Abstract
To study the efficacy of using amniotic membrane, balloon and intrauterine device (IUD) as barrier therapy to prevent re-adhesion after hysteroscopic adhesiolysis.
A total of 45 patients diagnosed with intrauterine adhesions in Changzhou Maternal and Child Health Hospital from June 2014 to December 2017 were included in this retrospective case control study. According to different postoperative isolation barrier methods, the patients were divided into group A (Foley balloon + fresh amniotic membrane Day1 + IUD Day7) (22 cases) and group B (Foley balloon Day1 + IUD Day7) (23 cases). Three months after the surgery, the second hysteroscopy was performed to observe the condition of the uterine cavity and the improvement of menstruation, and to monitor the thickness of the endometrium.
The efficacy of hysteroscopic procedure in group A was significantly higher than that of group B (P < .05). After 3 months of treatment, the improvement rate of menstruation was significantly higher in group A than in group B (P < .05). Endometrial thickness in both group A and B was significantly increased compared with that before the surgery (P < .05). The postoperative endometrium of group A was significantly thicker than that of group B (P < .05).
Amniotic membrane-mediated sequential double-barrier method is clinically feasible for preventing recurrent intrauterine adhesions.
Keywords: amniotic membrane, balloon, intrauterine adhesions
1. Introduction
Intrauterine adhesions are tiny, marginal, or complete occlusion of the uterine cavity during the repair of the endometrial basal layer damage which is also known as Asherman syndrome.[1] Endometrial basal layer damage can lead to infertility, recurrent miscarriage, abnormal uterine bleeding, amenorrhea, dysmenorrhea or abnormal placenta formation, as well as intrauterine hemorrhage and severe pelvic pain.[2] At present, intrauterine adhesions are mainly treated by hysteroscopic adhesiolysis and postoperative hormone therapy.[3] However, the recurrence rate of adhesions after hysteroscopic adhesiolysis is about 3% to 24%, and the recurrence rate of postoperative severe intrauterine adhesions can be as high as 63%.[4] Therefore, it is very important to prevent postoperative re-adhesion of the uterine cavity. In this study, we used fresh amniotic membrane-mediated sequential double-barrier isolation to prevent re-adhesion of the uterine cavity after hysteroscopic adhesiolysis and patients who did not receive amniotic membrane as the isolated barrier were used as the control to evaluate the efficacy of the treatment.
2. Materials and methods objects
From June 2014 to December 2017, a total of 45 patients aged 26 to 39 years were included in this retrospective case control study, who were diagnosed with intrauterine adhesions by hysteroscopy at Changzhou Maternal and Child Health Hospital Affiliated to Nanjing Medical University. According to the method used for preventing re-adhesion of the uterine cavity after hysteroscopic adhesiolysis, patients were divided into group A (Foley balloon + fresh amniotic membrane Day1 + IUD Day7) containing 22 cases, and group B (Foley balloon Day1 + IUD Day7) containing 23 cases.
3. Patient inclusion criteria
-
1.
Patients diagnosed with intrauterine adhesions according to the American Fertility Society classifications, 1988.[5]
-
2.
No other systemic diseases such as coagulation dysfunction, heart disease, liver and kidney dysfunction, and no contraindications to the use of estrogen and progesterone.
-
3.
Preoperative assessment of endocrine hormones was normal, with no other serious gynecological tumors or endocrine diseases.
-
4.
This study was approved by the ethics committee of Changzhou Maternal and Child Health Hospital of Nanjing Medical University our hospital and patient's consent was obtained.
4. Treatment method
4.1. Surgical methods
The 2 groups of patients received surgery within 3 to 7 days after menstruation was over. The surgery times in amenorrhea patients were not limited. Under continuous epidural anesthesia, the conditions of cervix and uterine cavity were detected hysteroscopically. Based on different circumstances, intrauterine adhesiolysis was performed using a needle electrode or a loop electrode, and the original uterine cavity anatomy was restored as much as possible. All surgery procedures for the enrolled patients were performed successfully. Forty five patients had no complications such as perforation, gas embolism, intraoperative bleeding, and water poisoning. No laparotomy or laparoscopy was performed during the operation, and no postoperative infection was found.
