Supplemental digital content is available in the text.
SIGNIFICANCE
This article summarizes the evidence for a higher prevalence of binocular vision dysfunctions in individuals with vision impairment. Assessment for and identification of binocular vision dysfunctions can detect individuals experiencing difficulties in activities including reading, object placement tasks, and mobility.
Comprehensive vision assessment in low vision populations is necessary to identify the extent of remaining vision and to enable directed rehabilitation efforts. In patients with vision impairment, little attention is typically paid to assessments of binocular vision, including ocular vergence, stereopsis, and binocular summation characteristics. In addition, binocular measurements of threshold automated visual fields are not routinely performed in clinical practice, leading to an incomplete understanding of individuals' binocular visual field and may affect rehabilitation outcomes.
First, this review summarizes the prevalence of dysfunctions in ocular vergence, stereopsis, and binocular summation characteristics across a variety of ocular pathologies causing vision impairment. Second, this review examines the links between clinical measurements of binocular visual functions and outcome measures including quality of life and performance in functional tasks. There is an increased prevalence of dysfunctions in ocular alignment, stereopsis, and binocular summation across low vision cohorts compared with those with normal vision. The identification of binocular vision dysfunctions during routine low vision assessments is especially important in patients experiencing difficulties in activities of daily living, including but not limited to reading, object placement tasks, and mobility. However, further research is required to determine whether addressing the identified deficits in binocular vision in low vision rehabilitative efforts directly impacts patient outcomes.
Irreversible vision impairment significantly and negatively affects participation in daily activities and quality of life.1–4 Low vision is defined as visual acuity worse than 6/18 and visual fields less than 20° in diameter in the better-seeing eye with best possible correction, whereas blindness is defined as visual acuity worse than 3/60 and visual fields less than 10° in diameter in the better-seeing eye with best possible correction.5–7 With an aging global population and associated increased prevalence of ocular disease,8,9 by 2040, worldwide prevalence of low vision and blindness is projected to reach 450 million and 82 million, respectively, and resultantly, there will be an increased demand for low vision services. For individuals with vision impairment, low vision rehabilitation can effectively improve reading performance, visual processing and motor skills, and performance in daily activities.10–12
In current low vision care models, rehabilitation efforts are directed by an initial comprehensive visual assessment to determine the extent of remaining visual function.13–15 Because patients with no light perception or gross bilateral vision impairment make up only a small proportion of the vision-impaired population,16 the majority of low vision patients may retain some level of binocularity.17,18 Nevertheless, in low vision assessments, little attention is typically paid to assessments of binocular vision, such as examination of the vergence system, stereopsis, and effects of binocular summation and inhibition.17,18 However, dysfunctions in binocular vision can contribute to symptoms of difficulty with both distance and near tasks and potentially affect success with low vision aids, which are routinely prescribed as part of the rehabilitation process.17,18 In addition, binocular measurements of threshold automated visual fields are not routinely performed in clinical practice, whereas more traditional monocular threshold perimetry may not adequately simulate real-world binocular viewing. Binocular suprathreshold techniques, on the other hand, use stimuli that may not detect more subtle deteriorations in the visual field; as such, both methods potentially provide an incomplete understanding of how individuals use their binocular visual field in real-world environments and therefore may affect outcomes of training programs designed to maximize the use of residual vision.
This article reviews the prevalence of binocular visual function anomalies in vision-impaired populations and the links between measurements obtained in clinical binocular vision assessments and outcome measures such as patient symptoms, quality of life, and performance in other functional tasks. The binocular visual functions covered by this topical review are ocular alignment, stereopsis, binocular summation and inhibition, and the binocular visual field. By identifying the prevalence and impacts of impaired binocular vision on quality of life in vision-impaired populations, we sought to highlight the relevance of examining binocular vision in patients with low vision by primary eye care practitioners before provision of low vision rehabilitation services.
LITERATURE SEARCH STRATEGY
Articles suitable for inclusion were identified using the National Institutes of Health's PubMed database using combinations of the following key words: “vision impairment” or “low vision,” “binocular” or “binocular vision,” “stereopsis” or “stereo*,” “summation” or “inhibition,” “visual field” or “binocular visual field,” or “integrated visual field.” The search was restricted to articles available in English only. Additional suitable articles were identified from reference lists in articles found using the original search. A total of 54 review articles, retrospective and prospective cohort studies, and case reports were included in this review, broken down into the following categories: 6 discussing vergence dysfunctions, 13 discussing stereopsis, 9 discussing binocular summation and inhibition, and 26 discussing binocular visual field assessment. Note that several studies discussed more than one of the aforementioned topics.
ASSESSMENTS OF BINOCULAR VISUAL PROCESSING
Examination of the Vergence System
With normal binocular vision, directing our gaze toward an object of interest results in stimulation of corresponding retinal locations, and these images are subsequently fused to form a single Cyclopean percept.19 In cases of dysfunctions of ocular alignment, including heterophoria and strabismus, the effort required to maintain the same visual direction for each eye can lead to symptoms of asthenopia, headaches, or blurred and double vision, which can affect prolonged reading performance in particular.20,21 In individuals with asymmetric vision impairment, an anomalous visual direction may develop because of reduced retinal stimulation and resultant poorer fixation stability in the worse-seeing eye.22,23 It is therefore unsurprising that dysfunctions of ocular alignment are relatively common in patients in the low vision population (Table 1), with Rundström and Eperjesi17 reporting that 73% of patients with visual acuities of 6/60 or better in both eyes complained of symptoms of diplopia or asthenopia. Therefore, if binocular dysfunctions are identified and considered in the rehabilitation process, patients can experience improved comfort and performance with low vision aids when reading.17,18
TABLE 1.
Summaries of nonreview articles investigating prevalence of vergence dysfunctions in low vision populations
| Study | Study design | Cohort | Tests used | Outcomes |
|---|---|---|---|---|
| Rundström and Eperjesi (1995)17 | Cross-sectional study | n = 30 n = 23 with AMD and n = 7 with other ocular pathologies (including glaucoma and lens opacity) |
Cover test with prism | n = 22 (73%) with symptoms consistent with binocular vision anomalies Most common symptoms: horizontal diplopia, jumbling of print, and asthenopia At least n = 22 (73%) with vergence anomalies Most common: exophoria and convergence insufficiency Trend between presence of symptoms and vergence anomalies not reported |
| Goldstein and Clahane (1966)24 | Retrospective case-control study | n = 14 with RP and n = 23 normals | Cover test with prism, prism fusional amplitudes | Greater prevalence of intermittent or constant strabismus in RP participants (n = 9 [64%] vs. n = 3 [13%]) and larger deviation in RP at distance (3.60 vs. 0.96Δ) and near (16.90 vs. 9.22Δ) Borderline difference in fusional amplitudes between RP and normals (distance divergence, 3.29 vs. 4.61Δ; distance convergence, 9.07 vs. 14.13Δ; near divergence, 10.21 vs. 13.43Δ; near convergence, 21.39 vs. 21.39Δ) Poorer awareness of induced diplopia in RP |
| Miyata et al. (2018)22 | Cross-sectional study | n = 119 with RP n = 83 with deviation ≤10Δ and n = 36 with deviation ≥10Δ |
Cover test with prism | Significant correlation (r = −0.39) between larger absolute horizontal deviation (exo or eso) at near and residual binocular visual fields measured with Goldmann perimetry Threshold of binocular visual field 40 cm2 can distinguish between patients with large (<40 cm2) and small horizontal deviations |
| Migliorini et al. (2015)25 | Cross-sectional study | n = 23 with RP | Cover test with prism | n = 12 (52%) with heterophoria, n = 8 (35%) with intermittent or constant strabismus; direction not stated Strabismus ≥10 Δ in n = 4 (17%) n = 15 (65%) showed restrictions in ocular motilities in at least one direction |
| Tarita-Nistor et al. (2012)23 | Case-control study | n = 12 with AMD and n = 16 normals | Eye tracker with deviation measured during binocular viewing | 75% of participants with AMD and 84% of normals showed deviation (heterophoria) >1Δ |
| Skrbek (2013)26 | Cross-sectional study | n = 12 with AMD n = 5 where binocular vision examination was possible, as other participants had extensive vision loss in one eye precluding binocular vision assessment |
MKR methods with polarization | n = 2 with esotropia and hypertropia, n = 1 with exotropia and hypertropia, n = 2 no strabismus observed Trend toward better vision in worse eye in participants where binocular vision examination was possible Adaptation to prism correction (n = 2 with esotropia) anecdotally reported to be faster than in individuals without vision impairment |
Articles are sorted by order of appearance within the text. Δ = prism diopters; MKR = Mess- und Korrektionsmethodik nach Haase; n = number of participants; r = correlation coefficient.
Peripheral vision loss disrupts normal peripheral fusion, which can result in sensory deviations in ocular alignment.22,24,27 Constant strabismus and intermittent strabismus, typically manifesting as exotropia, are more common in patients with retinitis pigmentosa (RP) compared with normal control subjects,22,24,25 and 65% of RP patients demonstrate restrictions in ocular motility in at least one direction.25 Furthermore, in RP, a larger degree of horizontal deviation significantly correlated with poorer visual acuity and a smaller remaining island of central vision as measured with Goldmann perimetry.22 Meanwhile, fusional amplitudes have been observed as borderline reduced in patients with RP.24 Of note is that patients with RP have been reported to display poorer awareness of diplopia compared with controls,24 so the presence of deviations in ocular alignment does not necessarily translate to patient symptoms.
Few studies have investigated the role of central vision impairment on ocular alignment. Tarita-Nistor et al.23 reported that most patients with AMD were exophoric as measured with an eye tracking device and did not significantly differ from normal participants in distribution of deviation types.23 Meanwhile, Skrbek26 observed both esodeviations and exodeviations in patients with AMD26; however, the translatability of these findings to all patients with AMD and low vision is affected by the very small sample size of five participants and measurement of ocular alignment with Mess- und Korrektionsmethodik nach Haase methods with polarization, which is not typically used in standard clinical practice. Furthermore, both studies did not stratify patients by AMD subtypes, and it is therefore difficult to infer the true distribution of deviation types in AMD from these studies alone. In addition, Skrbek26 found that prismatic correction resulted in improvement in binocular visual acuity in patients with esotropia and was anecdotally well tolerated by patients; although further studies with a larger sample size and using more objective measures of patient satisfaction with prismatic corrections are required, this study suggests that it may be worthwhile investigating and appropriately managing dysfunctions of binocularity in patients with central vision loss.
The usefulness of prism in managing ocular alignment dysfunctions in patients with central vision loss is complicated by the application of yoked prism in eccentric viewing, to direct images of interest from the damaged central fovea to a relatively intact paracentral retinal location or preferred retinal locus. Studies have reported that patients using eccentric viewing spectacles including yoked prisms demonstrate better visual acuities and performance on mobility tasks, in addition to subjective improvement in vision as self-reported by patients.28–30 However, ocular alignment characteristics or whether participants experienced symptoms suggestive of binocular dysfunction was not reported in these studies, and these factors may contribute to the discontinuation of eccentric viewing spectacles in 60% of patients after an average of 4.5 years.31 In circumstances where prismatic correction is considered to aid eccentric viewing and manage binocular vision anomalies, the appropriate prismatic prescription would need to account for both ocular alignment and preferred retinal locus characteristics.
