Abstract
Brain atrophy has been observed in perinatally HIV-infected patients (PHIV) despite initiation on combined antiretroviral treatment (cART), but neuroimaging studies are limited. We aimed to evaluate cortical thickness (CT) and subcortical gray matter (GM) volumes of PHIV youths with stable immunovirological situation and with a normal daily performance.
A prospective cross-sectional study was conducted. A total of 25 PHIV patients on cART and 25 HIV-negative (HIV-) controls matched by age, sex, level of education, and socioeconomic status underwent a magnetic resonance imaging scan. CAT12 toolbox was used to extract CT values from T1w images using parcellations from Desikan–Killiany atlas (DK40). To measure regional brain volumes, native segmented images were parceled in regions of interest according to the Neuromorphometrics Atlas. Neuropsychological assessment and psychopathological symptoms were documented.
Fifty participants were included (60% females, median age 20 years [interquartile range, IQR 19–23], 64% Whites). No differences regarding neuropsychological tests or psychopathological symptoms were found between groups (all P > .05). All participants presented an average performance in the Fluid Intelligence (FI) test (PHIV mean: −0.12, HIV- mean: 0.24), When comparing CT, PHIV-infected patients showed thinner cortices compared with their peers in fusiform gyrus (P = .000, P = .009), lateral-orbitofrontal gyrus (P = .006, P = .0024), and right parsobitalis gyrus (P = .047). Regarding subcortical GM volumes, PHIV patients showed lower right amygdala (P = .014) and left putamen (P = .016) volumes when compared with HIV- controls. Within the PHIV group, higher CD4 count was associated with higher volumes in right putamen (B = 0.00000038, P = .045). Moreover, increased age at cART initiation and lower nadir CD4 count was associated with larger volumes in left accumbens (B = 0.0000046, P = .033; B = −0.00000008, P = .045, respectively).
PHIV patients showed thinner cortices of areas in temporal, orbito-frontal and occipital lobes and lower volumes of subcortical GM volumes when compared with the HIV- control group, suggesting cortical and subcortical brain alterations in otherwise neuroasymptomatic patients. Nevertheless, larger and longitudinal studies are required to determine the impact of HIV on brain structure in PHIV patients and to further identify risk and protective factors that could be implicated.
Keywords: basal ganglia, cortical thickness, HIV, neuroimaging, perinatal, volume
1. Introduction
One of the most important consequences of the human immunodeficiency virus (HIV) infection is its impact on the central nervous system (CNS).[1] Fortunately, the cases of HIV encephalopathy and severe neurological complications have declined due to the introduction of combined antiretroviral therapy (cART). However, many patients continue to experience milder degrees of HIV-associated neurocognitive disorders (HAND).[2] In this regard, perinatally HIV (PHIV)-infected patients are more vulnerable than adults to displaying CNS alterations as the viral invasion occurs earlier in life.[3]
Additionally, some authors have described limited penetration of cART in the CNS[4] allowing the brain to act as a viral reservoir[5] and making the infection of CNS a generalized condition of HIV patients. It has also been shown that some antiretroviral drugs can sometimes cause neuropsychiatric adverse effects.[6,7] For the aforementioned reasons, finding a balance between the CNS penetration of antiretroviral treatment and possible neurotoxic effects is decisive for the well-being of HIV patients.
It is therefore essential to understand how HIV and cART might mediate in brain development, and to ensure an early detection of HAND. For these purposes, a variety of novel, noninvasive, neuroimaging techniques have been developed to support the quantitative characterization of the brain structure. Preliminary findings of this research in PHIV children and young adults has demonstrated that even in the cART era, there are alterations in microstructural brain regions.[1] However, the outcomes are not consistent,[9] being very difficult to isolate the effect caused by the different psychosocial backgrounds of the selected participants.
The most frequently reported findings have included lower total[3,8,10] or regional cortical and subcortical[8,11,12] gray matter (GM) volumes. Nevertheless, it is interesting that different results have been presented by other authors in recent years, showing increased regional or total GM volumes.[9,13,14] Moreover, although some authors did not find significant differences in cortical thickness (CT) between PHIV patients and HIV- control group,[1] other studies found thinning of certain cortical areas in PHIV patients.[12,15]
Commonly, within the PHIV population the alterations found in brain structure have been usually related to a poor immunovirological status and to the lack of an effective antiretroviral treatment.[9,14,17,18]
Taking into account all these factors and the previous results, the present study attempts to determine the characteristic pattern of cortical thinning and subcortical (total and regional) in a predominant viral suppressed perinatal HIV population with an average cognitive functioning and compare it with an HIV- control group strictly matched by age, sex, level of education, and socioeconomic status. Mindful of the need to restrict the number of statistical comparisons, we also studied the effect of HIV status on all main subregions of the basal ganglia (BG) volumes. In consonance with existing literature, we first hypothesize that the PHIV-infected group will show greater atrophy and thinner cortices in brain structure related to the HIV- controls. Secondly, we tested the hypothesis that a worse immunovirological status will be associated with smaller volumes of the BG since it has been reported to be particularly impacted.[11,12,14,16–18]
2. Materials and methods
2.1. Study design and participants
A cross-sectional study was performed between January 2017 and December 2017.
Initial sample participant number was 30 PHIV patients and 33 HIV-, but 13 participants were excluded (5 PHIV and 8 HIV- controls) due to outlier MRI image quality markers when compared with the sample average quality-assurance measurements.