4.2. Sequential double-barrier isolation method
Group A (Foley balloon + fresh amniotic membrane Day1 + IUD group Day7) containing 22 cases: The amniotic membrane was prepared on the day of surgery. A healthy postpartum woman with negative preoperative infectious indexes was selected as the donor. The woman was informed about the procedure and signed informed consent. Aseptically, the fresh amniotic membrane was bluntly dissected from the fetal surface of the placenta and cut into about 10 × 10 cm. The amniotic membrane was repeatedly washed with gentamycin and sterile normal saline until it was clean and transparent. After successful hysteroscopic adhesiolysis, 3 to 4 ml of normal saline was injected into a No.16 Foley catheter to form a balloon. The front tip of the catheter beyond the balloon was cut off, and the amniotic membrane was wrapped on the surface of the water-filled balloon (Fig. 1). The normal saline was withdrawn from the catheter and the balloon was placed in the uterine cavity. Saline was injected again to inflate the balloon to fix it in the uterine cavity to isolate the uterus walls. An external urinary drainage bag was used for drainage observation. The balloon catheter and the urinary drainage bag were removed 7 days later. The amniotic membrane remained in the uterine cavity, and a copper intrauterine device (IUD) was placed in the uterine cavity (Fig. 2). Group B (Foley balloon Day1+IUD Day7) containing 23 cases: after hysteroscopic adhesiolysis, the balloon was also placed in the uterine cavity, but it was not covered with the amniotic membrane. The balloon catheter was removed 7 days later and an IUD was placed in the uterine cavity.
Figure 1.
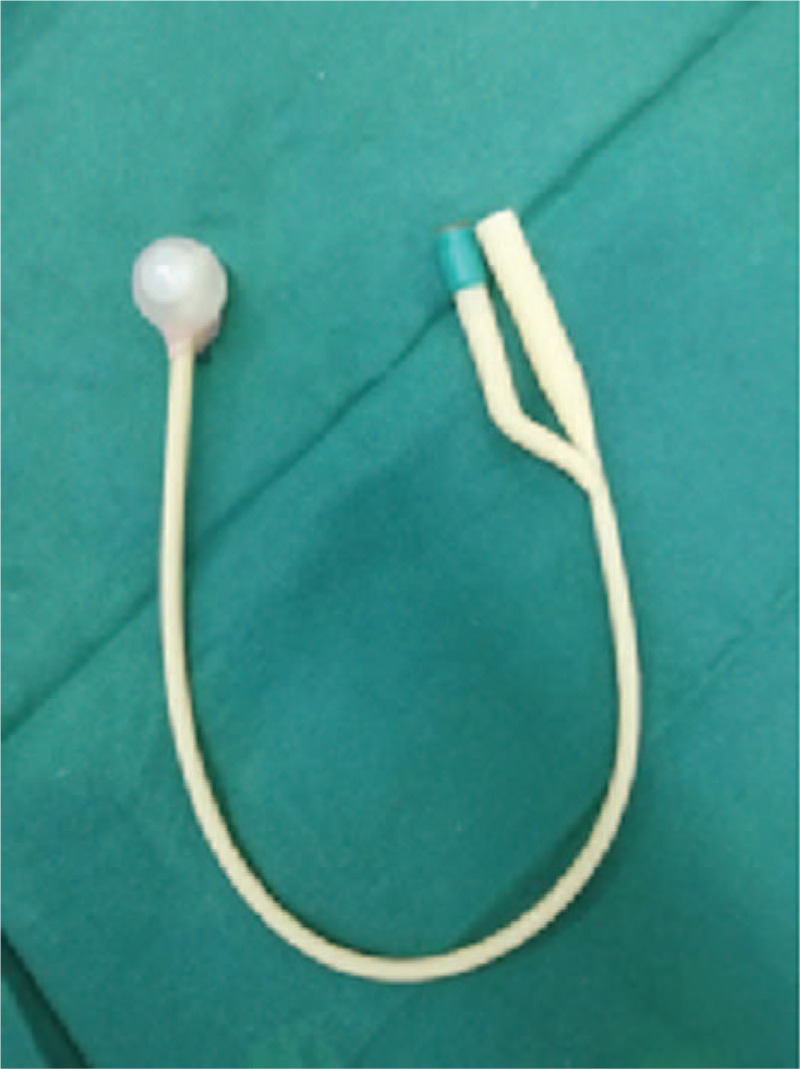
Foley catheter and amniotic membrane were placed in the uterine cavity right after the surgery (Note: Amniotic membrane was wrapped on the surface of the water-filled balloon).
Figure 2.

The balloon was removed on the 7th day postoperatively, and an IUD was placed in the uterine cavity.