Although these studies collectively demonstrate that vergence dysfunction is present in numerous ocular pathologies causing vision impairment, there are several limitations affecting the applicability of these findings to clinical practice. First, trends between extent of visual asymmetry and presence of anomalies in ocular alignment have not been explored by these studies, and although some have hinted that examination of the vergence system is not feasible in patients with very poor vision,17,26 there is no indication as to the visual acuity threshold or extent of visual asymmetry at which attempting a binocular vision examination is no longer feasible. Furthermore, of the few studies investigating both presence of deviations in ocular alignment and patient symptoms, trends could not be quantified because of small sample size26 or could not be explored entirely.17 Given that patients may be asymptomatic because of sensory suppression,24 this calls into question whether examining the vergence system in detail will translate to meaningful outcomes for the patient. In addition, although assessment of the vergence system in symptomatic patients may aid choice of appropriate low vision aids, no studies directly examined the impact of ocular motility dysfunctions management on the success of vision rehabilitation. Further studies investigating patient satisfaction and success upon prescription of low vision aids incorporating considerations of patients' binocular vision status are required, to guide whether examination of the vergence system should be routinely considered in the low vision population.
Stereopsis
The ability to locate objects in relation to one's own location is necessary for accurate interactions with the surrounding environment32 and is particularly important in reaching and placement tasks (for example, setting the table and putting away groceries) and navigation within the environment (for example, walking up and down steps and avoiding obstacles that may induce falls).33 Surrounding the horopter, along which locations in visual space stimulate corresponding retinal locations, is Panum’s fusional area where a single binocular vision is obtained, despite noncorresponding retinal locations being stimulated.19 This occurs as inputs that fall on disparate retinal locations between eyes are fused or combined to generate a perception of relative depth, a visual process known as stereopsis.
Stereopsis requires intact and reasonably symmetrical visual function between eyes.34–36 Because many ocular pathologies present asymmetrically, asymmetric inputs from corresponding retinal locations may affect stereopsis. Studies investigating stereopsis in simulated vision asymmetry and anisometropic amblyopia have reported poorer stereoacuity with greater differences in vision between the two eyes.35,37–39 Meanwhile, in simulated bilateral vision loss, a milder reduction in stereoacuity is often observed, although the extent to which stereopsis is degraded cannot be directly correlated with the extent of induced blur.39 It is therefore reasonable to predict that, in moderate, bilateral vision loss (6/15), stereopsis may be retained (Appendix Fig. A1C, available at http://links.lww.com/OPX/A480). On the other hand, in severe, bilateral vision loss, central stereopsis is likely to be markedly impaired or completely absent. In conjunction with the increased likelihood of binocular vergence anomalies including strabismus in the low vision population, which are associated with poorer stereopsis,35 it can be extrapolated that there is a notable proportion of the vision-impaired population with deficits in stereopsis, and this deficit may contribute to difficulties in activities involving accurate depth perception such as reaching and placement tasks and mobility.
Several studies24,40,41 have observed reduced stereoacuity and an increased prevalence of no stereopsis in patients with glaucoma and RP compared with those with no ocular pathology, despite normal or only mildly reduced visual acuities (Table 2). In particular, poor stereoacuity is associated with more advanced glaucoma, as more extensive visual field defects and those encroaching fixation cause sensory suppression in at least one eye,41,42 and has been correlated with poor quality-of-life summary scores.43 Meanwhile, absence of stereopsis in AMD patients with bilateral vision impairment has been associated with reduced overall quality of life, with these patients scoring particularly low on reading ability scores,44 but interestingly, no significant correlations between stereoacuity and visual ability scores were reported, suggesting that only the presence or absence of stereopsis affects functional visual abilities in AMD. In contrast, Tabrett and Latham45 did not find an association between presence of stereopsis and vision-related activity limitations; however, this may be due to differences in study cohorts or the relatively few participants in this study who demonstrated measurable stereopsis.
TABLE 2.
Summary of nonreview articles investigating stereopsis in low vision populations
| Study | Study design | Cohort | Tests used | Outcomes |
|---|---|---|---|---|
| Vingolo et al. (2020)40 | Case-control study | n = 26 with RP and n = 25 normals | Titmus stereotest, Lang stereotest, TNO stereotest | Significant reduction in stereoacuity across all stereotests in RP patients compared with controls (69.3 to 391.39 vs. 15.97 to 1150″) Although stereoacuity was significantly correlated with visual acuity and mean retinal sensitivity on microperimetry (correlations not stated), stereoacuity still reduced in RP patients with normal visual acuity |
| Goldstein et al. (1966)24 | Retrospective case-control study | n = 14 with RP and n = 23 normals | Titmus stereotest (fly and Wirt rings) | Stereopsis absent or reduced in RP patients compared with control, even in patients with visual acuity of at least 6/15 in each eye |
| Lin et al. (2018)41 | Cross-sectional study | n = 150 with glaucoma | Distance Randot stereotest | Only 35 participants (23.3%) demonstrated measurable distance stereoacuity Distance stereoacuity not a significant contributor to models correlating multiple measures of visual function with GQL-15 scores Stereoacuity significantly correlated with mean integrated visual field sensitivity (r = 0.278) |
| Lakshmanan and George (2013)42 | Cross-sectional study | n = 97 with glaucoma n = 11 mild glaucoma, n = 26 moderate glaucoma, and n = 60 advanced glaucoma |
Titmus stereotest (fly and Wirt rings) |
Significant correlation (r = 0.45) between stereoacuity and classification of glaucoma: better stereoacuity in mild glaucoma compared with severe glaucoma (median stereoacuity 40″ in mild glaucoma, 50″ in moderate glaucoma, and 60″ in advanced glaucoma) |
| Nelson et al. (2003)43 | Case-control study | n = 47 with glaucoma and n = 19 normals n = 18 mild glaucoma, n = 19 moderate glaucoma, and n = 10 advanced glaucoma |
Frisby stereotest | Poorer stereoacuity correlated with lower GQL-15 summary score No significant correlation between stereopsis and specific visual disability factors as per questionnaire |
| Cao and Markowitz (2014)44 | Case series | n = 27 with AMD | Frisby stereotest | Overall Functional Visual Abilities score higher in AMD participants with any level of stereopsis compared with those with no stereopsis (2.25 vs. 1.50) No significant correlation between stereoacuity and any visual ability score |
| Tabrett and Latham (2011)45 | Cross-sectional study | n = 100 with various ocular pathologies n = 57 with macular pathology including AMD, n = 11 with optic nerve pathology including glaucoma |
Frisby stereotest | n = 8 (8%) had measurable depth discrimination, and no correlation observed between presence/absence of depth discrimination and self-reported, vision-related activity limitation |
| Verghese et al. (2016)46 | Case-control study | n = 16 with AMD and n = 9 normals | Randot stereotest, custom laboratory-based stereoacuity test | Significant correlation (r = −0.78) between stereoacuity and improvement in reach-to-grasp and transport-to-place task performance under binocular viewing in participants with measurable stereopsis Significant correlation (r = −0.81) between overlap in binocular and monocular scotomas and improvement in performance under binocular viewing in AMD patients with no measurable stereopsis |
| Kotecha et al. (2009)47 | Case-control study | n = 16 with glaucoma and n = 16 normals | Titmus stereotest (fly only), Frisby stereotest |
Slightly poorer stereoacuity in glaucoma participants compared with normals (55 vs. 40″) Delays in initiating and performing reach-and-grasp tasks in glaucoma participants compared with normals; however, significant correlation between time to reach the object and stereoacuity were observed only (r = 0.43, P < .02) |
| Lamoureux et al. (2010)48 | Cross-sectional study | n = 127 with various ocular pathologies n = 47 with mild vision impairment, n = 64 with moderate vision impairment and n = 16 with severe vision impairment |
Titmus stereotest (fly only) |
n = 73 did not fall and n = 54 fell within study period No significant difference in prevalence of gross stereopsis in nonfalling and falling cohorts (n = 42 [58.3%] vs. n = 30 [41.7%]) |
| Lord and Dayhew (2001)49 | Prospective cohort study | n = 156, n = 45 with various ocular pathologies n = 19 with cataract, n = 21 with glaucoma, n = 5 with AMD |
Howard-Dolman apparatus, Frisby stereotest | n = 64 fell within study period and n = 32 fell multiple times during study period In participants with two or more falls compared with no falls or one fall only, significantly poorer depth perception and stereoacuity (depth perception, 5.76 vs. 1.99 cm and 1.98 cm; stereoacuity, 303 vs. 132 and 139,″ respectively) |
| Ivers et al. (2000)50 | Case-control study | n = 911 with hip fracture and n = 910 normals | Randot stereotest | Increased risk of hip fracture associated with no gross stereopsis (OR, 6.0, vs. stereopsis, 30 to 50″) Statistically significant trend between decreasing stereoacuity and risk of hip fracture (P < .0001) |
| Chew et al. (2010)51 | Case-control study | n = 108 Participants with low-fragility fractures and n = 108 normals | Frisby stereotest | Increased risk of fracture and falls associated with absence of gross stereopsis, defined as stereoacuity >600″ (OR, 3.603 and 2.112, respectively, vs. stereopsis, 55 to 600″) |
Articles are sorted by order of appearance within the text. ‘ = minutes of arc; “ = seconds of arc; GQL-15 = Glaucoma Quality of Life questionnaire; n = number of participants; OR = odds ratio; r = correlation coefficient.
The impact of reduced stereopsis in low vision on accurate object placement tasks has been reported in several studies, providing further insight on how reduced stereopsis affects performance in daily activities. In older subjects with macular disease and measurable stereopsis, binocular viewing condition was associated with a significant improvement in object placement tasks compared with monocular viewing, and the level of improvement was significantly correlated with stereoacuity.46 However, improvements under binocular viewing in patients without measurable stereopsis imply that monocular depth cues such as relative size may have contributed to these findings. Meanwhile, Kotecha et al.47 included a range of glaucoma participants with and without central visual field defects meeting the criteria of low vision and reported a significant correlation between stereoacuity and time to reach the object, but correlations with all other reach-to-grasp performance parameters were not significant. Overall, these studies suggest that stereoacuity may affect performance in object placement tasks; however, further studies with low vision cohorts with measurable stereopsis are required to confirm these findings.
In studies directly correlating stereopsis to the number of falls, there appears to be no consensus in the available literature. Although the majority of these do not specifically target the low vision population, older individuals are more likely to have ocular diseases causing low vision,8 reflected by the inclusion of participants with low vision in many studies, and are more likely to suffer significant morbidity secondary to falls.48,52 Several studies found that reduced depth perception and stereoacuity conferred a significant relative risk of multiple falls and hip fracture secondary to falls, independent of other demographic and physical risk factors,49–51,53 with an up to six times increased risk of multiple falls in participants with no measurable stereopsis.50 However, a study specifically investigating low vision participants failed to find a significant association between absence of stereopsis and increased falls risk.48 This may be due to their binary classification of “fallers” and “nonfallers,” rather than quantifying the number of falls as per other studies. An alternative explanation for this discrepancy is that several studies did not adjust for visual acuity,49,51 which was also correlated with increased risk of falls, and therefore, the risk of reduced stereopsis independent of other vision-related confounders is difficult to extrapolate from these studies alone.
