Abstract
Background:
Obstructive sleep apnea (OSA) is correlated with atrial fibrillation (AF). Over the past decade, there has been an increasing interest in the relationship between OSA with continuous positive airway pressure (CPAP) and progression or recurrence of AF.
Methods:
This investigation was an analysis of studies searched in the Cochrane Library, PubMed, EMBASE, EBSCO, OVID, and Web of Science databases from inception to July 2020 to evaluate the recurrence or progression of AF in CPAP users, CPAP nonusers, and patients without OSA.
Results:
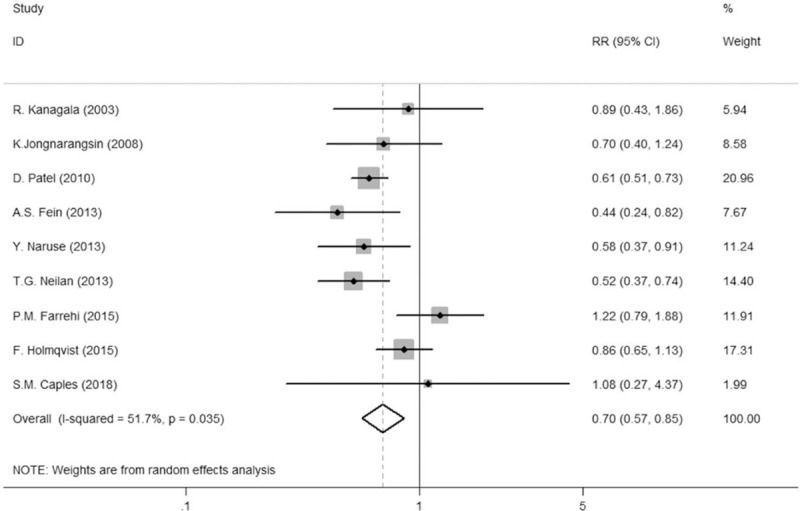
Nine studies with 14,812 patients were recruited. CPAP therapy reduced the risk of AF recurrence or progression by 63% in a random-effects model (24.8% vs 40.5%, risk ratio [RR] = 0.70, 95% confidence interval [CI] = 0.57–0.85, P = .035). Compared with non-OSA patients, AF recurrence or progression was much higher in CPAP nonusers (40.6% vs 21.1%, RR = 1.70, 95% CI = 1.19–2.43, P = .000). However, AF recurrence or progression in the CPAP group was similar to that in the non-OSA group (24.0% vs 21.1%, RR = 1.13, 95% CI = 0.87–1.47, P = .001). Begg correlation test and Egger regression test revealed no publication bias in this analysis.
Conclusions:
OSA is a salient factor in the progression or recurrence of AF. CPAP therapy for OSA may contribute to reduction of AF in patients for whom radiofrequency ablation or direct current cardioversion is not performed.
Trial Registration:
The protocol for this meta-analysis was registered on PROSPERO with a registration No. CRD42019135229.
Keywords: atrial fibrillation, continuous positive airway pressure, meta-analysis, obstructive sleep apnea
1. Introduction
Atrial fibrillation (AF) is a highly prevalent arrhythmia that may induce severe cardiovascular outcomes.[1–3] Epidemiological studies have showed that obstructive sleep apnea (OSA) is associated with an increased incidence and progression of coronary heart disease,[4] heart failure,[5] stroke,[6] and AF.[7] Recent studies strongly demonstrate that OSA could be an independent factor associated with AF.[8,9] The most common pathophysiological mechanisms between OSA and AF have been explored, such as hypoxia due to apnea, intrathoracic pressure changes, sympathetic nerve maladjustment, atrial remodeling, oxidative stress, systematic inflammation, vascular dysfunction, and neurohumoral activation.[10–12] Compared with patients without OSA, those with OSA are highly at high risk of developing AF and vice versa.[13] Currently, antiarrhythmic drugs and catheter ablation are primary treatments feasible for maintaining the sinus rhythm in patients with AF. However, other means to reverse AF to sinus rhythm are not generally acknowledged. Continuous positive airway pressure (CPAP) therapy has become the principal treatment for OSA since 1981.[14] However, it remains disputable whether CPAP should be preferred for treatment of AF.[15,16] This is largely because CPAP reverses atrial remodeling in patients with AF. Furthermore, platelet–lymphocyte ratio was considered as an independent indicator of cardiovascular disease (CVD) in OSA syndrome. CPAP treatment may impact platelet parameters and phenotype.[17] Nonetheless, the studies reporting such findings were usually single-center experiences with a small patient sample and divergent outcomes. A meta-analysis investigating the association between CPAP therapy for AF and OSA should therefore be conducted.
2. Methods
Relevant articles published in the Cochrane Library, PubMed, EMBASE, EBSCO, OVID, and Web of Science databases from inception to July 2020 were searched. Key words and related medical subject headings were searched for obstructive sleep apnea, atrial fibrillation, and continuous positive airway pressure. We also retrieved entry terms such as sleep apnea hypopnea syndrome, upper airway resistance sleep apnea syndrome, CPAP, airway pressure release ventilation, and airway pressure release ventilation mode. There was no language or other restrictions. Reference lists of reviews and relevant articles were also manually searched. In addition, we tried to email the researchers for potential original data. Detailed search strategy was shown in (Table S1, Supplemental Digital Content). Since all data were obtained from published articles, ethical approval was not necessary.
The following studies were included: randomized controlled trials (RCT), retrospective or prospective cohort studies, or case-control studies; studies comparing CPAP users with nonusers regarding OSA with AF; trials enrolling human participants aged >18 years diagnosed with OSA based on polysomnography or other criteria; and articles with data on OSA.