Twenty-five PHIV patients and 25 HIV- peers, matched by age, sex, educational level, and socioeconomic status participated in a cross-sectional study. Data were collected at 5 pediatric research centers that belong to the CoRISpe (Spanish National Cohort of Pediatric HIV) group.[19]
Patients with current brain infection, neurological or psychiatric disorder, or any congenital abnormality were not included in the sample. Nonetheless, 3 patients with history of encephalopathy were included since they all had normal neuropsychological performances at assessment.
The HIV- peers were selected from voluntary recruitment through the advertising of the study.
The Institutional Review Boards of each research center approved the study, and a written, informed consent was obtained from all participants in accordance with the Helsinki Declaration. When participants were underage, an assent form was signed by themselves, and their legal guardians provided the informed consent.
2.2. Disease markers in PHIV youth
In relation to the control of the infection, the following parameters were collected: CDC classification, encephalopathy, undetectable viral load (defined as viral load <50 copies/mL), time of undetectable viral load, viral load in detectable patients, total numbers and percentages of CD4 nadir and current CD4 viral load, CD4/CD8 ratio, cART history, and adherence to treatment. This information was obtained from the CoRISpe database.
2.3. Measures
A sociodemographic self-report semistructured questionnaire was created for this project. International Standard Classification of Education (ISCED) criteria were used to categorize the level of education attained by the participants.
Neuropsychological assessment assessed fluid intelligence (FI) through Kaufman Brief Intelligence Test (K-BIT) and Executive Functions (EF) measures by 10 neuropsychological tests (Table 1).
Table 1.
Neuropsychological assessment battery.
| Cognitive Domains | Subcomponents | Tool | Test |
| Intelligence | Fluid intelligence | Kaufman Brief Intelligence test[52] | Nonverbal (fluid) |
| Composite z score for executive function (EF10Z) | Processing speed | Stroop Test[53,54] | Stroop-Word |
| Stroop-Color | |||
| Wechsler Adult Intelligence Scale-4th edition (WAIS-IV)[55] | Coding subtest | ||
| Trail Making Test[56] | TMT-Part A | ||
| Decision-making, cognitive flexibility, verbal fluency, working memory, planning, inhibition | Trail Making Test[56] | TMT-Part B | |
| Semantical verbal fluency test[57] | Animals | ||
| Phonological verbal fluency test[57] | P | ||
| BADS[58] | Zoo Map | ||
| Stroop Test[53,54] | Stroop-Word-Color | ||
| Wechsler Adult Intelligence Scale-4th edition (WAIS-IV)[55] | Digits-Backward |
To assess psychopathological symptoms, 2 standardized evaluation tools were used State-Trait Anxiety Questionnaire, STAI[20] (Beck, Steer & Carbin, 1988) and Depression Inventory of Beck, BDI.[21]
2.4. Imaging acquisition protocol
Different MRI scanner systems were used at each hospital study site. For specific details of the acquisition parameters, see Supplementary material.
Image quality was assessed in 2 independent processes. Radiologist checked for the presence of any brain pathology, such as tumor, cyst, or any other lesion.
Additionally, image quality and processing experts checked for motion artifacts, low contrasts, incomplete whole brain coverage, low signal-to-noise ratio, and low resolution, excluding 5 PHIV patients and 8 HIV- controls as mentioned above. In a further analysis, all the acquisitions were correlated to determine the homogeneity of the image sample.
2.5. Image processing
2.5.1. Image analysis
The Computational Anatomy Toolbox (CAT12, http://dbm.neuro.uni-jena.de/cat/ version 1492), as an extension of SPM (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ version 7487), provides a standard processing pipeline for surface and volume-based morphometry. These pipelines allow the extraction of several morphometric parameters including CT and tissue volumes.
For surface data, the processing includes local adaptive segmentation of the T1-weighted images, topological correction,[22] spherical mapping,[23] spherical registration, CT, and central surface estimation.[24]
Mean CT values were calculated inside regions of interest (ROIs) defined by the Desikan-Killiany Atlas (DK40),[25] which is the most extensive in SBM studies and following the standard software procedure.
To measure brain volumes, native segmented images were parceled in ROIs according to the Neuromorphometrics atlas and tissue volumes (cubic millimeters) were estimated for each ROI and normalized by the total intracranial volume for each subject.
2.6. Statistical analysis
2.6.1. Sociodemographic, clinical, cognitive and Psychological assessment
Results were expressed as mean and standard deviation (SD), except for sociodemographical and immunological results which were expressed as median and interquartile ranges (IQRs) for quantitative variables or as frequencies and percentages for qualitative variables. To compare categorical variables Pearson χ2 or Fisher exact tests were used, whereas quantitative variables were compared using the Mann–Whitney U test. All tests with a P value <.05 were considered statistically significant. Raw scores on cognitive tests were transformed into a standard form (z scores), where the transformation was based on knowledge about the standardization sample mean and SD by each test. SPSS v. 24 was used for data analysis.
2.6.2. Neuroimaging analysis
Mean thickness values for all ROIs were compared between PHIV and HIV- to observe the intergroup differences. A 2-sample t test analysis was performed through CAT12 statistical models adjusting for age and sex as nuisance variables.[26] Results were subjected to a multiple comparison Holm-Bonferroni correction and a P value <.05 was considered statistically significant.