4.3. Postoperative treatment
Patients in both group A and B began to take estradiol valerate 6 mg qd orally for 21 days started on the night of surgery. On the 12th day, dydrogesterone 10 mg bid was given to the patients orally until before the menstruation. The patients continued to take the second cycle of the medicines on the fifth day of the next menstrual cycle for a total of 3 cycles.
The patient received the second hysteroscopy 3 months later to observe the condition of the uterine cavity. Therapeutic efficacy evaluation criteria:[6,7]
-
1.
Cured: Hysteroscopy showed normal uterine cavity morphology, and bilateral uterine horn and the opening of the fallopian tube were visible.
-
2.
Improved: The morphology of the uterine cavity was mostly normal, but part of the adhesions remained, and 1 or both uterine horns were not visible during hysteroscopy.
-
3.
Invalid: There was no significant improvement or re-adhesions occurred after hysteroscopic adhesiolysis.
The treatment was considered effective in cured patients and patients with improved conditions. Those who did not respond to the treatment were subjected to the second hysteroscopic adhesiolysis. The improvement of menstruation was recorded. The criteria for improvement were that the amenorrhea was cured and the menstruation was recovered or menstrual flow was increased compared with that before the surgery; while no improvement referred to that the menstruation was not recovered or menstrual flow was not obviously increased.[6,7] On the 14th day of the menstrual cycle, transvaginal ultrasound was performed to monitor the thickness of the endometrium. The time of transvaginal ultrasound for patients with amenorrhea was unrestricted. The endometrial thickness in patients with intermittent lower abdominal pain was monitored on the 14th day of abdominal pain.
4.4. Statistical analysis
SPSS 20.0 statistical analysis software was used for data analysis. Measured data were expressed as mean ± standard deviation (x ± s) and analyzed using t test. The count data were expressed as the rate and analyzed using the χ2 test. P < .05 was considered statistically significant.
5. Results
-
1.
Comparison of general clinical data between the 2 groups of patients
Group A had an average age of 31.64 ± 3.52 years, BMI of 22.3 ± 3.4, the number of uterine surgery 3, average pregnancy times of 3, amenorrhea of 4 cases, hypomenorrhea of 18 cases, intermittent lower abdominal pain of 5 cases, history of infertility of 4 cases. Group B had an average age of 31.70 ± 3.30 years, BMI of 23.1 ± 1.7, the number of uterine surgery 3, average pregnancy times of 3, amenorrhea of 6 cases, hypomenorrhea of 17 cases, intermittent lower abdominal pain of 7 cases, history of infertility of 6 cases. There were no significant differences between the 2 groups in terms of age, BMI (Body Mass Index), the number of uterine surgeries, preoperative menstrual changes, intermittent lower abdominal pain, history and type of infertility (P > .05) (Table 1), and the sub group analysis for of severe and moderate intrauterine adhesions cases are included in Table 2 and Table 3.
-
2.
Comparison of intraoperative observation between the 2 groups of patients
The average operation time in group A was 35.02 ± 4.82 minutes and the estimated blood loss was 27.32 ± 3.28 ml. The average operation time in group B was 34.86 ± 5.12 minutes and the estimated blood loss was 28.18 ± 3.12 ml.
There was no significant difference in operative time and estimated blood loss between the 2 groups of patients (P > .05) (Table 4).
-
3.
Comparison of clinical efficacy of the procedure between the 2 groups of patients
All patients received the second hysteroscopy 3 months later. In group A, the procedure was effective in 17 patents and ineffective in 5 patients, with an effective rate of 77.3%. The Group B procedure was effective in 11 patients and ineffective in 12 patients, with an effective rate of 47.8%. The efficacy of hysteroscopic procedure in group A was significantly higher than that in group B (P < .05) (Table 5).
-
4.
Comparison of the improvement in menstruation between group A and B
After 3 months of treatment, menstruation was improved in 18 patients in group A and no improvement was observed in 4 patients, with an improvement rate of 81.8%. In group B, improvement was observed in 11 cases and no improvement was observed in 12 cases, with an improvement rate of 47.8%. The menstruation improvement rate in group A was significantly higher than that of group B (P < .05) (Table 6).
-
5.
Comparison of the preoperative and postoperative endometrial thickness in group A and B
The preoperative and postoperative endometrial thickness were determined by transvaginal ultrasound in both group A and B.
Table 1.