The following studies were excluded: trials that focused only on central sleep apnea, trials that did not report arrhythmia measures before and after CPAP usage, and data from unpublished studies available as abstracts. Recurrence or progression of AF was considered as the outcome measure. The outcome was not restricted to any specific follow-up period.
2.1. Data abstraction and quality assessment
Structured data obtained from each study included the title, name of the first author, publication year, country where the research was conducted, demographic, and characteristic data of participants, exposure measurement, methods used to identify AF, OSA definition, and CPAP application. Progression of AF type was defined as paroxysmal AF at baseline (or “first detected/new onset”AF becoming paroxysmal AF at the subsequent follow-up) becoming persistent or permanent at the last follow-up or persistent AF at baseline (or “first detected/new onset” AF becoming persistent AF at next available follow-up) becoming permanent at the last follow-up.[18] The Newcastle–Ottawa scale was used to determine the quality of each study. Two investigators extracted data and appraised the study quality. Inconsistencies in any article retrieved were resolved through discussion with a senior investigator.
2.2. Statistical analysis
All statistical analyses were performed using Stata version 12.0 (Stata Corporation, College Station, TX). Heterogeneity among studies was assessed using Cochran Q test and expressed with the I2 value. A P value of the Q test of <.10 was considered statistically significant. A random-effect model was employed according to the DerSimonian–Laird method.[19] Publication bias was appraised using Begg correlation and Egger regression tests.[20] Meta-regression was performed to investigate the effect of various characteristics. Restricted estimation maximum likelihood (ReML) was conducted to evaluate the slope significance. Subgroup and sensitivity analyses were performed to adjust effect values.
The protocol for this meta-analysis was registered on PROSPERO with a registration No. CRD42019135229.
3. Results
3.1. Study samples
A total of 1225 citations were identified after merging the duplications; of these, 1142 were excluded. Therefore, 9 studies with 14,812 participants[15,16,18,21–26] that examined the correlation between CPAP treatment for OSA and the recurrence or progression of AF were included in the final analysis (Fig. 1). The majority of studies in this analysis involved radiofrequency ablation (RFA) and AF recurrence. However, one study applied medical management instead of CPAP usage as a rhythm-control strategy,[18] and whereas 2 selected direct current cardioversion of AF.[23,27] The study characteristics are presented in Table 1.
Figure 1.
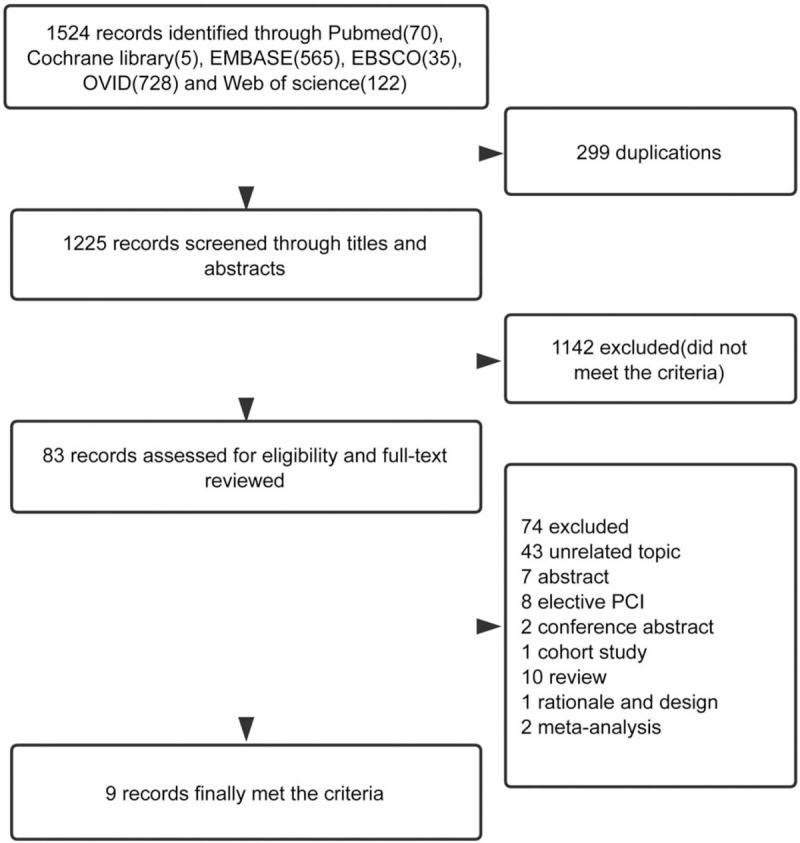
Flow chart of the meta-analysis.
Table 1.
Characteristics of the included studies.