2.6.3. HIV status and volumes for subcortical GM regions
These procedures resulted in the extraction of volumes for seven bilateral subcortical GM ROIs: the thalamus, putamen, pallidum, amygdala, accumbens, caudate, and hippocampus. Mean volumes inside ROIs were extracted and a linear regression analysis using the forward method was performed to study the effect of HIV-related clinical variables on BG volumes, covarying for age, and sex.
3. Results
3.1. Characteristics of study population
Twenty-five PHIV patients and 25 HIV- controls were enrolled. Sociodemographic characteristics were very similar between cases and controls: 60% were females with a median age of 20 years in both groups, 62% of the participants had low level of education, and 76% of the PHIV patients were Whites. No differences regarding FI, EF10Z, or psychopathological symptoms were found between groups (all P > .05, Table 2).
Table 2.
Means (standard deviation) of demographic, psychosocial, neurocognitive, and psychopathological characteristics.
| PHIV (n = 25) | HIV- (n = 25) | P | |
| Sex (female), % (n) | 60% (15) | 60% (15) | 1.000 |
| Age at assessment, % (n) | 20.60 (3.16) | 20.40 (2.84) | .815 |
| Level of education, % (n) | |||
| Low | 70.8% (17) | 63.6% (14) | .294 |
| Medium | 16.7% (4) | 4.5% (1) | |
| High | 12.5% (3) | 31.8% (7) | |
| Annual income, % (n) | |||
| <12.000 € | 21.7% (5) | 26.1% (6) | .175 |
| 12.000–16.000 € | 26.1% (6) | 34.8% (8) | |
| 16.001–20.000 € | 13% (3) | 17.4% (4) | |
| 20.001–26.000 € | 13% (3) | 13% (3) | |
| 26.001–30.000 € | 17.4% (4) | 8.7% (2) | |
| >30.000 € | 8.7% (2) | 0% (0) | |
| White, % (n) | 76% (19) | 52% (13) | .080 |
| STAI-STATE | 21.22 (12.99) | 15.45 (11.45) | .246 |
| STAI-TRAIT | 23.70 (12.40) | 21.45 (11.41) | .657 |
| BDI | 10.17 (9.60) | 10.05 (9.67) | .918 |
| Fluid intelligence∗ | -0.12 (0.73) | 0.24 (0.66) | .059 |
| Composite z score for executive function (EF10Z)∗ | 0.26 (0.44) | 0.17 (0.51) | .515 |
Regarding the PHIV group: 40% showed a previous event that led them to a CDC stage C3, among them 12% (N = 3) had encephalopathy. Median CD4 nadir was 11.9% (IQR 5–17); however, at baseline median CD4 was >500 cells/mm3 (687 cells/mm3 [IQR 497–830]). At the time of the assessment, 100% were under cART, 84% had viral load <50 copies/mL. Among patients with viral load above 50 copies/mL (N = 4), all had <1000 copies/mL being the median viral load in detectable patients of 416 copiesp/mL (IQR 185–530). Most of the patients have been receiving cART for long (median time on cART 17.1 years [IQR 14.8–18.5]), being the median time with undetectable viral load (uVL) of 10.8 years (IQR 6.8–13.1) (Table 3).
Table 3.
Clinical measures in PHIV patients (n [%] or median [IQR]).
| Age at HIV diagnosis, y | 0.50 (0.24–3.44) |
| CDC Stage C3 | 10 (40%) |
| Encephalopathy | 3 (12%) |
| NADIR CD4, cells/mm3 | 249 (84–343) |
| NADIR CD4 (%) | 11.9 (5–17) |
| CD4 count, cells/mm3 | 687 (497–830) |
| CD4 count (%) | 32.7 (30–39) |
| CD4/CD8 | 0.99 (0.69–1.42) |
| Age at treatment onset, y | 1.4 (0.3–4.2) |
| Age at onset on cART, y | 2.3 (1.4–5.4) |
| Total time of treatment with cART | 17.1 (14.8–18.5) |
| Number of cART regimens | 7 (5–9) |
| Patients with uVL | 21 (84%) |
| Time with uVL, y | 10.8 (6.8–13.1) |
| VL in detectable patients, copies/mL | 416 (185–530) |
| Type of CART | |
| 2 NRTIs + 1 NNRTI | 3 (12%) |
| 2 NRTIs + 1 PI | 3 (12%) |
| 2 NRTIs + 1 II | 9 (36%) |
| 1 PI + 1 II | 3 (12%) |
| Other combinations | 7 (28%) |
3.2. CT
Between-group statistical differences in thickness were observed in several areas defined by the DK40 atlas. Specifically, VIH patients present thinner cortex in the left (P = .000, t value = 4.766, z value = 4.274) and right (P = .009, t value = 3.942, z value = 3.639) fusiform gyri, the left (P = .006, t value = 4.068, z value = 3.740) and right (P = .024, t value = 3.627, z value = 3.383) lateral-orbitofrontal gyri, and the right pars orbitalis (P = .047, t value = 3.402, z value = 3.196) (Fig. 1). The opposite contrast (PHIV > HIV-) did not show any significant difference.
Figure 1.