The applicability of clinical measurements of stereopsis when considering patient mobility and risk of falls may be considered questionable, as stereoacuity measurements are typically performed at near. Although depth perception at distance may be measured using the Howard-Dolman apparatus, this is not commonly found in clinical practice, and no studies have investigated its use in low vision assessments. Furthermore, Fig. 1 and Appendix Table A1 (both available at http://links.lww.com/OPX/A480) highlight the change in object distance required to perceive a change in depth for near tasks compared with mobility and distance tasks; the large required changes in object distance for mobility and distance tasks suggest that monocular depth cues may play a greater role in identifying object distance compared with stereopsis afforded by binocularity.
FIGURE 1.
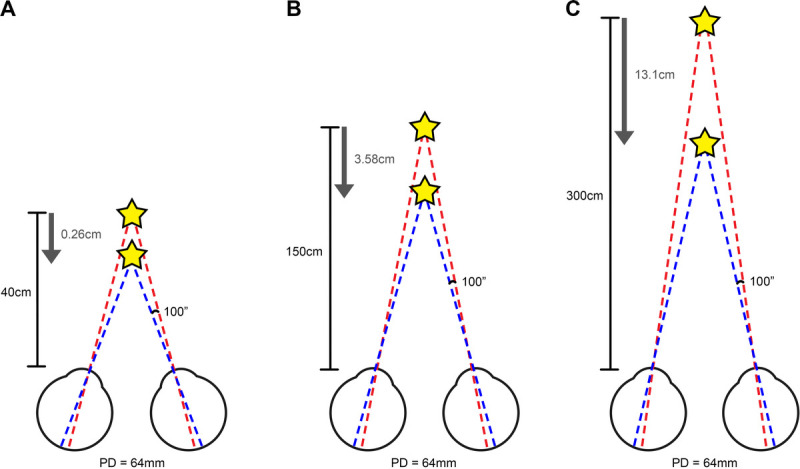
Simplified schematics depicting required change in object distance to detect change in depth with stereoacuity of 100″ and PD of 64 mm. A, For a typical near working distance of 40 cm, an object of interest (star) would only have to move 0.26 cm for change in depth to be detected. B, Conversely, at a typical working distance for mobility tasks of 150 cm (roughly equivalent to the distance between the eyes and feet), the object of interest would have to move 3.58 cm for a change in depth to be detected, for the same PD. C, With a greater working distance of 300 cm (roughly equivalent to looking across a room), the object of interest would have to move 13.1 cm for a change in depth to be detected. Please note that these numbers would vary depending on PD, stereoacuity, and working distance (see Appendix Table A1, available at http://links.lww.com/OPX/A480 for further information). ″ = seconds of arc; PD = pupillary distance.
Collectively, the most consistent threshold criterion across studies associated with reduced quality of life and increased risk of falls is absence of gross stereopsis, measured using the Titmus fly or Frisby stereotest. Although several studies have reported significant correlations between stereoacuity and other measures of visual function, such as visual acuity and visual field sensitivity,40,42 the relationships between presence of gross stereopsis and monocular or binocular visual acuity, visual field sensitivity, and intereye asymmetry in these visual parameters have not been explored, and therefore, gross stereopsis should be measured where possible rather than inferred based on other results of the clinical examination. Across studies, there is much variation in stereoacuity measurement technique, study cohorts, and outcome measures reported across studies, so further work investigating different methods of measuring stereoacuity and its relationship with performance on specific tasks and other quality-of-life measures is required to determine standardized criteria that may be applied to clinical settings.
There is no evidence suggesting that stereopsis can be improved with rehabilitative efforts in patients with low vision. Rather, the available literature implies that awareness of reduced stereopsis as measured in a low vision assessment may be helpful in directing rehabilitative toward areas where patients may have more difficulty, such as fine motor tasks, falls risk, and self-navigation around obstacles.
Binocular Summation and Inhibition
Most individuals with normal vision in each eye experience an improvement in visual acuity and contrast sensitivity with binocular vision versus monocular vision by a factor of √2,54–56 a phenomenon termed binocular summation. Theoretical models describing the mechanism of binocular summation include probability summation, that superior vision is expected binocularly as simultaneously presented inputs to each eye provide double the opportunity of a correct response over monocular viewing, and neural summation or integration of inputs from both eyes.54,56 However, with greater differences in monocular inputs between eyes, there is a greater likelihood of binocular inhibition occurring, where binocular vision is poorer than monocular vision in the better eye56; this is thought to arise because of unequal retinal illumination between eyes, otherwise known as Fechner paradox,57 and occurs with increasing likelihood with greater differences in monocular inputs between eyes. Because ocular diseases are often asymmetric in appearance, the resultant asymmetry in visual function is likely to disrupt the processes involved in binocular summation, and identifying binocular summation characteristics in a low vision assessment may direct recommendation of suitable low vision aids and rehabilitative care, depending on whether visual function is optimal with monocular or binocular vision.54
Binocular summation characteristics have been relatively well characterized in low vision populations with macular disease (Table 3). Greater differences in visual acuity between eyes do not confer a significant increase in prevalence of binocular inhibition of letter resolution acuity between individuals with AMD and a control group of a similar age (39 vs. 33%),58 implying that binocular summation characteristics vary between individuals and cannot be inferred from monocular measures, even though the linear relationship between binocular and monocular better eye measures approaching 1:1 indicates that differences between binocular and better eye acuities are often minimal.59–61 Nonetheless, this small difference may confer significant differences in binocular and monocular reading performance, with significant correlations between maximum reading speed and binocular ratios calculated for distance visual acuities implying that those AMD patients demonstrating binocular inhibition would have greater difficulty with binocular reading regardless of their distance visual acuity.62 This finding is further supported by Silvestri et al.,63 who reported that significantly poorer binocular reading speed in patients with AMD and Stargardt disease demonstrated binocular inhibition compared with those who demonstrated binocular summation. In the study of Silvestri et al.,63 patients with binocular inhibition also demonstrated significantly poorer binocular reading speed and reading acuity compared with better eye monocular viewing conditions. Whereas Tzaridis et al.60 observed reduced binocular gain in reading speed with greater interocular differences in reading speed in patients with macular telangiectasia, indicating that binocular inhibition was detrimentally influencing reading performance in this cohort, Kabanarou and Rubin59 did not find significant differences between binocular and better eye monocular viewing with respect to reading speed or significant relationships between interocular differences in visual acuity and change in reading speed. Rubin et al.61 found that, despite 38% of their cohort demonstrating binocular summation, binocular and monocular acuities were equally predictive of reading performance. These findings are likely due to the relatively low prevalence of binocular inhibition in these cohorts. Meanwhile, two studies that investigated contrast summation characteristics found an increased prevalence of binocular inhibition in bilateral AMD compared with participants with normal vision with gratings at low to medium spatial frequencies (45 to 62 vs. 10%),57,64 although this prevalence reduces to 9% when measured with the Pelli-Robson chart.59 Despite this variability between measurement techniques, there exists the possibility that individuals with AMD exhibiting binocular inhibition may have more difficulty in daily activities in low contrast environments.64
TABLE 3.
Summary of nonreview articles investigating binocular summation and inhibition
| Study | Study design | Cohort | Tests used (parameter) | Outcomes |
|---|---|---|---|---|
| Tarita-Nistor et al. (2006)58 | Case-control study | n = 17 with AMD, n = 38 normals | Multiple tumbling E tests at 12, 32, and 86% contrast (VA) | No significant difference in binocular ratios between groups at 12, 32, and 86 contrast levels No significant difference in proportions of participants experiencing binocular inhibition, equality, and summation between groups |
| Kabanarou and Rubin (2006)59 | Cross-sectional study | n = 22 with AMD | EDTRS chart (distance VA), MNREAD acuity chart (reading speed) | n = 14 (63.6%) demonstrated positive binocular gain and n = 3 (13.6%) demonstrated negative binocular gain in reading speed With reading speed plotted as a function of distance and reading VA, no significant differences in slopes between binocular and better eye (monocular) conditions Interocular differences in VA and reading acuity are poor predictors of binocular gain (r2 = 0.03 and 0.009, respectively) |
| Tzaridis et al. (2019)60 | Cross-sectional study | n = 68 with macular telangiectasia type 2 | ETDRS chart (distance VA), Radner reading charts (reading acuity and speed) | Greater interocular difference in reading speed correlated with reduced binocular gain in reading speed (r2 = 0.61) No significant correlation between binocular VA and interocular differences in VA |
| Rubin et al. (2000)61 | Cross-sectional study | n = 2520 older individuals, presence of ocular pathologies not reported n = 93 with VA >6/12 and n = 21 with VA >6/60 n = 261 with dissimilar VAs between eyes, although VA cutoff not defined |
EDTRS chart (distance VA), computerized reading display | With similar VAs between eyes, 38% show binocular summation and 10% show binocular inhibition, whereas, with dissimilar VAs between eyes, 20 to 29% show summation, and 19 to 23% show inhibition Inhibition on average was one letter Binocular acuity and better eye acuity were equally as predictive of reading performance across participants (r2 = 0.50 vs. 0.48, respectively) |
| Tarita-Nistor et al. (2013)62 | Cross-sectional study | n = 20 with AMD | ETDRS chart (distance VA), MNREAD acuity chart (reading speed) | Maximum reading speed significantly slower for patients with binocular inhibition compared with those with binocular summation or equality (mean, 42, 107, and 111 wpm, respectively) Significant correlation (r = 0.49) between binocular ratio and maximum reading speed |
| Silvestri et al. (2020)63 | Cross-sectional study | n = 42 with AMD, n = 29 with Stargardt disease | ETDRS chart (distance VA), MNREAD acuity chart (reading speed) | Maximum binocular reading speed significantly slower for patients with binocular inhibition compared with those with binocular summation (mean, 65 vs. 94 wpm, respectively), and borderline slower compared with those with equality (mean, 96 wpm) In the binocular inhibition group, binocular performance significantly poorer than better eye monocular performance for reading speed (65 vs. 81 wpm, respectively) and reading acuity (0.86 vs. 0.78 logMAR units, respectively) |
| Faubert and Overbury (2000)64 | Case-control study | n = 49 with AMD, n = 10 normals | Sine gratings (contrast sensitivity) | n = 27 (45%) of AMD participants showed poorer binocular contrast sensitivity than better eye contrast sensitivity, compared with n = 1 (10%) of normals |
| Valberg and Fosse (2002)57 | Case-control study | n = 13 with AMD, n = 10 normals | Sine gratings (contrast sensitivity) | Monocular to binocular ratios of contrast sensitivity are reduced in n = 12 (92%) of AMD participants |
| Gonzalez et al. (2004)65 | Case-control study | n = 17 with AMD, n = 38 normals | Multiple tumbling E tests at low, medium, and high contrast (distance VA) | Similar binocular ratios between AMD and normals No change in binocular ratios with reduced contrast |
Articles are sorted by order of appearance within the text. ETDRS = Early Treatment Diabetic Retinopathy Study; n = number of participants; r = correlation coefficient; r2 = coefficient of determination; VA = visual acuity; wpm = words per minute.
