Abstract
Objective:
The present study aimed to conduct a systematic review and meta-analysis to evaluate the relationships between ATP2B1 gene polymorphisms with blood pressure (BP) level and susceptibility to hypertension.
Methods:
PubMed, Web of Science, Embase and China National Knowledge Infrastructure (CNKI) Databases were systematically searched by 2 independent researchers to screen studies on ATP2B1 gene polymorphisms and BP related phenotypes. The records retrieval period was limited from the formation of the database to March 4, 2021. Pooled odds rations (ORs) or β and their 95% confidence intervals (95%CI) were calculated to assess the association between ATP2B1 gene polymorphisms and the risk of hypertension or BP levels. Publication bias and sensitivity analysis were conducted to find potential bias. All the statistical analysis were conducted with Stata version 11.0 software.
Results:
A total of 15 articles were ultimately included in the present study, including 15 polymorphisms of ATP2B1 gene. Nine articles (N = 65,362) reported the polymorphism rs17249754, and 7 articles(N = 91,997) reported rs2681472 (both loci were reported in 1 article). Meta-analysis showed that rs17249754 (G/A) and rs2681472 (A/G) were associated with the susceptibility to hypertension (rs17249754: OR = 1.19, 95%CI: 1.10–1.28; rs2681472: OR = 1.15, 95%CI: 1.12–1.17), and were positively associated with systolic BP (SBP) and diastolic blood pressure (DBP) (rs17249754: SBP, β=1.01, 95%CI: 0.86–1.16, DBP, β=0.48, 95%CI: 0.30–0.66; rs2681472: SBP, β=0.92, 95%CI: 0.77–1.07, DBP, β=0.50, 95%CI: 0.42–0.58) in the additive genetic model. Subgroup analysis stratified by race, population, sample size, and BP measurement method revealed that the association between A allele in rs2681472 polymorphism and risk of hypertension was slightly stronger in European (EUR) populations (OR = 1.16, 95%CI: 1.13–1.20) than in East Asians (OR = 1.14, 95%CI: 1.10–1.17). While in East Asians, relation between rs17249754 with risk of hypertension (OR = 1.19, 95%CI: 1.10–1.28) is stronger than rs2681472 (OR = 1.14, 95%CI: 1.10–1.17).
Conclusions:
Our study demonstrated that ATP2B1 gene polymorphism rs2681472 and rs17249754 were associated with BP levels and the susceptibility to hypertension.
Keywords: ATP2B1, gene, hypertension, meta-analysis, polymorphism
1. Introduction
Hypertension, defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg, is one of the most common cardiovascular diseases.[1] According to the Global Burden of Disease Study 2019, the number of deaths attributed to high SBP was 10.8 million.[2] Hypertension is a multifactorial disease caused by genetic and environmental factors, interactions between genes, and interactions between genes and acquired environmental factors.[3] Besides being a major risk factor for stroke, coronary heart disease, congestive heart failure, and chronic kidney disease, hypertension is one of the most important risk factors for morbidity and death.[4,5] Primary hypertension is a common but complex disease influenced by genetic, environmental, and lifestyle factors,[6,7] including age, obesity, and poor lifestyle (including high salt intake, smoking, alcohol consumption, and lack of physical activity).[5,8–12] Genetic factors play an important role in the development of hypertension and blood pressure (BP) levels; the heredity of BP is estimated to be approximately 40% to 60%.[13] Therefore, it is important to demonstrate the relationship between genetic mutations and BP phenotypes.
ATP2B1 is one of the most studied genes associated with the risk of hypertension or BP.[14–16]ATP2B1 is located on chromosome 12q21.3, encoding plasma membrane Ca2+-ATPase isoform 1 (also known as PMCA1), which is ubiquitous in tissues and transports Ca2+ into the cell, maintaining the normal intracellular Ca2+ level.[17,18] With the great achievements in hypertension-related genome-wide association studies (GWAS), many genetic risk factors for hypertension have been identified, including single nucleotide polymorphisms (SNPs) in the ATP2B1 gene. A large meta-analysis of GWAS performed by the Cohorts for Heart and Aging Research in Genome Epidemiology (CHARGE) consortium found that ATP2B1 polymorphism rs2681472 is significantly related to SBP, DBP, and risk of hypertension in Europe.[19] In East Asia, a Korean Association Resource (KARE)-based GWAS showed that rs17249754 near the ATP2B1 gene is closely related to SBP.[20] Later, the Japanese Millennium Genome Project for Hypertension found that ATP2B1 rs11105378 had the most significant association with the risk of hypertension.[21] In East Africa, a Ugandan GWAS based on young children and adolescents showed that ATP2B1 rs17249754 is associated with SBP and DBP.[22]
Based on GWAS, many confirmatory or replication studies have found that ATP2B1 gene polymorphisms are associated with blood pressure or the risk of hypertension.[23–25] However, these results have been inconsistent.[26,27] A case-control study in Spain found that the ATP2B1 polymorphisms rs17249754 and rs2681472 were not associated with the risk of hypertension.[28] A recent report from Lithuania showed that ATP2B1 rs2681472 was not associated with the risk of hypertension in children and adolescents.[29]
Considering the inconsistent and inclusive results in different studies regarding the associations between ATP2B1 polymorphisms and BP-related phenotypes, we conducted a systematic review and meta-analysis to comprehensively demonstrate the association between ATP2B1 polymorphisms and susceptibility to hypertension or BP levels in the present study, and calculated the pooled estimates to assess the associations.
2. Methods
2.1. Ethical approval
This meta-analysis was based entirely on previous published studies which had declared ethical approvals and no original clinical raw data was collected or utilized, thereby ethical approval was not required for this study.
2.2. Literature search strategy
PubMed, Embase, Web of Science, and China National Knowledge Infrastructure (CNKI) databases were searched from their establishment dates to March 4, 2021. The language was limited to Chinese or English, regardless of the region. Studies on ATP2B1 polymorphisms, blood pressure levels, and susceptibility to hypertension were collected. The search terms included any of the following: “ATP2B1,” “Plasma membrane calcium-transporting ATPase 1,” “PMCA,” “PMCA1,” or “plasma membrane Ca2+-ATPase 1b” as well as “hypertension,” “HTN,” “blood pressure,” “SBP,” or “DBP.” We systematically summarized the genetic polymorphisms in the ATP2B1 gene related to blood pressure phenotypes that have been reported in the literature and supplemented the relevant literature. Supplementary relevant literature was searched in PubMed's dbSNP database (rs2681472 and rs17249754, the 2 most-studied polymorphisms) (https://www.ncbi.nlm.nih.gov/snp). The present study was conducted according to the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 guidelines for meta-analysis and systematic review.[30]
2.3. Study selection
Studies were eligible if the following criteria were met:
-
1.
studies on the association of ATP2B1 gene polymorphisms with blood pressure phenotypes;
-
2.
studies that reported the following estimates: odds risk (OR) and 95% confidence interval (95%CI) or β and standard deviation (SD);
-
3.
high blood pressure in children or hypertension in adults were clearly defined.