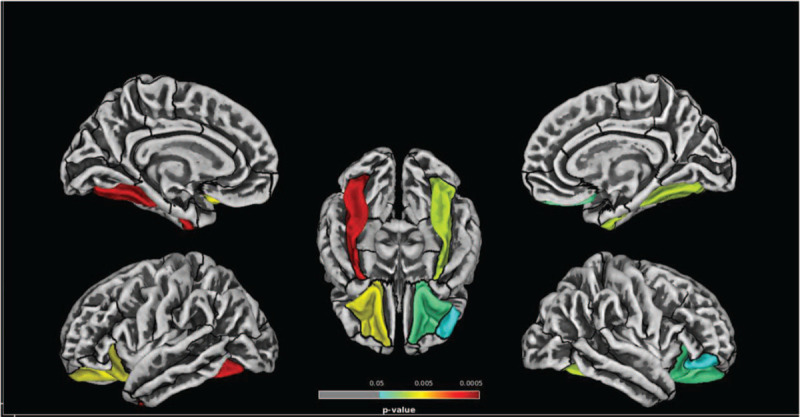
Significant differences in cortical thickness between healthy controls (HIV-) and pediatric HIV patients (PHIV) according to the DK40 atlas parcellation. As shown in the figure, patients present thinner cortex in the bilateral fusiform gyrus, the bilateral orbitofrontal gyrus and the right pars orbitalis (HIV- >PHIV; P < .05, Holm-Bonferroni corrected).
3.3. Volumes for subcortical GM regions
Regarding subcortical GM volumes, according to the Neuromorphometrics Atlas PHIV patients showed lower right amygdala (P = .014) and left putamen (P = .016) volumes when compared with HIV-. No differences were observed between groups for total GM, total white matter, total intracranial volume, or cerebrospinal fluid.
Within the PHIV group, higher CD4 count was associated with higher volumes in right putamen (B = 0.00000038, P = .045) (Fig. 2). Moreover, increased age of cART initiation and lower nadir CD4 count was associated with larger volumes in left accumbens (B = 0.0000046, P = 0.033; B = −0.00000008, P = 0.045, respectively).
Figure 2.
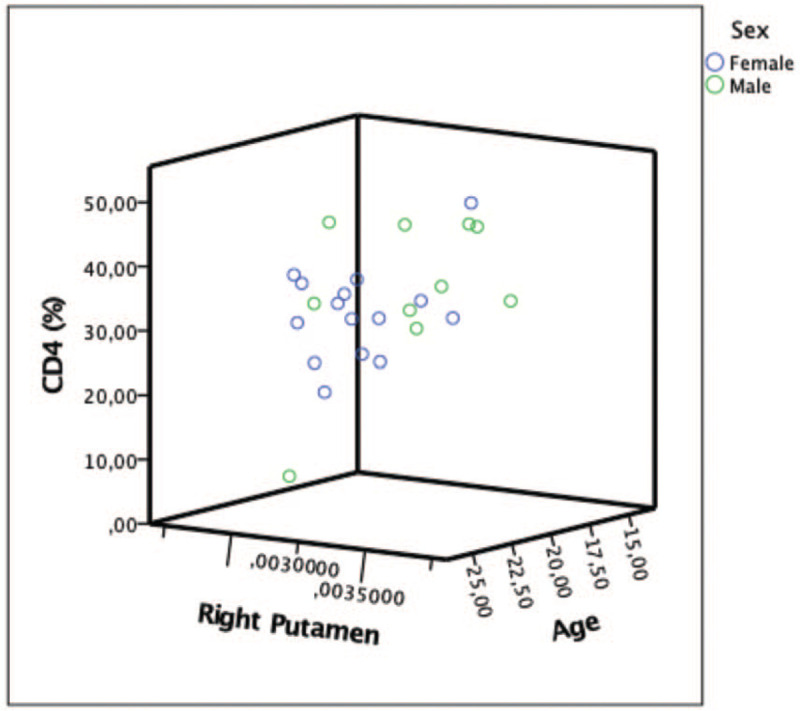
Positive association between CD4 and right putamen volumes (B = 0.00000038, P = .045).
4. Discussion
In this research, we used surface-based analysis to study CT and BG volumes in PHIV youths compared to a well-matched healthy control group using two popular atlases. The study detected differences in CT with thinning of different functional areas of the temporal, orbitofrontal, and occipital lobes and lower volumes of right amygdala and left putamen in otherwise well-controlled perinatally HIV-infected youths.
Cortical thinning is a biomarker of neurodegeneration. Recent studies have identified specific atrophy patterns in the most common neurodegenerative diseases. In Alzheimer disease (AD), prominent mesiotemporal and hippocampal atrophy is typically present.[27] Most consistent findings in Parkinson disease studies show atrophy mainly in the frontal regions.[27] In this regard, increased apparent brain aging, predicted using neuroimaging, has been observed in HIV-positive adults, despite undetectable viral load.[28]
Pathophysiologic mechanisms to explain these alterations in PHIV population could be an early HIV-related CNS damage, as well as ongoing low-grade viral replication and immune activation.[3]
The alterations of neuronal microstructure in the PHIV population found in the present study are supported by previous neuroimaging results. For example, in 2019 Yu et al[15] observed significantly thinner cortices in the temporal and orbitofrontal regions and thicker cortices in left occipital and right olfactory sulcus in HIV patients perinatally infected. Nevertheless, research regarding CT had shown conflicting outcomes in regard to the differences observed between PHIV-infected children and healthy control groups. Nwosu et al[10] found thicker cortex in patients compared to uninfected controls in a small left inferior lateral occipital region. Yadav et al[12] found regional CT decreases in bilateral postcentral and right superior temporal regions, although increases in bilateral medial frontal regions in 10 to 11-year-old PHIV children on cART. It should be noted that some studies did not show any differences in thickness between groups.[1,29]
The difference in thickness between PHIV patients and HIV- orbitofrontal regions could explain the alterations that are frequently shown in those patients whose executive functions are predominantly affected.[30–32] For example, diminished CT in the orbitofrontal area in adolescence correlates to impulsive behavior,[33] and an executive function known as inhibition, deficit of which has been widely described in PHIV patients.[34–36]
We also reported a significant thinning in the fusiform gyrus in PHIV patients compared to healthy controls. This area has been associated with: early atrophy in AD,[37] impact on executive function domains such as working memory[38] and impulsivity,[39] depression,[40] and drug use.[41–43]
With regard to subcortical GM volumes, PHIV patients in our study showed lower right amygdala and left putamen volumes when compared with HIV-. Reductions of amygdala and caudate have been previously reported in the HIV adult population,.[16,44] Similar findings have been described by Li et al[11] in PHIV adolescents, where loss of volume of right pallidum was described.