Comparison of general clinical data between group A and B.
| Average | Menstrual changes (n) | ||||||||
| Group | Case (n) | Age (x ± s years) | BMI | The number of curettage (n)∗ | pregnancy times(n)∗ | Amenorrhea | Hypomenorrhea | Intermittent lower abdominal pain (n) | History of infertility |
| Group A | 22 | 31.64 ± 3.52 | 22.3 ± 3.4 | 3 | 3 | 4 | 18 | 5 | 4 |
| Group B | 23 | 31.70 ± 3.30 | 23.1 ± 1.7 | 3 | 3 | 6 | 17 | 7 | 6 |
| P | 1.034 | 1.018 | 1.283 | 0.965 | 1.174 | 1.283 | |||
Table 2.
Analysis for severe intrauterine adhesions case.
| Average | Menstrual changes (n) | |||||||
| The number of severe intrauterine adhesions (n) | Age (x ± s years) | BMI | The number of curettage (n)∗ | pregnancy times(n)∗ | Amenorrhea | Hypomenorrhea | Intermittent lower abdominal pain (n) | History of infertility |
| 10 | 30.43 ± 3.61 | 22.7 ± 2.8 | 6 | 3 | 8 | 6 | 7 | 6 |
Table 3.
Analysis for moderate intrauterine adhesions case.
| Average | Menstrual changes (n) | |||||||
| The number of moderate intrauterine adhesions (n) | Age(x ± s years) | BMI | The number of curettage (n)∗ | pregnancy times(n)∗ | Amenorrhea | Hypomenorrhea | Intermittent lower abdominal pain (n) | History of infertility |
| 35 | 31.76 ± 4.28 | 23.2 ± 3.7 | 3 | 3 | 2 | 29 | 5 | 4 |
Table 4.
Comparison of the operative time and estimated blood loss between group A and B.
| Group | Case (n) | The operative time (min) | Estimated blood loss (ml) |
| Group A | 22 | 35.02 ± 4.82 | 27.32 ± 3.28 |
| Group B | 23 | 34.86 ± 5.12 | 28.18 ± 3.12 |
| p | 0.937 | 1.053 |
Table 5.
Comparison of clinical efficacy of operative hysteroscopy between group A and B.
| Efficacy of operative hysteroscopy | ||||
| Group | Case (n) | Effective (n) | Invalid (n) | Effective rate (%) |
| Group A | 22 | 17 | 5 | 77.3 |
| Group B | 23 | 11 | 12 | 47.8 |
| χ2 | 4.148 | |||
| p | 0.042 | |||
Table 6.
Comparison of the improvement in menstruation between group A and B.
| Improvement in menstruation | ||||
| Group | Case (n) | Improved (n) | No improvement (n) | Improvement rate (%) |
| Group A | 22 | 18 | 4 | 81.8 |
| Group B | 23 | 11 | 12 | 47.8 |
| χ2 | 4.284 | |||
| p | 0.038 | |||
Endometrial thickness in group A was 6.36 ± 1.40 mm before treatment, and 11.64 ± 1.69 mm after treatment. Endometrial thickness in group B was 6.48 ± 1.47 mm before treatment, and 9.74 ± 1.54 mm after treatment. The postoperative endometrium of both groups was significantly thicker than that before the surgery (P < .05). Comparison of the postoperative endometrial thickness between group A and B showed that the postoperative endometrium of group A was significantly thicker than that of group B (P < .05) (Table 7).
Table 7.
Preoperative and postoperative endometrial thickness in group A and B.
| Group | Preoperative thickness (mm) | Postoperative thickness (mm) | t value | P value |
| Group A (n = 22) | 6.36 ± 1.40 | 11.64 ± 1.69 | −26.447 | 0.000 |
| Group B (n = 23) | 6.48 ± 1.47 | 9.74 ± 1.54 | −18.094 | 0.000 |
| t | −0.262 | 3.848 | ||
| p | 0.795 | 0.000 |
6. Discussion
Common causes of intrauterine adhesions include curettage for abortion, postpartum hemorrhage, and hysteromyomectomy.[8,9] The occurrence of intrauterine adhesions is mainly related to uterine cavity operation. More than 90% of intrauterine adhesions are associated with curettage, and the second influencing factor is mainly infection.[6] Intrauterine adhesion-caused menstrual abnormalities, pelvic pain, infertility, and repeated abortions seriously affect the physical and mental health of women. The goal of the treatment of intrauterine adhesions is to remove adhesions, rebuild the morphology of the uterine cavity, restore uterine cavity size, repair uterine function, and increase the chance of pregnancy.[10,11] Hysteroscopy is currently the gold standard for the diagnosis and treatment of intrauterine adhesions. Hysteroscope provides a good field of vision for direct observation of the uterine cavity, thereby adhesions can be processed under direct view.[12]
The treatment of intrauterine adhesions is mainly through the combination treatment of hysteroscopic adhesiolysis, antiadhesion treatment, and endometrial regeneration and repair.[13,14] The application of estrogen after hysteroscopic adhesiolysis is beneficial for the repair of uterine wound, promotes endometrial regeneration, increases endometrial thickness and the volume of uterine cavity, and reduces the risk of recurrence of intrauterine adhesions.[15] However, how to effectively prevent the reformation of intrauterine adhesions after surgery is a hot issue in the treatment of intrauterine adhesions.