The exclusion criteria were as follows:
-
1.
studies related to the association of ATP2B1 gene polymorphisms with nonhypertensive phenotypes;
-
2.
studies lacking OR and 95%CI or β and SE;
-
3.
review and duplicate studies.
2.4. Data extraction and quality assessment
Literature retrieval, screening, and data extraction were completed independently by 2 researchers, in strict accordance with the established inclusion and exclusion criteria. Data extraction was checked for consistency by a third supervising researcher until the final consistent results of data extraction were obtained. The extracted information included:
-
1.
basic information of the included literature, including the first author and year of publication;
-
2.
basic information of the study subjects, including population (adults or children), ethnicity, number of cases and controls;
-
3.
mean SBP and DBP, method of blood pressure measurement (mercury sphygmomanometer or electronic sphygmomanometer);
-
4.
phenotypes of blood pressure and outcome indicators: hypertension (OR and 95%CI) and SBP/DBP (β and SE);
-
5.
risk alleles and covariates.
For the quality assessment, the 2 investigators used the Newcastle–Ottawa Scale (NOS)[31] to independently evaluate the quality of the included literature and cross-check the results. The full score was 9 points, and studies with a score of at least 5 points were included.[32]
2.5. Statistical analysis
Meta-analysis was performed using Stata version 11.0 (Stata Corp., College Station, TX) and OR with 95%CI or β with SE were pooled under the additive model. Heterogeneity test was performed using Cochrane Q test. When I2 ≥ 50% and P ≤ .05, it was considered to show moderate or high heterogeneity among the included studies, then the random effect model was selected. When I2 < 50% and P > .05, no significant heterogeneity existed between the studies and a fixed effect model was used.[33] Sensitivity analysis was used to evaluate the robustness of the results of the included studies. The effect values of the remaining studies were calculated and compared with the total effect estimates by eliminating one study after another, to measure the sensitivity and robustness of the results. If a study was excluded, there was a significant difference between the remaining results and the total effect value, showing the high sensitivity and low robustness of the results, and vice versa. Publication bias was tested using funnel plot and Egger test. Egger test sets the standardized effect value as Y (the dependent variable) and the precision of the effect value as X (the independent variable), establishing a linear regression equation to test publication bias. If the intercept term of the regression equation was close to 0, the publication bias was considered small, and P < .05, was considered as showing no publication bias. If publication bias existed, publication bias was corrected by conducting a trim and fill analysis.[34] In this study, the test level α was set at 0.05. Pairwise linkage disequilibrium (LD) was estimated using Haploview (version 4.2) (Broad Institute of MIT and Harvard, Cambridge, MA; http://www.broad.mit.edu/mpg/haploview/).
3. Results
3.1. Literature search
A total of 893 publications were retrieved from the PubMed, Embase, Web of Science, and CNKI databases. After screening the title and abstract of each article, 328 records were excluded as duplicate records, and 534 records were excluded as irrelevant articles. After assessing the full text, 3 records were removed for duplicate data and 13 for incomplete results. Finally, 15 articles were included in the present meta-analysis. A flowchart of the screening process is presented in Figure 1. A total of 15 polymorphisms of the ATP2B1 gene were reported for their associations with blood pressure phenotypes in these articles, namely rs17249754, rs2681472, rs2681492, rs10858911, rs1401982, rs2070759, rs2854371, rs3741895, rs11105357, rs957525, rs11105358, rs12579302, rs11105364, rs7136259, and rs11105354. Pairwise linkage disequilibrium (LD) among 15 SNPs in the ATP2B1 gene is shown in Figure 2. We found that rs17249754 and rs2681472 were in strong linkage disequilibrium (r2 = 0.95). We obtained 9 articles related to ATP2B1 rs17249754 and blood pressure and 7 articles related to rs2681472 (1 study involving both polymorphisms). A total sample size of 65,362 for rs17249754 and 91997 for rs2681472. Since Chen Liu,[35] Fumihiko Takeuchi,[24] Daniel Levy,[19] and Sandrita Simonyte[29] all reported 2 different studies in their papers, each of which could be considered as an independent study, 19 studies were included in this analysis.
Figure 1.
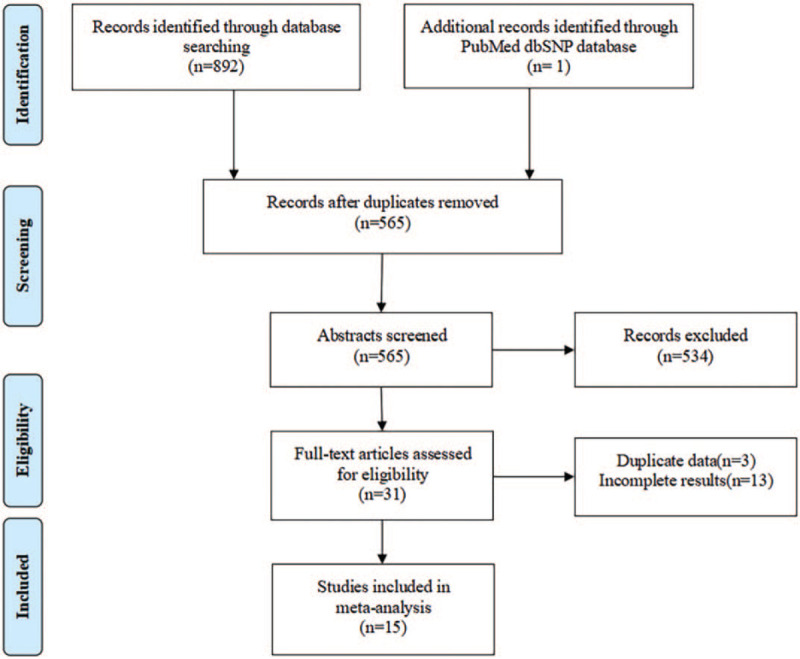
Flow diagram of the process of selection of articles.
Figure 2.