| Researcher | Farrehi et al[21] | Fein et al[21] | Holmqvist et al[18] | K. Jongnarangsin et al[22] | Kanagala et al[23] | Naruse et al[24] | Neilan et al[16] | Patel et al [25] | Caples et al[27] |
| Method | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort | RCT |
| Year | 2015 | 2013 | 2015 | 2008 | 2003 | 2013 | 2013 | 2010 | 2018 |
| Race | American | American | American | American | American | Japanese | American | American | American |
| Name of magazine | J Interv Card Electrophysiol | J Am Coll Cardiol | Am Heart J | J Cardiovasc Electrophysiol | Circulation | Heart Rhythm | J Am Coll Cardiol | Circ Arrhythm Electrophysiol | Int J Cardiol |
| Total | 247 | 92 | 10132 | 324 | 118 | 153 | 720 | 3000 | 34 |
| OSA | 94 | 62 | 1841 | 32 | 39 | 116 | 142 | 640 | 25 |
| Age, y | 62.7 | 57.6 | 69 | 59 | 65 | 60 | 57 | 51 | 64.1 |
| Male% | 81 | 74 | 69 | 81 | 81 | 88 | 81 | 74 | 56 |
| CPAP% | 34 | 51.6 | 51 | 56 | 31 | 70.7 | 50 | 49.2 | 48 |
| PAF% | 39 | 46 | 50 | 72 | — | — | 36 | 40 | — |
| LAD, mm | 45.3 | 55.2 | 46 | 48 | — | 41.4 | 43 | 45 | — |
| BMI, kg/m2 | 34.1 | 29.2 | 34 | 35 | 37 | 25.4 | 32.5 | 31 | 35.9 |
| LVEF% | 58 | 59.9 | 72 | 51 | 52 | — | 56 | 49 | 57.7 |
| HTN% | 61 | 67.8 | 87 | 72 | 78 | 66 | 63 | 35 | — |
| DM% | — | 19.4 | 42 | — | 22 | 23 | 23 | 18 | — |
| Non-OSA | 153 | 30 | 8291 | 292 | 79 | 37 | 578 | 2360 | 9 |
| Age, y | 62.4 | 58.5 | 76 | 57 | 67 | 58 | 56 | 57 | — |
| Male% | 69 | 72 | 55 | 75 | 65 | 73 | 72 | 78 | — |
| PAF% | 56 | 46.7 | 51 | 72 | — | — | 34 | 57 | — |
| LAD, mm | 43.2 | 55.9 | 44 | 43 | — | 36.1 | 56 | 42 | — |
| BMI, kg/m2 | 29.2 | 29.6 | 28 | 29 | 30 | 23.5 | 29 | 26 | — |
| LVEF% | 56 | 59.5 | 70 | 56 | 48 | — | 56 | 54 | — |
| HTN% | 54 | 65.6 | 82 | 44 | 48 | 38 | 48 | 45 | — |
| DM% | — | 18.6 | 27 | — | 12 | 22 | 13 | 12 | — |
3.2. Study quality
All of the included studies were cohort studies: 5 prospective,[16,18,21,23,24] 3 retrospective, and 1 RCT.[15,22,25,27] Sample sizes were large in all but 2 studies (Holmqvist et al and Patel et al). All included studies were grade high quality by the Newcastle–Ottawa scale (Table 2). These 9 studies were involved in the analysis (Table 3).
Table 2.
Quality assessment.
| First author | Farrehi et al, 2015[21] | Fein et al, 2013[15] | Holmqvist et al, 2015[18] | Jongnarangsin et al, 2008[22] | Kanagala et al, 2003[23] | Naruse et al, 2013[24] | Neilan et al, 2013[16] | Patel et al, 2010[25] | Caples et al, 2018[27] |
| Study | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort | RCT |
| Country | USA | USA | USA | USA | USA | Japan | USA | USA | USA |
| Total no. | 247 | 92 | 10132 | 324 | 118 | 153 | 720 | 3000 | 34 |
| Radiofrequency mode of AF | PVI | PVI | No ablation performed | PVI + CFAE | No ablation performed | PVI + linear ablation of the left atrial roof or CFAE or superior vena cava isolation | PVI + linear ablation of the left atrial roof or CAFE or superior vena cava isolation | PVI + linear ablation of the left atrial roof or CAFE | DC cardioversion |
| OSA definition | STOP-BANG questionnaire and AHI >5/h | Polysomnography and AHI >15/h | Clinician-defined OSA | Polysomnography and AHI >5/h | Polysomnogra phy | Polysomnography and AHI >15/h | AASM criteria | Polysomnography and AHI >15/h | Polysomnography and AHI >5/h |
| Medical therapy | First RFA = 142 or repeated RFA = 105 or 32 PVI (+) OSA (+) CPAP (+) 62 PVI (+) OSA (+) CPAP (−) 153 PVI (+) OSA (−) | 32 PVI (+) OSA (+) CPAP (+) 30PVI (+) OSA (+) CPAP (−) 30PVI (+) OSA (−) 22 treated medically PVI (+) OSA (+) CPAP (+) | 1067 OSA (+) CPAP (+) 774OSA (+) CPAP (−) 8291OSA (−) | 18 RFA (+) OSA (+) CPAP (+) 14 RFA (+) OSA (+) CPAP (−) 292 RFA (+) OSA (−) | 12 OSA (+) CPAP (+) 27 OSA (+) CPAP (−) 79 OSA (−) | 82 PVI (+) OSA (+) CPAP (+) 34 PVI (+) OSA (+) CPAP (−) 37PVI (+) OSA (−) | 71 PVI (+) OSA (+) CPAP (+) 71 PVI (+) OSA (+) CPAP (−) 578 PVI (+) OSA (−) | 315 PVI (+) OSA (+) CPAP (+) 325 PVI (+) OSA (+) CPAP (−) 2360 PVI (+) OSA (−) | 12 OSA (+) PAP (+) 13 OSA (+) CPAP (−) |
| Follow-up | 522 days | 12 mo | 2 y | 7 mo | 12 mo | 18 mo | 42 mo | 32 mo | 12 mo |
| AF recurrence evaluation | ECG + autotriggered event monitor | ECG + transtelephonic monitoring | ECG | ECG + autotriggered event monitor | By a physician or ECG | ECG or Holter | ECG or prolonged cardiac monitoring | Event monitor or Holter | PAP device and ECG |
| Quality | 8 | 9 | 8 | 8 | 8 | 9 | 9 | 9 | Jadad 3 |
| method | Prospective | Retrospective | Prospective | Retrospective | Prospective | Prospective | Prospective | Retrospective | RCT |
Table 3.