Within the PHIV group, we found higher volumes of right putamen in patients with higher CD4 count. The association between a low CD4 T-cell count and reduced subcortical regional volumes may indicate that prolonged immunosuppression could play and additional role in CNS damage, explaining the better brain development of well controlled patients. In line with this, Wade et al[18] described increased rates of volume growth in determined subcortical areas in children with higher CD4 counts and similarly, Cohen et al[3] published that longer time with low CD4 T-cell counts was associated with a lower total GM volume.
Furthermore, we found that a delay of cART initiation and lower nadir CD4 count was associated with larger volumes in left accumbens. According to our findings, different studies in the PHIV population showed an enlargement of caudate,[9] nucleus accumbens,[9,12] and putamen[14,45] when compared to a healthy control group. The authors postulated 2 principal hypotheses. First, although the exact pathophysiology for hypertrophy is not well understood, they suggest it could be related to stress-induced hypertrophy of medium spiny neurons resulting in an imperfect pruning. Secondly, BG hypertrophy may be also a result of neuroinflammation, since it is known to be an ongoing detectable process even after cART introduction.[46] Putamen hypertrophy in HIV+ adults has also been attributed to possible inflammation and dopaminergic system dysfunction.[47]
All these results, including the differences in CT between PHIV and negative controls, imply that an earlier and adequate continuous cART is probably the most important key for protection of the CNS.
Nevertheless, it is also important to highlight that although the PHIV showed lower subcortical GM volumes and thinner cortices, no significant differences were found in neurocognitive and neuropsychiatric evaluations, signposting the importance of performing complementary neuroimaging studies that could help detect more subtle neuroalterations not observed by psychometric evaluations.
These neuroimaging findings provide important data, constituting the first neuroimaging study measuring CT and subcortical GM volumes in the White-European PHIV population. Correlations with neuropsychological, psychological, and multiple HIV parameters in PHIV patients have been described. Moreover, it benefits from a meticulous selection of a healthy control group with very similar sociodemographic characteristics similar to the PHIV patients.
Although several studies reflect different results in brain volumes and CT when comparing by age, sex, socioeconomic status, and educational level,[48–50] most of the studies have been performed in African or Asian population where the background can be very different, being most patients CDC stage C, with significant differences in nutritional status and different sociodemographic characteristics. All that may justify the inconsistency of results in the PHIV neuroimaging studies.
This study is no exception in having its limitations, including the cross-sectional design of the study and the reduced number of participants. However, although our sample size is small, it can be considered as representative of younger perinatally HIV-infected adults receiving care in a developed setting, where all the patients are receiving cART and most of them show stable and good immunovirological situation for a long period of time.[51] Cohort studies are usually potentially limited by unmeasured differences, confounding but in our case this was controlled by recruiting a comparable healthy control population with a very similar socioeconomic status. Another limitation is the confounding effects derived from the differences in scanner and head coil parameters between sites. This limitation was minimized by strictly appraising the image quality, assessing the similarity between images from different scanners, thus controlling the comparability. The broad spectrum of findings can be explained since there are no studies using the same neuroimaging analysis tools and atlases, which makes it difficult to generalize and compare results. It is possible that the lack of consensus in terms of MRI analysis techniques and matching processes used are the reasons for the inconsistency of results in the neuroimaging field.
Finally, it is important to take into account that this cohort of young patients with PHIV infection comes from the pre-cART era, which implies that most of these patients received suboptimal treatments during the first years of their lives. There is a chance that the virus could have caused neurological damage during the crucial early years of brain development, highlighting the importance of an earlier and continuous treatment, but there is no way of knowing how or what damage has occurred nor whether it is reversible. Nevertheless, most of these young patients have been well controlled under cART for >10 years, rendering undetectable or very low detectable viral loads, and all of them exhibit a good daily performance and therefore they constitute a unique group. It could be also very interesting to compare this population with a group of early treated young PHIV adults matched by age, to determine the differences and therefore to try to further clarify the origin of this alterations and if they could be prevented.
5. Conclusion
Despite good control of HIV infection and no differences in neurocognitive evaluation, HIV vertically infected patients showed thinner cortices of the temporal, orbitofrontal, and occipital lobes and lower subcortical GM volumes, although the clinical significance/translation of these findings is still unclear. These results support the need to perform complementary neuroimaging studies that could help to detect more subtle neuroalterations not observed by psychometric evaluations. Moreover, longitudinal follow-up would be important to determine the HIV impact on brain structure in PHIV patients and whether these findings will have a clinical expression in the future.