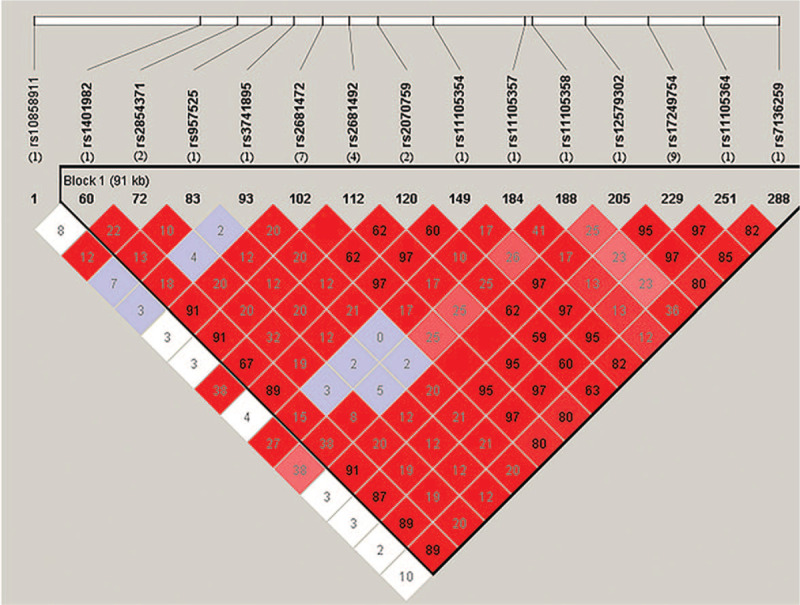
Pairwise linkage disequilibrium (LD) among 15 SNPs in ATP2B1 gene. The number below the SNPs was the number of papers reported the SNP in this meta-analysis. Data from 1000 Genomes Project (https://www.internationalgenome.org), we used the data of people of Chinese Han Ethnicity from Beijing in this project. LD was estimated using Haploview version 4.2 (http://www.broad.mit.edu/mpg/haploview/).
3.2. Characteristics of studies included
Among the 19 included studies, 13 studies were conducted in East Asia (including China, Japan, and Korea), 1 in West Asia, 4 in Europe, and 1 in a global population. Three studies involved children and adolescents, and 16 articles involved adults. The risk/nonrisk alleles for the rs17249754 polymorphism were G/A, and the risk/nonrisk alleles for the rs2681472 polymorphism were A/G or T/C. The quality of the literature was evaluated using the NOS case-control study quality assessment scale, and all quality assessment scores were more than 6 points. The basic characteristics of the included studies and the quality scores of these studies are shown in Table 1.
Table 1.
Characteristics of included studies in the meta-analysis and quality assessment.
| Sample size | |||||||||||||||
| Author | Year | Country | Ethnicity | Population | Cases | Controls | Technique for BP measurement | SBP (Mean±SD, mm Hg) | DBP (Mean±SD, mmHg) | Phenotype | OR (95%CI) or β(SE) | ATP2B1 SNP | Risk/Non risk allele | Adjustment | NOS |
| Xi B[16] | 2014 | China | East Asian | Children and adolescents | 619 | 2458 | Mercury sphygmomanometer | Cases:125.7±10.6Controls:101.6±10.3 | Cases:79.6±8.5Controls:63.7±7.8 | HTN | 1.25 (1.08,1.44) | rs17249754 | G/A | 1, 2, 4 | 8 |
| SBP | 0.48 (0.21) | 1, 2, 4, 6 | |||||||||||||
| DBP | -0.08 (0.18) | 1, 2, 4, 6 | |||||||||||||
| Liu C(BJ)[35] | 2010 | China | East Asian | Adults | 1414 | 181 | Electronic sphygmomanometer | 147.5 ± 24.4 | 82.8±11.5 | HTN | 1.29 (1.00,1.66) | rs2681472 | A/G | 2, 1, 5, 4 | 6 |
| SBP | 0.61 (0.86) | ||||||||||||||
| DBP | 0.53 (0.42) | ||||||||||||||
| Liu C(SH)[35] | 2010 | China | East Asian | Adults | 1264 | 369 | Electronic sphygmomanometer | 138.6 ± 23.8 | 80.5 ± 11.6 | HTN | 1.01 (0.82,1.24) | rs2681472 | A/G | 2, 1, 5, 4 | 6 |
| SBP | 0.14 (0.81) | ||||||||||||||
| DBP | 0.32 (0.40) | ||||||||||||||
| Takeuchi F(JPN)[24] | 2010 | Japan | East Asian | Adults | 3697 | 7283 | NA | NA | NA | SBP | 0.99 (0.18) | rs2681472 | A/G | 2, 3, 4 | 8 |
| DBP | 0.43 (0.11) | ||||||||||||||
| HTN | 1.10 (1.04,1.17) | ||||||||||||||
| Takeuchi F(EUR)[24] | 2010 | Japan | European | Adults | 63659 | NA | NA | NA | SBP | 0.85 (0.13) | rs2681472 | A/G | 2, 3, 4 | 8 | |
| DBP | 0.50 (0.08) | ||||||||||||||
| HTN | 1.16 (1.12,1.21) | ||||||||||||||
| Hong KW[36] | 2010 | Korea | East Asian | Adults | 2461 | 1473 | NA | 129.24 ± 17.69 | 82.70 ± 11.63 | SBP | 0.86 (0.30) | rs17249754 | G/A | 5, 2, 1, 4 | 8 |
| DBP | 0.42 (0.20) | ||||||||||||||
| HTN | 1.17 (1.05,1.29) | ||||||||||||||
| Wang Y[25] | 2013 | China | East Asian | Adults | 2831 | 1987 | Mercury sphygmomanometer | 133.1 ± 21.0 | 84.5 ± 12.3 | HTN | 1.20 (1.10,1.32) | rs17249754 | G/A | 1, 2, 3, 4 | 7 |
| SBP | 1.31 (0.44) | ||||||||||||||
| DBP | 0.82 (0.28) | ||||||||||||||
| HTN | 1.16 (1.06,1.28) | rs2681472 | A/G | ||||||||||||
| SBP | 0.88 (0.44) | ||||||||||||||
| DBP | 0.68 (0.28) | ||||||||||||||
| Tabara Y[21] | 2010 | Japan | East Asian | Adults | 2687 | 2719 | NA | NA | NA | SBP | 1.33 (0.23) | rs2681472 | A/G | 1, 2, 3, 4, 7 | 8 |
| DBP | 0.65 (0.14) | ||||||||||||||
| HTN | 1.21 (1.13,1.30) | ||||||||||||||
| Lin Y[27] | 2011 | China | East Asian | Adults | 1692 | 1168 | Mercury sphygmomanometer | Cases:157.41 ± 19.94Controls:109.91 ± 6.72 | Cases:91.77 ± 11.52Controls:66.09 ± 6.15 | SBP | -0.01 (0.50) | rs2681472 | A/G | 2, 1, 4 | 8 |
| DBP | 0.09 (0.29) | ||||||||||||||
| HTN | 1.01 (0.87,1.18) | ||||||||||||||