With the continuous development of biological science and technology, the methods commonly used in clinical prevention of re-adhesion include postoperative intrauterine injection of antiadhesion gel, intrauterine placement of IUD, balloon, amniotic membrane, and various biological antiadhesion membranes.[3,16,17] However, there are no standard guidelines for the prevention of the reformation of adhesions. Most clinicians empirically select 1 or 2 methods to prevent intrauterine adhesions based on the medical conditions of their hospitals and patient's conditions.
Placement of the IUD is a commonly used method to prevent re-adhesion of the uterine cavity. It helps to maintain the relative separation of the uterine cavity, but may lead to local inflammatory stimuli.[8] In addition, due to the limited contact area between the IUD and the endometrium, the anteroposterior wall and the lateral wall of the uterus cannot be completely isolated, thus the blank part outside the IUD is easy to re-adhere.
Water-filled balloon has high plasticity. The surface of the balloon can tightly attach to the endometrium, keeping the endometrial surface of the uterus fully separated. Therefore, the balloon can well isolate the anterior and posterior walls of the uterine cavity. The balloon catheter drains the intrauterine fluid and facilitates the repair of the endometrium. However, long-term placement of the balloon in the uterine cavity may cause infection. After the removal of the balloon, adhesions may reform in the uterine cavity.
Amniotic membrane is differentiated from trophoblast cells and contains a variety of biological active ingredients. It acts as a scaffold and a biological barrier to inhibit inflammatory reactions, fibrosis and scar formation, and promote cell growth. Amniotic epithelial cells do not express HLA antigens on the surface. Thus, almost no rejection occurs after the transplantation of amniotic membrane. Amniotic membrane contains a large number of mesenchymal stem cells, has multidirectional differentiation potential, and can promote the repair of endometrium.[10]
In this study, 2 methods were combined to prevent re-adhesion after hysteroscopic adhesiolysis of moderate-to-severe intrauterine adhesions, and Foley balloon and IUD were used sequentially using for the prevention of re-adhesion. In group A, after the removal of the intrauterine indwelling balloon 1 week after the surgery, the amniotic tissue remained in the uterine cavity to continue to exert its biological effects. The subsequently placed IUD was compatible with the morphology of the uterine cavity and it acts together with the remaining amniotic membrane in the uterine cavity to greatly reduce the chance of re-adhesion between the uterus walls after the balloon was removed. This antiadhesion treatment increased the time during which the endometrium was separated and allowed time for the postoperative artificial periodic use of estrogen to promote the repair of the endometrium. The results showed that there were statistically significant differences (P < .05) in improvement of menstruation and endometrial thickening between group A and B at the second hysteroscopy 3 months after the surgery, indicating that the amniotic membrane-mediated sequential double-barrier method is clinically feasible for preventing recurrent intrauterine adhesions and has certain antiadhesion effect. However, Foley balloon catheter is placed for too long, patients will be discomfort and pelvic infection. After the balloon is filled with water, the pressure in the uterine cavity is not easy to be controlled. The balloon compresses the uterine wall, causing endometrial ischemia and affecting endometrial repair.
7. Conclusion
In summary, the treatment plan for preventing re-adhesion after hysteroscopic adhesiolysis of moderate-to-severe intrauterine adhesion is diversified. Clinicians need to constantly improve the treatment plan and select better method based on patients’ symptoms. The effects of various barrier therapies on long-term outcomes of patients such as pregnancy outcomes require further follow-up observation. In the future, the sample size needs to be increased to further confirm the effectiveness and practicality of each treatment.
Author contributions
Conceptualization: Chaoying Wu.
Data curation: Chaoying Wu, Yishan Dong, Yong Li, Hefang Liu.
