:1198–1204. [PubMed] [Google Scholar]
- 33.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 α-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 34.Skiniotis G, Boulanger MJ, Garcia KC, Walz T. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat Struct Mol Biol. 2005;12:545–551. doi: 10.1038/nsmb941. [DOI] [PubMed] [Google Scholar]
- 35.Garbers C, Hermanns HM, Schaper F, et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23:85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Taga T, Narazaki M, Yasukawa K, et al. Functional inhibition of hematopoietic and neurotrophic cytokines by blocking the interleukin 6 signal transducer gp130. Proc Natl Acad Sci USA. 1992;89:10998–11001. doi: 10.1073/pnas.89.22.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirota H, Chen J, Betz UA, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida K, Taga T, Saito M, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guschin D, Rogers N, Briscoe J, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the JAKS in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16:843–862. doi: 10.1038/nrd.2017.201. [DOI] [PubMed] [Google Scholar]
- 43.Van Rompaey L, Galien R, van der Aar EM, et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol. 2013;191:3568–3577. doi: 10.4049/jimmunol.1201348. [DOI] [PubMed] [Google Scholar]
- 44.Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 Inhibitor, in a phase IIB study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68:2857–2866. doi: 10.1002/art.39808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kremer JM, Emery P, Camp HS, et al. A phase IIB study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2016;68:2867–2877. doi: 10.1002/art.39801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med. 2020;383:1511–1521. doi: 10.1056/NEJMoa2008250. [DOI] [PubMed] [Google Scholar]
- 47.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 48.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the JAK/STAT pathway. Cell. 2002;109(suppl):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 49.Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov. 2013;12:611–629. doi: 10.1038/nrd4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.German Clinical Trials Register A multi-centre, exploratory trial to assess the mechanisms of molecular activity, safety and tolerability of one dose level of FE 999301 by intravenous infusions in patients with active inflammatory bowel disease (IBD) http://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00010101
- 51.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 53.Bergmann J, Müller M, Baumann N, et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology. 2017;65:89–103. doi: 10.1002/hep.28874. [DOI] [PubMed] [Google Scholar]
- 54.Rabe B, Chalaris A, May U, et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111:1021–1028. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- 55.Kellum JA, Pike F, Yealy DM, Huang DT, Shapiro NI, Angus DC. Relationship between alternative resuscitation strategies, host response and injury biomarkers, and outcome in septic shock: analysis of the protocol-based care for early septic shock study. Crit Care Med. 2017;45:438–445. doi: 10.1097/CCM.0000000000002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molano Franco D, Arevalo-Rodriguez I, Roqué i Figuls M, Montero Oleas NG, Nuvials X, Zamora J. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst Rev. 2019;4 doi: 10.1002/14651858.CD011811.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 58.Roytblat L, Rachinsky M, Fisher A, et al. Raised interleukin-6 levels in obese patients. Obes Res. 2000;8:673–675. doi: 10.1038/oby.2000.86. [DOI] [PubMed] [Google Scholar]
- 59.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 60.Nilsonne G, Lekander M, Åkerstedt T, Axelsson J, Ingre M. Diurnal variation of circulating interleukin-6 in humans: a meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keustermans GC, Hoeks SB, Meerding JM, Prakken BJ, de Jager W. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods. 2013;61:10–17. doi: 10.1016/j.ymeth.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-α concentrations. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 63.Banks RE. Measurement of cytokines in clinical samples using immunoassays: problems and pitfalls. Crit Rev Clin Lab Sci. 2000;37:131–182. doi: 10.1080/10408360091174187. [DOI] [PubMed] [Google Scholar]
- 64.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pott GB, Chan ED, Dinarello CA, Shapiro L. α-1-Antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 68.Hedrick TL, Murray BP, Hagan RS, Mock JR. COVID-19: clean up on IL-6. Am J Respir Cell Mol Biol. 2020;63:541–543. doi: 10.1165/rcmb.2020-0277LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46:1105–1108. doi: 10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 76.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan H, Zhang L, Huang B, et al. Retrospective analysis of clinical features in 101 death cases with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.09.20033068. published online March 12. (preprint). [DOI] [Google Scholar]
- 81.Fu S, Fu X, Song Y, et al. Virologic and clinical characteristics for prognosis of severe COVID-19: a retrospective observational study in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.04.03.20051763. published online April 6. (preprint). [DOI] [Google Scholar]
- 82.Huang Y, Yang R, Xu Y, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.02.27.20029009. published online March 5. (preprint). [DOI] [Google Scholar]
- 83.Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020 doi: 10.1101/2020.02.10.20021832. published online Feb 12. (preprint). [DOI] [Google Scholar]
- 85.Xu Y, Li Y-r, Zeng Q, et al. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv. 2020 doi: 10.1101/2020.03.08.20031658. published online March 10. (preprint). [DOI] [Google Scholar]
- 86.Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020 doi: 10.1101/2020.02.26.20028191. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H, Wang X, Fu Z, et al. Potential factors for prediction of disease severity of COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.03.20.20039818. published online March 23. (preprint). [DOI] [Google Scholar]