| Qi Y[37] | 2013 | China | East Asian | Adults | 1009 | 756 | Mercury sphygmomanometer | Cases:147.31 ± 16.52Controls:109.76 ± 17.97 | Cases:89.09 ± 15.92Controls:71.37 ± 11.43 | HTN | 1.23 (1.07,1.41) | rs17249754 | G/A | 2, 1, 4 | 6 |
| Jamshidi J[26] | 2018 | Iran | West Asian | Adults | 200 | 200 | NA | Cases:140.59 ± 23.42Controls:113.22 ± 9.96 | Cases:84.86 ± 11.89Controls:72.12 ± 8.55 | HTN | 1.05 (0.54,2.02) | rs2681472 | T/C | 1, 2 | 6 |
| Kato N[38] | 2011 | Japan | East Asian | Adults | 7074 | 14644 | NA | NA | NA | SBP | 1.17 (0.13) | rs17249754 | G/A | NA | 8 |
| DBP | 0.58 (0.08) | ||||||||||||||
| Levy D (CHARGE)[19] | 2009 | America | European | Adults | 9980 | 19156 | NA | NA | NA | DBP | 0.64 (0.12) | rs2681472 | A/G | 1, 2, 3, 4 | 8 |
| HTN | 1.17 (1.10,1.24) | ||||||||||||||
| Levy D (Global)[19] | 2009 | America | Global | Adults | 34433 | NA | NA | NA | DBP | 0.36 (0.12) | rs2681472 | A/G | 1, 2, 3, 4 | 8 | |
| HTN | 1.14 (1.05,1.23) | ||||||||||||||
| Miyaki K[39] | 2011 | Japan | East Asian | Adults | 261 | 474 | Electronic sphygmomanometer | Cases:147.8 ± 13.2Controls:120.1 ± 10.3 | Cases:91.1 ± 8.6Controls:73.5 ± 8.0 | HTN | 2.12 (1.22,3.70) | rs17249754 | G/A | 2, 4, 8, 9 | 7 |
| Cho YS[20] | 2009 | Korea | East Asian | Adults | 1968 | 4451 | NA | NA | NA | SBP | 1.06 (0.20) | rs17249754 | G/A | 2, 1, 5 | 8 |
| DBP | 0.63 (0.14) | ||||||||||||||
| Lu X[40] | 2015 | China | East Asian | Adults | 8128 | 14768 | NA | NA | NA | SBP | 1.03 (0.15) | rs17249754 | G/A | 2, 3, 1, 4 | 8 |
| DBP | 0.52 (0.08) | ||||||||||||||
| HTN | 1.09 (1.05,1.12) | ||||||||||||||
| Simonyte S(Boys)[29] | 2018 | Lithuania | European | Children and adolescents | 143 | 170 | Electronic sphygmomanometer | 123.20 ± 16.13 | 64.34 ± 7.99 | HTN | 1.09 (0.67,1.75) | rs2681472 | A/G | 4, 10 | 8 |
| Simonyte S(Girls)[29] | 2018 | Lithuania | European | Children and adolescents | 94 | 239 | Electronic sphygmomanometer | 116.92 ± 12.61 | 65.61 ± 7.99 | HTN | 1.27 (0.75,2.17) | rs2681472 | A/G | 4, 10 | 8 |
3.3. Heterogeneity test and meta-analysis results
Heterogeneity analysis of rs17249754 in association with hypertension, SBP, and DBP revealed that the association of rs17249754 with hypertension and DBP was heterogeneous in different studies (I2 > 50%), which was analyzed using a random-effect model. The rs17249754 G allele was associated with increased susceptibility to hypertension (OR = 1.19, 95%CI: 1.10–1.28, P < .001) and was positively associated with both SBP (β = 1.01, 95%CI: 0.86–1.16, P < .001) and DBP (β = 0.48, 95%CI: 0.30–0.66, P < .001). The association between rs2681472 and the BP phenotype was less heterogeneous (I2 < 50%), and a fixed-effect model was chosen. The rs2681472 A allele was associated with an increased risk of hypertension (OR = 1.15, 95%CI: 1.12–1.17, P < .001), and SBP (β = 0.92, 95%CI: 0.77–1.07, P < .001) and DBP (β = 0.50, 95%CI: 0.42–0.58, P < .001) were positively correlated (Fig. 3).
Figure 3.
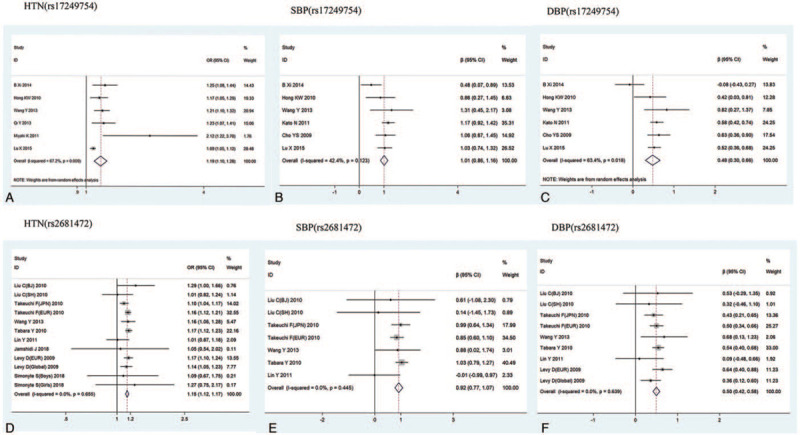
Meta-analysis of the association of ATP2B1 rs17249754 and rs2681472 with hypertension, SBP, and DBP.
3.4. Subgroup analysis
Subgroup analysis was performed by race, population, sample size, and blood pressure measurements. The association between the A allele in the rs2681472 polymorphism and the risk of hypertension was slightly stronger in European (EUR) populations (OR = 1.16, 95%CI: 1.13–1.20, P < .001) than in East Asians (OR = 1.14, 95%CI: 1.10–1.17, P < .001). The high heterogeneity of the ATP2B1 rs17249754 polymorphism with hypertension and DBP in different studies may be due to differences in blood pressure measurements and differences in the population (children, adolescents, or adults). In East Asians, the relationship between rs17249754 and risk of hypertension (OR = 1.19, 95%CI: 1.10–1.28, P < .001) was stronger than that between rs2681472 (OR = 1.14, 95%CI: 1.10–1.17, P < .001) (Table 2).
Table 2.