Data analysis.
| Researcher | Year | Total | OSA | CPAP | non-CPAP | CPAP AF recurrence | Non-CPAP AF recurrence | Non-OSA | Non-OSA AF recurrence |
| Farrehi et al[21] | 2015 | 247 | 94 | 32 | 62 | 17 | 27 | 153 | — |
| Fein et al[15] | 2013 | 84 | 62 | 32 | 30 | 9 | 19 | 30 | 10 |
| Holmqvist et al[18] | 2015 | 10,132 | 1841 | 602 | 411 | 94 | 75 | 5349 | 984 |
| K. | |||||||||
| Jongnarangsin et al[22] | 2008 | 324 | 32 | 18 | 14 | 9 | 10 | 292 | 108 |
| Kanagala et al[23] | 2003 | 118 | 39 | 12 | 27 | 5 | 22 | 79 | 42 |
| Naruse et al[24] | 2013 | 153 | 116 | 82 | 34 | 25 | 18 | 37 | 8 |
| Neilan et al[16] | 2013 | 720 | 142 | 71 | 71 | 25 | 48 | 578 | 172 |
| Patel et al[25] | 2010 | 3000 | 640 | 315 | 325 | 105 | 178 | 2360 | 519 |
| Caples et al[27] | 2018 | 34 | 25 | 12 | 13 | 3 | 3 | 9 | — |
3.3. Heterogeneity test
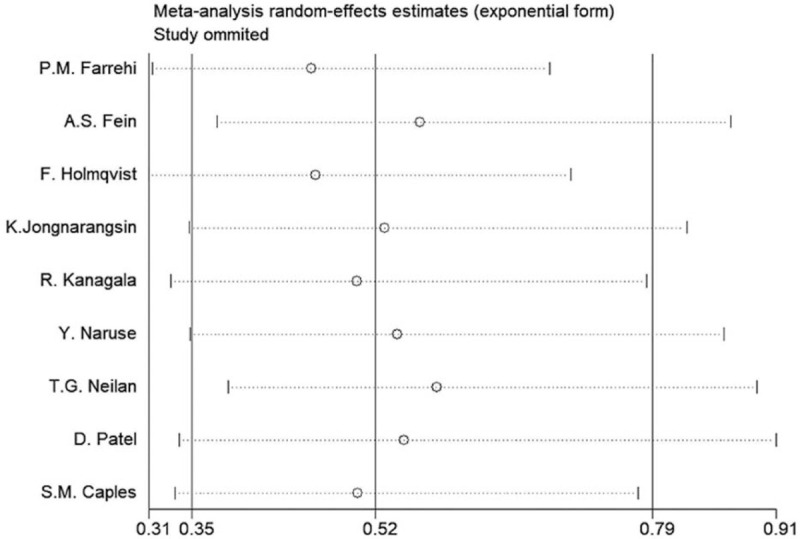
A significant heterogeneity was observed (Tau = 0.0465, P = .024, I2 = 56.6%, risk ratio [RR] = 0.69, 95% confidence interval [CI] = 0.56–0.85) (Fig. 2). A sensitivity analysis was conducted and showed that the study by Farrehi et al exerted the greatest heterogeneity to this meta-analysis (Fig. 3). This study was omitted, resulting in a decreased heterogeneity (Tau = 0.0131, P = .220, I2 = 27.3%, RR = 0.64, 95% CI = 0.54–0.76) (Figure S1, Supplemental Digital Content). The study by Farrehi et al was excluded because the population size of the CPAP group was smaller than that of the non-CPAP group, and the non-CPAP group included patients treated with 1 to 4 hours of CPAP per night. In addition, OSA was defined using the STOP-BANG questionnaire, which might not be accurate.
Figure 2.
Comparison of AF recurrence or progression in patients using a Forest plot. A pooled estimate of risk ratio (diamonds) and 95% confidence intervals (width of diamonds) summarizes the effect size using the random-effects model. 1 indicates no radiofrequency ablation, 2 indicates pulmonary venous isolation, and 3 indicates pulmonary venous isolation and complex fractionated atrial electrogram. CI = confidence interval, RR = risk ratio.
Figure 3.
Sensitivity analysis for the meta-analysis.
3.4. Publication bias
Nine studies were included to test the publication bias using the linear regression method and funnel plot (Figure S2, Supplemental Digital Content). Using Stata version 12.0, Egger bias yielded P = .884, and Begg test yielded P = .466, indicating no publication bias in this analysis.
3.5. Meta-regression
The meta-regression analysis based on the various characteristics such as study design, race, follow up, multiple factors, and disease condition besides radiofrequency mode did not impact the RR of AF recurrence or progression for the CPAP versus the non-CPAP group (Table 4).
Table 4.