Acknowledgments
This study would not have been possible without the collaboration of all the participants, medical and nursing staff, and data managers who have taken part in Neurocorispe Project of The Pediatric National AIDS Research Network of Spain (CORISPE). The authors acknowledge the selfless help of Trey C. Persson for his English language review. Manuela Martín-Bejarano and Beatriz Ruiz have contributed equally in this study.
Author contributions
Conceptualization: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Berta Zamora, Maria Luisa Navarro, Maria Isabel González-Tomé.
Data curation: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Ana Martínez de Aragon, Santiago Jiménez de Ory, Carlos Velo.
Formal analysis: Mario Gil-Correa, Helena Melero, Norberto Antonio Malpica, Santiago Jiménez de Ory.
Funding acquisition: Maria Isabel González-Tomé, Maria Luisa Navarro.
Investigation: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Ana Martínez de Aragon, Mario Gil-Correa, Helena Melero, Norberto Antonio Malpica, Sara Guillen, Pablo Rojo, Lola Falcon-Neyra, Alberto Alvarez, Pilar Fernández, Maria Luisa Lorente-Jareño, Jose Tomás Ramos, Talía Sainz, Maria Luisa Navarro, Maria Isabel González-Tomé.
Methodology: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Ana Martínez de Aragon, Santiago Jiménez de Ory, Berta Zamora, Sara Guillen, Pablo Rojo, Lola Falcon-Neyra, Alberto Alvarez, Pilar Fernández, Maria Luisa Lorente-Jareño, Jose Tomás Ramos, Talía Sainz, Carlos Velo, Maria Luisa Navarro, Maria Isabel González-Tomé.
Project administration: Maria Isabel González-Tomé, Maria Luisa Navarro.
Resources: Alberto Alvarez, Pilar Fernández, Maria Luisa Lorente-Jareño, Maria Luisa Navarro, Maria Isabel González-Tomé.
Software: Helena Melero, Norberto Antonio Malpica, Mario Gil.
Supervision: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Helena Melero, Norberto Antonio Malpica, Maria Luisa Navarro, Maria Isabel González-Tomé.
Validation: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Mario Gil-Correa, Helena Melero, Norberto Antonio Malpica, Maria Isabel González-Tomé.
Visualization: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Mario Gil-Correa, Helena Melero, Jose Tomás Ramos, Maria Luisa Navarro, Maria Isabel González-Tomé.
Writing – original draft: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Santiago Jiménez de Ory, Berta Zamora, Sara Guillen, Pablo Rojo.
Writing – review & editing: Beatriz Ruiz-Saez, Manuela Martín-Bejarano García, Mario Gil-Correa, Santiago Jiménez de Ory, Berta Zamora, Sara Guillen, Pablo Rojo, Lola Falcon-Neyra, Jose Tomás Ramos, Talía Sainz, Carlos Velo, Maria Luisa Navarro, Maria Isabel González-Tomé.
Footnotes
Abbreviations: BDI = Depression Inventory of Beck, BG = basal ganglia, cART = combined antiretroviral treatment, CNS = central nervous system, CT = cortical thickness, DK40 = Desikan–Killiany atlas, DK40 = Desikan–Killiany atlas, EF = Executive Functions, FI = fluid intelligence, GM = gray matter, HAND = HIV-associated neurocognitive disorders, HIV− = HIV negative, IQR = interquartile ranges, K-BIT = Kaufman Brief Intelligence Test, PHIV = perinatally HIV-infected, ROI = regions of interest, SD = standard deviation, SES = socioeconomic status, STAI = measures, State-Trait Anxiety Questionnaire, uVL = undetectable viral load.
How to cite this article: Ruiz-Saez B, García MM, Aragon AM, Gil-Correa M, Melero H, Malpica NA, Ory SJ, Zamora B, Guillen S, Rojo P, Falcon-Neyra L, Alvarez A, Fernandez P, Lorente-Jareño ML, Ramos JT, Sainz T, Velo C, Navarro ML, Gonzalez-Tomé MI. Effects of perinatal HIV-infection on the cortical thickness and subcortical gray matter volumes in young adulthood. Medicine. 2021;100:15(e25403).
Prior publication: Parts of this study was presented in the Conference on Retroviruses and Opportunistic Infections (CROI) in 2019 (March 4-7, Seattle) and 2020 (March 8-11, Boston).
Sources of support: This work was funded by the Instituto de Salud Carlos III, Acción Estratégica en Salud (FIS 15/00694) and Fondos FEDER of the UE. It was also supported by Fundación para la Investigación y Prevención de SIDA en España (FIPSE) (24691/07, 3608229/09, 240800/09, 361910/10 and 36-0910-1). CoRISpe is integrated in the Spanish AIDS Research Network (RIS), supported by the Instituto de Salud Carlos III (Grant no. RD06/0006/0034 and no. RD06/0006/0035); Red Temática de Investigación en SIDA (RED RIS) (RD16/0025/0017-ISCIII-FEDER, RD16/0025/0019-ISCIII-FEDER, RD16/0025/0024 -ISCIII-FEDER and RIS EPICLIN-12/2012). TS is funded by the Spanish Ministry of Science and Innovation- Instituto de Salud Carlos III and Fondos FEDER (Contratos Rio Hortega CM 16/0022 and Juan Rodés R16/00021, respectively). BRS was granted with a Río-Hortega specialized healthcare post-training contract (CM/00/22). This study is part of Beatriz Ruíz-Saez's PhD.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
z scores <1.0 SD indicate “borderline” cognitive impairment; 1.5 SD indicates minor cognitive impairment and 2.0 SD indicates major cognitive impairment.