Subgroup analysis of the association of ATP2B1 gene polymorphisms with hypertension.
| rs17249754 | rs2681472 | |||||||||||||||||||||||
| HTN | SBP | DBP | HTN | SBP | DBP | |||||||||||||||||||
| Subgroup types | n | OR(95%CI) | P | I2,% | n | β(95%CI) | P | I2,% | n | β(95%CI) | P | I2,% | n | OR(95%CI) | P | I2,% | n | β(95%CI) | P | I2,% | n | β(95%CI) | P | I2,% |
| Total | 6 | 1.19 (1.10,1.28) | <.001 | 67.2 | 6 | 1.01 (0.86,1.16) | <.001 | 42.4 | 6 | 0.48 (0.30,0.66) | <.001 | 63.4 | 12 | 1.15 (1.12,1.17) | <.001 | 0.00 | 7 | 0.92 (0.77,1.07) | <.001 | 0.00 | 9 | 0.50 (0.42,0.58) | <.001 | 0.00 |
| Ethnicities | ||||||||||||||||||||||||
| East Asian | 6 | 1.19 (1.10,1.28) | <.001 | 67.2 | 6 | 1.01 (0.86,1.16) | <.001 | 42.4 | 6 | 0.48 (0.30,0.66) | <.001 | 63.4 | 6 | 1.14 (1.10,1.17) | <.001 | 32.0 | 6 | 0.96 (0.77,1.14) | <.001 | 6.60 | 6 | 0.50 (0.39,0.61) | <.001 | 0.00 |
| West Asian | NA | NA | NA | 1 | 1.05 (0.54,2.03) | .885 | NA | NA | ||||||||||||||||
| European | NA | NA | NA | 4 | 1.16 (1.13,1.20) | <.001 | 0.00 | 1 | 0.85 (0.60,1.10) | <.001 | 2 | 0.54 (0.41,0.67) | <.001 | 0.00 | ||||||||||
| Others | NA | NA | NA | 1 | 1.14 (1.05,1.23) | .001 | NA | 1 | 0.36 (0.12,0.60) | .003 | ||||||||||||||
| Sample size | ||||||||||||||||||||||||
| <2000 | 2 | 1.51 (0.90,2.53) | .119 | 71.3 | NA | NA | 5 | 1.12 (0.97,1.29) | .122 | 0.00 | 2 | 0.36 (-0.79,1.52) | .540 | 0.00 | 2 | 0.42 (-0.15,0.99) | .147 | 0.00 | ||||||
| ≥2000 | 4 | 1.16 (1.08,1.24) | <.001 | 63.6 | 6 | 1.01 (0.86,1.16) | <.001 | 42.4 | 6 | 0.48 (0.30,0.66) | <.001 | 63.4 | 7 | 1.15 (1.12,1.18) | <.001 | 1.40 | 5 | 0.93 (0.78,1.08) | <.001 | 15.5 | 7 | 0.50 (0.42,0.58) | <.001 | 0.00 |
| Study population | ||||||||||||||||||||||||
| Children and adolescents | 1 | 1.25 (1.08,1.44) | .002 | 1 | 0.48 (0.07,0.89) | .022 | 1 | -0.08 (-0.43,0.27) | .657 | 2 | 1.17 (0.82,1.67) | .394 | 0.00 | NA | NA | |||||||||
| Adults | 5 | 1.18 (1.08,1.28) | <.001 | 69.1 | 5 | 1.09 (0.93,1.25) | <.001 | 0.00 | 5 | 0.56 (0.46,0.66) | <.001 | 0.00 | 10 | 1.15 (1.12,1.17) | <.001 | 0.00 | 7 | 0.92 (0.77,1.07) | <.001 | 0.00 | 9 | 0.50 (0.42,0.58) | <.001 | 0.00 |
| Measurement of BP | ||||||||||||||||||||||||
| Electronic sphygmomanometer | 1 | 2.12 (1.22,3.69) | .008 | NA | NA | 4 | 1.12 (0.97,1.30) | .121 | 0.00 | 2 | 0.36 (-0.79,1.52) | .540 | 0.00 | 2 | 0.42 (-0.15,0.99) | .147 | 0.00 | |||||||
| Mercury sphygmomanometer | 3 | 1.22 (1.14,1.31) | <.001 | 0.00 | 2 | 0.63 (0.26,1.01) | .001 | 65.5 | 2 | 0.34 (-0.54,1.23) | .443 | 86.3 | 2 | 1.12 (1.03,1.21) | .007 | 56.4 | 2 | 0.49 (-0.16,1.14) | .137 | 44.0 | 2 | 0.40 (0.00,0.79) | .050 | 53.3 |
| NA | 2 | 1.11 (1.04,1.18) | .001 | 39.6 | 4 | 1.08 (0.92,1.25) | <.001 | 0.00 | 4 | 0.55 (0.45,0.65) | <.001 | 0.00 | 6 | 1.15 (1.13,1.18) | <.001 | 0.00 | 3 | 0.96 (0.80,1.11) | <.001 | 0.00 | 5 | 0.50 (0.42,0.59) | <.001 | 0.00 |
3.5. Sensitivity analysis and publication bias
After excluding the studies one by one, we found that the effect values of the remaining studies were not significantly different from the total effect value, which could be because the sensitivity is relatively small and the combined result is relatively stable. The results of the meta-analysis were unlikely to be influenced by any single study (Fig. 4). A significant asymmetry in the funnel plots was observed between studies on rs17249754 and hypertension (Fig. 5A), suggesting the existence of publication bias. The publication bias between studies on rs17249754 and hypertension was statistically significant when subjected to Egger test (t = 10.13, P = .001). We then corrected the publication bias by conducting a trim and fill analysis (adjusted OR: 1.13, 95%CI: 1.05–1.21, P = .001), which was slightly lower than the precorrection effect value, but the direction of the effect estimates did not change. The funnel plots of studies on rs17249754 and SBP or DBP were symmetrically distributed (Fig. 5B-C), as well as the funnel plots of studies about rs2681472 and SBP, DBP, or risk of hypertension (Fig. 5D-F). In their Egger tests, the P values were all >.05, which suggested that there was no significant publication bias.
Figure 4.
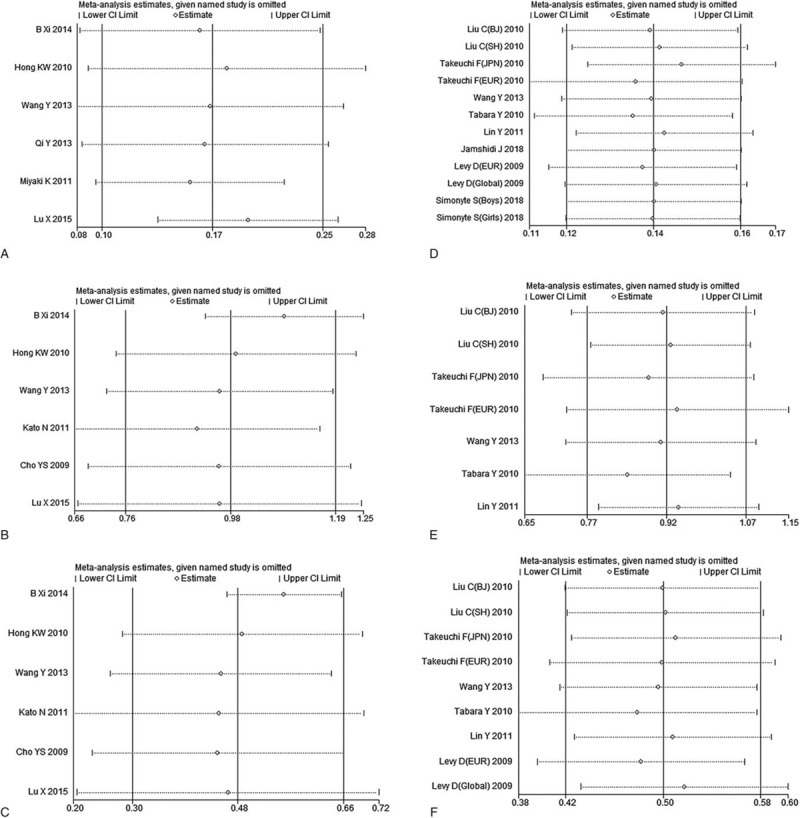
Sensitivity analysis of the association between ATP2B1 gene polymorphism and hypertension. A–C: the sensitivity analysis of the correlation between rs17249754 and hypertension, SBP and DBP, respectively; D–F: the sensitivity analysis of the correlation between rs2681472 and hypertension, SBP and DBP, respectively.