There are several concerns that affect the implications of binocular summation research on low vision rehabilitation. First, the techniques used to measure visual acuity and contrast sensitivity vary widely between studies, which in turn is likely to impact on the binocular summation effects observed. In AMD, when visual acuities were measured with a Tumbling E chart, binocular summation was preserved at all contrast levels, which does not appear consistent with studies investigating contrast sensitivity using gratings.65 In addition, studies investigating contrast sensitivity characteristics in AMD have used sine gratings,57,64 and the link with summation characteristics measured with letter contrast sensitivities, as is more often performed in clinical practice, has not been established. Furthermore, poor repeatability of clinically measured summation characteristics and poor correlation to self-reported binocular inhibition have been reported, which may be due to inadequate sensitivity of currently available clinical tests.66 Lastly, to date, there are no studies specifically investigating binocular summation characteristics in low vision cohorts outside of macular disease, and therefore, it is difficult to extrapolate the aforementioned findings to other low vision populations. Overall, although the concept of binocular summation and inhibition has potential to affect clinical practice patterns, this has not been addressed in the current literature, and therefore, further research systematically addressing patient outcomes and satisfaction with prescription of low vision aids and rehabilitation taking binocular summation characteristics into consideration is required before these concepts can be applied regularly into low vision assessments and rehabilitation.
Comment: Binocular Visual Dysfunction in Pediatric Low Vision Populations
The body of evidence describing binocular vision dysfunctions in low vision and blindness is focused on acquired degenerative causes of vision impairment, and therefore, adult cohorts have been primarily studied to date. Although participants younger than 18 years have been included in RP studies,22,40 it is difficult to determine whether pediatric RP cohorts demonstrate different degrees of binocular vision dysfunction, given that pooling of all participants was performed. However, differences in binocular summation characteristics in children and adolescents versus adult participants with prior optic neuritis,67,68 although not strictly including vision-impaired cohorts, suggest possible differences in binocular vision characteristics in pediatric and adult populations with the same ocular pathology. Furthermore, the vastly different range of ocular pathologies causing vision impairment in pediatric cohorts69,70 may manifest differently with respect to types and degrees of binocular vision dysfunction in older cohorts. Lastly, to date there are no studies that detail binocular vision dysfunctions specifically in causes of pediatric vision impairment. Given likely impacts on activities of daily living and quality of life,71 work in this field would be invaluable.
BINOCULAR MEASUREMENTS OF VISUAL FIELD EXTENT
Binocular Visual Fields
Visual field assessment enables the identification of regions of vision loss that could be contributing to difficulty in certain tasks and remaining vision that can be used in the rehabilitation process. Monocular threshold visual field assessments, typically performed to monitor the underlying ocular disease, do not directly translate to the habitual binocular setting and therefore are not directly applicable in low vision rehabilitation. Meanwhile, manual methods of measuring binocular visual fields, including Bjerrum screen and Goldman kinetic perimetry, are gross measurements of visual function that are less useful in identifying relative scotomas, as these methods rely on detection of stimuli that do not change in intensity. A similar problem affects the conventional Amsler grid, which has demonstrated significantly poorer sensitivity in identifying defective locations in the central visual field compared with threshold perimetry in a low vision cohort, with proposed modifications improving sensitivity not adopted into widespread practice.72 Similarly, although forming the standard of care mandated by driving and licensing authorities internationally,73,74 the suprathreshold nature of Esterman binocular visual field assessment imparts relatively poor sensitivity in identifying relative scotomas compared with monocular threshold perimetry.75–78 Therefore, although kinetic and Esterman visual field assessments provide a gross understanding of a patient's remaining visual function binocularly, particularly in the presence of very pronounced visual field loss, more subtle reductions in the visual field impacting difficulties in activities of daily living such as reading or mobility may be missed using these techniques alone.
Adapting existing monocular threshold visual field strategies to be performed binocularly has been explored previously,45,79 with these studies identifying that central locations in the visual field and locations 10 to 30° from fixation were significant predictors of vision-related activity limitation for reading and mobility tasks, respectively. A surrogate to binocular threshold tests that does not require additional visual field tests to be performed is the integrated visual field, which combines existing monocular threshold visual field results to create a composite map representing patient's binocular visual field sensitivities. Several methods of calculating pointwise integrated visual field thresholds have been described across different studies, with measurements using the higher visual field sensitivities between eyes as the “binocular” threshold value and using binocular summation equations to calculate binocular sensitivity demonstrating the best agreement with binocular threshold visual field results (Table 4).80 Although substantial agreement between integrated visual field and Esterman results has been reported in some studies,81,83,84 Xu et al.78 observed that mean visual field sensitivities calculated from integrated visual field results were able to distinguish moderate and severe glaucoma from early glaucoma subgroups, whereas Esterman scores could not distinguish early and moderate glaucoma. However, whether the more subtle binocular visual field defects detected with integrated visual field affect functionality and vision-related quality of life is of greater relevance, and there have been mixed results from this perspective, with some studies reporting superior correlations with integrated visual field82,85,86 and others finding better correlations with Esterman.77,87 Nevertheless, the integrated visual field has been used to identify patients with atypical performance on reach to grasp tasks including initiating movement and misestimation of object location,47 as well as identifying areas of the binocular visual field affecting self-reported function in activities such as reading, dining, and walking in patients with glaucoma88,89 (Fig. 2), and these may be beneficial to identify areas of potential difficulty based on individual patients' visual fields to target rehabilitative processes. However, because similar analyses have not been performed with Esterman visual field testing, head-to-head comparisons between Esterman and integrated visual field are required to identify whether Esterman visual field testing is sufficient to identify patients with these difficulties in daily activities or whether additional efforts to compute the integrated visual field are required. In addition, patients with greater visual field defects in integrated visual field have greater postural sway and more difficulty with laboratory mobility tasks90–92 and are at greater risk of falls,93 suggesting that integrated visual field can be used to identify patients with mobility difficulties that may benefit from fall prevention programs.
TABLE 4.
Summary of nonreview articles investigating use of binocular visual fields testing, including useful field of view, in low-vision populations
| Study | Study design | Cohort | Tests used | Outcomes |
|---|---|---|---|---|
| Tabrett and Latham (2011)45 | Cross-sectional study | n = 100 with vision impairment n = 54 with macular disease including AMD, n = 11 with optic neuropathy including glaucoma |
Binocular HFA 30-2 | Overall regression analyses showed that mean binocular thresholds 10 to 30° from fixation was a significant predictor of self-reported vision-related activity limitation in the subcategories of goals, all tasks, mobility, and visual information |
| Tabrett and Latham (2012)79 | Cross-sectional study | n = 100 with vision impairment n = 54 with macular disease including AMD, n = 11 with optic neuropathy including glaucoma |
Binocular HFA 30-2 | Greater visual field loss as per the binocular 30-2 associated with increased self-reported vision-related activity limitations Impairment in right side of central 5° best predicted activity limitations in reading, and impairment in central 10 to 20° best predicted activity limitations in mobility |
| Nelson-Quigg et al. (2000)80 | Cross-sectional study | n = 111 with glaucoma | Monocular HFA 30-2, binocular HFA 30-2 | IVFs calculated from monocular visual field results using best location and binocular summation were most similar to binocular visual field results, with 95% of predictions within 3 dB of binocular results IVFs calculated from mean sensitivities of monocular visual field results performed most poorly |
| Crabb et al. (2004)81 | Cross-sectional study | n = 65 with glaucoma | Binocular Esterman, monocular HFA 24-2, UFOV | Substantial agreement between Esterman test scores and IVF sensitivity values using pass/fail criteria (κ = 0.69) IVF has 100% sensitivity and 88% specificity using Esterman as comparative measure Comparisons of UFOV test results for 2% who failed IVF but passed Esterman versus other cohorts suggested Integrated Visual Field results more similar to those that failed both tests |
| Crabb and Viswanathan (1998)82 | Cross-sectional study | n = 48 with glaucoma | Binocular Esterman, monocular HFA 24-2 | IVF score (summary score describing number of defects <10 and <20 dB) showed better classification of participants with self-perceived visual difficulty compared with the Esterman Efficiency Score (AUROC, 0.79 vs. 0.70) |