CART = combination antiretroviral therapy, CDC = Centers for disease Control and Prevention, II = integrase inhibitor, NNRTI = non-nucleotide reverse transcriptase inhibitor, NRTIs = nucleotide reverse transcriptase inhibitors, PI = protease inhibitor, uVL = undetectable viral load, VL = viral load.
References
- [1].Hoare J, Fouche J-P, Phillips N, et al. Structural brain changes in perinatally HIV infected young adolescents in South Africa. AIDS 2018. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:03–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cohen S, Caan MWA, Mutsaerts H-J, et al. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology 2016;86:19–27. [DOI] [PubMed] [Google Scholar]
- [4].Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Van Rie A, Harrington PR, Dow A, et al. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatric Neurol 2007;11:01–9. [DOI] [PubMed] [Google Scholar]
- [6].González-Tomé MI, García-Navarro C, Ruiz-Saez B, et al. Sleep profile and self-reported neuropsychiatric symptoms in vertically HIV-infected adolescents on cART. J Pediatr Infect Dis 2018;13:300–7. [Google Scholar]
- [7].Du Plessis S, Perez A, Fouche J, et al. Efavirenz is associated with altered fronto-striatal function in HIV+ adolescents. J Neurovirol 2019;25:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lewis-de Los Angeles CP, Williams PL, Huo Y, et al. Lower total and regional grey matter brain volumes in youth with perinatally-acquired HIV infection: associations with HIV disease severity, substance use, and cognition. Brain Behav Immun 2017;62:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Paul R, Prasitsuebsai W, Jahanashad N, et al. Structural neuroimaging and neuropsychologic signatures of vertically acquired HIV. Pediatr Infect Dis J 2017. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nwosu EC, Robertson FC, Holmes MJ, et al. Altered brain morphometry in 7-year old HIV-infected children on early ART. Metab Brain Dis 2018;33:523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li J, Gao L, Wen Z, et al. Structural covariance of gray matter volume in HIV vertically infected adolescents. Sci Rep 2018;8:1182.Published 2018 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yadav SK, Gupta RK, Garg RK, et al. Altered structural brain changes and neurocognitive performance in pediatric HIV. NeuroImage Clin 2017;14:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sarma MK, Nagarajan R, Keller MA, et al. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage Clin 2014;4:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Randall SR, Warton CMR, Holmes MJ, et al. Larger subcortical gray matter structures and smaller corpora callosa at age 5 years in HIV infected children on early ART. Front Neuroanat 2017. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yu X, Gao L, Wang H, et al. Neuroanatomical changes underlying vertical HIV infection in adolescents. Front Immunol 2019;10:814.Published 2019 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ances BM, Ortega M, Vaida F, et al. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 2012;59:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wade BS, Valcour VG, Wendelken-Riegelhaupt L, et al. Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. Neuroimage Clin 2015;9:564–73. Published 2015 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wade BSC, Valcour VG, Puthanakit T, et al. Mapping abnormal subcortical neurodevelopment in a cohort of Thai children with HIV. NeuroImage Clin 2019;23:101810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Jose MI, Jiménez de Ory S, Espiau M, et al. Working groups of CoRISpe and HIV HGM BioBank. A new tool for the paediatric HIV research: general data from the Cohort of the Spanish Paediatric HIV Network (CoRISpe). BMC Infect Dis 2013;13:02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beck AT, Steer RA, Garbin MC. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100. [Google Scholar]
- [21].Guillén-Riquelme A, Buela-Casal G. Actualización psicométrica y funcionamiento diferencial del ítem en el State Trait Anxiety Inventory (STAI). Psicothema 2011;23:510–5. [PubMed] [Google Scholar]
- [22].Yotter RA, Nenadic I, Ziegler G, et al. Local cortical surface complexity maps from spherical harmonic reconstructions. Neuroimage 2011;56:961–73. [DOI] [PubMed] [Google Scholar]
- [23].Yotter RA, Thompson PM, Gaser C. Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J Neuroimaging 2011;21:e134–47. [DOI] [PubMed] [Google Scholar]
- [24].Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage 2013;65:336–48. [DOI] [PubMed] [Google Scholar]
- [25].Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006;31:968–80. [DOI] [PubMed] [Google Scholar]
- [26].Barnes J, Ridgway GR, Bartlett J, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage 2010;53:1244–55. [DOI] [PubMed] [Google Scholar]
- [27].Krajcovicova L, Klobusiakova P, Rektorova I. Gray matter changes in Parkinson's and Alzheimer's disease and relation to cognition. Curr Neurol Neurosci Rep 2019;19:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cole JH, Underwood J, Caan MW, et al. Increased brain-predicted aging in treated HIV disease. Neurology 2017;88:1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Andronikou S, Ackermann C, Laughton B, et al. Corpus callosum thickness on mid-sagittal MRI as a marker of brain volume: a pilot study in children with HIV-related brain disease and controls. Pediatr Radiol 2015;45:1016–25. [DOI] [PubMed] [Google Scholar]