Figure 5.
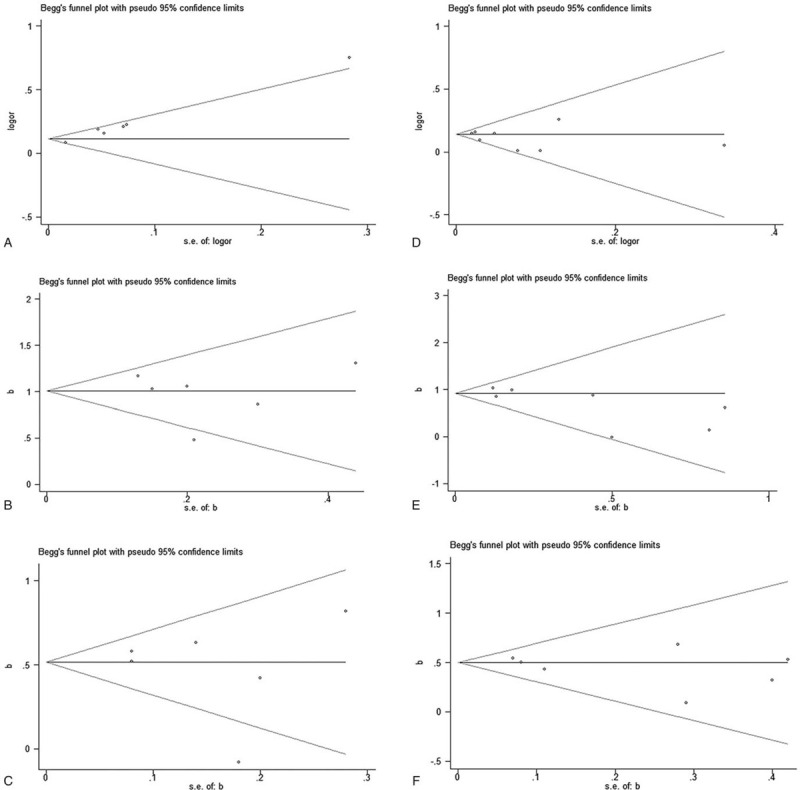
Funnel plots of publication bias for ATP2B1 gene polymorphism and hypertension-related studies. A–C: funnel plots of bias published in studies on rs17249754 and its correlation with hypertension, SBP and DBP. D–F: funnel plots of bias published in studies on rs2681472 and its correlation with hypertension, SBP and DBP.
4. Discussion
To our knowledge, our study is the first to comprehensively evaluate the association between ATP2B1 polymorphisms and blood pressure phenotypes. Fifteen polymorphisms of the ATP2B1 gene were reported regarding their associations with blood pressure phenotypes, with rs17249754 and rs2681472 being the most commonly reported in studies. The results of the meta-analysis indicated that G allele carriers of rs17249754 and A allele carriers of rs2681472 had significantly higher susceptibility to hypertension and higher BP levels than their counterparts.
The mechanism by which ATP2B1 affects blood pressure levels and the risk of hypertension is unclear, but an animal study in ATP2B1 knockout mice suggests that ATP2B1 may regulate blood pressure levels by altering calcium transport and vasoconstriction in vascular smooth muscle cells.[41] Additional experimental evidence has shown that the levels of ATP2B1 mRNA in smooth muscle cells of spontaneously hypertensive rats are higher than in normotensive rats.[42] Experiments in bladder smooth muscle cells have shown that ATP2B1 plays an important role in the elimination of calcium ions following carbachol stimulation or KCl depolarization.[43] These findings suggest that the mechanism by which ATP2B1 is involved in blood pressure regulation and influences the risk of hypertension is probably related to the level of the gene product. In addition, the associations between ATP2B1 rs17249754 and rs2681472 with the risk of hypertension may be related to sodium retention in the body. ATP2B1 gene polymorphism rs2681472 has been previously reported to be associated with salt sensitivity in Koreans.[44] A recent study found that Koreans carrying the G allele of the ATP2B1 rs17249754 polymorphism with a high sodium/potassium ratio and low calcium intake have a significantly increased risk of hypertension.[45] There have been many studies on the associations of ATP2B1 rs17249754 and rs2681472 with susceptibility to hypertension. The association of the ATP2B1 rs17249754 polymorphism with hypertension was first reported in Koreans,[20] and was subsequently confirmed in the GWAS conducted by CHARGE and Global Blood Pressure Genetics Consortium (Global BPgen).[19] In 2009, Levy et al first identified that the ATP2B1 rs2681472 polymorphism was associated with hypertension,[19] and subsequent studies in China[35] and Japan[24] in 2010 verified the association between rs2681472 and blood pressure phenotypes. The results of this meta-analysis showed that both rs17249754 and rs2681472 polymorphisms were associated with susceptibility to hypertension and were positively correlated with both SBP and DBP levels, which is consistent with the meta-analysis results of rs2681472 and hypertension analyzed by Xi et al[46] in the East Asian population.
To detect whether there are differences in the associations between ATP2B1 gene polymorphisms and blood pressure phenotypes in populations of different ages, races, or sample sizes, we performed subgroup analyses and found that associations between rs2681472 polymorphism risk allele A and risk of hypertension was slightly weaker in Asians than in EURs . Takeuchi et al also reported that the risk of hypertension in the Japanese population with the rs2681472 polymorphism A allele (OR = 1.10) is lower than that in EURs (OR = 1.16).[24] Because studies on the association between rs17249754 polymorphism and hypertension were limited to East Asians, the relationship between ATP2B1 rs17249754 and hypertension cannot be compared by ethnicity, but within East Asian populations, the relationship between rs17249754 and risk of hypertension is stronger than that between rs2681472. A replication study in China also found that the association between the risk of hypertension and rs17249754 (OR = 1.21) was higher than that with rs2681472 (OR = 1.16).[25] Subgroup analysis showed that the association between the G allele in rs17249754 polymorphism and risk of hypertension was stronger in children than in adults. However, because there was only 1 study that included children and adolescents with rs17249754, a direct comparison between children and adults should be cautiously interpreted, and the findings need to be validated by further research. For studies with a sample size of less than 2000, we did not find any significant associations. In other words, in some studies, rs17249754 and rs2681472 were not associated with the risk of hypertension or blood pressure levels, which is likely due to the limited statistical power of the small sample size.