| Chisholm et al. (2008)83 | Cross-sectional study | n = 60 with binocular paracentral scotomas of various origins | Monocular HFA 24-2, binocular Esterman, UFOV | Good agreement between IVF and Esterman fields in pass/fail classification regarding fitness to drive standards (κ = 0.84) One participant failed IVF but passed Esterman, whereas three passed IVF but failed Esterman, as defective locations were outside of the area tested in IVF No significant difference in UFOV scores in patients who passed and failed IVF and Esterman |
| Xu et al. (2019)78 | Cross-sectional study | n = 250 with glaucoma, n = 31 normal | Monocular HFA 30-2, binocular Esterman | In cases of glaucoma with asymmetric visual field loss, IVF MDs were significantly worse than better eye MDs; however, Esterman scores were more similar to better eye VFIs. IVF MDs were significantly worse in bilateral moderate glaucoma and unilateral or bilateral severe glaucoma, whereas Esterman scores were only significantly worse in bilateral severe glaucoma |
| Crabb et al. (2005)84 | Cross-sectional study | n = 59 with glaucoma | Binocular Esterman, monocular HFA 24-2 | Substantial agreement between Esterman test scores and IVF sensitivity values (κ = 0.81) IVF has 100% sensitivity and 86% specificity using Esterman as comparative measure |
| Bozzani et al. (2012)85 | Cross-sectional study | n = 132 with glaucoma | Monocular HFA 24-2 | Significant correlation between mean IVF sensitivity and VFQ-25 composite score (r = −0.71) and all subscales excluding general health and ocular pain (not stated) Significant correlations between mean IVF sensitivity and utility values calculated from EQ-5D, SF-6D, and TTO questionnaires (r = −0.25 to −0.47) |
| Chun et al. (2019)86 | Cross-sectional study | n = 826 with glaucoma | Monocular HFA 24-2 | Significant correlations between mean IVF sensitivities and VFQ-25 composite score (r2 = 0.176 to 0.181) and all subscales excluding near activities and ocular pain (r2 = 0.114 to 0.145) |
| Subhi et al. (2017)87 | Cross-sectional study | n = 50 with peripheral vision loss n = 23 with glaucoma, n = 14 with RP, n = 4 with retinal detachments, n = 9 with undisclosed pathology |
Binocular threshold extending across 120°, binocular suprathreshold (10 dB) extending across 120°, binocular Esterman, monocular HFA 24-2 | Significantly better AUROC for binocular threshold and suprathreshold summary scores compared with IVF MD for self-reported difficulty in walking and bumping into objects and people Significantly better AUROC for Esterman summary score compared with IVF MD for self-reported difficulty in bumping into objects and walking in high glare |
| Musch et al. (2017)77 | Cross-sectional study | n = 607 with glaucoma | Binocular Esterman, monocular HFA 24-2 | Weak to modest correlations between Esterman test scores and binocular MD approximated from monocular visual fields (r = 0.31 to 0.42 for different methods of calculating MD) Esterman test scores correlated better with VAQ total score and seven of nine subscales compared with binocular approximations (r = 0.14 to 0.25), and similar trends were observed in distance vision, peripheral vision, and driving subscales of NEI-VFQ (r not stated) |
| Kotecha et al. (2009)47 | Case-control study | n = 16 with glaucoma and n = 16 normals | Titmus stereotest (fly only), Frisby stereotest | Correlation between poorer IVF and delays in initiating reach-and-grasp movement in glaucoma participants (r = 0.55, P = .001) Initial misestimation of object position indicated by significant correlations between poorer IVF and faster time to reach maximum speed (r = 0.52, P < .002) and to reach deceleration (r = 0.37, P = .04). |
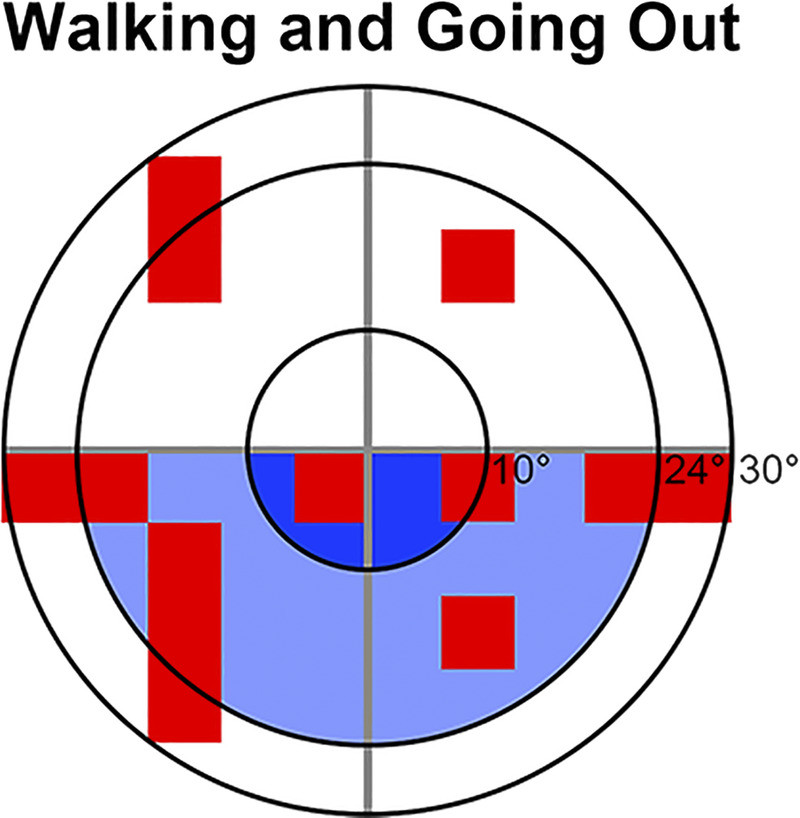
| Murata et al. (2013)88 | Cross-sectional study | n = 164 with glaucoma | Monocular HFA 30-2 | In IVFs across the central 60°, reduced sensitivities across the horizontal meridian corresponded to more difficulty in vision-related activities of daily living, particularly in reading and dining subcategories Difficulties in walking and going out subcategories corresponded to reduced sensitivities in the inferior hemifield and superior hemifield, respectively |
| Yamazaki et al. (2019)89 | Cross-sectional study | n = 172 with advanced glaucoma | Monocular HFA 24-2 and 10-2 | Worse IVF scores in the lower subfields of the 24-2 and 10-2 IVFs associated with poorer total disability index in Rasch analysis–derived person ability index Specific tasks affected by worse IVF scores were reading sentences, walking, going out, dining, and miscellaneous tasks |
| Turano et al. (2004)90 | Cross-sectional study | n = 1504 participants n = 136 with VA worse than 20/40 (cause unspecified) |
Monocular HFA single threshold (24 dB) 30-2, modified binocular Esterman without weighting | Greater visual field loss in the central 40° and in the inferior periphery correlated with slower walking speed: 0.8 cm/s for every 6 points missed in the central 40° and 0.6 cm/s for every 2 points missed in the inferior periphery Greater visual field loss in the central 40° correlated with increased number of bumps into obstacles: 13% increase for every 6 points missed in the central 40° |
| Black et al. (2008)91 | Cross-sectional study | n = 54 with glaucoma | Monocular HFA 24-2 and single threshold (24 dB) extending across 120° |
Significantly increased postural sway with eyes open in participants with poorer IVF MD and increased number of points missed on 120° binocular visual field Similar Spearman correlation coefficients between binocular 60 and 120° visual fields for all sway characteristics (r = 0.13 to 0.51) |
| Kotecha et al. (2012)92 | Case-control study | n = 24 with glaucoma and n = 24 normals | Monocular HFA 24-2 | Lower visual contribution and higher somatosensory contribution to sway in participants with glaucoma Poorer binocular MD correlated with reduced visual contribution and increased somatosensory contribution to sway in glaucoma participants |
| Ramulu et al. (2019)93 | Prospective cohort study | n = 225 with glaucoma or glaucoma suspect status | Monocular HFA 24-2 | IVF sensitivity not associated with higher rate of falls per year; however, with 5 dB worsening of IVF sensitivity, there were 33 and 45% higher rates of falls per step at home and away from home, respectively |
| Subhi et al. (2017)94 | Cross-sectional study | n = 52 with peripheral vision loss n = 22 with glaucoma, n = 21 with RP, n = 2 with vascular conditions, n = 2 with retinal detachments, n = 5 with undisclosed pathology |
Binocular HFA 30-2 and 60-4 | Significant correlations between self-reported mobility subscores and mean thresholds for central visual field (within 30° from fixation) and peripheral visual field (30 to 60° from fixation) (r2 = 0.61 and 0.63, respectively) In multiple regression analysis, inferior visual field (from 0 to 60° of fixation) best predicts mobility function |
| Fletcher et al. (2012)95 | Cross-sectional study | n = 153 with AMD | California central visual field test, Smith-Kettlewell reading test, MN read chart | Patients with binocular scotoma border within 2.5° of fixation had greater error rates in Smith-Kettlewell reading test compared with patients with no binocular scotoma border near fixation |
| Pardhan et al. (2017)33 | Case-control study | n = 17 with AMD, n = 17 with glaucoma (without low vision) and n = 10 normals | Monocular HFA 30-2 | Significantly longer movement time and reaction time in AMD patients compared with glaucoma and normals Significant correlations between poorer IVF scores within the central 5° and central 10°, and longer movement time (r = −0.49 for 5° and −0.45 for 10°), deceleration time (r = −0.51 for 5° and −0.44 for 10°), and velocity corrections (r = −0.44 for 5° and −0.40 for 10°) |
| Verghese et al. (2016)46 | Case-control study | n = 16 with AMD and n = 9 normals | Optos OCT/SLO | Significant correlation (r = −0.78) between stereoacuity and improvement in reach-to-grasp and transport-to-place task performance under binocular viewing in participants with measurable stereopsis Significant correlation (r = −0.81) between overlap in binocular and monocular scotomas and improvement in performance under binocular viewing in AMD patients with no measurable stereopsis |
| Tzaridis et al. (2019)60 | Cross-sectional study | n = 68 with macular telangiectasia type 2 | Radner reading charts (reading acuity and speed), MP1 microperimeter | Binocular gain in reading speed correlated with left eye scotoma size (r2 = 0.81), indicating increased binocular inhibition with larger left eye scotoma Binocular reading speed did not correlate with right eye scotoma size |
| Kabanarou et al. (2006)96 | Cross-sectional study | n = 29 with AMD | Infrared eye tracking, SLO | n = 20 demonstrated shift in gaze position from monocular to binocular viewing, with n = 17 demonstrating shift with worse eye monocular viewing only Significantly greater shift in gaze position from monocular to binocular viewing in worse eye monocular viewing compared with better eye monocular viewing (median, 5.6 vs. 1.2°; P < .001) Shift in gaze position from monocular to binocular viewing with worse eye predictive of the difference between worse and better eye PRLs (r2 = 0.59) |
| Tarita-Nistor et al. (2015)97 | Cross-sectional study | n = 9 with AMD and n = 5 normals | MP-1 microperimetry, infrared eye tracking | Monocular PRLs estimated with eye tracking yielded mean horizontal error of 0.2° and vertical error of 0.5° compared with PRLs measured with microperimetry Binocular PRLs fell within corresponding retinal locations and were similar to monocular PRLs in n = 8 AMD participants and all normal participants |
Articles are sorted by order of appearance within the text. κ = kappa coefficient; AUROC = area under receiver operator curve; EQ-5D = EuroQoL Index Tool; HFA = Humphrey Field Analyzer; HR = hazard ratio; IVF = integrated visual field; MD = mean deviation; n = number of participants; NEI-VFQ = National Eye Institute Visual Function Questionnaire; OCT = optical coherence tomographer; OR = odds ratio; PRL = preferred retinal locus; r = correlation coefficient; r2 = coefficient of determination; SF-6D = SF-36 (36-Item Short Form) using SF-D algorithm; SLO = scanning laser ophthalmoscope; TTO = Time Trade Off; UFOV = Useful Field of View; VA = visual acuity; VAQ = Visual Activities Questionnaire; VFI = visual field index; VFQ-25 = Visual Function Questionnaire.
FIGURE 2.