- [30].Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci 2000;20:6159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rich EL, Stoll FM, Rudebeck PH. Linking dynamic patterns of neural activity in orbitofrontal cortex with decision making. Curr Opin Neurobiol 2018;49:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huber B, Yeates M, Meyer D, et al. The effects of screen media content on young children's executive functioning. J Exp Child Psychol 2018;170:72–85. [DOI] [PubMed] [Google Scholar]
- [33].Pehlivanova M, Wolf DH, Sotiras A, et al. Diminished cortical thickness is associated with impulsive choice in adolescence. J Neurosci 2018;38:2471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Malee KM, Chernoff MC, Sirois PA, et al. Impact of perinatally acquired HIV disease upon longitudinal changes in memory and executive functioning. J Acquir Immune Defic Syndr 2017;75:455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nichols SL, Chernoff MC, Malee KM, et al. Executive functioning in children and adolescents with perinatal HIV infection and perinatal HIV exposure. J Pediatric Infect Dis Soc 2016;5: suppl 1: S15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kerr SJ, Puthanakit T, Malee KM, et al. Increased risk of executive function and emotional behavioral problems among virologically well-controlled perinatally HIV-infected adolescents in Thailand and Cambodia. J Acquir Immune Defic Syndr 2019;82:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Parker TD, Slattery CF, Zhang J, et al. Cortical microstructure in young onset Alzheimer's disease using neurite orientation dispersion and density imaging. Hum Brain Mapp 2018;39:3005–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Owens MM, Duda B, Sweet LH, et al. Distinct functional and structural neural underpinnings of working memory. Neuroimage 2018;174:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 2014;47:578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Couvy-Duchesne B, Strike LT, de Zubicaray GI, et al. Lingual gyrus surface area is associated with anxiety-depression severity in young adults: a genetic clustering approach. eNeuro 2018;5: ENEURO.0153-17.2017. Published 2018 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mashhoon Y, Sava S, Sneider JT, et al. Cortical thinness and volume differences associated with marijuana abuse in emerging adults. Drug Alcohol Depend 2015;155:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thames AD, Kuhn TP, Williamson TJ, et al. Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV- adults. Drug Alcohol Depend 2017;170:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang L. Lower total and regional grey matter brain volumes in youth with perinatally-acquired HIV infection: associations with HIV disease severity, substance use, and cognition. Brain Behav Immun 2017;62:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Thames AD, Foley JM, Wright MJ, et al. Basal ganglia structures differentially contribute to verbal fluency: evidence from Human Immunodeficiency Virus (HIV)-infected adults. Neuropsychologia 2012;50:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Blokhuis C, Mutsaerts HJ, Cohen S, et al. Higher subcortical and white matter cerebral blood flow in perinatally HIV-infected children. Medicine (Baltimore) 2017;96:e5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Anthony IC, Ramage SN, Carnie FW, et al. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 2005;64:529–36. [DOI] [PubMed] [Google Scholar]
- [47].Castelo JMB, Courtney MG, Melrose RJ, et al. Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Arch Neurol 2007;64:1275–80. [DOI] [PubMed] [Google Scholar]
- [48].Hair NL, Hanson JL, Wolfe BL, et al. Association of child poverty, brain development, and academic achievement. JAMA Pediatr 2015;169:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci 2015;18:773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Piccolo LR, Merz EC, He X, et al. Pediatric imaging, neurocognition, genetics study. age-related differences in cortical thickness vary by socioeconomic status. PLoS One 2016;11:e0162511.Published 2016 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].UNAIDS data 2018. Global and regional data. [Google Scholar]
- [52].Kaufman AS, Kaufman NL. Manual del Test breve de inteligencia de Kaufman (K-BIT). [Manual for the Kaufman Brief Intelligence Test]. Madrid, Spain: TEA Ediciones; 2000. [Google Scholar]
- [53].Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–62. [Google Scholar]
- [54].Peña-Casanova J, Qui-ones-Ubeda S, Gramunt-Fombuena N, et al. Spanish multicenter normative studies (NEURONORMA Project): norms for the Stroop color-word interference test and the Tower of London-Drexel. Arch Clin Neuropsychol 2009;24:413–29. [DOI] [PubMed] [Google Scholar]
- [55].Wechsler D. WAIS-IV. Escala de inteligencia de Wechsler para adultos-IV. Manual de aplicación y corrección. 2012;Madrid: NCS Pearson, Inc. Edición original, 2008. [Google Scholar]
- [56].Tamayo F, Casals-Coll M, Sanchez-Benavides G, et al. Spanish normative studies in a young adult population (NEURONORMA young adults Project): norms for the verbal span, visuospatial span, Letter-Number Sequencing, Trail Making Test and Symbol Digit Modalities Test. Neurologia 2012;27:319–29. [DOI] [PubMed] [Google Scholar]
- [57].Peña-Casanova J, Quiñones-Ubeda S, Gramunt-Fombuena N, et al. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for verbal fluency tests. Arch Clin Neuropsychol 2009;24:395–411. [DOI] [PubMed] [Google Scholar]
- [58].Wilson BA, Alderman N, Burguess PW, Emslie H, Evans JJ. In: Behavioural Assessment of the Dysexecutive Syndrome (BADS) Souza Ricardo O., Schmidt Sergio L., translators. Bury St Edmunds, U.K.: Thames Valley Test Company; Rio de Janeiro: Cognição; 1996. [Google Scholar]