- 88.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 89.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson T, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 92.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 94.Alladina JW, Levy SD, Hibbert KA, et al. Plasma concentrations of soluble suppression of tumorigenicity-2 and interleukin-6 are predictive of successful liberation from mechanical ventilation in patients with the acute respiratory distress syndrome. Crit Care Med. 2016;44:1735–1743. doi: 10.1097/CCM.0000000000001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 96.Sinha P, Delucchi KL, McAuley DF, O'Kane CM, Matthay MA, Calfee CS. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med. 2020;8:247–257. doi: 10.1016/S2213-2600(19)30369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44:1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gadotti AC, de Castro Deus M, Telles JP, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pierce CA, Preston-Hurlburt P, Dai Y, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sinha P, Calfee CS, Cherian S, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med. 2020;8:1209–1218. doi: 10.1016/S2213-2600(20)30366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson JG, Simpson LJ, Ferreira AM, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abcejo AS, Andrejko KM, Raj NR, Deutschman CS. Failed interleukin-6 signal transduction in murine sepsis: attenuation of hepatic glycoprotein 130 phosphorylation. Crit Care Med. 2009;37:1729–1734. doi: 10.1097/CCM.0b013e31819dee81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2020;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parr JB. Time to reassess tocilizumab's role in COVID-19 pneumonia. JAMA Intern Med. 2021;181:12–15. doi: 10.1001/jamainternmed.2020.6557. [DOI] [PubMed] [Google Scholar]
- 111.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa954. published online July 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roche Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. July 29, 2020. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm
- 116.Roche Roche's phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. Sept 18, 2020. https://www.roche.com/investors/updates/inv-update-2020-09-18.htm
- 117.Park M. US trial investigating sarilumab for COVID-19 stopped. July 7, 2020. https://www.empr.com/home/news/drugs-in-the-pipeline/sarilumab-interleukin-6-antagonist-covid19-mechanical-ventilation/
- 118.Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perlmutter DH, May LT, Sehgal PB. Interferon β2/interleukin 6 modulates synthesis of α1-antitrypsin in human mononuclear phagocytes and in human hepatoma cells. J Clin Invest. 1989;84:138–144. doi: 10.1172/JCI114133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carrell RW, Jeppsson JO, Laurell CB, et al. Structure and variation of human α1-antitrypsin. Nature. 1982;298:329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- 121.Lee CT, Fein AM, Lippmann M, Holtzman H, Kimbel P, Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981;304:192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- 122.Döring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150:S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 123.McCarthy C, Dunlea DM, Saldova R, et al. Glycosylation repurposes alpha-1 antitrypsin for resolution of community-acquired pneumonia. Am J Respir Crit Care Med. 2018;197:1346–1349. doi: 10.1164/rccm.201709-1954LE. [DOI] [PubMed] [Google Scholar]
- 124.Matheson NR, Wong PS, Schuyler M, Travis J. Interaction of human α-1-proteinase inhibitor with neutrophil myeloperoxidase. Biochemistry. 1981;20:331–336. doi: 10.1021/bi00505a016. [DOI] [PubMed] [Google Scholar]
- 125.Ogushi F, Fells GA, Hubbard RC, Straus SD, Crystal RG. Z-type alpha 1-antitrypsin is less competent than M1-type alpha 1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987;80:1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bergin DA, Reeves EP, Hurley K, et al. The circulating proteinase inhibitor α-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007116. [DOI] [PubMed] [Google Scholar]
- 127.Bergin DA, Reeves EP, Meleady P, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jonigk D, Al-Omari M, Maegel L, et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci USA. 2013;110:15007–15012. doi: 10.1073/pnas.1309648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O'Brien ME, Fee L, Browne N, et al. Activation of complement component 3 is associated with airways disease and pulmonary emphysema in alpha-1 antitrypsin deficiency. Thorax. 2020;75:321–330. doi: 10.1136/thoraxjnl-2019-214076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O'Dwyer CA, O'Brien ME, Wormald MR, et al. The BLT1 inhibitory function of α-1 antitrypsin augmentation therapy disrupts leukotriene B4 neutrophil signaling. J Immunol. 2015;195:3628–3641. doi: 10.4049/jimmunol.1500038. [DOI] [PubMed] [Google Scholar]
- 131.Joosten LA, Crişan TO, Azam T, et al. Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty arthritis by reducing release and extracellular processing of IL-1β and by the induction of endogenous IL-1Ra. Ann Rheum Dis. 2016;75:1219–1227. doi: 10.1136/annrheumdis-2014-206966. [DOI] [PubMed] [Google Scholar]
- 132.Janciauskiene SM, Nita IM, Stevens T. α1-Antitrypsin, old dog, new tricks. α1-Antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem. 2007;282:8573–8582. doi: 10.1074/jbc.M607976200. [DOI] [PubMed] [Google Scholar]
- 133.McElvaney OJ, Carroll TP, Franciosi AN, et al. Consequences of abrupt cessation of alpha1-antitrypsin replacement therapy. N Engl J Med. 2020;382:1478–1480. doi: 10.1056/NEJMc1915484. [DOI] [PubMed] [Google Scholar]
- 134.National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf [PubMed]
- 135.Bhimraj A, Morgan RL, Shumaker AH, et al. IDSA Guidelines on the treatment and management of patients with COVID-19. April 11, 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ [DOI] [PMC free article] [PubMed]
- 136.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.02.11.21249258. published online Feb 11. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.The REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2100433. published online Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 140.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 141.Taquet M, Luciano S, Geddes J, Harrison P. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lim WS, Emberson JR, Mafham M, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;394:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McElvaney OJ, Hobbs BD, Qiao D, et al. A linear prognostic score based on the ratio of interleukin-6 to interleukin-10 predicts outcomes in COVID-19. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 145.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 146.Shankar-Hari M, Fan E, Ferguson ND. Acute respiratory distress syndrome (ARDS) phenotyping. Intensive Care Med. 2019;45:516–519. doi: 10.1007/s00134-018-5480-6. [DOI] [PubMed] [Google Scholar]