Formal analysis: Chaoying Wu, Yishan Dong, Yong Li, Hefang Liu.
Investigation: Chaoying Wu.
Resources: Yishan Dong, Yong Li.
Software: Yishan Dong, Yong Li.
Validation: Chaoying Wu, Yishan Dong.
Writing – original draft: Chaoying Wu.
Writing – review & editing: Chaoying Wu.
Footnotes
Abbreviations: BMI = Body Mass Index, HLA = human leukocyte antigen, IUD = intrauterine device.
How to cite this article: Wu C, Dong Y, Li Y, Liu H. The efficacy of amniotic membrane-mediated sequential double-barrier therapy for the treatment of postoperative intrauterine adhesions. Medicine. 2021;100:15(e25416).
The authors have received no funding for this article.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update 2014;20:262–78. [DOI] [PubMed] [Google Scholar]
- [2].Healy MW, Schexnayder B, Connell MT, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol 2016;215:267–75. e7. [DOI] [PubMed] [Google Scholar]
- [3].Chen Y, Liu L, Luo Y, et al. Effects of aspirin and intrauterine balloon on endometrial repair and reproductive prognosis in patients with severe intrauterine adhesion: a prospective cohort study. Biomed Res Int 2017;2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang JH, Chen CD, Chen SU, et al. The influence of the location and extent of intrauterine adhesions on recurrence after hysteroscopic adhesiolysis. BJOG 2016;123:618–23. [DOI] [PubMed] [Google Scholar]
- [5].The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies and intrauterine adhesions. Fertil Steril 1988;49:944–55. [DOI] [PubMed] [Google Scholar]
- [6].Xiao S, Wan Y, Xue M, et al. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet 2014;125:121–4. [DOI] [PubMed] [Google Scholar]
- [7].Hanstede MM, van der Meij E, Goedemans L, et al. Results of centralized Asherman surgery, 2003-2013. Fertil Steril 2015;104:1561–8. [DOI] [PubMed] [Google Scholar]
- [8].Lin X, Wei M, Li TC, et al. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: a cohort study. Eur J Obstet Gynecol Reprod Biol 2013;170:512–6. [DOI] [PubMed] [Google Scholar]
- [9].Ugboaja JO, Oguejiofor CB, Igwegbe AO. Clinico-hysteroscopic analysis of severe intrauterine adhesions among Nigerian infertile women. Pan Afr Med J 2017;28:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gan L, Duan H, Xu Q, et al. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 2017;19:603–16. [DOI] [PubMed] [Google Scholar]
- [11].Xu W, Zhang Y, Yang Y, et al. Effect of early second-look hysteroscopy on reproductive outcomes after hysteroscopic adhesiolysis in patients with intrauterine adhesion, a retrospective study in china. Int J Surg 2018;50:49–54. [DOI] [PubMed] [Google Scholar]
- [12].Baradwan S, Shafi D, Baradwan A, et al. The effect of endometrial thickness on pregnancy outcome in patients with Asherman's syndrome post-hysteroscopic adhesiolysis. Int J Womens Health 2018;10:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hooker AB, de Leeuw R, van de Ven PM, et al. Prevalence of intrauterine adhesions after the application of hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: short-term outcomes of a multicenter, prospective randomized controlled trial. Fertil Steril 2017;107:1223–31. e3. [DOI] [PubMed] [Google Scholar]
- [14].Yan Y, Xu D. The effect of adjuvant treatment to prevent and treat intrauterine adhesions: a network meta-analysis of randomized controlled trials. J Minim Invasive Gynecol 2018;25:589–99. [DOI] [PubMed] [Google Scholar]
- [15].Guo J, Li TC, Liu Y, et al. A prospective, randomized, controlled trial comparing two doses of oestrogen therapy after hysteroscopic adhesiolysis to prevent intrauterine adhesion recurrence. Reprod Biomed Online 2017;35:555–61. [DOI] [PubMed] [Google Scholar]
- [16].Bosteels J, Weyers S, D’Hooghe TM, et al. Anti-adhesion barrier gels following operative hysteroscopy for treating female infertility: a systematic review and meta-analysis. Gynecol Surg 2014;11:113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Di Spiezio Sardo A, Calagna G, Scognamiglio M, et al. Prevention of intrauterine post-surgical adhesions in hysteroscopy. A systematic review. Eur J Obstet Gynecol Reprod Biol 2016;203:182–92. [DOI] [PubMed] [Google Scholar]