There was significant heterogeneity among the studies included in this study regarding the rs17249754 polymorphism in relation to the risk of hypertension and DBP. The heterogeneity may be due to differences in the blood pressure measurement method and the different populations of studies. For the rs2681472 polymorphism, there is a weaker heterogeneity among the studies on rs2681472 polymorphism, which may be because the studies were mostly from Asian populations (including Japanese and Chinese, except for the study by Daniel Levy[19]), and probably there was better consistency in studies of the same ethnicity.
Using a sensitivity analysis, we found that the results of this meta-analysis were stable and reliable. Begg and Egger tests showed that there was a significant publication bias in the included studies on rs17249754 polymorphism and the risk of hypertension, which may be because positive results are easier to publish than negative or insignificant results. Therefore, there is still publication bias when analyzing the influence of the rs17249754 polymorphism on the risk of hypertension. However, the meta-analysis of rs17249754 and blood pressure was corrected by trim and fill analysis, which is the most appropriate method to handle data with publication bias, and yields the pooled effect adjusted for the funnel plot asymmetry;[34] however, the pooled estimates of rs17249754's influence on the risk of hypertension should also be interpreted with caution.
There are some limitations within this study. First, the current study populations related to rs17249754 polymorphism, hypertension and blood pressure levels are mostly Asian adults, and there are few relevant studies on EURs, Americans, and Africans, as well as studies regarding children. Therefore, it is not possible to compare the relationship between the rs17249754 polymorphism and the risk of hypertension among different ethnic groups. Second, the languages of the included studies were limited to English or Chinese, and there may be some literature in other languages not included in this study. Third, since most of the current studies mainly reported the results under the additive genetic model, few studies have reported dominant genetic models, recessive genetic models, heterozygous models, homozygous models, or other genetic models. It is not possible to perform a meta-analysis of these genetic models. When only the additive model was considered, the results of this study may not be applicable. Fourth, in this study, because only the rs17249754 and rs2681472 polymorphisms of ATP2B1 were subjected to meta-analysis, the interaction between each polymorphism, and between genetic polymorphisms and the environment cannot be controlled.
5. Conclusion
In summary, our meta-analysis of the ATP2B1 rs17249754 polymorphism (G/A) and rs2681472 polymorphism (A/G) implies that they are associated with the risk of hypertension, SBP, and DBP, and could be genetic biomarkers for hypertension. However, the relationship between other polymorphisms of the ATP2B1 gene and susceptibility to hypertension in a globally representative sample remains to be further investigated. Furthermore, genetic risk factors are difficult to modify, but lifestyle factors are relatively easy to modify. In the future, gene-lifestyle or gene-environment interactions with representative samples are needed to improve recommendations of the precise interventions for hypertension.
Author contributions
Acquisition of data: Ming Xie, Yuan Zeng, Shuqian Yuan.
Data curation: Ming Xie.
Design: Yide Yang, Ming Xie.
Literature search: Ming Xie, Yuan Zeng.
Manuscript: Ming Xie.
Revision of manuscript: Yide Yang, Chanjuan Zheng, Yanhui Dong, Quanyuan He.
Statistical analyses: Ming Xie, Yuan Zeng.
Validation: Shuqian Yuan, Yuan Zeng.
Writing – original draft: Ming Xie.
Writing – review & editing: Yide Yang, Chanjuan Zheng, Yanhui Dong, Quanyuan He.
Footnotes
Abbreviations: BP = blood pressure, DBP = diastolic blood pressure, EUR = European, HTN = hypertension, SBP = systolic blood pressure.
How to cite this article: Xie M, Yuan S, Zeng Y, Zheng C, Yang Y, Dong Y, He Q. ATP2B1 gene polymorphisms rs2681472 and rs17249754 are associated with susceptibility to hypertension and blood pressure levels: a systematic review and meta-analysis. Medicine. 2021;100:15(e25530).
This work was supported by the National Natural Science Foundation of China (Grant No. 81903336 to Yide Yang), the Hunan Provincial Natural Science Foundation of China (Grant No. 2019JJ50376 to Yide Yang) and the China Postdoctoral Science Foundation (National Postdoctoral Program for Innovative Talent, BX20200019 to Yanhui Dong). The funders had no role in the design, analysis, or writing of this article.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
BJ = Beijing, EUR = European, HTN = hypertension, JPN = Japanese, SH = Shanghai, Adjustment:1: sex, 2: age, 3: age2, 4: body mass index (BMI), 5: region, 6: hypertension status, 7: cohort, 8: salt intake levels, 9: energy intake levels, 10: waist; NA: not available.
HTN = hypertension, NA = not available.