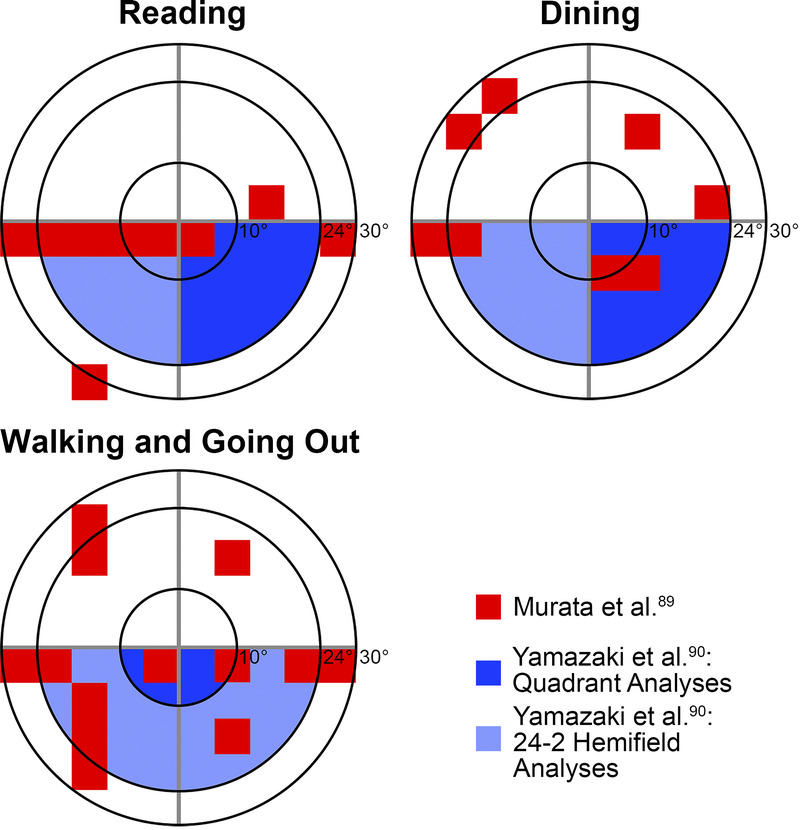
Schematic demonstrating locations within the central 60° most important for reading (top left), dining (top right), and walking and going out (bottom left) in patients with glaucoma calculated using the IVF, based on Murata et al.88 and Yamazaki et al.89 (shaded in red and blue, respectively). The smallest to largest concentric rings indicate 10, 24, and 30° from fixation, respectively. Note that both studies were conducted in Japan where reading direction may be different, and similar studies with Caucasian populations have not yet been conducted. IVF = integrated visual field.
The integrated visual field is not without its limitations, the most notable of which is that the area of the visual field tested is smaller compared with the binocular Esterman and kinetic perimetry. Although several studies have observed equivalent correlations between mean visual field indices and mobility factors when comparing the central 60°, which is within 30° of fixation, and the central 120° of the visual field,90,91,94 Subhi et al.94 reported that the inferior visual field up to 60° from fixation was the best predictor of self-reported mobility. However, given that this area also includes the inferocentral visual field (that is 0 to 30° from fixation), further studies specifically investigating the roles of the inferocentral and inferior peripheral visual field on mobility are required before additional recommendations on threshold testing outside of the central 60° can be made. Furthermore, at this stage, automated perimeters do not automatically calculate the integrated visual field, and the further steps required to calculate individual visual field thresholds across the visual field may be considered a cumbersome addition to the low vision assessment. Perhaps in the future, with greater uptake of the integrated visual field within clinical practice, automated algorithms to calculate the integrated visual field would be included in commercially available devices, increasing the accessibility of integrated visual field to practicing low vision clinicians.
Relatively little research has focused on the applicability of visual field assessment on characterizing binocular scotomas in central vision loss. This is surprising considering that increasing central scotoma size, when measured binocularly, has been reported to significantly affect reading performance independent of visual acuity measurements.95 One study to adopt an integrated visual field-like approach33 reported significant correlations between poorer integrated visual field scores in the central 10° and several reach-to-grasp parameters, consistent with previous work identifying that the presence of binocular central scotomas affects performance in reach-to-grasp tasks.98 A more common approach in central field loss is to use monocular microperimetry results to designate tested locations as part of or outside of the binocular scotoma to estimate binocular scotoma size,46 as poor fixation stability in central visual field loss generally precludes standard automated perimetry from being performed. Verghese et al.46 reported that a greater difference between the binocular scotoma and monocular scotoma of the better-seeing eye resulted in poorer performance in object placement tasks, which the authors postulated was due to the effects of rivalry when the difference was greater. Interestingly, although Tzaridis et al.60 did not compute the binocular scotoma size from microperimetry, in patients with macular telangiectasia and central vision loss, they observed worse binocular gain in reading speeds with larger left eye scotomas but not right eye scotomas; the authors hypothesized that left eye scotomas, projecting to the right side of the visual field, interrupt the perceptual span required for fluent reading more so than right eye scotomas. However, in light of the unavailability of binocular microperimetry methods to date, logistical concerns including variations in binocular and monocular viewing directions and therefore location of preferring retinal loci potentially affect the precision of mapping binocular scotomas from monocular scotomas using microperimetry.23,96 Whereas Kabanarou et al.96 observed significant shifts in gaze position between worse-seeing eye monocular viewing and binocular viewing in 20 of 29 AMD participants, implying that corrections for differences in gaze are required before mapping binocular scotomas from monocular perimetry, a small study by Tarita-Nistor et al.97 demonstrated that preferred retinal loci did not differ significantly between monocular and binocular viewing conditions in nine AMD participants. As such, larger-scale studies applying integrated visual field concepts to central threshold visual field testing, incorporating possible differences in gaze position with monocular versus binocular viewing, and their correlations with near activities are required before determining its utility in clinical low vision assessments.
COMMENT: ADDITIONAL GAPS IN THE LITERATURE
Patients with central vision impairment often complain of difficulties with facial recognition, yet there is little literature exploring the role of binocular visual function on facial recognition. Although the presence of central visual field defects has been correlated with increased difficulty in recognizing faces in patients with AMD and glaucoma,99,100 these studies used monocular visual field results or inferred binocular visual field performance from better eye visual fields only; it is therefore difficult to judge how methods of assessing the binocular visual field may contribute to our understanding of difficulties with facial recognition. Moreover, studies comparing acuity measures, both monocularly and binocularly, to performance on facial recognition tasks showed variable results,61,101 and therefore, the role of binocular summation on facial recognition ability is equally contentious. Tejeria et al.101 found that self-reported facial recognition did not correlate with performance on in-office facial recognition tasks, which may confound the ability of clinical measures to obtain meaningful information on how patients recognize faces in real-world environments. It would be worthwhile if future studies could explore the potential role of clinically measured binocular visual functions on real-world facial recognition in low vision cohorts.
The majority of studies investigating binocular vision dysfunctions and measurement of the binocular visual field in vision impairment have concentrated on AMD, glaucoma, and RP cohorts as key ocular pathologies resulting in low vision. However, diabetic retinopathy is another significant contributor to nonreversible vision impairment with previously reported substantial impacts on quality of life,102,103 yet there were no studies to date that specifically investigated binocular visual processes in diabetic retinopathy. Although several studies included patients with diabetic retinopathy,17,45,48,79 these were pooled with the remainder of the cohort before analysis, and analysis by cause of vision impairment was not performed, so at face value, how diabetic retinopathy may affect binocular vision distinct from other ocular pathologies could not be discerned. Particularly, as vision impairment in diabetic retinopathy may manifest as both central and/or peripheral vision loss, studies specifically exploring how binocular vision dysfunctions and binocular visual field measures impact visual function in diabetic retinopathy would be of value and may aid low vision rehabilitation in this cohort.
Finally, how addressing identified binocular vision dysfunctions and binocular visual field defects, via the appropriate choice of low vision aid and rehabilitation, affects rehabilitation success, patient satisfaction, and overall quality of life has not been explored in detail, forming a significant gap in the available evidence. Only one study by Skrbek26 has reported improved binocular visual acuity and anecdotal acceptance of prism in AMD patients with esotropia. Further research on the choice of low vision aids and rehabilitation, guided by clinical measures of binocular vision, is necessary to determine whether binocular vision assessment can translate to improved quality of life in vision-impaired populations.
CONCLUSIONS
The literature has consistently described a higher prevalence of dysfunctions in ocular alignment, stereopsis, and binocular summation across a variety of ocular pathologies causing vision impairment. Reading performance in low vision cohorts can be affected by the presence of ocular alignment dysfunctions, binocular summation characteristics, and central visual field defects measured binocularly, and mobility and accurate object placement are affected by stereopsis and peripheral visual field defects as measured binocularly. Furthermore, the absence of stereopsis and binocular visual field deficits can identify patients with greater self-reported reductions in quality of life. Therefore, the identification of these binocular vision dysfunctions and deficits in the binocular visual field during routine low vision assessments should be applied during rehabilitation. However, the level of vision impairment or asymmetry in vision impairment at which assessments of binocularity are no longer practical has not been established. In addition, research regarding the direct impact of rehabilitative efforts targeting binocular vision dysfunctions on patient outcomes is still an emerging area of research interest and would be invaluable to facilitate delivery of optimal, best-practice care for patients with low vision in rehabilitation processes.
Supplementary Material
Footnotes
Supplemental Digital Content: Appendix Table A1, available at http://links.lww.com/OPX/A480, shows the required change in object distance to detect change in depth with varying stereoacuities for pupillary distance (PD) of 64 mm and working distances of 40, 150, and 300 cm, as per Fig. 1. These values are under the theoretical assumption that other monocular cues to depth do not play a role; where larger changes in object distance are required before stereopsis computes a change in depth, it is far more likely that changes in depth are computed with smaller changes in object depth owing to monocular cues in real-life scenarios.
Appendix Figure A1 (available at http://links.lww.com/OPX/A480). Simplified schematics depicting potential deficits in stereopsis in various levels of visual impairment when fixating on an object of interest (star). The black dotted line indicates the horopter and the surrounding area bordered by the solid black lines indicate Panum’s fusion area, with location from fixation on the star labeled above. The white-to-gray color scale within Panum’s fusion area depicts maximum stereoacuity to no measurable stereopsis. (A) With normal bilateral visual function, there is bifoveal fixation with the principal visual directions (red dotted lines) on the object of interest, resulting in the retinal images to fall on corresponding retinal locations and accurate computation of central stereopsis. In normal participants, stereoacuity decreases with increasing eccentricity and is measurable out to 14° from fixation.37 (B) In asymmetric central vision impairment with resultant moderately poor vision in one eye, the asymmetry in retinal inputs results in reduced stereoacuity. (C) In bilateral mild visual impairment, given the similarity of retinal inputs between eyes, stereoacuity is likely to be reduced, albeit less markedly than in B. (D) In bilateral severe central vision impairment, central stereopsis is absent.
Funding/Support: None of the authors have reported funding/support.
Conflict of Interest Disclosure: JT, MK, and AL receive salary support from Guide Dogs New South Wales/Australian Capital Territory, and JM is an employee of Guide Dogs New South Wales/Australian Capital Territory. JH and VK are recipients of Australian Government Research Training Program scholarships and PhD scholarships provided by Guide Dogs New South Wales/Australian Capital Territory. Guide Dogs New South Wales/Australian Capital Territory played no role in the conceptualization of this article, and the authors have no proprietary interest in any of the materials mentioned in this article.
Author Contributions and Acknowledgments: Conceptualization: JT, MK, AL; Data Curation: JT, JH, VK; Investigation: JT; Methodology: JT; Project Administration: JT; Resources: MK; Visualization: JT; Writing – Original Draft: JT; Writing – Review & Editing: JT, JH, VK, JM, MK, AL.
The authors would like to thank Emma Bartley and Kelly Prentice (Guide Dogs New South Wales/Australian Capital Territory) for their advice and article feedback.
Supplemental Digital Content: Direct URL links are provided within the text.
Contributor Information
Janelle Tong, Email: jtong@cfeh.com.au.
Jessie Huang, Email: jhuang@cfeh.com.au.
Vincent Khou, Email: vkhou@cfeh.com.au.