Summary of the meta-analysis results.
| Random-effects model | Fixed-effects model | Heterogeneity | ||||||
| Analysis | N | Reference | RR (95%) | P | RR (95%) | P | I2 | Ph |
| Study design | 9 | 15–16,18, 21–26 | 0.52 | (.35–0.79) | 0.54 | (.45–.66) | 64.9% | 0.004 |
| Retrospective | 3 | 15,22,25 | 0.39 | (.29–0.53) | 0.39 | (.29–.53) | 0.0% | 0.574 |
| Prospective | 5 | 16,18,21, 23,24 | 0.62 | (.34–1.12) | 0.69 | (.53–.89) | 70.6% | 0.009 |
| RCT | 1 | 26 | 1.11 | (.18–6.97) | 1.11 | (.18–6.97) | — | — |
| Stage | 9 | |||||||
| Recurrence | 8 | 16,18, 21–26 | 0.47 | (.31–.71) | 0.43 | (.34–.55) | 47.6% | 0.064 |
| Progression | 1 | 18 | 0.83 | (.59–1.16) | 0.83 | (.59–1.16) | — | — |
| Therapy | 9 | |||||||
| No | 3 | 18,23,26 | 0.84 | (.61–1.15) | 0.84 | (.61–1.15) | 0.0% | 0.953 |
| PVI | 2 | 15,21 | 0.59 | (.10–3.71) | 0.71 | (.36–1.38) | 86.0% | 0.008 |
| PVI + CFAE | 4 | 16,22,24, 25 | 0.38 | (.29–.50) | 0.38 | (.29–.50) | 0.0% | 0.706 |
3.6. Heterogeneity test in subgroup analysis
In the subgroup analysis, 7 studies were used to compare the AF recurrence in the CPAP, non-CPAP, and non-OSA groups. The heterogeneity significantly decreased to zero (Tau P = .99, I2 = 0%) from P = .000, I2 = 91.9%, RR = 1.79, 95% CI = 1.19–2.43 between non-CPAP and non-OSA groups, after a subgroup analysis on radiofrequency mode (Fig. 4). The result of the heterogeneity test between the CPAP and non-OSA groups was significantly reduced to zero from Tau2 = 0.0752, P = .001, and I2 = 72.3% (Fig. 5; Fig. S3, Supplemental Digital Content), with a subgroup analysis on radiofrequency mode or AF stage. Given the wide variety of study designs, the random-effect model was therefore adopted in both comparisons.
Figure 4.
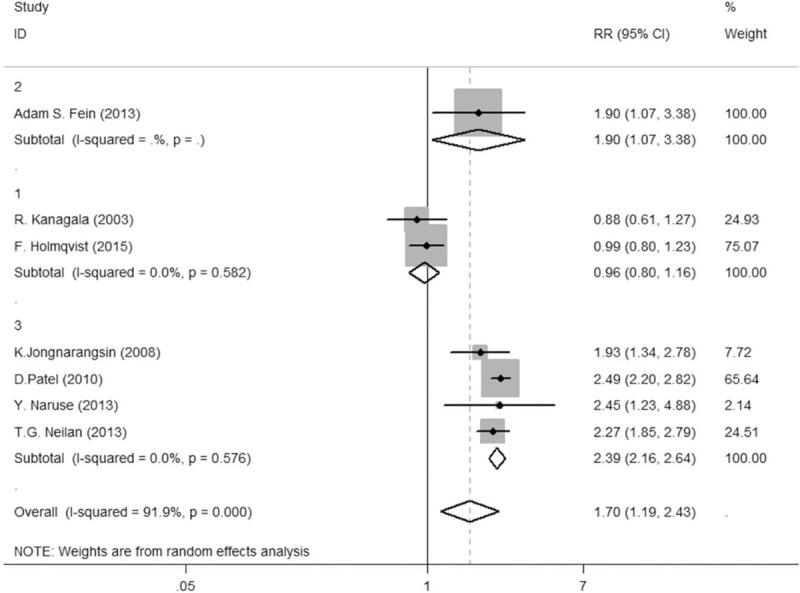
Comparison between CPAP group and non-CPAP group. CPAP = continuous positive airway pressure.
Figure 5.
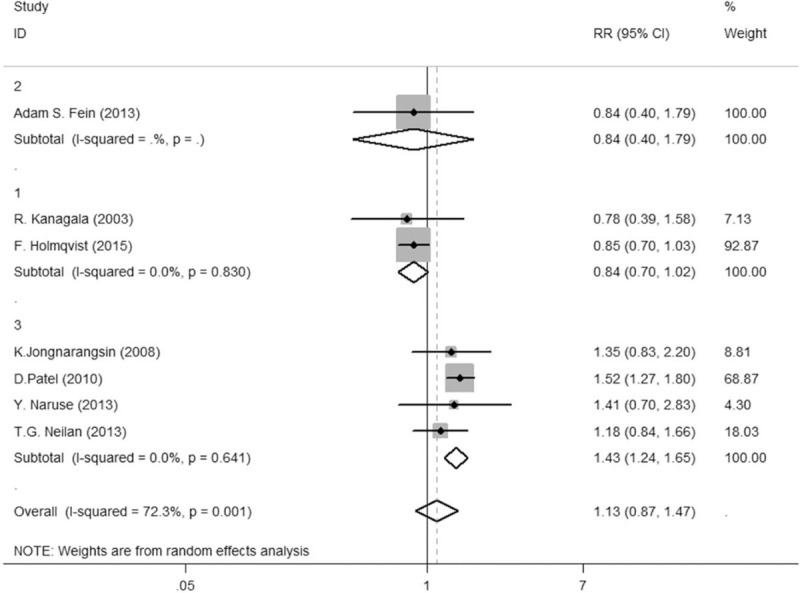
Comparison between non-CPAP group and non-OSA group. Figure 3.3 Comparison between CPAP group and non-OSA group. CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea.
3.7. Subgroup statistical analysis
Overall, 1176 patients who sustained CPAP treatment, 987 treated with a non-CPAP regimen and 8887 without OSA. A random-effect model was administered to calculate the RR values of the two groups and to draw forest plots and demonstrated that the AF recurrence in the CPAP group was lower than that in the non-CPAP group (24.8% vs 40.5%, RR = 0.70, P = .035, 95% CI = 0.57–0.85), but was significantly higher in the non-CPAP group than that in the non-OSA group (40.6% vs 21.1%, RR = 1.70, P = .000, 95% CI = 1.19–2.43), and the AF recurrence in the CPAP group was approximately higher than that in the non-OSA group (24.0% vs 21.1%, RR = 1.13, P = .001, 95% CI = 0.87–1.47).