References
- [1].Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Giornale Italiano Di Cardiologia 2018;19:3s–73s. [DOI] [PubMed] [Google Scholar]
- [2].GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li AL, Peng Q, Shao YQ, et al. The interaction on hypertension between family history and diabetes and other risk factors. Scientific Reports 2021;11:4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cameron NA, Molsberry R, Pierce JB, et al. Pre-pregnancy hypertension among women in rural and urban areas of the United States. J Am Coll Cardiol 2020;76:2611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valenzuela PL, Carrera-Bastos P, Gálvez BG, et al. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol 2020;18:251–75. [DOI] [PubMed] [Google Scholar]
- [6].Muñoz M, Pong-Wong R, Canela-Xandri O, et al. Evaluating the contribution of genetics and familial shared environment to common disease using the UK Biobank. Nat Genet 2016;48:980–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vaura F, Kauko A, Suvila K, et al. Polygenic risk scores predict hypertension onset and cardiovascular risk. Hypertension 2021;77:1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bigazzi R, Zagato L, Lanzani C, et al. Hypertension in high school students: genetic and environmental factors: the HYGEF study. Hypertension 2020;75:71–8. [DOI] [PubMed] [Google Scholar]
- [9].Kim W, Go TH, Kang DO, et al. Age and sex dependent association of uric acid and incident hypertension. Nutr Metab Cardiovasc Dis 2021;31:1200–8. [DOI] [PubMed] [Google Scholar]
- [10].Mollerup PM, Lausten-Thomsen U, Fonvig CE, et al. Reductions in blood pressure during a community-based overweight and obesity treatment in children and adolescents with prehypertension and hypertension. J Hum Hypertens 2017;31:640–6. [DOI] [PubMed] [Google Scholar]
- [11].Schneider RH, Salerno J, Brook RD. 2020 International Society of Hypertension global hypertension practice guidelines-lifestyle modification. J Hypertens 2020;38:2340–1. [DOI] [PubMed] [Google Scholar]
- [12].Shen Y, Chang C, Zhang J, et al. Prevalence and risk factors associated with hypertension and prehypertension in a working population at high altitude in China: a cross-sectional study. Environ Health Prev 2017;22:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension 2003;41:1196–201. [DOI] [PubMed] [Google Scholar]
- [14].Hirawa N, Fujiwara A, Umemura S. ATP2B1 and blood pressure: from associations to pathophysiology. Curr Opin Nephrol Hypertens 2013;22:177–84. [DOI] [PubMed] [Google Scholar]
- [15].Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xi B, Shen Y, Zhao X, et al. Association of common variants in/near six genes (ATP2B1, CSK, MTHFR, CYP17A1, STK39 and FGF5) with blood pressure/hypertension risk in Chinese children. J Hum Hypertens 2014;28:32–6. [DOI] [PubMed] [Google Scholar]
- [17].Sun XM, Yang M, Jiang CX. Association of ATP2B1 gene polymorphism with incidence of eclampsia. Eur Rev Med Pharmacol Sci 2019;23:10609–16. [DOI] [PubMed] [Google Scholar]
- [18].Long Y, Xia JY, Chen SW, et al. ATP2B1 gene silencing increases insulin sensitivity through facilitating Akt activation via the Ca [2+]/calmodulin signaling pathway and Ca [2+]-associated eNOS activation in endothelial cells. Int J Biol Sci 2017;13:1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009;41:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cho YS, Go MJ, Kim YJ, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 2009;41:527–34. [DOI] [PubMed] [Google Scholar]
- [21].Tabara Y, Kohara K, Kita Y, et al. Common variants in the ATP2B1 gene are associated with susceptibility to hypertension: the Japanese millennium genome project. Hypertension 2010;56:973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lule SA, Mentzer AJ, Namara B, et al. A genome-wide association and replication study of blood pressure in Ugandan early adolescents. Mol Genet Genomic Med 2019;7:e00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hong KW, Jin HS, Lim JE, et al. Recapitulation of two genomewide association studies on blood pressure and essential hypertension in the Korean population. J Hum Genet 2010;55:336–41. [DOI] [PubMed] [Google Scholar]
- [24].Takeuchi F, Isono M, Katsuya T, et al. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 2010;121:2302–9. [DOI] [PubMed] [Google Scholar]
- [25].Wang Y, Zhang Y, Li Y, et al. Common variants in the ATP2B1 gene are associated with hypertension and arterial stiffness in Chinese population. Mol Biol Rep 2013;40:1867–73. [DOI] [PubMed] [Google Scholar]
- [26].Jamshidi J, Asnaashari A, Alipoor R, et al. ATP2B1 rs2681472 and STK39 rs35929607 polymorphisms and risk of hypertension in Iranian population. Med J Islamic Republic Iran 2018;32:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin Y, Lai X, Chen B, et al. Genetic variations in CYP17A1, CACNB2 and PLEKHA7 are associated with blood pressure and/or hypertension in She ethnic minority of China. Atherosclerosis 2011;219:709–14. [DOI] [PubMed] [Google Scholar]
- [28].Valencia DM, Naranjo CA, Parra MV, et al. Association and interaction of AGT, AGTR1, ACE, ADRB2, DRD1, ADD1, ADD2, ATP2B1, TBXA2R and PTGS2 genes on the risk of hypertension in Antioquian population. Biomedica 2013;33:598–614. [DOI] [PubMed] [Google Scholar]
- [29].Simonyte S, Kuciene R, Dulskiene V, et al. Association between ATP2B1 and CACNB2 polymorphisms and high blood pressure in a population of Lithuanian children and adolescents: a cross-sectional study. BMJ Open 2018;8:e019902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur Journal Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [32].Stang A, Jonas S, Poole C. Case study in major quotation errors: a critical commentary on the Newcastle–Ottawa scale. Eur J Epidemiol 2018;33:1025–31. [DOI] [PubMed] [Google Scholar]
- [33].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [35].Liu C, Li H, Qi Q, et al. Common variants in or near FGF5, CYP17A1 and MTHFR genes are associated with blood pressure and hypertension in Chinese Hans. J Hypertens 2011;29:70–5. [DOI] [PubMed] [Google Scholar]
- [36].Hong KW, Go MJ, Jin HS, et al. Genetic variations in ATP2B1, CSK, ARSG and CSMD1 loci are related to blood pressure and/or hypertension in two Korean cohorts. J Hum Hypertens 2010;24:367–72. [DOI] [PubMed] [Google Scholar]
- [37].Qi Y, Zhao H, Wang Y, et al. Replication of the top 10 most significant polymorphisms from a large blood pressure genome-wide association study of northeastern Han Chinese East Asians. Hypertens Res 2014;37:134–8. [DOI] [PubMed] [Google Scholar]
- [38].Kato N, Takeuchi F, Tabara Y, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 2011;43:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miyaki K, Htun NC, Song Y, et al. The combined impact of 12 common variants on hypertension in Japanese men, considering GWAS results. J Hum Hypertens 2012;26:430–6. [DOI] [PubMed] [Google Scholar]
- [40].Lu X, Wang L, Lin X, et al. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet 2015;24:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kobayashi Y, Hirawa N, Tabara Y, et al. Mice lacking hypertension candidate gene ATP2B1 in vascular smooth muscle cells show significant blood pressure elevation. Hypertension 2012;59:854–60. [DOI] [PubMed] [Google Scholar]
- [42].Okuyama Y, Hirawa N, Fujita M, et al. The effects of anti-hypertensive drugs and the mechanism of hypertension in vascular smooth muscle cell-specific ATP2B1 knockout mice. Hypertens Res 2018;41:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang Y, Wilson C, Cartwright EJ, et al. Plasma membrane Ca [2+] -ATPase 1 is required for maintaining atrial Ca [2+] homeostasis and electrophysiological stability in the mouse. J Physiol 2017;595:7383–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rhee MY, Yang SJ, Oh SW, et al. Novel genetic variations associated with salt sensitivity in the Korean population. Hypertens Res 2011;34:606–11. [DOI] [PubMed] [Google Scholar]
- [45].Daily JW, Kim BC, Liu M, et al. People with the major alleles of ATP2B1 rs17249754 increases the risk of hypertension in high ratio of sodium and potassium, and low calcium intakes. J Hum Hypertens 2017;31:787–94. [DOI] [PubMed] [Google Scholar]
- [46].Xi B, Tang W, Wang Q. Polymorphism near the ATP2B1 gene is associated with hypertension risk in East Asians: a meta-analysis involving 15 909 cases and 18 529 controls. Blood Pressure 2012;21:134–8. [DOI] [PubMed] [Google Scholar]