Jodi Martin, Email: jmartin@guidedogs.com.au.
Michael Kalloniatis, Email: mkalloniatis@cfeh.com.au.
REFERENCES
- 1.Tseng VL, Coleman AL. Reducing the Burden of Unilateral Vision Impairment and Blindness in Australia. JAMA Ophthalmol 2018;136:248–9. [DOI] [PubMed] [Google Scholar]
- 2.Lamoureux EL, Hassell JB, Keeffe JE. The Determinants of Participation in Activities of Daily Living in People with Impaired Vision. Am J Ophthalmol 2004;137:265–70. [DOI] [PubMed] [Google Scholar]
- 3.West SK Rubin GS Broman AT, et al. How Does Visual Impairment Affect Performance on Tasks of Everyday Life? Arch Ophthalmol 2002;120:774–80. [DOI] [PubMed] [Google Scholar]
- 4.Cypel MC Salomao SR Dantas PEC, et al. Vision Status, Ophthalmic Assessment, and Quality of Life in the Very Old. Arq Bras Oftalmol 2017;80:159–64. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Report on Vision. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 6.Bourne RR Flaxman SR Braithwaite T, et al. Magnitude, Temporal Trends, and Projections of the Global Prevalence of Blindness and Distance and Near Vision Impairment: A Systematic Review and Meta-analysis. Lancet Glob Health 2017;5:e888–97. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor P, Keeffe J. Focus on Low Vision. Melbourne, Australia: Centre for Eye Research; 2007. [Google Scholar]
- 8.Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Seeing into Old Age: Vision Function beyond Acuity. Optom Vis Sci 1999;76:141–58. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz A. The Prevalence and Consequences of Vision Impairment in Later Life. Top Geriatr Rehabil 2004;20:185–95. [Google Scholar]
- 10.Binns AM Bunce C Dickinson C, et al. How Effective Is Low Vision Service Provision? A Systematic Review. Surv Ophthalmol 2012;57:34–65. [DOI] [PubMed] [Google Scholar]
- 11.Wittich W, Canuto A, Overbury O. Overcoming Barriers to Low-vision Rehabilitation Services: Improving the Continuum of Care. Can J Ophthalmol 2013;48:463–7. [DOI] [PubMed] [Google Scholar]
- 12.Wolffsohn J, Cochrane AL. Design of the Low Vision Quality-of-life Questionnaire (LVQOL) and Measuring the Outcome of Low-vision Rehabilitation. Am J Ophthalmol 2000;130:793–802. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz SN. Principles of Modern Low Vision Rehabilitation. Can J Ophthalmol 2006;41:289–312. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson ME, Shahid KS. Low Vision Rehabilitation: An Update. Saudi J Ophthalmol 2018;32:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon K Bonfanti A Pearson V, et al. Comprehensive Vision Rehabilitation. Can J Ophthalmol 2015;50:85–6. [DOI] [PubMed] [Google Scholar]
- 16.Kalloniatis M, Johnston AW. Visual Characteristics of Low Vision Children. Optom Vis Sci 1990;67:38–48. [DOI] [PubMed] [Google Scholar]
- 17.Rundström MM, Eperjesi F. Is There a Need for Binocular Vision Evaluation in Low Vision? Ophthalmic Physiol Opt 1995;15:525–8. [PubMed] [Google Scholar]
- 18.Uzdrowska M, Crossland M, Broniarczyk-Loba A. Is Binocular Vision Worth Considering in People with Low Vision? Klin Oczna 2014;116:49–51. [PubMed] [Google Scholar]
- 19.Kalloniatis M, Luu C. Space perception. In: Kolb H, Fernandez E, Nelson R, eds. The Organization of the Retina and Visual System. Salt Lake City, UT: University of Utah Health Sciences Center; 2005. [Google Scholar]
- 20.Sheedy JE, Saladin JJ. Association of Symptoms with Measures of Oculomotor Deficiencies. Am J Optom Physiol Opt 1978;55:670–6. [DOI] [PubMed] [Google Scholar]
- 21.Cacho-Martinez P Canto-Cerdan M Carbonell-Bonete S, et al. Characterization of Visual Symptomatology Associated with Refractive, Accommodative, and Binocular Anomalies. J Ophthalmol 2015;2015:895803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyata M Oishi A Ogino K, et al. Relationship between Ocular Deviation and Visual Function in Retinitis Pigmentosa. Sci Rep 2018;8:14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarita-Nistor L Brent MH Steinbach MJ, et al. Fixation Patterns in Maculopathy: From Binocular to Monocular Viewing. Optom Vis Sci 2012;89:277–87. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JH, Clahane AC. The Role of the Periphery in Binocular Vision. Am J Ophthalmol 1966;62:702–6. [DOI] [PubMed] [Google Scholar]
- 25.Migliorini R Comberiati AM Galeoto G, et al. Eye Motility Alterations in Retinitis Pigmentosa. J Ophthalmol 2015;2015:145468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skrbek M. Binocular Refraction in Patients with Age-related Macular Degeneration. Coll Antropol 2013;37:153–6. [PubMed] [Google Scholar]
- 27.Reche-Sainz JA Gómez de Liaño R Toledano-Fernández N, et al. Binocular Vision in Glaucoma. Arch Soc Esp Oftalmol 2013;88:174–8. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg R Faye E Fischer M, et al. Role of Prism Relocation in Improving Visual Performance of Patients with Macular Dysfunction. Optom Vis Sci 1989;66:747–50. [DOI] [PubMed] [Google Scholar]
- 29.Verezen CA, Volker-Dieben HJ, Hoyng CB. Eccentric Viewing Spectacles in Everyday Life, for the Optimum Use of Residual Functional Retinal Areas, in Patients with Age-related Macular Degeneration. Optom Vis Sci 1996;73:413–7. [DOI] [PubMed] [Google Scholar]
- 30.Al-Karmi R, Markowitz SN. Image Relocation with Prisms in Patients with Age-related Macular Degeneration. Can J Ophthalmol 2006;41:313–8. [DOI] [PubMed] [Google Scholar]
- 31.Verezen CA Meulendijks CFM Hoyng CB, et al. Long-term Evaluation of Eccentric Viewing Spectacles in Patients with Bilateral Central Scotomas. Optom Vis Sci 2006;83:88–95. [DOI] [PubMed] [Google Scholar]
- 32.Giaschi D Narasimhan S Solski A, et al. On the Typical Development of Stereopsis: Fine and Coarse Processing. Vision Res 2013;89:65–71. [DOI] [PubMed] [Google Scholar]
- 33.Pardhan S Scarfe A Bourne R, et al. A Comparison of Reach-to-grasp and Transport-to-place Performance in Participants with Age-related Macular Degeneration and Glaucoma. Invest Ophthalmol Vis Sci 2017;58:1560–9. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor AR, Tidbury LP. Stereopsis: Are We Assessing It in Enough Depth? Clin Exp Optom 2018;101:485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levi DM, Knill DC, Bavelier D. Stereopsis and Amblyopia: A Mini-review. Vision Res 2015;114:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell D. A Review of the Concept of “Panum's Fusional Areas”. Am J Optom Arch Am Acad Optom 1966;43:387–401. [PubMed] [Google Scholar]
- 37.Wallace DK Lazar EL Melia M, et al. Stereoacuity in Children with Anisometropic Amblyopia. J AAPOS 2011;15:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odell NV Hatt SR Leske DA, et al. The Effect of Induced Monocular Blur on Measures of Stereoacuity. J AAPOS 2009;13:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larson WL, Bolduc M. Effect of Induced Blur on Visual Acuity and Stereoacuity. Optom Vis Sci 1991;68:294–8. [DOI] [PubMed] [Google Scholar]
- 40.Vingolo EM Limoli PG Steigerwalt RD Jr., et al. Abnormal Stereopsis and Reduced Retinal Sensitivity in Patients with Retinitis Pigmentosa. Int Ophthalmol 2020;40:179–84. [DOI] [PubMed] [Google Scholar]
- 41.Lin S Mihailovic A West SK, et al. Predicting Visual Disability in Glaucoma with Combinations of Vision Measures. Transl Vis Sci Technol 2018;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakshmanan Y, George RJ. Stereoacuity in Mild, Moderate and Severe Glaucoma. Ophthalmic Physiol Opt 2013;33:172–8. [DOI] [PubMed] [Google Scholar]
- 43.Nelson P Aspinall P Papasouliotis O, et al. Quality of Life in Glaucoma and Its Relationship with Visual Function. J Glaucoma 2003;12:139–50. [DOI] [PubMed] [Google Scholar]
- 44.Cao KY, Markowitz SN. Residual Stereopsis in Age-related Macular Degeneration Patients and Its Impact on Vision-related Abilities: A Pilot Study. J Optom 2014;7:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabrett DR, Latham K. Factors Influencing Self-reported Vision-related Activity Limitation in the Visually Impaired. Invest Ophthalmol Vis Sci 2011;52:5293–302. [DOI] [PubMed] [Google Scholar]
- 46.Verghese P Tyson TL Ghahghaei S, et al. Depth Perception and Grasp in Central Field Loss. Invest Ophthalmol Vis Sci 2016;57:1476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotecha A O'Leary N Melmoth D, et al. The Functional Consequences of Glaucoma for Eye-hand Coordination. Invest Ophthalmol Vis Sci 2009;50:203–13. [DOI] [PubMed] [Google Scholar]
- 48.Lamoureux E Gadgil S Pesudovs K, et al. The Relationship between Visual Function, Duration and Main Causes of Vision Loss and Falls in Older People with Low Vision. Graefes Arch Clin Exp Ophthalmol 2010;248:527–33. [DOI] [PubMed] [Google Scholar]
- 49.Lord SR, Dayhew J. Visual Risk Factors for Falls in Older People. J Am Geriatr Soc 2001;49:508–15. [DOI] [PubMed] [Google Scholar]
- 50.Ivers RQ Norton R Cumming RG, et al. Visual Impairment and Risk of Hip Fracture. Am J Epidemiol 2000;152:633–9. [DOI] [PubMed] [Google Scholar]
- 51.Chew FL Yong CK Ayu SM, et al. The Association between Various Visual Function Tests and Low Fragility Hip Fractures among the Elderly: A Malaysian Experience. Age Ageing 2010;39:185–91. [DOI] [PubMed] [Google Scholar]
- 52.Timmis MA, Pardhan S. Patients with Central Visual Field Loss Adopt a Cautious Gait Strategy during Tasks That Present a High Risk of Falling. Invest Ophthalmol Vis Sci 2012;53:4120–9. [DOI] [PubMed] [Google Scholar]
- 53.Lord SR. Visual Risk Factors for Falls in Older People. Age Ageing 2006;35(Suppl. 2):ii42–5. [DOI] [PubMed] [Google Scholar]
- 54.Tarita-Nistor L Gonzalez EG Markowitz SN, et al. Binocular Function in Patients with Age-related Macular Degeneration: A Review. Can J Ophthalmol 2006;41:327–32. [DOI] [PubMed] [Google Scholar]
- 55.Schneck ME Haegerstrom-Portnoy G Lott LA, et al. Monocular vs. Binocular Measurement of Spatial Vision in Elders. Optom Vis Sci 2010;87:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gagnon RW, Kline DW. Senescent Effects on Binocular Summation for Contrast Sensitivity and Spatial Interval Acuity. Curr Eye Res 2003;27:315–21. [DOI] [PubMed] [Google Scholar]
- 57.Valberg A, Fosse P. Binocular Contrast Inhibition in Subjects with Age-related Macular Degeneration. J Opt Soc Am (A) 2002;19:223–8. [DOI] [PubMed] [Google Scholar]
- 58.Tarita-Nistor L Gonzalez EG Markowitz SN, et al. Binocular Interactions in Patients with Age-related Macular Degeneration: Acuity Summation and Rivalry. Vision Res 2006;46:2487–98. [DOI] [PubMed] [Google Scholar]
- 59.Kabanarou SA, Rubin G. Reading with Central Scotomas: Is There a Binocular Gain? Optom Vis Sci 2006;83:798–6. [DOI] [PubMed] [Google Scholar]
- 60.Tzaridis S Herrmann P Charbel Issa P, et al. Binocular Inhibition of Reading in Macular Telangiectasia Type 2. Invest Ophthalmol Vis Sci 2019;60:3835–41. [DOI] [PubMed] [Google Scholar]
- 61.Rubin GS Munoz B Bandeen-Roche K, et al. Monocular versus Binocular Visual Acuity as Measures of Vision Impairment and Predictors of Visual Disability. Invest Ophthalmol Vis Sci 2000;41:3327–34. [PubMed] [Google Scholar]
- 62.Tarita-Nistor L Brent MH Markowitz SN, et al. Maximum Reading Speed and Binocular Summation in Patients with Central Vision Loss. Can J Ophthalmol 2013;48:443–9. [DOI] [PubMed] [Google Scholar]
- 63.Silvestri V Sasso P Piscopo P, et al. Reading with Central Vision Loss: Binocular Summation and Inhibition. Ophthalmic Physiol Opt 2020;40:778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faubert J, Overbury O. Binocular Vision in Older People with Adventitious Visual Impairment: Sometimes One Eye Is Better Than Two. J Am Geriatr Soc 2000;48:375–80. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez EG, Markowitz M, Steinbach MJ. Vision Loss from Macular Degeneration: An Optimal Way to Measure What Remains. Poster presented at the University Health Network Research Day; October 31, 2004; Toronto, Ontario, Canada.