3.8. Publication bias in subgroups
In the analysis of publication bias between the non-OSA and non-CPAP groups, Egger bias yielded P = .580 and Begg test yielded P = .548. In the analysis of publication bias between the non-OSA and CPAP groups, Egger bias yielded of P = .964 and Begg test yielded of P = .764. Therefore, no publication bias was observed in these subgroups.
4. Discussion
Our meta-analysis showed that AF recurrence in the CPAP group was lower than that in the non-CPAP group. Publication bias tests showed a reliable reduction in AF recurrence in patients with OSA and AF who received CPAP treatment. Similarly, the results revealed that AF recurrence in the non-CPAP group was significantly higher than that in the non-OSA group, indicating that patients with OSA who do not receive CPAP treatment have a higher possibility of having AF recurrence or progression than without OSA patients among all patients with AF. Conversely, AF recurrence was similar between the CPAP and non-OSA groups, with no significant difference between the 2 groups after combining the effect. The results of analysis of AF recurrence were similar between patients with OSA treated with CPAP as a regimen and patients without OSA. The findings show that patients with OSA have a 1.9-fold increased risk of AF recurrence compared with those without OSA. CPAP treatment for OSA is associated with a 0.6-fold reduction of AF recurrence or progression.
Emerging research highlights the complex interrelationships between sleep-disordered breathing and CVD, providing opportunities, and challenges for clinical research.[26] A previous meta-analysis focused on the association between CPAP treatment for OSA and recurrent AF after RFA.[28–31] CPAP treatment for OSA has been considered potentially beneficial for patients with AF even if RFA or direct current cardioversion is not performed. A majority of studies have evaluated AF recurrence after RFA. Patients with AF have an increased risk of OSA and those with OSA have an increased risk of AF.[32]
Some studies have identified possible pathophysiological mechanisms to explain the relationship between OSA and AF over the years. Oxidative stress seems to be one of the main causes of endothelial injury in OSA patients. Hypoxia may cause endothelial cell damage in patients with OSA. CPAP treatment could improve the clinical characteristics of patients, reduce the serum levels of nuclear factor-κB (NF-κB), hypoxia-inducible factor-1α (HIF-1α).[33] NF-κB is an essential initiator of inflammation and HIF-1α was the main factor to maintain the homeostasis of oxygen metabolism. Both can regulate gene transcription and posttranslational protein modification.[34,35] Intermittent hypoxia induced by repeated interruption of ventilation in rats can cause connexin disorders and atrial conduction abnormalities associated with atrial fibrosis.[36] The incidence of spontaneous atrial premature beats was shown to be significantly shortened due to obstructive respiratory events, representing an effective trigger factor for spontaneous AF in both OSA human and pig models.[37,38] Similar arrhythmogenic electrophysiological changes were noted in rat models of obesity and OSA.[39] Furthermore, repeated obstructive respiratory events may result in mechanical atrial dilatation, atrial wall expansion, intermittent hypoxemia, and hypercapnia, sympathetic nerve activation, and subsequent hemodynamic fluctuations during and after apnea. Interestingly, serum HIF-1α levels might have significant diagnostic and even prognostic value in both OSA and CVD including AF.[40,41] These factors also cause the causes of structural remodeling and myocardial injury. Atrial dilation might shorten the atrial instability, slow down conduction, and increase the number of interatrial conduction blockades in isolated Langendorf perfused rabbit hearts.[42] Negative fluctuation in intrathoracic pressure during upper respiratory inhalation in OSA induces changes in left atrial extension and transmural pressure gradients, particularly in the thin-walled atrium, through pressure, volumetric load, and sympathetic tone changes.[43] Chronic complications such as obesity and hypertension may play a key role in the progression of structural atrial matrix remodeling. Atrial electrical and structural remodeling are crucial factors in the AF pathogenesis.
CPAP is currently the best therapeutic treatment for moderate-to-severe OSA.[44,45] Systolic and diastolic abnormalities in patients with OSA may be reversed as early as 3 months after initiating of CPAP therapy, which increasingly ameliorates cardiovascular remodeling for >1 year.[46] CPAP therapy may promote more homogeneous atrial conduction in patients with OSA, which may mitigate the long-term risk of atrial arrhythmias.[47] Neilan et al[16] demonstrated that CPAP treatment is associated with lower blood pressure, LV mass, and LA size in patients with paroxysmal rather than persistent AF. Fein et al[15] showed that the effect of CPAP treatment in patients without RFA was similar to that of RFA in CPAP nonuser patients with OSA. Despite the findings by Holmqvist et al,[18] data on CPAP usage in similar registries were lacking, and the population with progressive AF in CPAP therapy was still found to be smaller than that without progressive AF. Fein et al's study[15] revealed no significant difference in the frequency of repeat ablations among CPAP users, CPAP nonusers, and patients without OSA. CPAP could ameliorate endothelial dysfunction and enhance the release of nitric oxide, an influential vasodilator in atherosclerosis and CVD development. Additionally, CPAP could mitigate the systemic inflammation and oxidative stress, another potential mechanism of CVD. A larger LA size is also associated with chronic AF progression, one of the predictors of surgical failure. All these factors may constitute the mechanisms of how CPAP treatment reduces recurrent AF exposure after catheter ablation.[48]
According to the current data, CPAP therapy may be associated with a reduced recurrence or progression in patients with AF without RFA. These results suggest that CPAP therapy reverses the electrical and/or structural remodeling associated with OSA, thereby decreasing the incidence of AF recurrence. Future studies investigating the effects of CPAP on atrial and ventricular remodeling in patients with AF should consider the clinical significance of these findings. CPAP therapy can provide homogeneous conduction in the atrium and ventricle, which might reduce the risk of atrial and ventricular arrhythmias in the long-term. To date, no study has compared the status of patients with AF before and after RFA who have undergone CPAP therapy and further research in this field is needed to confirm CPAP, OSA, AF, and RFA in future randomized controlled trials.
4.1. Limitations
The strength of this study is that we comprehensively searched multiple databases including the Cochrane Library, PubMed, EMBASE, EBSCO, and Web of Science. However, this review has some potential limitations. First, we only included observational studies rather than randomized studies, and our analysis demonstrated relevance other than causality. Second, the inclusion and exclusion criteria ablation strategies, types of AF, degrees of OSA, and even the existence of occult OSA differ among the included studies; these factors lead to heterogeneity in this meta-analysis. Nevertheless, subgroup and sensitivity analyses were performed to adjust for potential publication bias and confirm the outcome stability. In addition, a majority of the included patients were from the study by Holmqvist et al, which is the primary, original source of heterogeneity.
5. Conclusion
This meta-analysis found that OSA is a significant factor in the progression or recurrence of AF. CPAP therapy, which may be beneficial for patients with AF, may be a potential treatment for patients with AF besides medication, RFA, and direct current cardioversion.
Author contributions
MZ and WM designed the study. XYL and XBZ were involved in the selection of publications and data collection for the meta-analysis. XMX reviewed the selected studies. XYL, JD, CC, LM and JYL participated in data analysis. XYL and XBZ wrote the core manuscript, and all authors reviewed and approved of the final manuscript.
Conceptualization: Wei Mao, Min Zhu.
Data curation: Xinbin Zhou.
Formal analysis: Jin Dai, Chen Chen, Lan Ma, Jiaying Li.
Funding acquisition: Xinbin Zhou, Wei Mao.
Methodology: Jin Dai, Chen Chen, Lan Ma, Jiaying Li.
Resources: Xiaoming Xu.
Writing – original draft: Xinyao Li.
Writing – review & editing: Xinbin Zhou.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, CPAP = continuous positive airway pressure, CVD = cardiovascular disease, HIF-1α = hypoxia-inducible factor-1α, NF-κB = nuclear factor-κB, OSA = obstructive sleep apnea, ReML = restricted estimation maximum likelihood, RFA = radiofrequency ablation.
How to cite this article: Li X, Zhou X, Xu X, Dai J, Chen C, Ma L, Li J, Mao W, Zhu M. Effects of continuous positive airway pressure treatment in obstructive sleep apnea patients with atrial fibrillation: A meta-analysis. Medicine. 2021;100:15(e25438).
XL and XZ contributed the same in this study.
License to publish: This is an open access article licensed under a Creative Commons Attribution 4.0 International License.
Ethics approval and consent to participate: Not applicable because all data were obtained from published studies. No ethics approval was involved in this meta-analysis.
Consent for publication: Not applicable.
Availability of data and materials: All data are presented within the manuscript.
The authors report no conflicts of interests.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
BMI = body mass index, CPAP = continuous positive airway pressure, HTN = hypertension; DM, diabetes mellitus, LAD = left atrial diameter, LVEF = left ventricular ejection fraction, OSA = obstructive sleep apnea, PAF = paroxysmal atrial fibrillation.
AHI = apnea-hypopnea index, CFAE = complex fractionated Atrial electrogram, PVI = pulmonary venous isolation, RCT = randomized controlled trial, RFA = radiofrequency ablation.
AF = atrial fibrillation, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea.
References
- [1].Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- [2].Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet (London, England) 2005;365:1046–53. [DOI] [PubMed] [Google Scholar]
- [3].Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment. JAMA Cardio 2018;3:532–40. [DOI] [PubMed] [Google Scholar]
- [4].Peled N, Abinader EG, Pillar G, et al. Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol 1999;34:1744–9. [DOI] [PubMed] [Google Scholar]
- [5].Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- [6].Brown DL, Shafie-Khorassani F, Kim S, et al. Sleep-disordered breathing is associated with recurrent ischemic stroke. Stroke 2019;50:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Drager LF, McEvoy RD, Barbe F, et al. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation 2017;136:1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kendzerska T, Gershon AS, Atzema C, et al. Sleep apnea increases the risk of new hospitalized atrial fibrillation: a historical cohort study. Chest 2018;154:1330–9. [DOI] [PubMed] [Google Scholar]
- [9].Wong JK, Mariano ER, Doufas AG, et al. Preoperative treatment of obstructive sleep apnea with positive airway pressure is associated with decreased incidence of atrial fibrillation after cardiac surgery. J Cardiothor Vasc An 2017;31:1250–6. [DOI] [PubMed] [Google Scholar]
- [10].Linz D, Linz B, Hohl M, et al. Atrial arrhythmogenesis in obstructive sleep apnea: therapeutic implications. Sleep Med Rev 2016;26:87–94. [DOI] [PubMed] [Google Scholar]
- [11].May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest 2017;151:225–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tung P, Anter E. Atrial fibrillation and sleep apnea: considerations for a dual epidemic. J Atr Fibrillation 2016;8:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gorenek B, Pelliccia A, Benjamin EJ, et al. European heart rhythm association (EHRA)/european association of cardiovascular prevention and rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the heart rhythm society (HRS) and asia pacific heart rhythm society (APHRS). Europace 2017;19:190–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Owen JE, Benediktsdottir B, Gislason T, et al. Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep 2019;42:UNSzsy199. [DOI] [PubMed] [Google Scholar]