- 66.McElvanney A, Moseley MJ, Jones HJ. Binocular Inhibition of Visual Performance in Patients with Cataract. The Influence of Test Reliability. Acta Ophthalmol 1994;72:606–11. [DOI] [PubMed] [Google Scholar]
- 67.Pineles SL Birch EE Talman LS, et al. One Eye or Two: A Comparison of Binocular and Monocular Low-contrast Acuity Testing in Multiple Sclerosis. Am J Ophthalmol 2011;152:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waldman AT Hiremath G Avery RA, et al. Monocular and Binocular Low-contrast Visual Acuity and Optical Coherence Tomography in Pediatric Multiple Sclerosis. Mult Scler Relat Disord 2013;3:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pham C Sheth SJ Keeffe JE, et al. New Trends in Childhood Vision Impairment in a Developed Country. J AAPOS 2017;21:496–8. [DOI] [PubMed] [Google Scholar]
- 70.Chong C, McGhee CNJ, Dai SH. Causes of Childhood Low Vision and Blindness in New Zealand. Clin Exp Ophthalmol 2019;47:165–70. [DOI] [PubMed] [Google Scholar]
- 71.Decarlo DK McGwin G Jr. Bixler ML, et al. Impact of Pediatric Vision Impairment on Daily Life: Results of Focus Groups. Optom Vis Sci 2012;89:1409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achiron LR Witkin NS McCarey B, et al. The Illuminated High Contrast Macular Grid: A Pilot Study. J Am Optom Assoc 1995;66:693–7. [PubMed] [Google Scholar]
- 73.Bron AM Viswanathan AC Thelen U, et al. International Vision Requirements for Driver Licensing and Disability Pensions: Using a Milestone Approach in Characterization of Progressive Eye Disease. Clin Ophthalmol 2010;4:1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silveira S Jolly N Heard R, et al. Current Licensing Authority Standards for Peripheral Visual Field and Safe On-road Senior Aged Automobile Driving Performance. Clin Experiment Ophthalmol 2007;35:612–20. [DOI] [PubMed] [Google Scholar]
- 75.Jampel HD Schwartz A Pollack I, et al. Glaucoma Patients' Assessment of Their Visual Function and Quality of Life. J Glaucoma 2002;11:154–63. [DOI] [PubMed] [Google Scholar]
- 76.Noe G Ferraro J Lamoureux E, et al. Associations between Glaucomatous Visual Field Loss and Participation in Activities of Daily Living. Clin Experiment Ophthalmol 2003;31:482–6. [DOI] [PubMed] [Google Scholar]
- 77.Musch DC Niziol LM Gillespie BW, et al. Binocular Measures of Visual Acuity and Visual Field versus Binocular Approximations. Ophthalmology 2017;124:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu J Lu P Dai M, et al. The Relationship between Binocular Visual Field Loss and Various Stages of Monocular Visual Field Damage in Glaucoma Patients. J Glaucoma 2019;28:42–50. [DOI] [PubMed] [Google Scholar]
- 79.Tabrett DR, Latham K. Important Areas of the Central Binocular Visual Field for Daily Functioning in the Visually Impaired. Ophthalmic Physiol Opt 2012;32:156–63. [DOI] [PubMed] [Google Scholar]
- 80.Nelson-Quigg JM, Cello K, Johnson C. Predicting Binocular Visual Field Sensitivity from Monocular Visual Field Results. Invest Ophthalmol Vis Sci 2000;41:2212–21. [PubMed] [Google Scholar]
- 81.Crabb DP Fitzke FW Hitchings RA, et al. A Practical Approach to Measuring the Visual Field Component of Fitness to Drive. Br J Ophthalmol 2004;88:1191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crabb DP, Viswanathan AC. Integrated Visual Fields: A New Approach to Measuring the Binocular Field of View and Visual Disability. Graefes Arch Clin Exp Ophthalmol 2005;243:210–6. [DOI] [PubMed] [Google Scholar]
- 83.Chisholm CM Rauscher FG Crabb DC, et al. Assessing Visual Fields for Driving in Patients with Paracentral Scotomata. Br J Ophthalmol 2008;92:225–30. [DOI] [PubMed] [Google Scholar]
- 84.Crabb DP Viswanathan AC McNaught AI, et al. Simulating Binocular Visual Field Status in Glaucoma. Br J Ophthalmol 1998;82:1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bozzani FM Alavi Y Jofre-Bonet M, et al. A Comparison of the Sensitivity of EQ-5D, SF-6D and TTO Utility Values to Changes in Vision and Perceived Visual Function in Patients with Primary Open-angle Glaucoma. BMC Ophthalmol 2012;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chun YS Sung KR Park CK, et al. Vision-related Quality of Life According to Location of Visual Field Loss in Patients with Glaucoma. Acta Ophthalmol 2019;97:e772–9. [DOI] [PubMed] [Google Scholar]
- 87.Subhi H Latham K Myint J, et al. Functional Visual Fields: A Cross-sectional UK Study to Determine Which Visual Field Paradigms Best Reflect Difficulty with Mobility Function. BMJ Open 2017;7:e018831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murata H Hirasawa H Aoyama Y, et al. Identifying Areas of the Visual Field Important for Quality of Life in Patients with Glaucoma. PLoS One 2013;8:e58695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamazaki Y Sugisaki K Araie M, et al. Relationship between Vision-related Quality of Life and Central 10 Degrees of the Binocular Integrated Visual Field in Advanced Glaucoma. Sci Rep 2019;9:14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turano K Broman AT Bandeen-Roche K, et al. Association of Visual Field Loss and Mobility Performance in Older Adults: Salisbury Eye Evaluation Study. Optom Vis Sci 2004;81:298–307. [DOI] [PubMed] [Google Scholar]
- 91.Black AA Wood JM Lovie-Kitchin JA, et al. Visual Impairment and Postural Sway among Older Adults with Glaucoma. Optom Vis Sci 2008;85:489–97. [DOI] [PubMed] [Google Scholar]
- 92.Kotecha A Richardson G Chopra R, et al. Balance Control in Glaucoma. Invest Ophthalmol Vis Sci 2012;53:7795–801. [DOI] [PubMed] [Google Scholar]
- 93.Ramulu PY Mihailovic A West SK, et al. Predictors of Falls per Step and Falls per Year at and Away from Home in Glaucoma. Am J Ophthalmol 2019;200:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subhi H Latham K Myint J, et al. Functional Visual Fields: Relationship of Visual Field Areas to Self-reported Function. Ophthalmic Physiol Opt 2017;37:399–408. [DOI] [PubMed] [Google Scholar]
- 95.Fletcher DC, Schuchard RA, Renninger LW. Patient Awareness of Binocular Central Scotoma in Age-related Macular Degeneration. Optom Vis Sci 2012;89:1395–8. [DOI] [PubMed] [Google Scholar]
- 96.Kabanarou SA Crossland MD Bellmann C, et al. Gaze Changes with Binocular versus Monocular Viewing in Age-related Macular Degeneration. Ophthalmology 2006;113:2251–8. [DOI] [PubMed] [Google Scholar]
- 97.Tarita-Nistor L Eizenman M Landon-Brace N, et al. Identifying Absolute Preferred Retinal Locations during Binocular Viewing. Optom Vis Sci 2015;92:836–72. [DOI] [PubMed] [Google Scholar]
- 98.Timberlake GT Omoscharka E Quaney BM, et al. Effect of Bilateral Macular Scotomas from Age-related Macular Degeneration on Reach-to-grasp Hand Movement. Invest Ophthalmol Vis Sci 2011;52:2540–50. [DOI] [PubMed] [Google Scholar]
- 99.Glen FC Crabb DP Smith ND, et al. Do Patients with Glaucoma Have Difficulty Recognizing Faces? Invest Ophthalmol Vis Sci 2012;53:3629–37. [DOI] [PubMed] [Google Scholar]
- 100.Wallis TSA Taylor CP Wallis H, et al. Characterization of Field Loss Based on Microperimetry Is Predictive of Face Recognition Difficulties. Invest Ophthalmol Vis Sci 2013;55:142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tejeria L Harper RA Artes PH, et al. Face Recognition in Age Related Macular Degeneration: Perceived Disability, Measured Disability, and Performance with a Bioptic Device. Br J Ophthalmol 2002;86:1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lamoureux EL Tai ES Thumboo J, et al. Impact of Diabetic Retinopathy on Vision-specific Function. Ophthalmology 2010;117:757–65. [DOI] [PubMed] [Google Scholar]
- 103.Fenwick EK Pesudovs K Rees G, et al. The Impact of Diabetic Retinopathy: Understanding the Patient's Perspective. Br J Ophthalmol 2011;95:774–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