- [15].Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62:300–5. [DOI] [PubMed] [Google Scholar]
- [16].Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013;2:e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gabryelska A, Łukasik ZM, Makowska JS, et al. Obstructive sleep apnea: from intermittent hypoxia to cardiovascular complications via blood platelets. Front Neurol 2018;9:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 2015;169:647–54.e642. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JPTGS. Cochrane handbook for systematic reviews of interventions version 5 1 0 2011. [Google Scholar]
- [20].Easterbrook PJBJ, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet (London, England) 1991;337:867–72. [DOI] [PubMed] [Google Scholar]
- [21].Farrehi PM, Brien LMO, Bas HD, et al. Occult obstructive sleep apnea and clinical outcomes of radiofrequency catheter ablation in patients with atrial fibrillation. J Interv Card Electrophysiol 2015;43:279–86. [DOI] [PubMed] [Google Scholar]
- [22].Jongnarangsin K, Chugh A, Good E, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2008;58:668–72. [DOI] [PubMed] [Google Scholar]
- [23].Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589–94. [DOI] [PubMed] [Google Scholar]
- [24].Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10:331–7. [DOI] [PubMed] [Google Scholar]
- [25].Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010;3:445–51. [DOI] [PubMed] [Google Scholar]
- [26].Abumuamar AM, Mollayeva T, Sandor P, et al. Efficacy of continuous positive airway pressure treatment in patients with cardiac arrhythmia and obstructive sleep apnea: what is the evidence? Clin Med Insights Ther 2017;9:1–0. [Google Scholar]
- [27].Caples SM, Mansukhani MP, Friedman PA, et al. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol 2019;278:133–6. [DOI] [PubMed] [Google Scholar]
- [28].Li L, Wang ZW, Li J, et al. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace 2014;16:1309–14. [DOI] [PubMed] [Google Scholar]
- [29].Shukla A, Aizer A, Holmes D, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol 2015;1:41–51. [DOI] [PubMed] [Google Scholar]
- [30].Congrete S, Bintvihok M, Thongprayoon C, et al. Effect of obstructive sleep apnea and its treatment on atrial fibrillation recurrence after radiofrequency catheter ablation: a meta- analysis. J Evid Based Med 2018;11:145–51. [DOI] [PubMed] [Google Scholar]
- [31].Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol 2015;116:1767–73. [DOI] [PubMed] [Google Scholar]
- [32].Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49:565–71. [DOI] [PubMed] [Google Scholar]
- [33].Gabryelska A, Szmyd B, Szemraj J, et al. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J Clin Sleep Med 2020;16:1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lu D, Li N, Yao X, et al. Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Bosn J Basic Med Sci 2017;17:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gabryelska A, Szmyd B, Panek M, et al. Serum hypoxia-inducible factor-1α protein level as a diagnostic marker of obstructive sleep apnea. Pol Arch Intern Med 2020;130:158–60. [DOI] [PubMed] [Google Scholar]
- [36].Iwasaki YK, Kato T, Xiong F, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol 2014;64:2013–23. [DOI] [PubMed] [Google Scholar]
- [37].Linz DHM, Nickel A, Mahfoud F, et al. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension 2013;62:767–74. [DOI] [PubMed] [Google Scholar]
- [38].Linz DHM, Ukena C, Mahfoud F, et al. Obstructive respiratory events increase premature atrial contractions after cardioversion. Eur Respir J 2015;45:1332–40. [DOI] [PubMed] [Google Scholar]
- [39].Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012;9:1409–16.e1401. [DOI] [PubMed] [Google Scholar]
- [40].Gabryelska A, Białasiewicz P. Hitting two birds with one stone: the potential role of serum hypoxia-inducible factor-1α protein levels in obstructive sleep apnea-related cardiovascular disease. Authors’ reply. Pol Arch Intern Med 2020;130:162. [DOI] [PubMed] [Google Scholar]
- [41].Patoulias D, Katsimardou A, Kalogirou MS, et al. Hitting two birds with one stone: the potential role of serum hypoxia-inducible factor-1α protein levels in obstructive sleep apnea-related cardiovascular disease. Pol Arch Intern Med 2020;130:161–2. [DOI] [PubMed] [Google Scholar]
- [42].Ravelli FAM. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation 1997;96:1686. [DOI] [PubMed] [Google Scholar]
- [43].Anter E, Di Biase L, Contreras- Valdes FM, et al. Atrial substrate and triggers of paroxysmal atrial fibrillation in patients with obstructive sleep apnea. Circ Arrhythm Electrophysiol 2017;10:e005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lorenzi-Filho G, Almeida FR, Strollo PJ. Treating OSA: current and emerging therapies beyond CPAP. Respirology 2017;22:1500–7. [DOI] [PubMed] [Google Scholar]
- [45].Aslan G, Afsar B, Siriopol D, et al. Cardiovascular effects of continuous positive airway pressure treatment in patients with obstructive sleep apnea: a meta-analysis. Angiology 2018;69:195–204. [DOI] [PubMed] [Google Scholar]
- [46].Goudis CA, Ketikoglou DG. Obstructive sleep and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Int J Cardiol 2017;230:293–300. [DOI] [PubMed] [Google Scholar]
- [47].Bayir PT, Demirkan B, Bayir O, et al. Impact of continuous positive airway pressure therapy on atrial electromechanical delay and P-wave dispersion in patients with obstructive sleep apnea. Ann Noninvasive Electrocardiol 2014;19:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bradley TDHM, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest 2001;119:1827–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


